Microencapsulation of Chia Seed Oil (Salvia hispanica L.) in Spray and Freeze-Dried Whey Protein Concentrate/Soy Protein Isolate/Gum Arabic (WPC/SPI/GA) Matrices †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPC/SPI/GA Dispersions
2.3. Zeta Potential and Turbidity Measurements
2.4. Differential Scanning Calorimetry of Dispersions
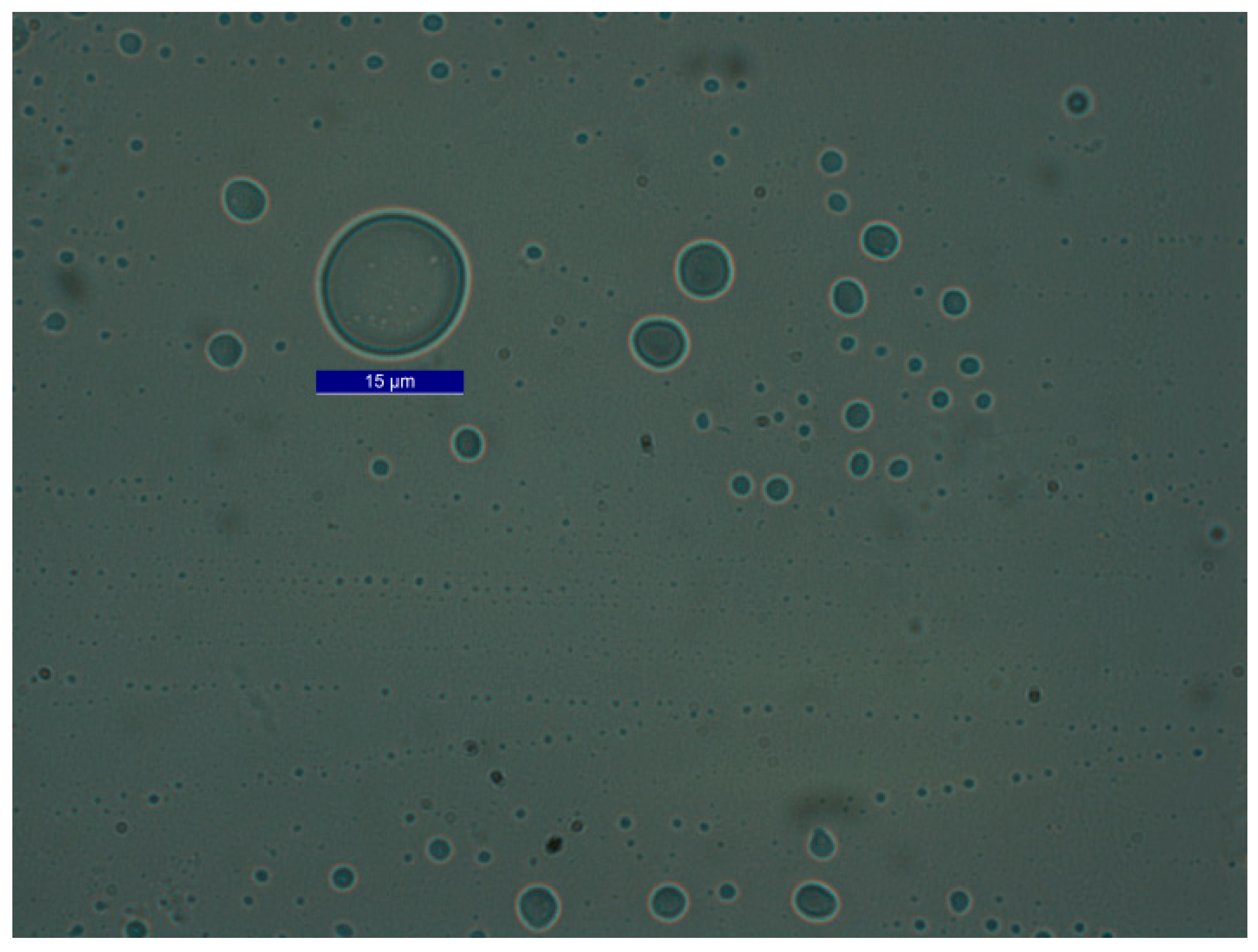
2.5. Emulsion Preparation and Characterization
2.6. Spray and Freeze-Drying Processes
2.7. Powder Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- González, A.; Martínez, M.L.; Paredes, A.; Leon, A.; Ribotta, P.D. Study of the preparation process and variation of wall components in chia (Salvia hispanica L.) oil microencapsulation. Powder Technol. 2016, 301, 868–875. [Google Scholar] [CrossRef]
- Timilsena, Y.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Microencapsulation of chia seed oil using chia seed protein isolate⿿chia seed gum complex coacervates. Int. J. Boil. Macromol. 2016, 91, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Digestion behaviour of chia seed oil encapsulated in chia seed protein-gum complex coacervates. Food Hydrocoll. 2017, 66, 71–81. [Google Scholar] [CrossRef]
- Anandharanakrishnan, C.; Ishwarya, S. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Chichester, West Sussex, UK, 2015; ISBN 978-1-118-86419-7. [Google Scholar]
- Burgos-Díaz, C.; Wandersleben, T.; Marqués, A.M.; Rubilar, M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr. Opin. Colloid Interface Sci. 2016, 25, 51–57. [Google Scholar] [CrossRef]
- Martínez, M.L.; Marín, M.A.; Faller, C.M.S.; Revol, J.; Penci, M.C.; Ribotta, P.D. Chia (Salvia hispanica L.) oil extraction: Study of processing parameters. LWT Food Sci. Technol. 2012, 47, 78–82. [Google Scholar] [CrossRef]
- Canalis, M.S.B.; Valentinuzzi, M.C.; Acosta, R.H.; Leon, A.E.; Ribotta, P.D. Effects of Fat and Sugar on Dough and Biscuit Behaviours and their Relationship to Proton Mobility Characterized by TD-NMR. Food Bioprocess Technol. 2018, 11, 953–965. [Google Scholar] [CrossRef]
- Us-Medina, U.; Julio, L.; Segura-Campos, M.R.; Ixtaina, V.Y.; Tomás, M.C. Development and characterization of spray-dried chia oil microcapsules using by-products from chia as wall material. Powder Technol. 2018, 334, 1–8. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.A. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, H.; Wang, J.; Sun, B. Effect of processing conditions on the morphology and oxidative stability of lipid microcapsules during complex coacervation. Food Hydrocoll. 2019, 87, 637–643. [Google Scholar] [CrossRef]
- Rodriguez, E.S.; Julio, L.; Henning, C.; Diehl, B.W.; Tomás, M.C.; Ixtaina, V.Y. Effect of natural antioxidants on the physicochemical properties and stability of freeze-dried microencapsulated chia seed oil. J. Sci. Food Agric. 2018, 99, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Anandharamakrishnan, C.; Rielly, C.D.; Stapley, A.G. Effects of Process Variables on the Denaturation of Whey Proteins during Spray Drying. Dry. Technol. 2007, 25, 799–807. [Google Scholar] [CrossRef]

| ΔH J/g | T Onset °C | T Peak °C | T Endset °C | |
|---|---|---|---|---|
| HAP-treated WPC A | 1.10–1.30 | 71.30–72.40 | 76.50–77.70 | 81.30–81.50 |
| WPC/SPI/GA mixture | 0.74–0.96 | 71.60–72.60 | 77.40–78.00 | 84.00–84.30 |
| WPC/SPI/GA mixture-c.c B | 0.53–0.67 | 81.30–81.50 | 85.90–86.00 | 91.30–92.60 |
| MC % Wet Basis | aw | AI | EE % Dry Basis | WI | YI | OSI h | |
|---|---|---|---|---|---|---|---|
| FD A | 3.70–4.30 | 0.216–0.220 | 17.00–39.70 | 65.60–77.70 | 18.70–31.40 | 30.30–36.70 | 6.45–12.04 |
| SD B | 2.91–4.01 | 0.214–0.257 | 0.14–0.16 | 71.30–80.07 | 61.30–64.10 | 15.20–16.22 | 12.05–15.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordón, M.G.; Barrera, G.N.; Penci, M.C.; Bori, A.; Caballero, V.; Ribotta, P.; Martínez, M.L. Microencapsulation of Chia Seed Oil (Salvia hispanica L.) in Spray and Freeze-Dried Whey Protein Concentrate/Soy Protein Isolate/Gum Arabic (WPC/SPI/GA) Matrices. Proceedings 2020, 53, 22. https://doi.org/10.3390/proceedings2020053022
Bordón MG, Barrera GN, Penci MC, Bori A, Caballero V, Ribotta P, Martínez ML. Microencapsulation of Chia Seed Oil (Salvia hispanica L.) in Spray and Freeze-Dried Whey Protein Concentrate/Soy Protein Isolate/Gum Arabic (WPC/SPI/GA) Matrices. Proceedings. 2020; 53(1):22. https://doi.org/10.3390/proceedings2020053022
Chicago/Turabian StyleBordón, María Gabriela, Gabriela Noel Barrera, Maria C. Penci, Andrea Bori, Victoria Caballero, Pablo Ribotta, and Marcela Lilian Martínez. 2020. "Microencapsulation of Chia Seed Oil (Salvia hispanica L.) in Spray and Freeze-Dried Whey Protein Concentrate/Soy Protein Isolate/Gum Arabic (WPC/SPI/GA) Matrices" Proceedings 53, no. 1: 22. https://doi.org/10.3390/proceedings2020053022
APA StyleBordón, M. G., Barrera, G. N., Penci, M. C., Bori, A., Caballero, V., Ribotta, P., & Martínez, M. L. (2020). Microencapsulation of Chia Seed Oil (Salvia hispanica L.) in Spray and Freeze-Dried Whey Protein Concentrate/Soy Protein Isolate/Gum Arabic (WPC/SPI/GA) Matrices. Proceedings, 53(1), 22. https://doi.org/10.3390/proceedings2020053022





