Abstract
This research aimed to explore the influence of climate factors, especially in the three weeks prior to harvest, on the reaction of key phytonutrients in industrial tomatoes used for juice thermal processing and their stability. The cultivation was performed in two areas with differing climatic conditions. In the region with higher temperatures and rainfall, the levels and stability of carotenoids were lower compared to the area characterized by warm temperatures and minimal rainfall during both the growth and harvest phases of the tomatoes. The extraction of cold-break (CBE) tomatoes from relatively cool and wet environments resulted in a loss of total carotenoids, particularly lycopene, amounting to 66% and 58% of the initial raw tomato content in 2018 and 2019, respectively, while a markedly reduced loss of 10% was observed after the CBE of tomatoes from the warmer and drier region in both years (36% and 35%). In contrast, hot-break extraction (HBE) demonstrated a higher stability of lycopene compared to CBE, with losses of 43% and 53% in 2018 and 2019, respectively. Additionally, the stability of lycopene in HBE did not show significant differences between the cultivation sites. Climatic conditions influenced the accumulation of geometrical isomers and oxidized forms of lycopene and β-carotene, especially in tomatoes grown in areas with higher rainfall and lower temperatures. A similar trend in response was noted for β-carotene, lutein, phytoene, and phytofluene, as well as total and individual tocopherols. Regarding vitamin C, the environmental factors had no meaningful impact on the vitamin content in tomato fruits; however, its stability during processing, especially with hot-break extraction, was considerably influenced by the climatic conditions of the cultivation site, with p values ranging from <0.01 to <0.001 across different products in various years. The content and stability of phytonutrients in pomace, the by-product from tomato juice processing, were also assessed. In conclusion, tomato fruits and processed products that boast high phytonutrient levels and stability during thermal processing can be achieved through cultivation in conditions of low rainfall and relatively high temperatures, particularly in the three weeks leading up to harvest.
1. Introduction
Tomatoes (Solanum lycopersicon L.) and their products are essential components of contemporary diets, providing significant amounts of vitamins, minerals, and antioxidants while also being comparatively easy to obtain. They can be consumed in various forms, including fresh, cooked, concentrated, and dried. Ripe tomatoes are rich in valuable phytochemicals, enhancing their nutritional significance and their role as functional foods or food ingredients. Of particular interest among these phytochemicals are carotenoids, which have been linked to a decrease in the likelihood of several cancer types [1,2] and neurodegenerative disorders [3]. Additionally, polyphenols and vitamin C are vital in combatting oxidative stress [4], while the alkaloids found in tomatoes exhibit antimicrobial properties [5]. Recent research has increasingly focused on the advantageous effects of these phytonutrients in both humans and animals. Juice processing stands out as the most recognized and economically vital method for preserving tomatoes, surpassing the need for fresh consumption and exportation. In situations where fresh tomatoes are unavailable, such as during winter, tomato juice is commonly used to fill the nutritional gap left by the absence of bioactive nutrients like lycopene, polyphenols, tocopherols, and vitamin C. Thus, post-harvest processing must be carefully selected and managed to create juices that retain their quality and nutritional value. Several factors likely influence the levels of phytonutrients in industrial tomatoes, as well as in other vegetables, including variety, harvest, the ripening stage, agronomic practices, geographic location, and environmental conditions [6,7]. The nutritional value of tomato fruits can be maximized through the implementation of appropriate cultural and agricultural practices, such as choosing the right site for cultivation [8].
The technology utilized in tomato processing is another element that can affect the quality characteristics and nutritional properties of the end products. It is important to examine how the abiotic factors of the growing season interact with the technological parameters used during processing, and to select an appropriate technology that minimizes or reduces the loss of quality attributes and nutritional features. To create tomato products of exceptional quality, the raw materials should already possess a high content of quality components. Generally, cold-break extraction (CBE) performed at 50–60 °C and hot-break extraction (HBE) conducted at 90 °C are the most employed methods for producing tomato juice [9].
The heat treatment applied in cold-break extraction (CBE) and hot-break extraction (HBE) serves multiple purposes. On the one hand, it aims to deactivate microorganisms and enzymes, while, on the other, it seeks to soften the tissue to achieve the desired sensory qualities [10]. The cold-break extraction method results in better color, stock, and certain organoleptic features (taste, flavor), but it also incurs higher waste loss. In contrast, the HBE technique typically yields improved results in terms of yield, microbial condition, enzyme deactivation, and removal of absorbed gases; however, it can lead to a greater loss of quality characteristics like color, flavor, and nutritional content [11]. In the domestic canned food sector, heat treatment is commonly applied at temperatures ranging from 80 to 90 °C [12]. Through processing, the bioactive compounds mentioned earlier undergo significant changes because of heat treatments. To monitor the compositional shifts in phytochemicals, it is important to utilize an analytical protocol that allows for efficient and accurate separation and detection of individual compounds and their isomers and derivatives. In Hungary, industrial tomatoes are grown in various regions that experience different climatic conditions [13].
The abiotic factors that are likely to affect quality components in tomatoes and determine the stability of this vegetable during post-harvest and processing include temperature, sunlight duration, and rainfall. Another research group from our university examined the biological and physiological characteristics, as well as the response to microbial inoculation in the soil, of two industrial varieties, one of which is the focus of our current study [14,15].
The primary aims of this investigation were to assess how climate variables at the cultivation site before harvest influence the content and stability of carotenoids, tocopherols, and vitamin C during postharvest thermal processing of tomato juice using effective and reproducible liquid chromatographic methods.
2. Materials and Methods
2.1. Chemicals Used in the Determinations
All analytical-grade chemicals and HPLC-grade organic solvents were sourced from Merck Group Ltd. (Budapest, Hungary). Standard compounds including lycopene, lutein, β-carotene, 8-β-apo-carotenal, ascorbic acid, α-tocopherol (α-toc), α-tocopherol acetate (α-tocAc), α-tocopherol palmitate (α-toc-Es), β-tocopherol (β-toc), and γ-tocopherol (γ-toc) were obtained from Sigma-Aldrich through Merck, Budapest, Hungary. The authentic standard for α-tocopherol hydroquinone (α-tocHQ) was freshly prepared following the method outlined in [16].
2.2. Cultivation of Tomato
The outdoor cultivation of the industrial tomato (Solanum lycopersicon L. var. UG812 J hybrid, supplied by Orosco Ltd., United Genetics Seeds Co., Hollister, CA, USA) took place in the years 2018 and 2019 across two experimental farms belonging to the Institute of Horticulture at the Hungarian University of Agricultural and Life Sciences. These two locations exhibit differing climatic conditions. The first location, Szárítópuszta in Gödöllő, is distinguished by high levels of precipitation coupled with low temperatures during the vegetable growing season. In contrast, the second location, Szarvas in Southeast Hungary, is characterized by lower precipitation and higher temperatures throughout the vegetable cultivation period (as shown in Table 1). The soil composition at both sites is sandy clay loam, with a distribution of 34% clay, 29% sand, and 33% silt, along with a humus content of 3% and a pH level of approximately 6.68. The Arany’s binding value (Ka) ranges from 28 to 42, indicating medium water-holding capacity, as well as adequate absorption and drainage capabilities. Irrigation was managed through a drip system during the growing season, according to initial climate forecasts provided by the local meteorological authorities regarding anticipated air temperatures and precipitation.

Table 1.
Meteorological data of Location-1 (Szárítópuszta) and Location-2 (Szarvas) during the growing seasons of 2018 and 2019.
The planting arrangement comprised twin rows of 120 cm × 40 cm, each row measuring 25 m in length, with 20 cm spacing maintained between the tomatoes for both years and sites. Information regarding the provision of water and nutrients, along with other agronomic requirements, can be referenced in [14], where yield, physiological, and biological characteristics were assessed. As part of an extensive research initiative, we concentrated on the post-harvest technology for tomato fruits obtained from plants grown in standard tomato farming conditions, without any supplementary treatments. Tomatoes designated for processing were picked at the final developmental stage during late August and early September when mature red fruits made up the majority (90%) of the plants. Any immature, infected, or damaged fruits were eliminated. The collected tomatoes were divided into three replicates from each site and across both growing seasons.
2.3. Thermal Extraction of Tomato Juice
Fully mature tomatoes with a rich red hue were freshly picked from no fewer than 40 plants, with each extraction being replicated three times. The extraction of juice from tomatoes was conducted at the Institute of Food Science and Technology at the Hungarian University of Agricultural and Life Sciences in Budapest, Hungary. The processing steps involved washing and shredding, followed by cold-break and hot-break extraction (CBE; HBE) and pasteurization. For the hot-break extraction, the shredded tomato mixture was heated to 90 °C for a duration of 15 min, whereas the cold-break extraction was conducted at 60 °C for 30 min using stainless-steel open cookers. The pomace samples, which contained skins and seeds from both CBE and HBE, were separated using rotary roller sieves. The dry content in the pomace varied, measuring 32% for CBE and 36% for HBE, which was assessed by evaporating the water at 105 °C for 24 h until a constant weight was achieved. The pomace samples were then packaged in nylon bags under vacuum and stored at −20 °C until they were analyzed chemically. The juices were placed in plastic bottles, pasteurized at 100 °C for 15 min, and stored at −20 °C if they were not analyzed immediately. The raw tomato fruit (control) was processed in a meat mincer to prevent foaming and minimize oxidation. The homogenate was kept at −20 °C until the analysis of carotenoids and tocopherols. To analyze vitamin C, a part of the freshly harvested tomato was taken and cut into small pieces, which were thoroughly mixed and subjected to immediate extraction and HPLC analysis of the vitamin.
2.4. Analysis of Carotenoids, Tocopherols, and Vitamin C
2.4.1. Extraction of Carotenoids and Tocopherols
To obtain the fat-soluble components, 5 g of whole tomato or pomace and 10 g of juice were placed in a mortar crucible and ground with the incorporation of 1 g of ascorbic acid and quartz sand. Twenty milliliters of methanol were added to the macerate to facilitate water binding. The methanol fraction was then transferred into a 100 mL Erlenmeyer flask equipped with a stopper. The remaining solids were further crushed and extracted by progressively adding 50 mL of a 1:6 mixture of methanol and 1,2-dichloroethane. This extract was combined with the methanol fraction. One milliliter of water was included to enhance pigment solubility in the less polar solvent, aiding in the separation of the two phases. After mechanically shaking for 15 min, the two phases were separated in a separatory funnel. The lower phase, which contained pigments dissolved in the less polar solvent, was dried using anhydrous sodium sulfate and transferred to a round-bottom flask. The solvent was then evaporated under vacuum at 40 °C until dry using a vacuum-controlled evaporator (Ingots RVO-400, INGOS Ltd., Prague, Czech Republic). The residues were then re-dissolved in 10 mL of HPLC-grade acetone for injections onto the HPLC column for carotenoid determination. For tocopherol analysis via HPLC, the residues were re-dissolved in 5 mL of a solvent mixture composed of 55:35:10 isopropanol–acetonitrile–methanol, followed by the addition of 5 mL of methanol to achieve a clear extract.
2.4.2. Extraction of Vitamin C
For ascorbic acid determination, freshly picked tomato fruits were chopped into small pieces using a stainless-steel knife, and 10 g were immediately extracted with a 3% metaphosphoric acid solution. In the case of juices and by-products (pomaces), 5–10 g from each were taken right after processing for analysis. The samples were ground in a crucible mortar along with quartz sand, gradually adding 30–50 mL of a 3% metaphosphoric acid solution. The mixture was quantitatively moved to a stopper-equipped Erlenmeyer flask and shaken for 15 min. The supernatant was then filtered using Hahnemüehle DF 400-125-type filter paper. The filtrate underwent further purification by being passed through a Whatman 0.22 µm cellulose acetate syringe filter before being injected onto the HPLC column [17].
2.4.3. HPLC Determinations
The HPLC analyses were performed using Hitachi Chromaster HPLC equipment, which included a Model 5110 Pump, Model 5430 Diode Array detector, Model 5440 Fluorescence detector, and Model 5210 autosampler. EZChrom Elite software (OpenLab version 1.2) was utilized for separation and data analysis. Carotenoids were separated on a core C-30, 2.6 µ, 150 × 4.6 mm column (Accucore from Thermo Scientific, Greater Mumbai, MA, USA) employing a gradient elution of Tert-butyl-methyl ether (TBME) (A) in methanol with 2% water (B) following a recently established protocol [18]. The gradient elution commenced with 100% B, shifted to 30% A in B over 25 min, maintained isocratic conditions for 5 min, and transitioned to 100% B within 5 min. The eluted carotenoid compounds were measured by DAD across a wavelength range of 190 to 600 nm.
Tocopherols were separated using a Nucleodur C18, 100 A°, 3 µ, 250 × 4.6 mm column (Macherey Nagel, Dürer, Germany) with a gradient elution involving water (A), methanol (B), and a mixture composed of 55:35:10 isopropanol–acetonitrile–methanol (C), starting with 8% A in B and transitioning to 100% B in 3 min, then to 10% B in C over 25 min, and finally returning to 8% A in B in 5 min [19]. The separated tocopherols were identified using the fluorescent detector set to EX: 295 nm and EM: 325 nm.
The identification of carotenoids relied on comparing retention times and spectral properties with those of known standards like lutein, β-carotene, and lycopene. When standard materials were unavailable, the compounds were identified through their mass spectra obtained via LC-MS/MS, spectral attributes, and retention behavior [20]. The quantitative assessment of carotenoids utilized β-8-apocarotenal as an internal standard that was added to the samples. For quantification, the area of each compound was integrated at its peak absorbance wavelength. In the case of tocopherols, identification was achieved using external standards for the various tocopherol analogs.
Vitamin C (L-ascorbic acid) was separated using a Nataulis aqua column (Machery Nagel, Dürer, Germany), 3 µ, 150 × 4.6 mm, with a gradient elution of acetonitrile (A) and 0.01 M KH2PO4 (B). The separation process began with 2% A in B, shifted to 30% A in B over 15 min, maintained an isocratic state for 5 min, and concluded with a return to 2% A in B over 5 min. The separated compounds were detected by DAD within the range of 190 to 400 nm. The identification and quantification of L-ascorbic acid were performed using a calibration curve created from standard solutions. Under the specified conditions, L-ascorbic acid exhibited an absorption maximum at 262 nm, where the peak area was integrated.
2.5. Statistical Analysis
For the statistical evaluation, we utilized IBM SPSS statistics V31 with results presented as mean value ± standard deviation. We employed repeated measures ANOVA for climate 1 and climate 2, CBE, and HBE, followed by post hoc analysis (Fisher’s test) for an in-depth comparison of measurement data among replicate samples for each tomato product.
3. Results and Discussion
3.1. Content and Stability of Carotenoids
The HPLC method utilized offered outstanding separation of the primary carotenoids along with their geometric isomers and derivatives within a span of 35 min (Figure 1). The profile features five geometric isomers of lycopene, two isomers of β-carotene, and various mono- and di-epoxides of carotenoids. Furthermore, besides the two dominant carotenoids, ɣ-carotene, neurosporene, lycoxanthin, ʆ-carotene, and the less detectable phytoene and phytofluene were effectively separated and identified in all the tomato products analyzed. The main artifacts of lycopene and β-carotene identified were the 9- and 13-cis-isomers and their oxidized forms. All-trans lycopene constituted 85–87% of the overall carotenoids, coinciding with the tomatoes that are high in lycopene and commonly used in tomato industry This study concentrated on both the total carotenoids and the key individual compounds connected to quality, specifically all-trans lycopene and its principal derivatives, as well as β-carotene, which serves as the primary provitamin A carotenoid.

Figure 1.
HPLC profile of tomato carotenoids on C-30, core, 3 µ, 150 × 4.6 mm column and gradient elution of TBME in methanol containing 2% water. Peak identification; 1: lutein; 2,3: β-carotene di-epoxides; 4: β-carotene mono-epoxides; 5,6: Lycopene diepoxides; 7: z-dimethoxy lycopene; 8: Lycopene monoepoxide; 9: lycoxanthin; 10: z-β-carotene; 11: β-carotene; 12: 15-z-lycopene; 13: neurosporene; 14: z-lycopene; 15: γ-carotene; 16: 9-z-lycopene; 17: all-trans-lycopene.
The extraction and sample preparation of carotenoids were conducted in conditions that minimize the formation of artifacts (utilizing brown glassware, including antioxidants, and employing vacuum evaporation). The detection of cis-isomers and oxygen-containing derivatives in fresh raw tomatoes indicates that these compounds are produced de novo during fruit ripening through enzyme-driven pathways, which involve isomerase, hydroxylase, and dioxygenase [21,22]. The factors likely influencing the content and stability of carotenoids and their derivatives after tomato harvest may include the levels of essential nutrients in the growing media [23], as well as the effects of thermal processing and improper storage [24,25].
The total carotenoid concentration in the industrial variety utilized in this study ranged from 3500 to 4800 µg g−1 dry matter (equivalent to 175−230 µg g−1 fresh weight) across two distinct locations and seasons. This level falls within the range observed in various industrial varieties [26,27], exceeding the 75−147 µg g−1 fresh weight reported by Temitope et al. [28], but significantly lower than the 3310 µg g−1 fresh weight indicated by Mayeaux et al. [29].
Across both growing seasons (Figure 2), the carotenoid content diminished in the dry matter of juice derived from CBE and HBE due to the removal of seeds and skins (including adhered pulp), which represent the largest share of the dry matter in tomato fruits. The peel and pulp have been noted to contain the highest concentrations of carotenoids, particularly lycopene [30,31]. Vinha et al. [32] indicated that peeling results in a 60 to 80% reduction in lycopene. In 2018, the decline in carotenoids in juice from CBE for tomatoes from Location-1 was 67%, compared to 35% for those from Location-2, in relation to the initial levels in the raw materials. A comparable percentage of decline was observed in 2019 (56% versus 32%). The significant drop in carotenoids with CBE, particularly for tomatoes from Location-1 in 2018, is likely attributed to various biochemical factors influenced by climatic variables, in addition to the removal of peels and seeds.
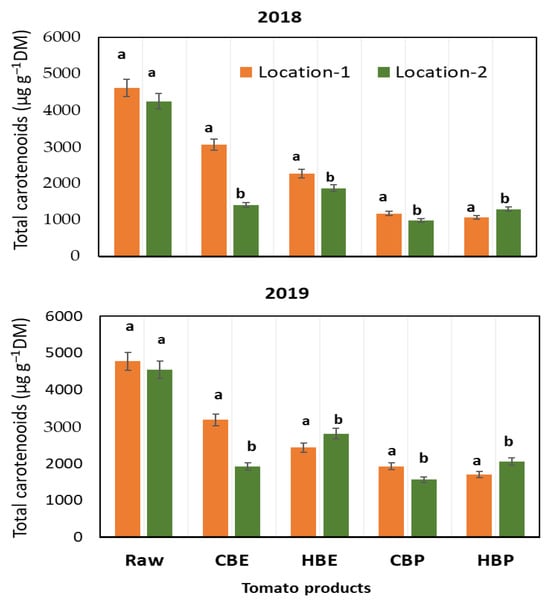
Figure 2.
Content (µg g−1 dm) of total carotenoids in different juice and pomace products of tomato cultivated in 2018 and 2019. The values of two locations with the same letter are statistically insignificant (p > 0.05). Raw: raw tomato; CBE: cold-break extraction; HBC: hot-break extraction; CBP: cold-break pomace; HBP: hot-break pomace.
The reduction in total carotenoids attributed to HBE was 57% for tomatoes from Location-1, and 51% for those from Location-2 in 2018, whereas a smaller loss was recorded in 2019, with no notable difference observed between the two locations regarding the percentage of carotenoid loss (47% and 48%). These findings suggest that cultivation under elevated temperatures and low rainfall, particularly three weeks prior to harvest, enhances the stability of carotenoids during gentle thermal processing such as CBE juice. Additionally, seasonal climate fluctuations may slightly affect the response of carotenoids under mild processing conditions.
In the HBE method, while the process at high temperatures led to a greater retention of carotenoids, especially in 2019, compared to that in CBE juice with p < 0.05, the percentage loss of carotenoids, in relation to the initial content, was marginally higher in juice from tomatoes cultivated under elevated air temperatures and low rainfall, compared to those from less severe climate conditions (57% versus 51%). Notably, the percentage loss of carotenoids in HBE from Location-2 is significantly greater (p < 0.01) than the % loss observed in CBC across both years of cultivation (51% and 48% versus 36% and 35%). It should be noted that the percentage loss of carotenoids in HBE can be influenced by various factors. On one hand, the high-temperature treatment can deactivate certain biochemical agents that degrade carotenoids [33] and help detach the bound carotenoids from the peels, allowing them to dissolve in the juice [34]; on the other hand, thermal degradation of carotenoids is anticipated concurrently. Thus, the overall variation in ca rotenoids in HBE results from the interplay of these chemical and physical influences.
The by-product of juice processing is the pomace, which consists of seeds and peels of tomato after juice extraction. The carotenoid content of such a by-product depends on the amount of tomato pulp adhered to the fruit skin. The content of carotenoids in the pomace is also influenced by the severity of heating in the extraction technology and centrifugation applied to separate the pomace from the juice. Like juices, as a function of climate variation, the content of carotenoids in pomaces was significantly higher (p < 0.05) in 2019, the relatively wet and cool season, than that in 2018, the dry and warm season. As regards the effect of climate variables of the cultivation location, it was found that such variables slightly significantly (p ≤ 0.05) affected the total carotenoid content in the pomaces from the two processing methods. Unexpectedly, the concentration of carotenoids in pomace from HBE was markedly higher than that from CBE (p < 0.01), most probably due to heat-catalyzed disruption of cell walls and membranes that makes easier the extraction of bound carotenoids from tomato peels in the analytical procedure. Carotenoid content has been reported to be higher in thermally processed tomato products including the by-product pomace than in fresh raw tomatoes [35].
Table 2 and Table 3 present the concentrations of specific carotenoid compounds found in the raw materials, juices, and pomaces. The differences in climate conditions between the cultivation locations led to varying responses of individual carotenoids to the thermal processing of juice. In 2018, the tomatoes grown in the climate conditions of Location-2 exhibited higher levels of lycoxanthin, lutein, phytofluene, Z-13-lycopene, and epoxides of β-carotene compared to those from Location-1; however, the levels of other carotenoids were not significantly affected by these climate variations. In 2019, a notable shift in environmental factors, especially regarding precipitation, air temperature, and sunlight exposure three weeks prior to harvest, influenced the carotenoid content differently at the various locations (as shown in Table 3). Fruits from Location-1 had greater amounts of β-carotene, Z-dimethoxy lycopene, Z-β-carotene di-epoxide, lutein, phytoene, and phytofluene than those from Location-2, which had higher concentrations of lutein and one epoxide of β-carotene.

Table 2.
Carotenoid content (µg g−1 dm) of different products from juice processing of tomato cultivated in different locations having different climate parameters in 2018. The values are means ± standard deviation, n = 3.

Table 3.
Carotenoid composition and content (µg g−1 dm) of different products from juice processing of tomato cultivated in different locations in 2019. Values are means ± standard deviation, n = 3.
The various climate factors, along with seasonal changes, influenced how different carotenoids responded to the thermal processing of tomato juice. Lycopene, the predominant carotenoid in tomatoes, demonstrated greater stability against CBE and HBE in fruits harvested from Location-1 during both the 2018 and 2019 growing seasons. Other carotenoids such as γ-carotene, β-carotene, Z-dimethoxy lycopene, cis-lycopene isomers, and phytoene exhibited better stability during CBE of tomatoes from Location-1, while only the epoxide derivatives of β-carotene showed increased stability due to the climate conditions at Location-2 in 2018. In general, the stability of most individual carotenoids during the high temperatures of HBE was not significantly affected by the climate variations between the two locations, except for lycopene in tomatoes from Location-1, lutein in tomatoes from Location-2 (in 2018), and lycopene, γ-carotene, hydroxy phytoene in tomatoes from Location-1, as well as phytoene in tomatoes from Location-2 (in 2019). It is noteworthy that the relatively cool and wet conditions of 2019, combined with climate factors prior to harvest, led to a significant increase in the accumulation of 13Z-lycopene in all juices and pomaces from tomatoes grown at Location-2. This observation is particularly significant from both biological and nutritional perspectives, as the cis isomers of lycopene have been shown to possess greater bioavailability than trans-lycopene in the human body [36].
In terms of how climate variables at the cultivation location affect the content and stability of pomace, it is noted that only in HBP from tomatoes cultivated under Location-2 conditions was there a significant increase in the stability of certain carotenoids, particularly in 2019. The pomace produced by both juice extraction methods contains significantly high levels of essential carotenoids such as lycopene, β-carotene, lutein, and phytoene, making it particularly valuable for human nutrition, animal feed, and the chemical industry.
3.2. Response of Tocopherols
The reversed-phase HPLC method was utilized to analyze tocopherols from non-hydrolyzed tomato extracts. The HPLC effectively separated the primary tocopherol compounds (Figure 3). The predominant compounds include α-tocopherol, its acylated ester, and α-tocopherol hydroquinone. The latter compound is produced naturally through the oxidation of vitamin E to quinone, followed by reduction by hydrogen donors such as vitamin C and phenolic compounds. A notable amount of ɣ-tocopherol was detected in the pomaces, which have a higher concentration of seeds, the primary source of ɣ-tocopherol in tomatoes. The reversed-phase HPLC profile resembles that observed in the HPLC-FL analysis of tocopherols from fresh tomatoes [37].
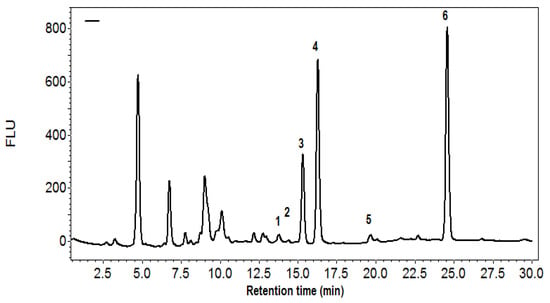
Figure 3.
HPLC profile of tomato tocopherols separated on Nucleosil C18, ec, 3 µ, 250 × 4.6 mm column with gradient elution of methanol, 2-propanol, acetonitrile (10:55:35) in 8% water in methanol; 1: γ-tocopherol, 2: β-tocopherol; 3: α-tocopherol hydroquinone; 4: α-tocopherol; 5: α-tocopherol acetate; 6: α-tocopherol ester.
The variations in total and individual tocopherols due to seasonal changes and the climate of the two locations are illustrated in Figure 4 and Table 4. The tocopherol content in the industrial tomato variety examined in this study is significantly higher than the 22−47 µg g−1 dry weight reported by Seybold et al. [38] and the 291 µg g−1 dry weight documented by Gharbi et al. [39] for various industrial tomatoes. In 2018, the raw materials collected from Location-1 had considerably lower tocopherol levels (p < 0.05) compared to those gathered from Location-2, which experienced contrasting climatic conditions.
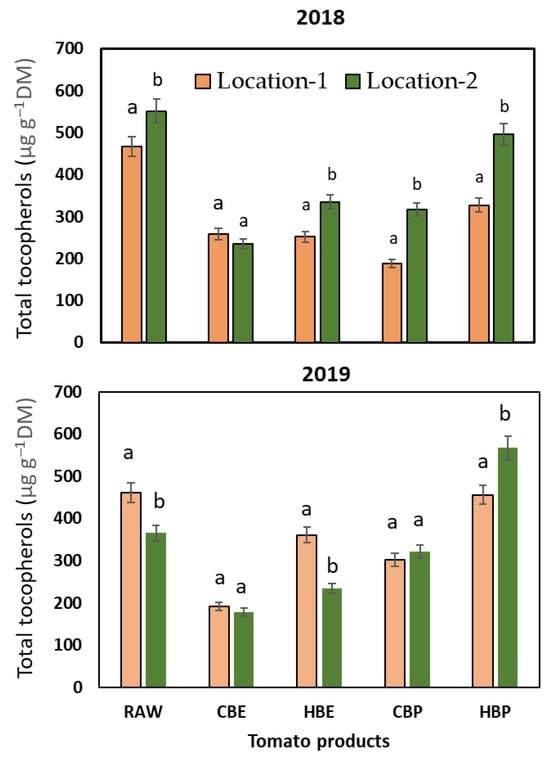
Figure 4.
Content (µg g−1 dm) of total tocopherols in different juice and pomace products of tomato cultivated in 2018. RAW: raw tomato; CBE: cold-break extracted juice; HBE: hot-break extracted juice; CBP: cold-break pomace; HBP: hot-break pomace. The values for tomato tocopherols from two locations with the same letter are statistically insignificant (p > 0.05).

Table 4.
Effect of processing on the tocopherol content (µg g−1 dm) of juices and pomace from tomatoes cultivated in different locations having different climate parameters. Values are means ± standard deviation, n = 3.
Like carotenoid concentrations, the tocopherol levels in juice produced by hot-water extraction (HBE) saw a substantial decline of 59% and 58% for tomatoes from Location-1 and Location-2, respectively. With HBE, the tocopherol loss reduced to 39%, but this was observed only in the juice derived from tomatoes harvested from Location-2. The percentage of loss remained the same for HBE juice made from tomatoes from Location-1. As previously discussed regarding carotenoids, the elevated temperature utilized in HBE might promote the extraction of tocopherols from the peels into the juice and/or inhibit certain tocopherol-degrading biochemical factors (biotic influences). In terms of the pomaces, there was a significant increase in tocopherol levels, especially in the pomace produced from the HBE of tomatoes from both locations. The high tocopherol content in the pomaces is likely attributable to the substantial amounts of peels (skin plus attached pulp) and seeds, which are abundant in tocopherols, predominantly the γ-analogue.
In 2019, the seasonal trends indicating increased rainfall and lower temperatures influenced the stability of tocopherols during cold and hot beverage extraction processes at the two locations. For instance, contrary to 2018, the highest concentration of tocopherols was observed in both the raw materials and HBE juice from Location-1. Regarding pomaces, there were no significant differences in the tocopherol content among CBP products from various locations. A notable rise in tocopherol concentration, alongside a significant disparity between the growing locations, was observed in pomace from HBE. The overall tocopherol levels in HBP derived from tomatoes grown in the climate of Location-1 were significantly lower (p < 0.05) than those from tomatoes cultivated in Location-2.
The CBE of tomatoes grown in Location-2 produced juice and pomace that contained 59% less tocopherol compared to the juice from HBE (p < 0.01). Conversely, juice from tomatoes grown in Location-1 exhibited a different trend. The behavior of tocopherols resembles that of carotenoids since both are produced through the same pathways and are found in the membranes of the chromoplasts [40]. The likely explanations for the elevated levels of tocopherols in HBE juice and pomace may involve the breaking down of cell walls and membranes, allowing for the release of bound tocopherols, as well as the thermal inactivation of tocopherol-oxidizing enzymes. The vitamin E and its esters present in HBE juice and pomace from tomatoes cultivated in Location-2, which experienced low precipitation and high air temperatures, were significantly higher (p < 0.01) than the corresponding levels from tomatoes in Location-1, thereby reinforcing the beneficial impact of limited water availability and uptake by plants on tocopherol synthesis and stability in tomatoes.
In the year 2019, it is notable that while the level of precipitation significantly declined in Location-1, it conversely rose in Location-2, leading to a trend in the changes related to HBE and CBE that differed from that of 2018. The tocopherol levels in both raw materials and CBE juice were greater in Location-1 compared to Location-2, although there were no significant differences in the amounts of the primary tocopherols found in other products across the two locations. This observation reinforces the idea that the concentration of vitamin E components is greatly influenced by the environmental conditions of the industrial tomato cultivation sites.
The elevated levels of tocopherols found in the pomace samples can likely be attributed to the significant presence of pulp in the peels and the high quantity of seeds, which are the source of ɣ-tocopherol [41]. The concentration of the oxidation product of vitamin E (α-tocHQ) was notably higher in the raw tomato and CBE pomace collected from Location-2 during the dry season of 2018. In 2019, when seasonal changes resulted in increased rainfall and a decrease in air temperature, the levels of α-tocHQ were greater in HBE and CBP derived from tomatoes at Location-2, while notably higher amounts of vitamin E and its fatty acid esters were observed in raw tomato and CBE from Location-1.
3.3. Response of Vitamin C
Even though there were significant differences in environmental factors between the two locations, the concentration of vitamin C in the raw materials used for juice processing did not show notable variation between the two locations in 2018. In 2019, seasonal fluctuations, particularly the rainfall occurring three weeks prior to the harvest in Location-1, resulted in significantly greater vitamin C content in raw tomatoes (p < 0.05) compared to levels observed in 2018 (Figure 5). These findings suggest that ample water supply before harvesting is essential for enhancing the accumulation of the water-soluble vitamins in tomato fruits. The vitamin C levels in raw tomatoes sourced from the cultivation sites ranged from 1140 to 1508 µg g−1 dm. This range is comparable to the ascorbic acid content found in ripe fruits of various other cultivars [42,43].

Figure 5.
Content (µg g−1 dm) of vitamin C in different juice and pomace products of tomato cultivated in 2018 and 2019. RAW: raw tomato; CBE: cold-break extracted juice; HBE: hot-break extracted juice; CBP: cold-break pomace; HBP: hot-break pomace. The values for vitamin C in tomato from two locations with the same letter are statistically insignificant (p > 0.05).
Due to sensitivity to heat and molecular oxygen, significant degradation of vitamin C was anticipated because of thermal processing. Throughout both growing seasons, the vitamin C level considerably increased (p < 0.05) in the juice derived from cold-break extraction (CBE) of tomatoes grown in location-1. This rise is likely attributed to the removal of peel and seed components, which contain lower amounts of vitamin C; particularly in 2019, an increase was noted only in Location-1, where water supply was abundant. Interestingly, hot-break extraction (HBE) during the relatively dry and warm growing season of 2018 resulted in a complete loss of vitamin C in the juice and pomace from tomatoes harvested in both locations, highlighting a significant decrease in vitamin stability when subjected to high-temperature thermal extraction. This trend was similarly observed in the pomace from CBE. In 2019, however, the trend shifted due to higher precipitation levels compared to 2018. The variation in seasonal abiotic factors led to noticeable changes in the stability of vitamin C during the thermal extraction process, particularly in Location-1. The stability of vitamin C in juice and pomace from HBE was notably greater at Location-1 compared to the same products from Location-2. In 2019, the concentration of vitamin C was two times and five times higher in juice and pomace from HBE than in the equivalent products from Location-2, respectively (p < 0.001). A study reported an 80% loss of vitamin C during the preparation of puree from red ripe tomatoes cultivated under standard outdoor conditions [44].
In this study, we noted a complete loss (100%) in 2018 and a significant decline in tomato products, excluding juice, from CBE in 2019, which was linked to the decreased water availability (from both rainfall and irrigation) for tomato crops, particularly during the dry and warm growing seasons, just before the harvest.
In several studies [45,46,47], the preservation of vitamin C has been attributed to varying levels of activity in ascorbic acid oxidase within tomatoes, whereas another study [48] related the stability of vitamin C to the activity and concentration of peroxidase enzymes found in tomato juice. An additional factor that might explain the consistent levels of vitamin C in juices is the heightened activity of stabilizing and/or regenerating enzymes like mono-dehydro-ascorbic acid reductase, catalase, and superoxide dismutase, which catalyze the regeneration of L-ascorbic acid and provide robust antioxidant protection against its oxidation [49,50]. Conversely, the substantial reduction in vitamin C concentration, particularly in juice and pomace from HBE, could be linked to heat-assisted release of certain degrading biochemical components that are attached to the fruit skin and seed endosperm [50]. Three peroxidase isoenzymes have been characterized from the plasma membranes and cell walls of tomato fruits [51]. Peroxidase activity has been observed to develop in the micropylar area of the endosperm in imbibed tomato seeds [52]. These enzymes are known to be relatively heat-stable and can be chemically or thermally eluted while being capable of oxidizing ascorbic acid. Factors such as elevated air temperatures have been documented to hinder the recycling of ascorbic acid [53], and intense UV radiation from sunlight can diminish both the content and stability of bioactive compounds, including vitamin C, in tomatoes [54]. All the biotic factors can be influenced by the climatic variables present during the cultivation season and just before the harvest of tomato fruits.
4. Conclusions
The results obtained from this study provide new data about how the climate variables, particularly 3 weeks before harvest, and seasonal variations in different cultivation locations affect the content of phytonutrients and their stability during postharvest processing of tomato juice. The stability and degradation of phytochemicals can be significantly affected by the seasonal variation and the abiotic factors of the cultivation location, particularly air temperature and precipitation, 3 weeks before harvest. The increased global warming and water deficiency in tomato cultivation territories may negatively impact on the stability of phytonutrients in tomato products, making it necessary to take into consideration the interaction between abiotic factors during the cultivation period, the type of processing to be used, as well as selection of tomato cultivars that adapt to climate changes in order to produce tomato products with outstanding quality.
Author Contributions
H.G.D.: Supervision, Writing—original draft, Correspondence, S.R. and A.A.A.: Analysis, Methodology, M.M.: Data curation, Investigation, L.H.: Conceptualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Flagship Research Groups Program of the Hungarian University of Agriculture and Life Sciences.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WS | water supply |
| CBE | cold-break extraction |
| HBE | hot-break extraction |
| α-Toc | alpha-tocopherol |
| α-TocHQ | alpha-tocopherol hydroquinone |
| α-TocES | alpha-tocopherol ester |
| DM | dry matter |
References
- Giovannucci, E.T. Tomatoes, Tomato-Based Products, Lycopene, and Cancer: Review of the Epidemiologic Literature. J. Natl. Canc. Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762–26772. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- George, S.A.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Meulebroek, L.V.; Vanhaecke, L.; De Swaef, T.; Steppe, K.; De Brabander, H. U-HPLC-MS/MS to quantify liposoluble antioxidants in red-ripe tomatoes, grown under different stress levels. J. Agric. Food Chem. 2012, 60, 566–573. [Google Scholar] [CrossRef]
- Krebbers, B.; Master, A.M.; Hoogerwerf, S.W. Combined high-pressure and thermal treatments for processing tomato puree: Evaluation of microbial inactivation and quality parameters. Innov. Food Sci. Emerg. Technol. 2003, 4, 377–385. [Google Scholar] [CrossRef]
- Goodman, C.L.; Fawcett, S.; Barringer, S.A. Flavour, viscosity, and color analyses of hot and cold break tomato juices. J. Food Sci. 2002, 67, 404–408. [Google Scholar] [CrossRef]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; de Vos, R. Changes in antioxidant s and metabolite profile during the production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroglu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef]
- Illés, G.; Fonyó, T.; Pásztor, L.; Bakacsi, Z.S.; Laborczi, A. Agroclimate 2 project of compilation of digital soil-type map of Hungary. Erdészettudomány Közlemények (For. Sci. Commun.) 2016, 6, 17–24. [Google Scholar]
- Nemeskéri, E.; Horváth, K.Z.; Andryei, B.; Ilahy, R.; Takács, S.; Neményi, A.; Pék, Z.; Helyes, L. Impact of plant growth-promoting rhizobacteria inoculation on the physiological response and productivity traits of field-grown tomatoes in Hungary. Horticulturae 2022, 8, 641. [Google Scholar] [CrossRef]
- Bulgan, A.; Horváth, K.Z.; Duah, S.A.; Takács, S.; ÉgeI, M.; Szuvandzsiev, P.; Neményi, A. Use of plant growth promoting rhizobacteria (PGPRs) in the mitigation of water deficiency of tomato plants (Solanum lycopersicum L.). J. Centr. Eur. Agric. 2021, 22, 167–177. [Google Scholar]
- Kruk, J.; Szymanska, R.; Krupinska, K. Tocopherol quinone content of green algae and higher plants revised by a new highly sensitive fluorescence detection method using HPLC–Effects of high light stress and senescence. J. Plant Physiol. 2008, 165, 1238–1247. [Google Scholar] [CrossRef]
- Daood, H.G.; Biacs, P.A.; Dakar, M.; Hajdú, F. Ion-pair chromatography and photo mode-array detection of vitamin C and organic acids. J. Chromatogr. Sci. 1994, 32, 481–487. [Google Scholar] [CrossRef]
- Daood, H.G.; Ráth, S.; Palotás, G.; Halász, G.; Hamow, K.; Helyes, L. Efficient HPLC separation on a core C30 column with MS2 characterization of isomers, derivatives, and unusual carotenoids from tomato products. J. Chromatogr. Sci. 2022, 60, 336–347. [Google Scholar] [CrossRef]
- Daood, H.G.; Biacs, P.A. Simultenous determination of Sudan dyes and carotenoids in red pepper and tomato products by HPLC. J. Choromatogr. Sci. 2005, 43, 461–465. [Google Scholar] [CrossRef][Green Version]
- Hsu, K.C.; Tan, F.J.; Chi, H.J. Evaluation of microbial inactivation and physiochemical properties of pressurized tomato juice during refrigerated storage. LWT 2008, 41, 367–375. [Google Scholar] [CrossRef]
- Schwartz, K.; Bertelsen, G.; Nissen, L.R.; Gardner, P.T.; Heinonen, M.I.; Hopia, A.; Huynh-Ba, T.; Lambelet, P.; McPhail, D.; Skibsted, L.H.; et al. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of an antioxidant assay based on radical scavenging, lipid oxidation, analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 212, 319–328. [Google Scholar] [CrossRef]
- Bramely, P.M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Deli, J.; Osz, E. Carotenoid 5,6-, 5,8- and 3,6-epoxides. Arkivoc 2004, 7, 150–168. [Google Scholar] [CrossRef]
- Gama, J.J.T.; Tadiotti, A.C.; de Sylos, C.M. Comparison of carotenoid content in tomato, tomato pulp, and ketchup by liquid chromatography. Alim. Nutr. Araraquara 2006, 17, 353–358. [Google Scholar]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in Antioxidant Content of Tomato Fruits in Response to Cultivar and Nutrient Solution Composition. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Sharma, S.K.; Maguer, M. L Kinetics of lycopene degradation in tomato pulp solids under different processing and storage conditions. Food Res. Int. 1996, 29, 309–315. [Google Scholar] [CrossRef]
- Martinéz-Hernández, G.B.; Boluda-Aguilar, M.; Taboada-Rodréguez, A.; Soto-Jover, S.; Marín-Iniesta, F.; López Gómez, A. Processing, packaging and storage of tomato products: Influence on the lycopene content. Food Eng. Rev. 2016, 8, 52–75. [Google Scholar] [CrossRef]
- Temitope, A.O.; Eloho, A.P.; Olubunmi, I.D. Lycopene content in tomatoes (Lycopersicon esculentum Mill): Effect of thermal heat and its health benefits. Fresh Prod. 2009, 3, 40–43. [Google Scholar]
- Mayeaux, M.; Xu, Z.; King, I.M.; Prinyawiwatkul, W. Effect of cooking conditions on the lycopene content in tomatoes. J. Food Sci. 2006, 71, 461–464. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Chandra, H.M.; Ramalingam, S. Antioxidant potentials of skin, pulp and seed fractions of commercially important tomato cultivars. Food Sci. Technol. 2011, 20, 15–21. [Google Scholar] [CrossRef]
- Vinha, A.F.; Alves, R.C.; Barreira, S.V.P.; Castro, A.; Costa, A.S.G.; Olivieira, M.B.P.P. Effect of peel and seed removal on the nutritional value and antioxidant activity of tomato (Lycopersicon esculentum L.) fruits. LWT 2014, 55, 197–202. [Google Scholar] [CrossRef]
- Angaman, D.M.; Renato, M.; Azcón-Bieto, J.; Boronat, A. Oxygene consumption and lipoxygenase in isolated tomato fruit chromoplasts. J. Plant Sci. 2014, 2, 5–8. [Google Scholar]
- Urbonaviciene, D.; Viskelis, P.; Viskelis, J.; Jankauskiene, J.; Bobinas, C. Lycopene and β-carotene in non-blanched and blanched tomatoes. J. Food Agric. Environ. 2012, 10, 142–146. [Google Scholar]
- Tonucci, L.H.; Holden, J.M.; Beecher, G.R.; Khachik, F.; Davis, C.S.; Mulokozi, G. Carotenoid content of thermally processed tomato-based food products. J. Agric. Food Chem. 1995, 43, 579–586. [Google Scholar] [CrossRef]
- Nascimento, A.A.G.; Vasconcelos, A.G.; Souza, G.; Oliveira, A.; de Souza de Almeida Leite, G.J.R.; Pintado, M. Bioavailability, anticancer potential, and chemical data of lycopene: An overview and technological prospecting. Antioxidants 2022, 11, 360. [Google Scholar] [CrossRef]
- D’Evoli, L.; Lombardi-Boccia, G.; Lucarini, M. Influence of heat treatments on carotenoid content of cherry tomatoes. Foods 2013, 2, 352–363. [Google Scholar] [CrossRef]
- Seybold, C.; Fröhlich, K.; Bitch, R.; Otto, K.; Böhm, V. Change in content of carotenoids and vitamin E during tomato processing. J. Agric. Food Chem. 2004, 52, 7005–7010. [Google Scholar] [CrossRef]
- Gharbi, S.; Renda, G.; La Barbera, L.; Amri, M.; Messina, C.M.; Santulli, A. Tunisian tomato by-products, as a potential source of natural bioactive compounds. Nat. Prod. Res. 2017, 31, 626–631. [Google Scholar] [CrossRef]
- Quadrana, L.; Almeida, J.; Otalza, S.N.; Duffy, T.; Corredo da Silva, J.V.; de Godoy, F.; Asis, F.; Bermúdez, L.; Fernie, A.R.; Carrari, F.; et al. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol. Biol. 2013, 81, 309–325. [Google Scholar] [CrossRef]
- Abushita, A.A.; Daood, H.G.; Biacs, P. Change in carotenoids and antioxidant vitamin in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef]
- Chanforan, C.; Loonis, M.; Mora, N.; Caris-Veyrat, C.; Dufour, C. The impact of industrial processing on health-beneficial tomato microconstituents. Food Chem. 2012, 134, 1786–1795. [Google Scholar] [CrossRef]
- Hdider, C.; Ilahy, R.; Tlili, I.; Lenucci, S.C.; Dalessandro, G. Effect of maturity on the antioxidant content and antioxidant activity of high-pigment tomato cultivars grown in Italy. Food 2013, 7, 1–7. [Google Scholar]
- Yahia, E.M.; Contreras-Padilia, M.; Gonzalez-Aguilar, G. The ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation, and senescence. LWT 2001, 34, 452–457. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R. Increasing ascorbic acid and salinity tolerance of cherry tomato plants by suppressed expression of the ascorbate oxidase gene. Agronomy 2019, 9, 51–64. [Google Scholar] [CrossRef]
- Munyaka, A.W.; Makule, E.E.; Oey, I.; Loey, A.V.; Hendrickx, M. Thermal stability of L-ascorbic acid and ascorbic acid oxidase in Broccoli. J. Food Sci. 2010, 75, C336–C340. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Sekine, Y.; Watanabe, N.; Barrett, D.M. Thermal inactivation of pectin methylesterase, polygalacturonase and peroxidise in tomato juice. J. Agric. Food Chem. 2002, 50, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability, and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Stevens, R.; Page, D.; Gouble, B.; Garchery, C.; Zamir, D.; Causs, M. Tomato fruit ascorbic acid content is liked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ. 2008, 31, 1086–1096. [Google Scholar] [CrossRef]
- Melliduo, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Andrews, J.; Adams, S.R.; Burton, K.S.; Evered, C.E. Subcellular localization of peroxidase in tomato fruit skin and the possible implications of the regulation of fruit growth. J. Exp. Bot. 2002, 53, 2185–2191. [Google Scholar] [CrossRef]
- Morohashi, Y. Peroxidise activity develops in the micropylar endosperm of tomato seeds prior to radical protrusion. J. Exp. Bot. 2002, 53, 1643–1650. [Google Scholar] [CrossRef]
- Massot, C.; Bancel, D.; Lauri, F.L.; Truffault, V.; Baldet, P.; Stevens, R.; Gautier, H. High-temperature inhibits recycling and light stimulation of the ascorbate pool in tomato despite increased expression of biosynthesis genes. PLoS ONE 2013, 8, e84474. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioproc. Technol. 2011, 4, 1463–1472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).