Abstract
This research aimed to compare the apparent viscosity and the degree of fragmentation/aggregation produced in dispersions of xanthan gum and chia mucilage during the gastrointestinal tract by using an in vitro digestion. Both soluble fibers exhibited pseudoplastic behavior, independent of the concentration and stage of digestion (oral, gastric or intestinal). The viscosity decreased from the oral to intestinal stage in all the concentrations, produced mainly by the “dilution effect” by the addition of digestive fluids. The particle size of xanthan gum increased drastically in the gastric stage mainly due to the decrease in pH, but at intestinal level returned to its original pattern, while particle size and pattern of mucilage during all the stages of digestion remained unchanged, maintaining its integrity. In general terms, since chia mucilage and xanthan gum maintain their viscosity and integrity through the gastrointestinal tract, they could be used as functional ingredients improving the functionality of foods.
1. Introduction
According to several authors and health organizations, diets high in dietary fiber have a higher incidence in the prevention of many major non-communicable diseases compared to diets lower in this component [1,2,3,4,5]. Many of the beneficial effects have been explained by their behavior at gastrointestinal level. According to EFSA (European Food Safety Authority), dietary fiber corresponds to non-digestible carbohydrates plus lignin, including all carbohydrate components occurring in foods that are non-digestible in the human small intestine and pass into the large intestine. Based on their solubility, dietary fiber can be classified into water soluble (pectins, gums, mucilages, etc.) and insoluble fractions (cellulose, lignin, etc.); both types have different molecular characteristics and physiological effects on the gastrointestinal tract [6]. Soluble dietary fiber (DSF) intake is generally associated with slow transit through the stomach and increasing of the small intestine transit time; this behavior is related with its ability to form viscous solutions [7]. In addition, soluble fibers are fermented by the colonic microbiota, releasing different levels of short chain fatty acids (SCFAs) and play a critical role in the composition and metabolic activity of the microbiome, which affects the intestinal health and ultimately the immune system and the body’s ability to resist some chronic diseases [8].
Chia seed has been described as a source of soluble fiber, its mucilage has the ability to retain large amounts of water and produce viscous dispersions, even at low concentrations [9]. On the other hand, xanthan gum is a commercial soluble fiber obtained by fermentation of Xanthomonas campestris, is soluble in cold water and in solution exhibits high pseudoplastic flow [10].
The objective of this study was to evaluate comparatively the viscosity and degree of aggregation/fragmentation changes produced during the in vitro digestion of mucilage from chia seeds and xanthan gum.
2. Materials and Methods
2.1. Materials
Chia seeds were provided by Benexia (Functional Products Trending S.A., Santiago, Chile); the crude mucilage was obtained from chia seeds by using the method proposed by Muñoz et al. (2012) and xanthan gum was purchased from Sigma-Aldrich [11]. To perform the in vitro digestion, the enzymes and reagents were purchased from Sigma-Aldrich and Merck. The comparisons among means were performed using one-way ANOVA (Analysis of Variance) and the significant differences were determined by the Tukey test (p < 0.05). All these tests were performed using the software, Statgraphics Centurion XV.I.
2.2. In Vitro Digestion
Suspensions of the two DSF at low, medium and high concentrations (0.3, 0.5 and 1.0% w/w) were subjected to in vitro digestion simulating the gastrointestinal conditions (oral, gastric and intestinal). To perform the experiments, the standardized static in vitro digestion protocol proposed by Minekus et al. (2014) was used [12]. The simulated gastrointestinal fluids such as salivary (SSF), gastric (SGF) and intestinal (SIF) and the enzymes were prepared according to the same protocol.
2.3. Steady Shear Flow Behavior
The apparent viscosity to each soluble fiber dispersion was determined without digestion as control and before and after each digestion stage by applying an increasing shear rate from 0.1 to 100 s−1 in triplicate using a Rotational Rheometer, RheolabQC (Anton Paar GmbH, Austria-Europe). The rheometer was equipped with a double gap concentric cylinder and a Peltier temperature plate set at 37 °C, simulating body temperature. The flow behavior index (n) and consistency index (k) values were obtained by fitting to the Power Law model (Equation (1)):
where η is the shear viscosity (Pa s), k is the consistency index (Pa s−1), γ is the shear rate (s−1) and n is the fluid behavior index (dimensionless).
η = kγ (n−1)
2.4. Determination of Degree of Aggregation/Fragmentation
The degree of aggregation/fragmentation was determined at each stage of in vitro digestion in terms of particle size distribution in six-fold, by laser light scattering with a Malvern Mastersizer 2000 (Malvern Instruments, Worcestershire, UK) software version 5.6, using water at 25 °C as solvent.
3. Results
3.1. Steady Shear Flow Behaviour
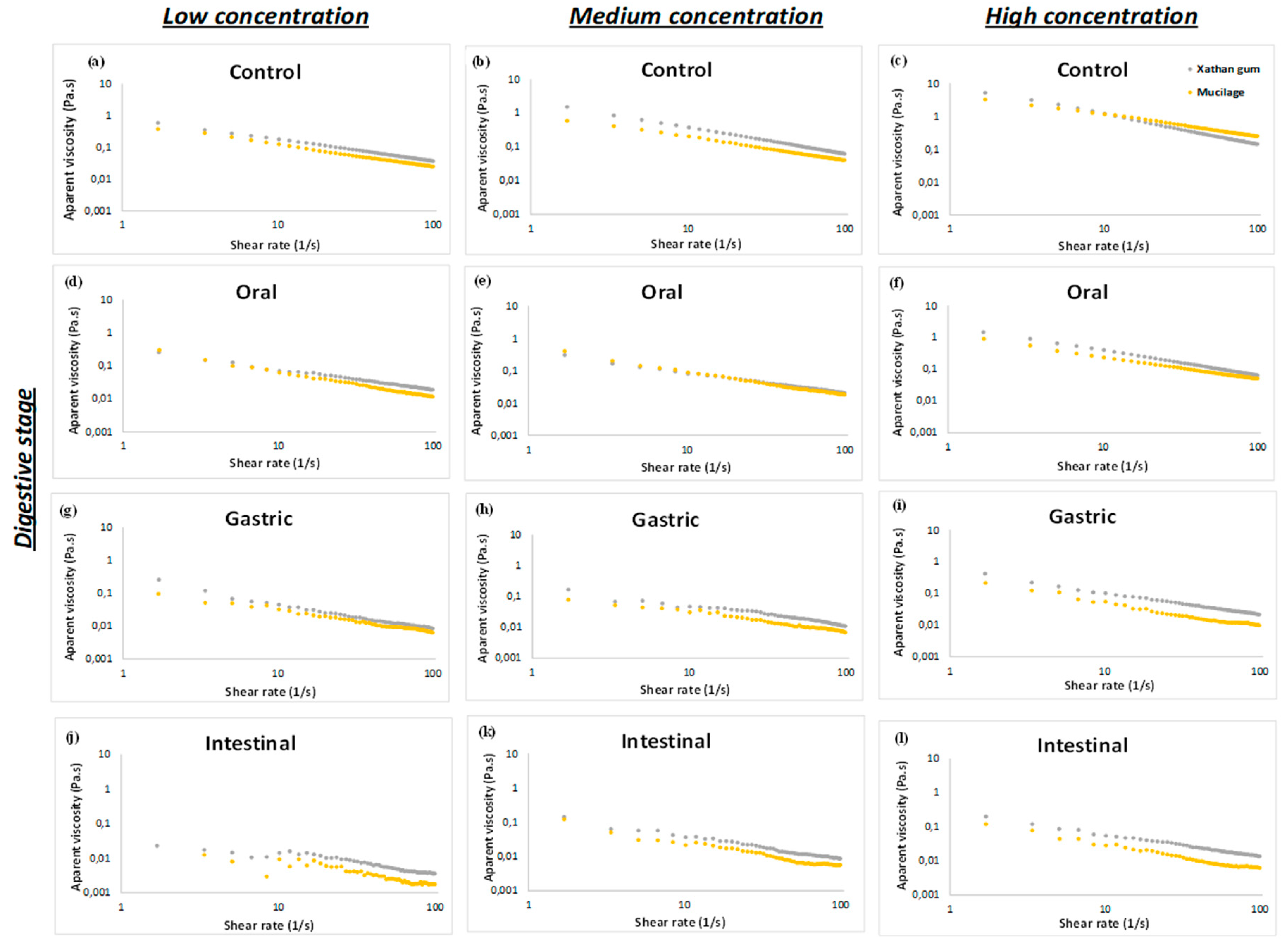
The steady flow behavior, consistency (k), and flow index (n) behavior of the mucilage and xanthan gum at low, medium, and high concentration, before and during each stage of in vitro digestion can be seen in Figure 1 and Table 1, respectively. All the dispersions show non-Newtonian behavior, with decreasing viscosity with increasing shear rate, also known as pseudoplasticity or shear-thinning behavior (Figure 1). The apparent viscosity to the samples without digestion (Figure 1a–c) show a directly proportional relationship with the concentration and k increase as concentration increased. Similar behavior was reported by Timilsena et al., (2015) where the rheological properties of the purified chia seed polysaccharide were evaluated [13]. Moreover, the apparent viscosity of mucilage and xanthan gum decreased from the oral to intestinal stage in all the concentrations (Figure 1d–l), caused mainly by the addition of digestive fluids (SSF, SGF and SIF) and was less affected by the pH changes and ionic strength. Similar behavior was previously observed by Lazaro et al. (2018) and Fabek et al. (2014) when different soluble fibers were subjected to in vitro digestion [14,15]. According to Vuksan et al. (2011), many of the beneficial physiological effects produced by the dietary fiber intake are associated with their capacity to hydrate and increase the viscosity of the human digesta. In this study, both DSF provided viscosity at gastrointestinal level, therefore, their use in food matrices would help to improve functionality [16].
Figure 1.
Apparent viscosity during in vitro digestion.

Table 1.
Power of Law parameters for xanthan gum and mucilage from chia seed during in vitro digestion.
3.2. Degree of Aggregation/Fragmentation
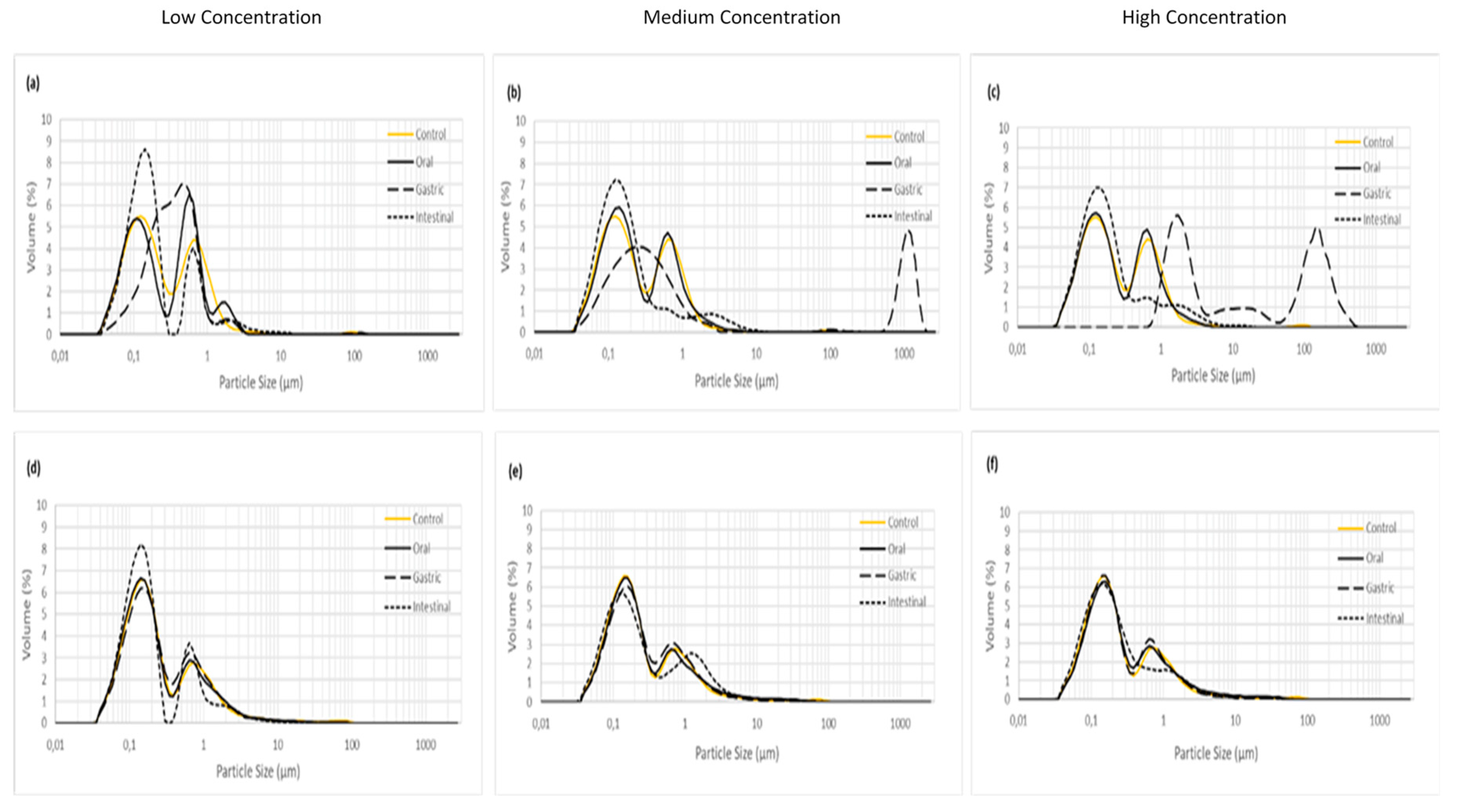
Figure 2 shows the distribution of the particle size of the mucilage and xanthan gum during the in vitro digestion. Figure 2a–c shows the changes in particle size of xanthan gum at the three concentrations. In this case, xanthan gum did not show differences in the bimodal distribution between control and oral stage for the three concentrations, but at gastric level the particle size distribution changed; the curve moves to the right which indicates increasing size.
Figure 2.
Degree of aggregation/fragmentation during in vitro digestion, (a–c) correspond to xanthan gum and (d–f) correspond to mucilage from chia seed.
This behavior has been previously explained as the decrease of the intermolecular electrostatic repulsion at low pH [10], which allows the expansion of the fiber chains. Finally, at intestinal level, the particle size of xanthan gum returned to the original size mainly due to the increase in pH, at the same time as the concentration increases the conformation becomes monomodal. On the other hand, the particle size and pattern at the three concentrations of mucilage during all the stages of digestion remained unchanged (Figure 2d–f). This behavior has not been previously reported and could indicate a better physiological response when the mucilage from chia seed is ingested. Furthermore, the gastric empty will be slower by using mucilage than xanthan gum and possibly could reduce the nutrient absorption through the intestinal mucosa [17].
4. Conclusions
In this study both soluble fibers, mucilage and xanthan gum, had the ability to retain viscosity through the gastrointestinal tract, which could indicate their ability to modulate certain physiological responses enhancing functionality when they are added into the food matrix. In addition, the aptitude of these fibers to maintain their structure suggests that they can be used to develop structured foods as a strategy to modulate the digestive process, delaying the gastric emptying.
Acknowledgments
This work was supported by the following grant: Ia ValSe-Food-CYTED (119RT0567); the National Fund for Scientific Development and Technological, Project FONDECYT 11150307, Chile and the Fundación para la Innovación Agraria PYT-2018-0261 FIA.
References
- FAO (Food and Agriculture Organization); WHO (World Health Organization). Report of a Joint FAO/WHO Expert Consultation, Diet, Nutrition and the Prevention of Chronic Disease; Technical Report Series; FAO; WHO: Geneva, Switzerland, 2003; No. 916 (TRS 916). [Google Scholar]
- Chawla, R.; Patil, G. Soluble Dietary Fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA J. 2010, 8. [Google Scholar] [CrossRef]
- Gidley, M.J. Hydrocolloids in the digestive tract and related health implications. Curr. Opin. Colloid Interface Sci. 2013, 18, 371–378. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2016, 57, 3543–3564. [Google Scholar] [CrossRef]
- Repin, N.; Cui, S.W.; Goff, H.D. Rheological behavior of dietary fibre in simulated small intestinal conditions. Food Hydrocoll. 2018, 76, 216–225. [Google Scholar] [CrossRef]
- Taghipoor, M.; Barles, G.; Georgelin, C.; Licois, J.; Lescoat, P. Digestion modeling in the small intestine: Impact of dietary fiber. Math. Biosci. 2014, 258, 101–112. [Google Scholar] [CrossRef]
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-Arribas, M.V.; Muñoz, L.A. Understanding the impact of chia seed mucilage on human gut microbiota by using the dynamic gastrointestinal model simgi®. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Bercea, M.; Morariu, S.; Dascalu, M. Some properties of xanthan gum in aqueous solutions: Effect of temperature and pH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Munoz, L.; Cobos, A.; Díaz, O.; Aguilera, J. Chia seeds: Microstructure, mucilage extraction and hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised staticin vitrodigestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.; Adhikari, R.; Kasapis, S.; Adhikari, B. Rheological and microstructural properties of the chia seed polysaccharide. Int. J. Boil. Macromol. 2015, 81, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, H.; Puente, L.; Zúñiga, M.C.; Muñoz, L.A. Assessment of rheological and microstructural changes of soluble fiber from chia seeds during an in vitro micro-digestion. LWT 2018, 95, 58–64. [Google Scholar] [CrossRef]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Vuksan, V.; Jenkins, A.L.; Rogovik, A.L.; Fairgrieve, C.D.; Jovanovski, E.; Leiter, L.A. Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br. J. Nutr. 2011, 106, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, G.M.; Kostlan, K.; Singh, R. Particle Size Distribution of Brown and White Rice during Gastric Digestion Measured by Image Analysis. J. Food Sci. 2013, 78, E1383–E1391. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).