Development and Characterization of Functional O/W Emulsions with Chia Seed (Salvia hispanica L.) by-Products †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Preparation of Emulsions
2.2.2. Droplet Size
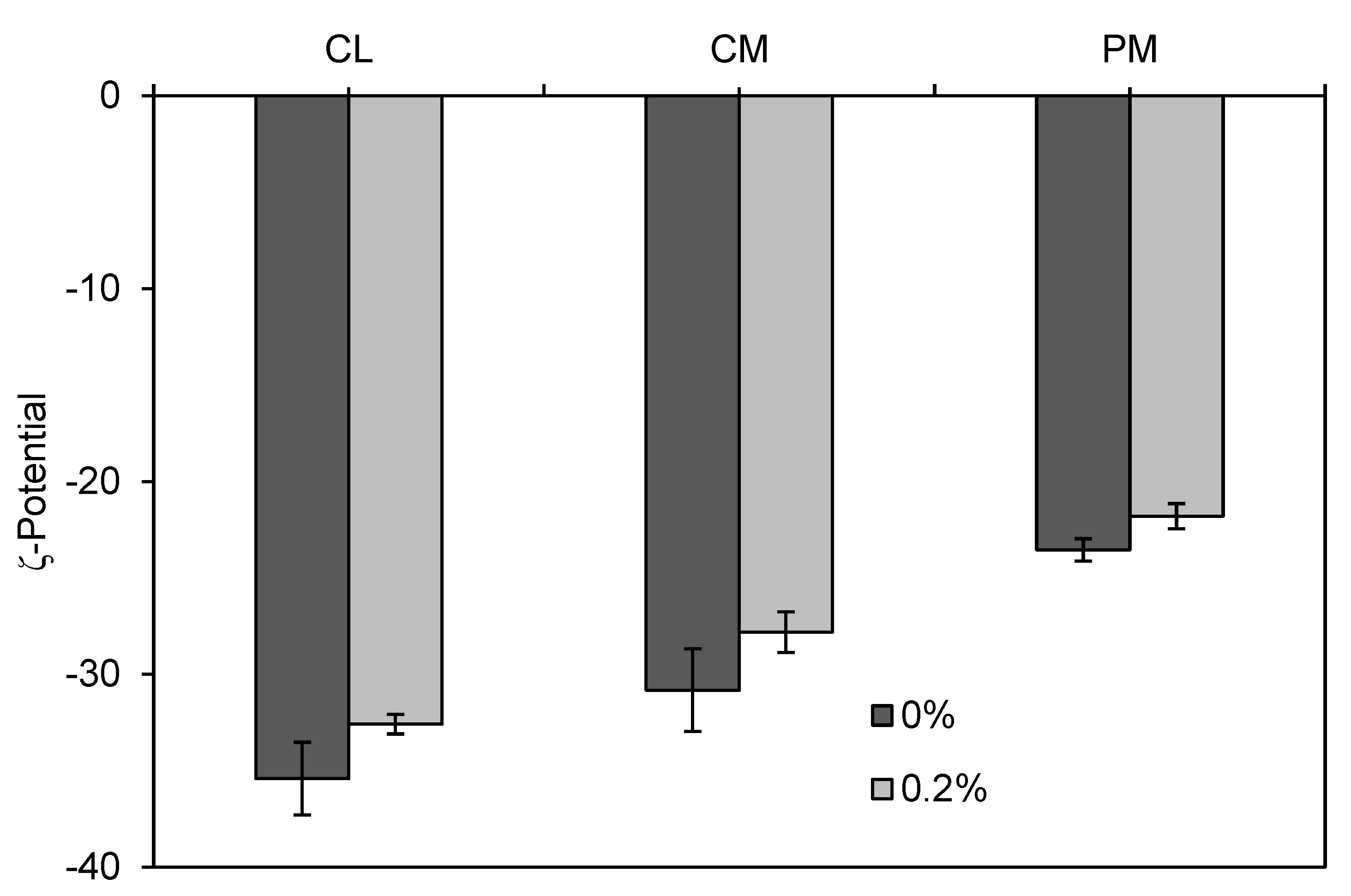
2.2.3. ζ-potential
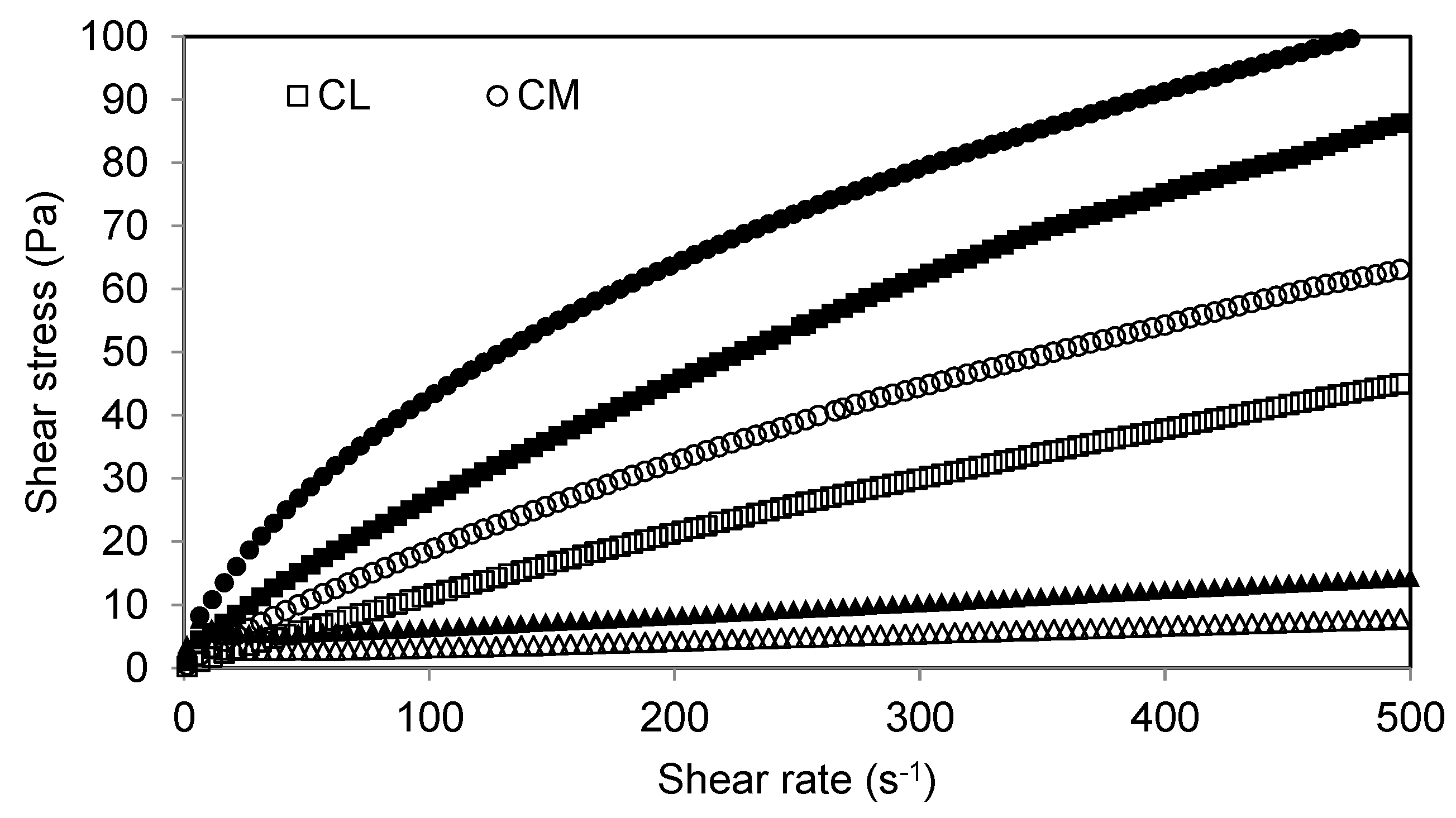
2.2.4. Rheological Properties
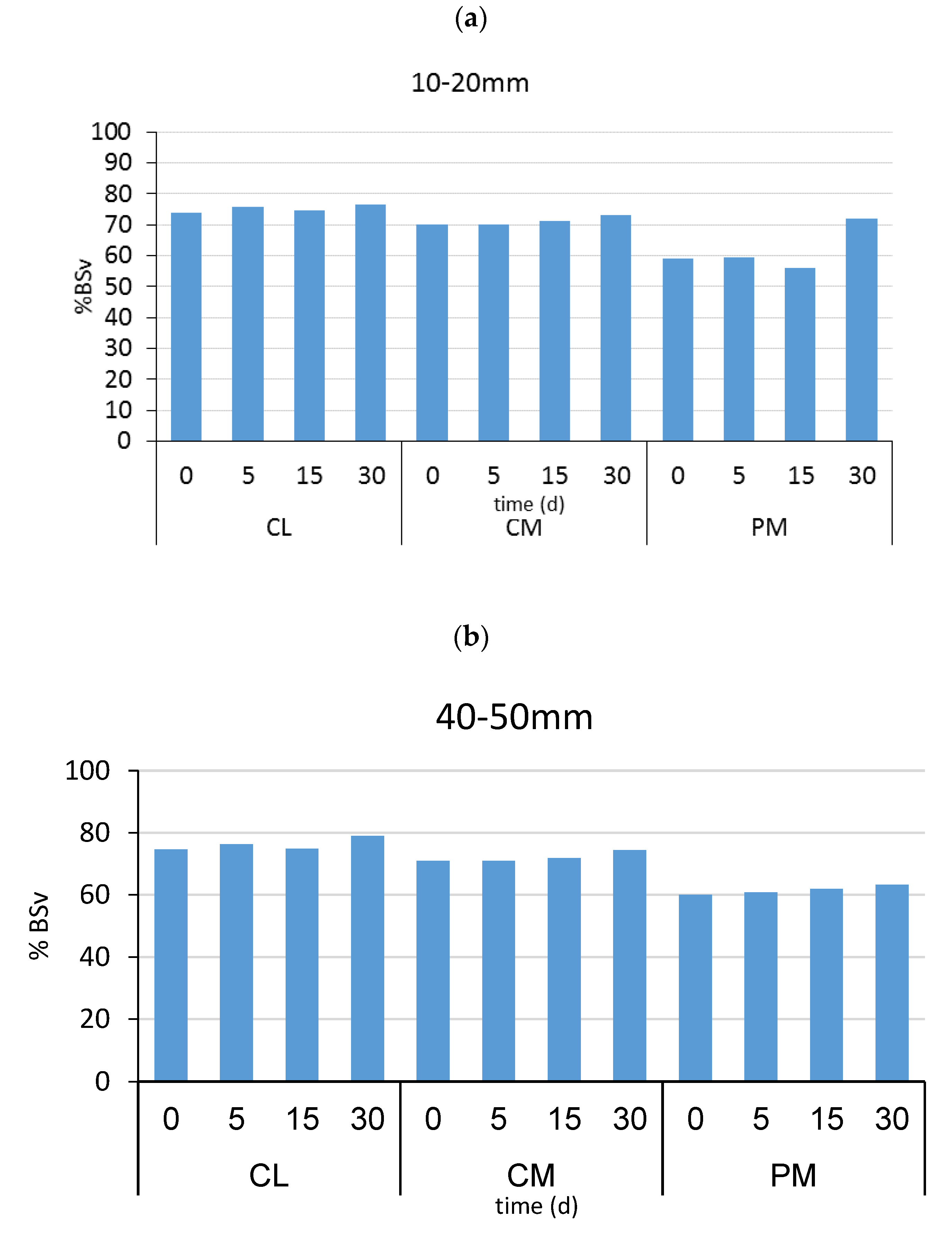
2.2.5. Emulsion Stability
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Sandoval-Oliveros, M.R.; Paredes-López, O. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem. 2012, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ovando, A.; Betancur-Ancona, D.; Chel-Guerrero, L. Physicochemical and functional properties of a protein-rich fraction produced by dry fractionation of chia seeds (Salvia hispanica L.). CyTA-J. Food 2012, 11, 75–80. [Google Scholar] [CrossRef]
- Mann, J.I.; Cummings, J.H. Possible implications for health of the different definitions of dietary fibre. Nutr. Metab. Cardiovas. 2009, 19, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Julio, L.M.; Ixtaina, V.Y.; Fernández, M.A.; Sánchez Torres, R.M.; Wagner, J.R.; Nolasco, S.M.; Tomás, M.C. Chia seed oil-in-water emulsions as potential delivery systems of ω-3 fatty acids. J. Food Eng. 2015, 162, 48–55. [Google Scholar] [CrossRef]
- Capitani, M.; Spotorno, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT-Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- Pan, L.; Tomás, M.; Añon, M. Effect of sunflower lecithins on the stability of water-in-oil and oil-in-water emulsions. J. Surfactants Deterg. 2002, 5, 135–143. [Google Scholar] [CrossRef]
- Day, L.; Xu, M.; Hoobin, P.; Burgar, I.; Augustin, M. Characterisation of fish oil emulsions stabilised by sodium caseinate. Food Chem. 2007, 105, 469–479. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0849320232. [Google Scholar]
| Sample | Chia Oil | Aqueous Phase Composition % wt/wt | ||||
|---|---|---|---|---|---|---|
| Chia Protein-Rich Fraction | Sodium Caseinate | Lactose | Malodextrin | Chia Mucilage | ||
| CL | 10 | - | 10 | 10 | - | - |
| CM | 10 | - | 10 | - | 10 | - |
| PM | 10 | 10 | - | - | 10 | - |
| CL + Mg | 10 | - | 10 | 10 | - | 0.2 |
| CM + Mg | 10 | - | 10 | - | 10 | 0.2 |
| PM + Mg | 10 | 10 | - | - | 10 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julio, L.M.; Ixtaina, V.Y.; Tomás, M.C. Development and Characterization of Functional O/W Emulsions with Chia Seed (Salvia hispanica L.) by-Products. Proceedings 2020, 53, 20. https://doi.org/10.3390/proceedings2020053020
Julio LM, Ixtaina VY, Tomás MC. Development and Characterization of Functional O/W Emulsions with Chia Seed (Salvia hispanica L.) by-Products. Proceedings. 2020; 53(1):20. https://doi.org/10.3390/proceedings2020053020
Chicago/Turabian StyleJulio, Luciana M., Vanesa Y. Ixtaina, and Mabel C. Tomás. 2020. "Development and Characterization of Functional O/W Emulsions with Chia Seed (Salvia hispanica L.) by-Products" Proceedings 53, no. 1: 20. https://doi.org/10.3390/proceedings2020053020
APA StyleJulio, L. M., Ixtaina, V. Y., & Tomás, M. C. (2020). Development and Characterization of Functional O/W Emulsions with Chia Seed (Salvia hispanica L.) by-Products. Proceedings, 53(1), 20. https://doi.org/10.3390/proceedings2020053020





