Abstract
Flavonoids are essential secondary metabolites in plants, predominantly found in flowers, leaves, and fruits. They mainly include anthocyanins, proanthocyanidins, and flavonols. Transcription factors are a crucial family of proteins in plants, playing a significant role in regulating the biosynthesis of secondary metabolites. This review introduces flavonoids and explores the characteristics and biological functions of MYB transcription factors. It establishes a phylogenetic tree using Arabidopsis thaliana MYB transcription factors as an example, which includes 17 subgroups (S1–S17). The subgroups related to flavonoids are primarily concentrated in S17, further classified into A, C, D, E, and F. The review also discusses the different regulatory roles of MYB transcription factors in flavonoid synthesis in monocotyledonous and dicotyledonous plants. This knowledge provides a theoretical foundation for further studies on the diverse regulatory functions of MYB transcription factors in flavonoid biosynthesis across the plant kingdom.
1. Introduction
During the long process of natural selection and evolution, plants have developed unique gene expression profiles and regulatory mechanisms to adapt to constantly changing environments [1]. Gene expression and silencing are tightly controlled to regulate diverse physiological processes throughout development, operating at multiple interdependent levels—including DNA modification, transcription, and translation [2]. Among these, transcriptional regulation is the most complex. It involves both cis-acting elements and trans-acting factors, which bind to specific nucleotide sequences and interact to precisely modulate the expression of structural genes, thereby executing regulatory functions [3]. MYB transcription factors represent one of the largest and most widespread families of regulatory proteins in plants and play key roles in the biosynthesis of numerous secondary metabolites. Recent genomic studies in safflower have shown that overexpression of R2R3-MYB genes significantly upregulates structural genes involved in flavonoid biosynthesis and enhances flavonol accumulation [4]; similarly, whole-genome identification in the medicinal orchid Dendrobium officinale revealed that certain R2R3-MYB members negatively regulate flavonoid synthesis by repressing key enzyme genes [5]. However, the field of MYB-mediated flavonoid regulation still faces considerable challenges. For instance, within a given species, multiple MYB homologs may regulate distinct branches of flavonoid metabolism or respond to different signals via functional differentiation. Moreover, understanding how epigenetic modifications of MYB genes and their target genes influence flavonoid synthesis and accumulation remains a major unresolved question. Flavonoids, an important class of plant secondary metabolites, are abundantly present in flowers, leaves, and fruits [6]. In Arabidopsis thaliana, early biosynthetic genes (EBGs) are activated by functionally redundant R2R3-MYB regulators (MYB11, MYB12, and MYB111), whereas late biosynthetic genes (LBGs) require the assembly of a ternary MYB–bHLH–WD40 (MBW) complex. In contrast, in monocot species such as maize (Zea mays), the MBW complex activates both EBGs and LBGs uniformly. Similar regulatory distinctions have been observed between other dicot and monocot species, suggesting fundamental differences in the flavonoid (particularly anthocyanin) regulatory networks between these two groups of plants [7]. By examining the characteristics and biological functions of MYB transcription factors, constructing a phylogeny of MYBs involved in flavonoid biosynthesis, and comparing their regulatory roles in monocots (e.g., rice and maize) and dicots (e.g., Arabidopsis thaliana), this study aims to provide a theoretical foundation for understanding the functional diversity and evolutionary divergence of MYB-mediated flavonoid regulation across the plant kingdom.
2. Flavonoid Biosynthetic Pathways
Flavonoids, which represent one of the primary classes of secondary metabolites in plants, mainly comprise anthocyanins, proanthocyanidins, and flavonols [8]. Among these, flavonols are the earliest evolved flavonoids, with their biosynthetic origins traceable to bryophytes, such as physcomitrium patens and marchantia polymorpha [9]. Proanthocyanidins have been identified in ferns, whereas anthocyanins are believed to have originated prior to the divergence of gymnosperms and angiosperms [10].
Anthocyanins are one of the principal pigments found in flowers, leaves, stems, fruits, and other tissues and organs of plants. Their primary function is to impart diverse colors, contributing to the vibrant pigmentation observed in various plant species. Stable anthocyanins are typically localized within vesicles of epidermal cells [11]. The coloration of anthocyanins is determined by the number of hydroxyl groups on the B-ring; an increased number of hydroxyl groups results in a bluer hue. Proanthocyanidins are widely distributed in various plant tissues, including tree bark, flowers, seeds, and fruit peels. Maynard et al. (1967) first isolated four polyphenolic compounds from grape skin and grape seed extracts that yielded red anthocyanidins upon acid hydrolysis under heating; these were subsequently designated collectively as proanthocyanidins [12]. The regulation of proanthocyanidin biosynthesis in plants is more complex than that of anthocyanins. Nevertheless, their synthesis is primarily controlled by MYB transcription factors. In most species, proanthocyanidin accumulation is regulated by multiple MYB transcription factors that exhibit functional divergence or act in concert through specific regulatory sequences [13]. Flavonols represent the most abundant and ubiquitous subclass of flavonoid compounds. Common examples include quercetin, kaempferol, isorhamnetin, and myricetin, which are present in various plant organs such as roots, stems, leaves, flowers, and fruits [14]. They often co-localize with anthocyanins and contribute to color development in plant tissues while also acting as a defense mechanism against environmental stressors such as insect herbivory, UV radiation, and bacterial infection [15].
In current horticultural crop studies focusing on secondary metabolism, the metabolic pathways underlying flavonoid biosynthesis have been characterized in greater detail (Figure 1). As a conserved and pivotal component of the phenylpropanoid pathway across plant taxa, flavonoid biosynthesis is governed by two primary gene categories: structural genes, further classified into early and late subsets [16]. In the anthocyanin branch, enzymes such as CHS, CHI, F3H, F3′H, and F3′5′H constitute the EBGs, while DFR, ANS/LDOX, and UFGT are designated as LBGs [17]; regulatory genes exemplified by transcription factors (Table 1). Key enzymes in the anthocyanin pathway are coordinately regulated by the MBW transcriptional activation complex, comprising members from the MYB, bHLH, and WD40 repeat protein families, as well as additional TFs such as WRKY and NAC [16]. The synthesis of flavonoids begins with phenylalanine within the phenylalanine pathway, where phenylalanine aminolyase serves as the initial enzyme. Phenylalanine Ammonia-Lyase (PAL)catalyzes the conversion of phenylalanine into cinnamic acid, subsequently processed by cinnamic acid-4-hydroxylase and 4CL4-coumarate coenzyme A ligase to yield 4-coumaroyl coenzyme A. Chalcone synthase then acts on malonyl coenzyme A and 4-coumaroyl coenzyme A, producing chalcone. While chalcone isomerase (CHI) is not mandatory for this reaction in plants, its absence slows down the process significantly, showcasing tissue-specific expression. Dihydroflavonols are generated from 4,5,7-trihydroxyflavanones catalyzed by flavanone 3-hydroxylase, a common substrate for flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase. F3H plays a regulatory role in the synthesis of flavonols and anthocyanin products and is a key enzyme in the metabolic pathway of flavonoid substances with a central position in the whole flavonoid metabolism process. Dihydroxyflavonol reductase, reliant on NADPH, catalyzes the formation of colorless anthocyanins from flavonols. Its expression varies across species and tissues. These colorless anthocyanins are later oxidized by anthocyanin synthase into colored anthocyanins. Anthocyanins, inherently unstable, undergo modification by uridine diphosphate glucosyltransferase, leading to glycosylation, hydroxylation, and methylation, ultimately producing stable, colored anthocyanins. Alternatively, anthocyanin reductase and colorless anthocyanin reductase facilitate the conversion of unmodified colorless anthocyanins or anthocyanins into proanthocyanidins [18] (Figure 1).
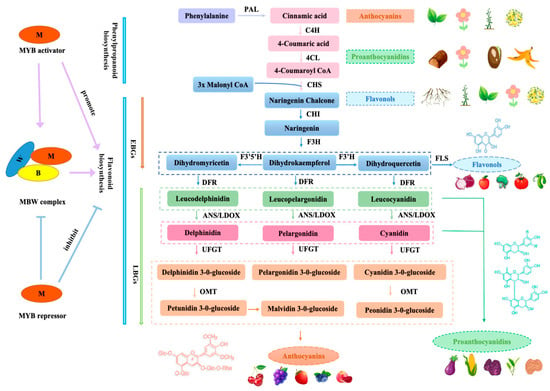
Figure 1.
Simplified model for the flavonoid biosynthetic pathway and the function of MYB transcription factors.

Table 1.
Classification and functions of EBGs and LBGs.
Abbreviations are as follows: EBGs, early biosynthetic genes; LBGs, late biosynthetic genes; PAL, phenylalanine ammonium lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumaroyl-CoA synthase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′5′-hydroxylase; FLS, flavonol synthase; DFR, dihydroflavonol reductase; ANS, anthocyanindin synthase; LDOX, leucoanthocyanidin dioxygenase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase; UFGT, UDP-Glc:flavonoid 3-O-glucosyltransferase; OMT, O-methyl transferase.
Biosynthetic pathways exhibit considerable taxonomic variation across plant lineages, reflecting divergent evolutionary trajectories and ecological adaptations. Although core enzymatic mechanisms are often conserved, key differences emerge through gene family expansion, alterations in enzyme substrate specificity, and lineage-specific transcriptional regulation—particularly involving MYB transcription factors [19]. These variations give rise to distinct profiles of specialized metabolites, such as differences in flavonoid composition between monocots and dicots [20].
Although monocots and dicots share the core biochemical pathway for flavonoid biosynthesis, significant divergences exist between them in terms of metabolic profile, regulatory mechanisms, and compound specificity. With regard to product accumulation, dicots produce a wide diversity of anthocyanins, such as pelargonidin, cyanidin, and delphinidin, resulting in a broad spectrum of flower colors [21]. In contrast, monocots—particularly those in the Poaceae family—exhibit more conservative floral pigmentation, predominantly accumulating cyanidin-3-glucoside, which confines their coloration to red and purple hues [22]. At the molecular regulatory level, although both groups rely on the MYB-bHLH-WD40 (MBW) transcriptional complex to control gene expression in this pathway, key MYB transcription factors in monocots show low homology to those in dicots, indicating distinct evolutionary trajectories [23]. Furthermore, monocots are notably specialized in the accumulation of characteristic metabolites such as C-glycosyl flavonoids (e.g., orientin and isoorientin), which are less common in dicots and contribute to enhanced antioxidant capacity and stress adaptation [23]. These differences collectively reflect the metabolic diversity that has emerged through adaptation to distinct ecological and developmental constraints during the evolution of these two major plant groups.
3. Structural Characteristics and Origin of MYB Transcription Factors
The functional diversification of transcription factors is primarily driven by gene duplication and subsequent functional divergence [24]. Duplicated paralogs may undergo subfunctionalization—where ancestral functions are partitioned among copies—or neofunctionalization, in which one copy acquires a novel function, thereby expanding functional diversity. Additionally, mutations in cis-regulatory elements, alterations in protein interaction interfaces, and structural domain rearrangements further promote functional innovation in DNA-binding specificity, regulatory activity, and signal responsiveness [25]. These evolutionary mechanisms ultimately enable transcription factors to regulate a broader array of biological processes and enhance organismal adaptability to environmental challenges [26]. Dedicated databases have been developed to catalog transcription factors across species—examples include the Plant Transcription Factor Database (PlantTFDB) and the Grass Transcription Factor Database (Grassius)—with well-characterized families such as MYB, WRKY, NAC, and AP2/ERF being widely recognized as key regulators of these metabolic pathways [27].
3.1. Structural Characteristics and Classification of MYB Transcription Factors
MYB transcription factors are named after their highly conserved DNA-binding domain, the MYB domain, which typically comprises 1–4 tandem and partially repeated R units (R1, R2, R3, and in rare cases R4). Each R unit consists of approximately 50–53 amino acids that form a helix-turn-helix (HTH) motif capable of binding DNA. Key tryptophan residues—spaced at roughly 18-amino acid intervals—form a hydrophobic core essential for stabilizing the HTH conformation. Substitutions of these tryptophans, particularly the first tryptophan in the R3 repeat, with other aromatic or hydrophobic residues such as phenylalanine or leucine, are often observed and may influence DNA-binding specificity [28].
Based on the number and arrangement of R repeats, MYB proteins are classified into four major categories: (1) 1R-MYB (MYB-related) proteins contain a single R repeat and are subdivided into R3-type, R1/R2-type, and other variants. R3-type members—such as CAPRICE (CPC), TRIPTYCHON (TRY), and MYBL2—participate in cell fate determination and secondary metabolism. R1/R2-type proteins, including LHY and CCA1, play key roles in circadian rhythm regulation [29].
(2) R2R3-MYB proteins contain two adjacent R repeats and represent the largest MYB subfamily in plants. They feature a conserved N-terminal DNA-binding domain and a divergent C-terminal region that often contains transcriptional activation or repression domains, enabling diverse regulatory functions [30].
(3) R1R2R3-MYB (3R-MYB) proteins comprise three R repeats and form an evolutionarily ancient group with limited membership. For instance, only five 3R-MYB genes are found in Arabidopsis and tobacco, and four to five in rice [31].
(4) 4R-MYB proteins, containing four R repeats, are the rarest class. These proteins consist of four tandem R1/R2-like repeats and are encoded by very few genes across plant species. Their functional roles remain poorly characterized [31].
Among these, R2R3-MYB and R3-MYB family members are notably involved in the regulation of anthocyanin biosynthesis, with many R3-MYBs acting as transcriptional repressors in this pathway [32].
3.2. Origin and Differentiation of MYB Transcription Factors
Two principal models have been proposed to explain the evolution of MYB genes in plants. The first model posits that the MYB DNA-binding domain originated over a billion years ago. According to this view, MYB proteins containing two or three repeats emerged through sequence duplication and amplification events. Differential expansion of these genes across lineages accounts for the striking abundance of MYB transcription factors in plants—for example, the hundreds of R2R3-MYB genes found in Arabidopsis—compared to the more limited repertoires in animals and bacteria. This model further suggests that R2R3-MYB genes arose through the evolutionary loss of the R1 repeat from ancestral R1R2R3-MYB genes [33]. An alternative model proposes that R1R2R3-MYB transcription factors evolved through the acquisition of an additional R1 repeat, forming three-repeat proteins from simpler precursors [34]. Despite their differences, both models agree that MYB genes originated from single-repeat ancestral genes prior to the evolutionary divergence of plants and animals.
We generated a phylogenetic tree utilizing Arabidopsis MYB transcription factors as a model. The Arabidopsis MYB transcription factor family members were sourced from TAIR (https://www.Arabidopsis.org/, accessed on 5 February 2024), while those specifically associated with flavonoid biosynthesis were retrieved from the NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 8 February 2024). A total of 126 Arabidopsis R2R3-MYBs and 93 R2R3-MYBs linked to flavonoid biosynthesis were aligned using MAFFT, and subsequently, an ML tree was constructed employing IQ-TREE 2. This analysis delineated a significant Arabidopsis MYB family comprising 17 subgroups that regulate diverse metabolic pathways. In this study, the MYB transcription factors of primary interest, which are involved in the regulation of flavonoid biosynthesis, were positioned on Section S17 in Figure 2 to facilitate more detailed and focused analysis. R2R3 MYBs, which control flavonoids, have been discovered in an increasing variety of plants, and these gene homologs are frequently believed to control the same pathway [35]. In order to analyze the MYB gene related to flavonoids more clearly, we have subdivided it according to its different functions [36]. Flavonoid-related MYBs are divided into five clades, containing flavonol-related, proanthocyanin-related, anthocyanin-related, and repressor (Figure 2). While some R2R3 MYBs specifically control the expression of a single gene, others influence multiple genes and aspects of the flavonoid pathway.
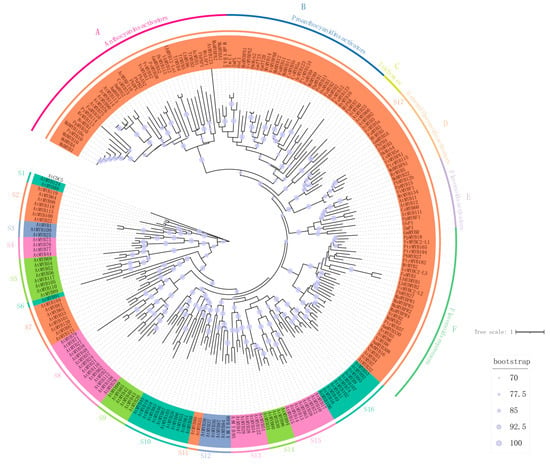
Figure 2.
Phylogenetic tree of the R2R3-MYB genes in Arabidopsis thaliana [37] and functionally characterized genes related to flavonoid biosynthesis in plants. The tree was constructed by the maximum likelihood (ML) method using IQ-TREE 2, and ModelFinder was used to choose the best model. The tree was rooted using AtCDC5 as the outgroup. Bootstrap values ≥ 50% are shown as dark blue dots in the phylogenetic tree. The candidate R2R3-MYB proteins are clustered into 17 subfamilies (designated as S1 to S17). And the S17 subfamily is further divided into six groups, namely A (anthocyanin activators), B (proanthocyanidin activators), C (trichomes), D (general flavonoid activators), E (flavonol activators), and F (flavonoid repressors).
To enable a more precise investigation of the S17 subgroup, we performed correlation analyses of key parameters, yielding the following technical data. Multiple sequence alignment was performed with MAFFT v7.525 (129 sequences (S17), 1131 sites), comprising 277 conserved sites (24.5%), 298 singleton sites (26.4%), and 556 parsimony-informative sites (49.1%). AliStat v1.16 analysis indicated an average p-distance of 0.543, corresponding to ~45.7% mean identity. Phylogenetic inference was conducted in IQ-TREE v3.0.1 under the best-fit substitution model JTT+R7 selected by ModelFinder (BIC). Branch support was assessed with 1000 ultrafast bootstrap replicates, and values are shown on the consensus ML tree.
MYB transcription factors exhibit both evolutionary convergence and divergence in their functions. Evolutionary convergence refers to the process whereby MYB genes from distinct evolutionary lineages and independent origins gradually develop similar molecular functions through long-term natural selection and adaptation to shared physiological demands, such as responses to environmental stress or regulation of specific branches of flavonoid metabolism. For example, OsMYB12 in monocot rice and AtMYB12 in dicot Arabidopsis thaliana, despite belonging to divergent plant clades without direct evolutionary connections, both activate key promoters of flavonoid biosynthesis genes to regulate the accumulation of flavonols, demonstrating functional convergence [38,39]. In contrast, evolutionary divergence occurs when MYB genes derived from a common ancestor acquire functional specificity over time in different species or lineages due to accumulated genetic variations, ecological niche differentiation, and distinct selective pressures. For instance, an ancestral MYB gene initially involved in basal flavonoid regulation may have diverged in gymnosperms to control defensive flavonoid compounds in conifer needles, while in angiosperms, it further specialized into subtypes regulating distinct metabolites such as anthocyanins or proanthocyanidins [30]. Some MYBs even evolved from transcriptional activators into repressors, fine-tuning the balance of flavonoid metabolic networks. This dynamic interplay of convergence and divergence not only shapes the functional diversity of the MYB family but also underpins plants’ adaptation to varied ecological environments and enables precise regulation of metabolic pathways [40]. It is central to understanding the widespread yet functionally specialized distribution of key secondary metabolic pathways, such as flavonoid biosynthesis, across the plant kingdom.
4. The Regulation of Flavonoid Synthesis by MYB Transcription Factors
The evolutionary divergence between monocots and dicots is characterized by several distinct genomic and phenotypic patterns, driven by mechanisms such as lineage-specific whole-genome duplications, differential expansion of gene families, and functional diversification of key regulatory components [41]. For example, subfunctionalization of MADS-box genes has led to profound differences in floral architecture, while divergent expansion and expression of transcription factor families—including MYB and NAC—underpin variations in stress adaptation and secondary metabolism [42]. Structural innovations, such as the scattered vascular bundles characteristic of monocots and the robust secondary growth typical of many dicots, further illustrate how developmental and ecological constraints have shaped independent evolutionary trajectories. Together, these patterns highlight the deep phylogenetic and adaptive changes that have generated the morphological, physiological, and molecular distinctions defining these two major angiosperm groups.
MYB transcription factors in plants stand out from their animal and yeast counterparts due to their remarkable structural and functional conservation. They serve as pivotal players in a diverse array of biological processes, exerting a unique influence on plant growth. Engaging in multiple facets of plant development, MYB transcription factors govern cell fate, morphogenesis, and various developmental stages. They also oversee primary and specialized metabolic pathways, as well as orchestrate responses to both biotic and abiotic stresses. Notably, MYB transcription factors are actively involved in plant flavonoid metabolic pathways, regulating flower, fruit, and seed color, among others, in monocotyledonous plants such as rice and maize, and in dicotyledonous plants such as Arabidopsis thaliana. EBGs in Arabidopsis are activated by functionally redundant R2R3MYB regulatory genes (MYB11, MYB12, and MYB111), and the initiation of LBGs requires the MYB regulatory factor and bHLH factor, as well as the WD40 protein to make up the ternary MBW complex. In contrast, in monocotyledonous maize and rice, the MBW complex can activate both EBGs and LBGs as a single unit; therefore, we suggest that the regulatory mechanism of flavonoid synthesis in dicotyledons is different from that in monocotyledons (Figure 3). In Arabidopsis, the early synthetic genes EBGs associated with flavonoid synthesis are regulated by AtMYB12/PFG1 [43], and the late synthetic genes, LBGs, are mainly regulated by AtMYB75/PAP1, AtMYB90/PAP2, AtMYB5, AtMYB123/AtTT2, AtMYB113, AtMYB114 [44,45,46,47], AtMYB3, AtMYB4, AtMYB7, and MYB32 overexpression inhibited both early synthesis genes EBGs and late synthesis genes, LBGs [48,49,50], AtMYBL2 and AtCPC also inhibited the control of flavonoid production [51,52]. In monocotyledonous plants, of all the MYB genes present in the model plant rice, OsP1 (PERICARP COLOUR1) and OsMYB3/Kala3 are overexpressed to increase the transcriptional level of EBGs, and OsC1 (COLOURESS1) is overexpressed to increase the transcriptional level of LBGs, promoting the production of anthocyanins [53,54,55]. In another model plant, maize, ZmP1 is overexpressed to increase the transcriptional level of EBG [56], and ZMC1 and ZMPL are overexpressed to increase the transcriptional level of EBGs and LBGs, which promote the production of flavonoids [57,58].
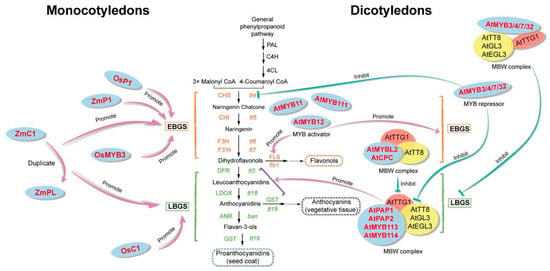
Figure 3.
MYB regulation of the flavonoid biosynthetic pathway in monocotyledons and dicotyledons.
Activation-type and repression-type MYB transcription factors can exhibit both functional paralogy and convergent evolution, enhancing the complexity and adaptability of regulatory networks in plants. Functional paralogy often arises from gene duplication events, where paralogous genes diverge to assume opposing roles—one activating and one repressing the same pathway—as observed in anthocyanin biosynthesis regulators such as AtMYB75 and AtMYB27 in Arabidopsis [59]. Meanwhile, convergent evolution is reflected in the independent emergence of repressive motifs in non-homologous MYB repressors across distantly related species, enabling analogous suppression of flavonoid biosynthesis in both monocots and eudicots [60]. These evolutionary mechanisms collectively fine-tune metabolic responses and contribute to phenotypic diversity under environmental constraints.
4.1. Involvement of MYB Transcription Factors in Monocotyledonous Plants in Flavonoid Metabolism, Regulating the Color of Flowers, Fruits, etc.
In monocotyledonous plants, MYB transcription factors regulate flavonoid biosynthesis, which is more typical of rice and maize. Among all MYB genes present in rice, an R2R3 type (C1) regulates anthocyanin biosynthesis, OsC1(COLOURLESS1), OsP1(PERICARP COLOUR1), and OsMYB3/Kala3 overexpressed in rice plants trigger anthocyanin production through augmentation of the transcript level of late ABP genes [53,54,55] (Table 2). The maize ZmMYBC1 gene, which is the first MYB-like gene to be discovered in plants, is ectopically expressed in tissue cells and interacts with the bHLH protein to jointly elicit the manifestation of the structural enzyme genes DFR and 3GT in the anthocyanin synthesis pathway, leading to massive synthesis and accumulation of anthocyanin in pigment-free maize tissue cells [56]. ZMP1 and ZMPL bind to and activate transcription of the A1 gene required for 3-deoxy flavonoid to promote the production of flavonoid compounds [57,58] (Table 2).
In other monocotyledons, MYB transcription factors promote or inhibit flavonoid production (Table 3). In lily, LhMYB6 and LhMYB12 interact with bHLH transcription factors to activate the expression of DFR and the formation of spots on tepals [61], LhMYB12-Lat promotes anthocyanin synthesis [62], overexpression of LhR3MYB1 and LhR3MYB2 actually inhibits anthocyanin biosynthesis [63]; In orchids, the OgMYB1 was associated with anthocyanin synthesis in the flowers of Mandarin orchids [64]; In Tulip, TfMYB3, TfMYB4 and TfMYB5 interact with TfbHLH1, TfMYB5 activates the promoter of TfCHS1, while TfMYB3 and TfMYB4 promote the promoter activity of TfANS1 were participated in the regulating of anthocyanin biosynthesis [65]; In Phalaenopsis, PeMYB2, PeMYB11 and PeMYB12 regulate the anthocyanin synthesizing Enzymes F3′5′H, DFR1, and ANS3 to accumulate anthocyanin synthesis [66]; In Bananas, MaMYBPA1 and MaMYBPA2 interact with MaANS to promote flavonoid synthesis [67,68], MaMYBA1 and MaMYBA2 function as components of transcription factor complexes with bHLH and WD40 proteins to activate anthocyanin biosynthesis [67], while MaMYBPR1/MaMYB4, MaMYBPR2, MaMYBPR3 and MaMYBPR4 inhibit the synthesis of anthocyanins [68,69]; In Onions, AcMYB1 bounds to the promoters of anthocyanidin synthase (AcANS) and flavonoid 3-hydroxylase 1 (AcF3H1) and activates their expression [70]; In Dendrobium officinale, DhMYB2 and DhbHLH1 interact and are expressed together with DhDFR and DhANS, promote anthocyanin accumulation [71]. In Freesia hybrida, the R2R3-MYB factor FhMYB5 regulates the biosynthesis of anthocyanins and proanthocyanidins, FhMYB27 and FhMYBx inhibit anthocyanin synthesis by activating MBW; in contrast, FhPAP1 promotes anthocyanin synthesis [72]. In Daffodils, the NtMYB2 and NtMYB3 from Narcissus R2R3-MYB inhibit the biosynthesis of anthocyanin and flavonoids [73].

Table 2.
MYB transcription factors involved in the regulation of flavonoid compounds in model plants.
Table 2.
MYB transcription factors involved in the regulation of flavonoid compounds in model plants.
| Species (Family) | Gene | Type | Metabolism | Function | Accession | Reference |
|---|---|---|---|---|---|---|
| Arabidopsis thaliana (Brassicaceae) | AtMYB5 | R2R3-MYB | Flavonoids, Proanthocyanidins | Activation | AT3G13540.1 | [54] |
| AtMYB11/PFG2 | R2R3-MYB | Flavonols | Activation | AT3G62610.1 | [74] | |
| AtMYB12/PFG1 | R2R3-MYB | Flavonols | Activation | AT2G47460.1 | [43] | |
| AtMYB75/PAP1 | R2R3-MYB | Anthocyanins | Activation | AT1G56650.1 | [45] | |
| AtMYB90/PAP2 | R2R3-MYB | Anthocyanins | Activation | AT1G66390.1 | [45] | |
| AtMYB111/PFG3 | R2R3-MYB | Flavonols | Activation | AT5G49330.1 | [74] | |
| AtMYB113 | R2R3-MYB | Anthocyanins | Activation | AT1G66370.1 | [44] | |
| AtMYB114 | R2R3-MYB | Anthocyanins | Activation | AT1G66380.1 | [44] | |
| AtMYB123/AtTT2 | R2R3-MYB | Proanthocyanidins | Activation | AT5G35550.1 | [46] | |
| AtMYB3 | R2R3-MYB | Flavonoids | Inhibition | AT1G22640.1 | [75] | |
| AtMYB4 | R2R3-MYB | Flavonoids | Inhibition | AT4G38620.1 | [48] | |
| AtMYB7 | R2R3-MYB | Flavonols | Inhibition | AT2G16720.1 | [76] | |
| AtMYB32 | R2R3-MYB | Flavonoids | Inhibition | AT4G34990.1 | [50] | |
| AtMYBL2 | R3-MYB | Anthocyanins | Inhibition | AT1G71030.1 | [52] | |
| AtCPC | R3-MYB | Anthocyanins | Inhibition | AT2G46410.1 | [75] | |
| Oryza sativa (Poaceae) | OsC1(COLOURLESS1) | R2R3-MYB | Anthocyanins | Activation | LOC_Os06g10350.1 | [54] |
| OsP1(PERICARP COLOUR1) | R2R3-MYB | Flavonols | Activation | LOC_Os03g19120.1 | [55] | |
| OsMYB3/Kala3 | R2R3-MYB | Anthocyanins | Activation | LOC_Os03g29614.1 | [53] | |
| Zea mays (Poaceae) | ZmC1(COLOURLESS1) | R2R3-MYB | Anthocyanins | Activation | Zm00001d044975_P001 | [56] |
| ZmPL(PURPLE LEAF) | R2R3-MYB | Anthocyanins | Activation | Zm00001d037118_P001 | [58] | |
| ZmP1(PERICARP COLOUR) | R2R3-MYB | Flavonols | Activation | Zm00001d028850_P001 | [57] |

Table 3.
MYB transcription factors involved in the regulation of flavonoid compounds in monocotyledon plants.
Table 3.
MYB transcription factors involved in the regulation of flavonoid compounds in monocotyledon plants.
| Species (Family) | Gene | Type | Metabolism | Function | Accession | Reference |
|---|---|---|---|---|---|---|
| Lilium spp. (Liliaceae) | LhMYB6 | R2R3-MYB | Anthocyanins | Activation | BAJ05399.1 | [61] |
| LhMYB12 | R2R3-MYB | Anthocyanins | Activation | BAJ05398.1 | [61] | |
| LhMYB12-Lat | R2R3-MYB | Anthocyanins | Activation | BAO04194.1 | [62] | |
| LhR3MYB1 | R3-MYB | Anthocyanins | Inhibition | BBG71951.1 | [63] | |
| LhR3MYB2 | R3-MYB | Anthocyanins | Inhibition | BBG71952.1 | [63] | |
| Oncidium spp. (Orchidaceae) | OgMYB1 | R2R3-MYB | Anthocyanins | Activation | ABS58501.1 | [64] |
| Tulipa fosteriana (Liliaceae) | TfMYB3 | R2R3-MYB | Anthocyanins | Activation | AHY20034.1 | [65] |
| TfMYB4 | R2R3-MYB | Anthocyanins | Activation | AHY20035.1 | [65] | |
| TfMYB5 | R2R3-MYB | Anthocyanins | Activation | AHY20036.1 | [65] | |
| Phalaenopsis aphrodite (Orchidaceae) | PeMYB2 | R2R3-MYB | Anthocyanins | Activation | AIS35919.1 | [66] |
| PeMYB11 | R2R3-MYB | Anthocyanins | Activation | AIS35928.1 | [66] | |
| PeMYB12 | R2R3-MYB | Anthocyanins | Activation | AIS35929.1 | [66] | |
| Musa acuminata (Musaceae) | MaMYBPA1 | R2R3-MYB | Proanthocyanidins | Activation | Ma03_g07840.1 | [68] |
| MaMYBPA2 | R2R3-MYB | Anthocyanins, Proanthocyanidins | Activation | Ma10_g17650.1 | [67,68] | |
| MaMYBA1 | R2R3-MYB | Anthocyanins | Activation | Ma06_g05960.1 | [67] | |
| MaMYBA2 | R2R3-MYB | Anthocyanins | Activation | Ma09_g27990.1 | [67] | |
| MaMYBPR1/MaMYB4 | R2R3-MYB | Anthocyanins, Proanthocyanidins | Inhibition | Ma03_g21920.1 | [68] | |
| MaMYBPR2 | R2R3-MYB | Anthocyanins | Inhibition | Ma06_g11140.1 | [68] | |
| MaMYBPR3 | R2R3-MYB | Anthocyanins | Inhibition | Ma08_g16760.1 | [68] | |
| MaMYBPR4 | R2R3-MYB | Anthocyanins | Inhibition | Ma10_g19970.1 | [68] | |
| Allium cepa (Amaryllidaceae) | AcMYB1 | R2R3-MYB | Anthocyanins | Activation | AQP25672.1 | [70] |
| Dendrobium spp. (Orchidaceae) | DhMYB2 | R2R3-MYB | Anthocyanins | Activation | AQS79852.1 | [71] |
| Freesia hybrida (Iridaceae) | FhMYB5 | R2R3-MYB | Anthocyanins, Proanthocyanidins | Activation | QAX87835.1 | [72] |
| FhPAP1 | R2R3-MYB | Anthocyanins | Activation | QJW70307.1 | [72] | |
| FhMYB27 | R2R3-MYB | Anthocyanins | Inhibition | QJW70308.1 | [72] | |
| FhMYBx | R3-MYB | Anthocyanins | Inhibition | QJW70309.1 | [72] | |
| Narcissus tazetta (Amaryllidaceae) | NtMYB2 | R2R3-MYB | Anthocyanins | Inhibition | ATO58377.1 | [73] |
| NtMYB3 | R2R3-MYB | Flavonoids | Inhibition | AGO33166.1 | [73] |
4.2. Involvement of MYB Transcription Factors in Dicotyledonous Plants in Flavonoid Metabolism, Regulating the Color of Flowers, Fruits, etc.
In dicotyledonous plants, many R2R3 MYBs have been shown to play an important role in flavonoid biosynthesis, among which Arabidopsis is a very representative class and a focus of research (Table 2). In Arabidopsis, the early synthesis genes (EBGs) related to flavonoid synthesis are promoted and regulated by AtMYB12/PFG1, AtMYB11/PFG2, and AtMYB111/PFG3 [43], and the LBGs related to flavonoid synthesis are promoted and regulated by AtMYB75/PAP1, AtMYB90/PAP2, AtMYB5, and AtMYB123/AtTT2 [44,45]. In the study of the synthesis pathways of flavonoids such as Arabidopsis anthocyanins, proanthocyanidins, and flavonols, some MYB transcription factors, AtMYB123/AtTT2 and AtMYB5 bind to the promoters of different key enzyme genes to promote flavonols synthesis [77,78], AtMYB11/PFG2 and AtMYB111/PFG3, three members of subfamily 7 of the MYB family in Arabidopsis, can activate structural enzyme genes related to flavonol synthesis in different plant parts of Arabidopsis to regulate flavonol synthesis and control the accumulation of flavonol analogs in various tissues or organs of the plant [74], while AtMYB3 inhibits the synthesis of flavonoids in Arabidopsis thaliana [79], AtMYB4 and AtMYB32 interact with the bHLH transcription factors TT8, GL3 and EGL3 and thereby interfere with the transcriptional activity of the MBW complexes to inhibit the synthesis of anthocyanins [48,49,50], AtMYB7 inhibits the flavonol biosynthesis by controlling DFR and UGT [76], ectopic expression of AtMYBL2 or of a chimeric repressor that is a dominant negative form of AtMYBL2 suppressed the expression of the biosynthesis of anthocyanin [51,52], anthocyanin synthesis genes are down-regulated in AtCPC overexpressing plants [75].
In addition to its role in Arabidopsis, there is a large amount of new evidence indicating the role of R2R3 MYBs in transcriptional regulation of flavonoid metabolism in other aspects (Table 4). In grapes, the VvMYBF1 can directly activate the structural enzyme genes related to flavonol [80], the VvMYBA1 and VvMYBA2 promote anthocyanin synthesis by activating UFGT, CHS3 and LDOX [81], VvMYBPA1 and VvMYBPA2 activate the promoters of LAR and ANR to promote the synthesis of anthocyanins [82,83], VvMYB5a and VvMYB5b activate the grapevine promoters of several structural genes of the flavonoid pathway to regulate of the flavonoid [84], VvMYBPAR induces proanthocyanidin synthase to promote proanthocyanidin accumulation [85], VvMYBC2-L1, VvMYBC2-L2 and VvMYBC2-L3 inhibit anthocyanin synthesis by affecting PAL, C4H, CHS, DFR, and UFGT [86,87]. In poplar, PtrMYB134 and PtrMYB115 regulate proanthocyanidin biosynthesis by activating key flavonoid genes through a transcription factor complex composed of bHLH and WD-40 proteins [88], whereas PtrMYB165 and PtrMYB194 inhibit the activation of flavonoid promoters and reduce anthocyanin and proanthocyanidin synthesis by interacting with bHLH131 [89], overexpression of PtrMYB57 and PtrMYB182 led to a decrease in anthocyanin and PA accumulation in poplar, demonstrating that PtrMYB57 and PtrMYB182 play a negative role in the regulation of anthocyanin and PA biosynthesis in poplar [90,91], PtrRML1 controls inhibition of anthocyanins and synthesis such as DFR and UF3GT [92]. In Medicago truncatula, MtPAR and MtTT8 regulate genes encoding enzymes of the flavonoid–PA pathway via a activation of WD40-1 to regulate proanthocyanidin biosynthesis [93,94], MtMYB5 and MtMYB14 interact and activate the promoters of anthocyanidin reductase and leucoanthocyanidin reductase [95], MtMYB134 activate AtCHS and AtFLS1 to promote flavonol biosynthesis [96], ectopic expression of MtLAP1 transcription factor activates the anthocyanin [97], overexpression of MtMYB2 abolishes anthocyanin and PA accumulation in Arabidopsis thaliana seeds [98]; In soybean, GmTT2A, GmTT2B and GmMYB5A induce the accumulation of proanthocyanidins and anthocyanins [99], Ectopic expression of GmMYBA2 conferred the enhanced accumulation of delphinidin and cyanidin types of anthocyanins in W1t and w1T backgrounds, GmMYBR is an inhibitor of anthocyanins [100]. In strawberry, FaMYB123 interacts with FabHLH3 to regulate anthocyanin [101], FaMYB5 is an R2R3-MYB activator involved in the composition of MBW to regulate the biosynthesis of anthocyanin and proanthocyanidin [102], FaMYB9 and FaMYB11 activate the proanthocyanidin reductase gene promoter and promote proanthocyanidin accumulation [103], FaMYB10 regulates the expression of most of the EBGs and the LBGs to involve in anthocyanin production [104], the FaMYB1 of strawberry significantly inhibited the synthesis of anthocyanins and flavonols [105]. In rose, RcMYB1 activates its own gene promoter and those of other EBGs and LBGs; overexpression of RcMYB1 promotes anthocyanin accumulation [106]. In tea trees, CsMYB5a, CsMYB5b, and CsMYB5e promote the gene expression of CsLAR and CsANR, CsMYB5f and CsMYB5g only upregulate the gene expression of CsLAR but not CsANR to regulate proanthocyanidin biosynthesis [107], CsMYB75 promotes the expression of CsGSTF1 to improve anthocyanin synthesis [108]. In apple, the MdMYB1 gene from apple and validated it in Arabidopsis and isolated grapevine somatic cells and found that MdMYB1 is capable of ectopic expression for the synthesis of anthocyanin and has abundant polymorphisms in its promoter region, overexpression of MdMYB3 has resulted in transcriptional activation of several anthocyanins and flavonol pathway genes, including CHS, CHI, UFGT and FLS [109], MdMYB9, MdMYB10 and MdMYB11 interact with MdbHLH3, bind to the promoters of ANS, ANR, and LAR, and promote anthocyanin synthesis [110,111], MdMYB12 interacts with bHLH3 and bHLH33 and played an essential role in proanthocyanidin synthesis [112], MdMYB22 activate flavonol pathways by combining directly with the flavonol synthase promoter. MdMYB24L positively regulates the transcription of MdDFR and MdUFGT and promotes anthocyanin accumulation [113], MdWRKY11 binds to W-box cis-elements in the MdMYB10 and MdUFGT promoters and promotes anthocyanin synthesis in apple [114], MdMYB110a binds to the promoter of CHS and promotes anthocyanin synthesis [115], MdMYBA bound to an anthocyanidin synthase (MdANS) promoter region to control the increase in anthocyanins [116], the interaction between MdMYBPA1 and MdbHLH33 forms a complex, promote the production of proanthocyanidin [117], MdbHLH33 in callus overexpressing MdMYB16 and found that it weakened the inhibitory effect of MdMYB16 on anthocyanin synthesis [118]. In kiwifruit, AcMYBF110 and AcbHLH1, AcbHLH4, AcbHLH5 and AcWDR1 together activate the kiwifruit anthocyanin synthesis promoter and regulate anthocyanin accumulation [119], AcMYB123 activates promoters of AcANS and AcF3GT1 that encode the dedicated enzymes for anthocyanin biosynthesis to affect the accumulation of anthocyanins [120]. In tomato, the SlMYB75/AN2 regulates chlorophyll, carotenoids, and flavonoids [121], and SlMYBATV plays a repressive role in anthocyanin biosynthesis in tomato [122]. In cucumber, CsMYB60 activates CsFLS and CsLAR to promote biosynthesis of flavonols and proanthocyanidins in cucumber [123]. In peony flowers, PsMYB58 interacted with PsbHLH1 and PsbHLH3 to enhance anthocyanin accumulation in various organs [124]. In carrot, DcMYB113 induces DcbHLH3, which controls anthocyanin biosynthesis gene expression and promotes anthocyanin biosynthesis [125,126].

Table 4.
MYB transcription factors involved in the regulation of flavonoid compounds in dicotyledonous plants.
5. Prospects
MYB transcription factors are central regulators of the flavonoid biosynthesis pathway in both monocot and dicot species. Comparative analyses between model monocots and the dicot Arabidopsis thaliana have revealed fundamental divergences in regulatory circuitry. In Arabidopsis, EBGs are primarily activated by functionally redundant R2R3-MYB proteins, while LBGs require the assembly of MBW complexes for transcriptional activation. In contrast, monocots exhibit a more integrated regulatory model in which the MBW complex directly coordinates both EBGs and LBGs. This functional plasticity suggests that the MBW module has undergone lineage-specific evolution, leading to distinct architectural logics in flavonoid pathway regulation. Despite progress in characterizing the MBW complex, major knowledge gaps persist regarding the evolutionary mechanisms and molecular determinants underlying these regulatory divergences. Key unanswered questions include how the MBW complex acquired extended target gene specificity in monocots, the role of cis-regulatory element evolution and chromatin accessibility in shaping regulatory divergence, and the contribution of non-MYB factors and post-translational modifications to pathway fine-tuning in a lineage-specific manner. Addressing these questions is critical not only for understanding the evolutionary rewiring of metabolic networks but also for harnessing MYB regulators in biotechnology. Targeted manipulation of MYBs—through overexpression, silencing, or genome editing—holds substantial promise for tailoring flavonoid profiles to enhance crop resilience, nutritional content, and visual traits. Examples include increasing antioxidant levels in food crops, modifying flower and fruit pigmentation, and reducing antinutrient accumulation. Thus, elucidating the mechanistic basis of MYB regulatory divergence offers a foundation for predictive metabolic engineering across economically important plant species.
Author Contributions
Z.S., H.L., Q.L., T.B. and J.N. conceived and designed the review. H.L. wrote the manuscript, and Z.S., H.L., Q.L., T.B. and J.N. proposed revisions to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 32071731), and the Personnel Startup Project of the Scientific Research and Development Foundation of Liaocheng University (Grant No. 318042402).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 103–114. [Google Scholar] [CrossRef]
- Islam, K.; Rawoof, A.; Ahmad, I.; Dubey, M.; Momo, J.; Ramchiary, N. Capsicum chinense MYB Transcription Factor Genes: Identification, Expression Analysis, and Their Conservation and Diversification With Other Solanaceae Genomes. Front. Plant Sci. 2021, 12, 265–280. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Zhang, Y.; Meng, H.; Wang, K.; Sun, Q.; Li, X.; Dong, H.; Chen, L.; He, F. Bioinformatics analysis of Myelin Transcription Factor 1. Technol. Health Care 2021, 29, 441–453. [Google Scholar] [CrossRef]
- Tan, Z.; Lu, D.; Li, L.; Su, X.; Sun, Y.; Wang, L.; Yu, Y.; Wan, X.; Xu, L.; Li, C. Comprehensive analysis of safflower R2R3-MYBs reveals the regulation mechanism of CtMYB76 on flavonol biosynthesis. Ind. Crops Prod. 2025, 15, 227–244. [Google Scholar] [CrossRef]
- Zhao, C.; Hou, H.; Wu, J.; Zhu, Y.; Shao, Q.; Lv, A. DcMYB30 negatively function in drought tolerance of Dendrobium catenatum by modulating flavonoid biosynthesis. Plant Physiol. Biochem. 2025, 11, 199–221. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, L.; Wang, H.; Chen, X.; Wang, Y.; Yang, H.; Wei, C.; Wan, X.; Xia, T. The R2R3-MYB, bHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct. Integr. Genom. 2013, 13, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 377–392. [Google Scholar] [CrossRef]
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The Evolution of Flavonoid Biosynthesis: A Bryophyte Perspective. Front. Plant Sci. 2020, 11, 7–21. [Google Scholar] [CrossRef]
- Wilkins, O.; Nahal, H.; Foong, J.; Provart, N.J.; Campbell, M.M. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009, 149, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Yu, C.; Wang, C.; Jin, Y.; Zhang, H. MYB transcription factor PdMYB118 directly interacts with bHLH transcription factor PdTT8 to regulate wound-induced anthocyanin biosynthesis in poplar. BMC Plant Biol. 2020, 20, 173–197. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Ma, D.; Constabel, C.P. Complex regulation of proanthocyanidin biosynthesis in plants by R2R3 MYB activators and repressors. Recent Adv. Polyphen. Res. 2021, 7, 207–225. [Google Scholar]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A Multifunctional Flavonol in Biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, Q.; Aniya; Chen, J.; Jin, Z. Copigmentation effect of flavonols on anthocyanins in black mulberry juice and their interaction mechanism investigation. Food Chem. 2023, 399, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.B.; Li, Y.H.; Shu, X.C.; Pu, Y.T.; Wang, X.J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Li, K.; Yang, D.; Liu, N.; Zhang, L.; Zhao, L.; Zhang, X.; Liu, Y.; Gao, L.; et al. Roles of the 2-Oxoglutarate-Dependent Dioxygenase Superfamily in the Flavonoid Pathway: A Review of the Functional Diversity of F3H, FNS I, FLS, and LDOX/ANS. Molecules 2021, 26, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Q.; Liu, C.; Zhang, N.; Xu, W. Flavonoids as key players in cold tolerance: Molecular insights and applications in horticultural crops. Hortic. Res. 2025, 12, 366–380. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L.; Zhong, Y. Structure, evolution, and roles of MYB transcription factors proteins in secondary metabolite biosynthetic pathways and abiotic stresses responses in plants: A comprehensive review. Front. Plant Sci. 2025, 16, 844–860. [Google Scholar] [CrossRef]
- Patyka, M.; Khablak, S.; Patyka, T.; Bondareva, L.; Dolia, M.; Spychak, V.; Lykholat, Y. Evolution of immune mechanisms in monocots and dicots in response to microbial pathogens and abiotic stressors. Biosyst. Divers. 2025, 33, 531–545. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Tian, B.; Shi, G.; Liu, C.; Guo, J.; Cao, G.; Wei, F. Metabolome and transcriptome analyses of anthocyanin biosynthesis reveal key metabolites and candidate genes in purple wheat (Triticum aestivum L.). Physiol. Plant 2023, 175, 921–936. [Google Scholar] [CrossRef]
- Yang, T.; Wu, X.; Wang, W.; Wu, Y. Regulation of seed storage protein synthesis in monocot and dicot plants: A comparative review. Mol. Plant 2023, 16, 145–167. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y.; Liao, Y.; Gong, Y.; Yang, J. Unveiling the evolution of VIP1 subgroup bZIP transcription factors in plants and the positive effects of BdiVIP1A on heat stress response in Brachypodium distachyon. Plant Cell Rep. 2025, 44, 179–195. [Google Scholar] [CrossRef]
- Kuzmin, E.; Taylor, J.S.; Boone, C. Retention of duplicated genes in evolution. Trends Genet. 2022, 38, 59–72. [Google Scholar] [CrossRef]
- Clapier, C.R. Sophisticated Conversations between Chromatin and Chromatin Remodelers, and Dissonances in Cancer. Int. J. Mol. Sci. 2021, 22, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, L.; Jiang, W. Understanding AP2/ERF Transcription Factor Responses and Tolerance to Various Abiotic Stresses in Plants: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 93–108. [Google Scholar] [CrossRef]
- Thakur, S.; Vasudev, P.G. MYB transcription factors and their role in Medicinal plants. Mol. Biol. Rep. 2022, 49, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Hua, Q.; Chen, C.; Zhang, Z.; Zhang, R.; Zhao, J.; Hu, G.; Chen, J.; Qin, Y. Genome-Wide Characterization of R2R3-MYB Transcription Factors in Pitaya Reveals a R2R3-MYB Repressor HuMYB1 Involved in Fruit Ripening through Regulation of Betalain Biosynthesis by Repressing Betalain Biosynthesis-Related Genes. Cells 2021, 10, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, J.; Xia, Y.; Zhang, L.; Du, H. Evolution and functional diversification of R2R3-MYB transcription factors in plants. Hortic. Res. 2022, 9, 58–74. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Gu, G.; Yuan, J.; Shen, S.; Jin, L.; Lin, Z.; Lin, J.; Xie, X. Genome-wide identification and expression analysis of the R2R3-MYB gene family in tobacco (Nicotiana tabacum L.). BMC Genom. 2022, 23, 432–458. [Google Scholar] [CrossRef]
- LaFountain, A.; Yuan, Y. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Lipsick, J.S. One billion years of Myb. Oncogene 1996, 13, 223–235. [Google Scholar] [PubMed]
- Jiang, C.; Gu, J.; Chopra, S.; Gu, X.; Peterson, T. Ordered origin of the typical two- and three-repeat Myb genes. Gene 2004, 22, 326–349. [Google Scholar] [CrossRef]
- Lucas, C.W.; Maximilian, L.; Stacey, D.S. MYB regulator of ‘colorless’ flavonols underlies the evolution of red flowers in Iochroma (Solanaceae). G3 2025, 230, jkaf230. [Google Scholar]
- Li, P.; Xia, E.; Fu, J.; Xu, Y.; Zhao, X.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.; Zhao, J. Diverse roles of MYB transcription factors in regulating secondary metabolite biosynthesis, shoot development, and stress responses in tea plants (Camellia sinensis). Plant J. Cell Mol. Biol. 2022, 110, 1144–1165. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Abdirad, S.; Ghaffari, M.R.; Majd, A.; Irian, S.; Soleymaniniya, A.; Daryani, P.; Koobaz, P.; Shobbar, Z.S.; Farsad, L.K.; Yazdanpanah, P.; et al. Genome-Wide Expression Analysis of Root Tips in Contrasting Rice Genotypes Revealed Novel Candidate Genes for Water Stress Adaptation. Front. Plant Sci. 2022, 13, 79–92. [Google Scholar] [CrossRef]
- Schilbert, H.M.; Glover, B.J. Analysis of flavonol regulator evolution in the Brassicaceae reveals MYB12, MYB111 and MYB21 duplications and MYB11 and MYB24 gene loss. BMC Genom. 2022, 23, 604–617. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB Transcription Factors and Its Regulation in Secondary Cell Wall Formation and Lignin Biosynthesis during Xylem Development. Int. J. Mol. Sci. 2021, 22, 90–104. [Google Scholar] [CrossRef]
- Ma, L.; Liu, K.W.; Li, Z.; Hsiao, Y.Y.; Qi, Y.; Fu, T.; Tang, G.D.; Zhang, D.; Sun, W.H.; Liu, D.K.; et al. Diploid and tetraploid genomes of Acorus and the evolution of monocots. Nat. Commun. 2023, 14, 61–75. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Kasahara, R.D. An Overview on MADS Box Members in Plants: A Meta-Review. Int. J. Mol. Sci. 2024, 25, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 2005, 138, 83–96. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.; Lloyd, A. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. Cell Mol. Biol. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Borevitz, J.; Xia, Y.; Blount, J.; Dixon, R.; Lamb, C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 2000, 12, 383–2394. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Jond, C.; Debeaujon, I.; Caboche, M.; Lepiniec, L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 2001, 13, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.; Wheeler, J.; Heazlewood, J.; Li, S.F.; Parish, R.W. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004, 40, 979–995. [Google Scholar] [CrossRef]
- Li, X.; Zhong, M.; Qu, L.; Yang, J.; Liu, X.; Zhao, Q.; Liu, X.; Zhao, X. AtMYB32 regulates the ABA response by targeting ABI3, ABI4 and ABI5 and the drought response by targeting CBF4 in Arabidopsis. Plant Sci. 2021, 310, 983–996. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Guan, M.; Zhao, C.; Geng, P.; Zhao, Q. Arabidopsis MYB4 plays dual roles in flavonoid biosynthesis. Plant J. Cell Mol. Biol. 2020, 101, 637–652. [Google Scholar] [CrossRef]
- Zimmermann, I.; Heim, M.; Weisshaar, B.; Uhrig, J. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. Cell Mol. Biol. 2004, 40, 22–34. [Google Scholar] [CrossRef]
- Matsui, K.; Umemura, Y.; Ohme-Takagi, M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 2008, 55, 954–967. [Google Scholar] [CrossRef]
- Dubos, C.; Le Gourrierec, J.; Baudry, A.; Huep, G.; Lanet, E.; Debeaujon, I.; Routaboul, J.; Alboresi, A.; Weisshaar, B.; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2008, 55, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, H.; Zhao, M.; Yang, Z.; Zhou, Z.; Guo, Y.; Lin, Y.; Chen, H. OsMYB3 is a R2R3-MYB gene responsible for anthocyanin biosynthesis in black rice. Mol. Breed. 2021, 41, 51–65. [Google Scholar] [CrossRef]
- Upadhyaya, G.; Das, A.; Ray, S. A rice R2R3-MYB (OsC1) transcriptional regulator improves oxidative stress tolerance by modulating anthocyanin biosynthesis. Physiol. Plant. 2021, 173, 2334–2349. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhu, H.; Huang, C.; Liu, C.; Chang, Y.; Kong, Z.; Zhou, Z.; Wang, G.; Lin, Y.; et al. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol. 2019, 223, 705–721. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.; Saedler, H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 684–699. [Google Scholar] [CrossRef]
- Grotewold, E.; Drummond, B.; Bowen, B.; Peterson, T. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 1994, 76, 543–553. [Google Scholar] [CrossRef]
- Cone, K.; Cocciolone, S.; Burr, F.; Burr, B. Maize anthocyanin regulatory gene pl is a duplicate of c1 that functions in the plant. Plant Cell 1993, 5, 1795–1805. [Google Scholar] [PubMed]
- Higgins, I.J.; Choudury, S.G.; Husbands, A.Y. Mechanisms driving functional divergence of transcription factor paralogs. New Phytol. 2025, 247, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Lou, Q.; Hao, L.; Qi, G.; Tian, Y.; Pu, X.; He, C.; Wang, Y.; Xu, W.; Xu, Z.; et al. Comparative genomics reveal the convergent evolution of CYP82D and CYP706X members related to flavone biosynthesis in Lamiaceae and Asteraceae. Plant J. 2022, 109, 1305–1318. [Google Scholar] [CrossRef]
- Yamagishi, M.; Shimoyamada, Y.; Nakatsuka, T.; Masuda, K. Two R2R3-MYB Genes, Homologs of Petunia AN2, Regulate Anthocyanin Biosyntheses in Flower Tepals, Tepal Spots and Leaves of Asiatic Hybrid Lily. Plant Cell Physiol. 2010, 13, 55–69. [Google Scholar] [CrossRef]
- Yamagishi, M.; Toda, S.; Tasaki, K. The novel allele of the LhMYB12 gene is involved in splatter-type spot formation on the flower tepals of Asiatic hybrid lilies (Lilium spp.). New Phytol. 2014, 201, 1009–1020. [Google Scholar] [CrossRef]
- Sakai, M.; Yamagishi, M.; Matsuyama, K. Repression of anthocyanin biosynthesis by R3-MYB transcription factors in lily (Lilium spp.). Plant Cell Rep. 2019, 38, 609–622. [Google Scholar] [CrossRef]
- Chiou, C.; Yeh, K. Differential expression of MYB gene (OgMYB1) determines color patterning in floral tissue of Oncidium Gower Ramsey. Plant Mol. Biol. 2008, 66, 379–388. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, Y.; Tang, D. Isolation and characterization of R2R3-MYB and basic helix–loop–helix (bHLH) transcription factors involved in anthocyanin biosynthesis in tulip tepals. Acta Physiol. Plant. 2020, 42, 56–71. [Google Scholar] [CrossRef]
- Hsu, C.; Chen, Y.; Tsai, W.; Chen, W.; Chen, H. Three R2R3-MYB transcription factors regulate distinct floral pigmentation patterning in Phalaenopsis spp. Plant Physiol. 2015, 168, 175–191. [Google Scholar] [CrossRef]
- Busche, M.; Pucker, B.; Weisshaar, B.; Stracke, R. Three R2R3-MYB transcription factors from banana (Musa acuminata) activate structural anthocyanin biosynthesis genes as part of an MBW complex. BMC Res. Notes 2023, 16, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.; Naik, J.; Stracke, R.; Pandey, A. Interplay between R2R3 MYB-type activators and repressors regulates proanthocyanidin biosynthesis in banana (Musa acuminata). New Phytol. 2022, 236, 1108–1127. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, S.; Yang, Q.; Gao, H.; Sheng, O.; Bi, F.; Li, C.; Dong, T.; Yi, G.; He, W.; et al. MaMYB4, an R2R3-MYB Repressor Transcription Factor, Negatively Regulates the Biosynthesis of Anthocyanin in Banana. Front. Plant Sci. 2020, 11, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Schwinn, K.E.; Ngo, H.; Kenel, F.; Brummell, D.A.; Albert, N.W.; McCallum, J.A.; Pither-Joyce, M.; Crowhurst, R.N.; Eady, C.; Davies, K.M. The onion (Allium cepa L.) R2R3-MYB gene MYB1 regulates anthocyanin biosynthesis. Front. Plant Sci. 2016, 7, 1865–1880. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, J.; Ding, L.; Huang, M.; Huang, S.; Yang, G.; Yin, J. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals. Plant Physiol. Biochem. 2017, 112, 335–345. [Google Scholar] [CrossRef]
- Xiaotong, S.; Deyu, Z.; Ruifang, G.; Meng, Q.; Liudi, Z.; Jia, Z.; Yanan, W.; Qi, Z.; Niu, Z.; Guoyun, X.; et al. Molecular insights into TT2-type MYB regulators illuminate the complexity of floral flavonoids biosynthesis in Freesia hybrida. Hortic. Res. 2025, 12, 52–68. [Google Scholar]
- Anwar, M.; Wang, G.; Wu, J.; Waheed, S.; Allan, A.; Zeng, L. Ectopic Overexpression of a Novel R2R3-MYB, NtMYB2 from Chinese Narcissus Represses Anthocyanin Biosynthesis in Tobacco. Molecules 2018, 23, 781–795. [Google Scholar] [CrossRef]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. Cell Mol. Biol. 2007, 50, 660–677. [Google Scholar] [CrossRef]
- Zhu, H.; Fitzsimmons, K.; Khandelwal, A.; Kranz, R. CPC, a single-repeat R3 MYB, is a negative regulator of anthocyanin biosynthesis in Arabidopsis. Mol. Plant 2009, 2, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Fornalé, S.; Lopez, E.; Salazar-Henao, J.; Fernández-Nohales, P.; Rigau, J.; Caparros-Ruiz, D. AtMYB7, a new player in the regulation of UV-sunscreens in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Guo, Q.; Li, H.; Yuan, G.; Gui, Q.; Xiao, Y.; Liao, M.; Yang, L. Integrated transcriptomic and WGCNA analyses reveal candidate genes regulating mainly flavonoid biosynthesis in Litsea coreana var. sinensis. BMC Plant Biol. 2024, 24, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Brisou, G.; Dalmais, M.; Thévenin, J.; van der Wal, F.; Latrasse, D.; Suresh Devani, R.; Benhamed, M.; Dubreucq, B.; Boualem, A.; et al. TT2The Seed Development Factors and Regulate Heat Stress Response in. Genes 2021, 12, 46–59. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, K.; Sun, Z.; Yan, M.; Chen, C.; Zhang, X.; Tang, Y.; Wu, Y. LNK1 and LNK2 Corepressors Interact with the MYB3 Transcription Factor in Phenylpropanoid Biosynthesis. Plant Physiol. 2017, 174, 1348–1358. [Google Scholar] [CrossRef]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.; Walker, A.; Robinson, S.; Bogs, J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef]
- Poudel, P.R.; Azuma, A.; Kobayashi, S.; Koyama, K.; Goto-Yamamoto, N. VvMYBAs induce expression of a series of anthocyanin biosynthetic pathway genes in red grapes (Vitis vinifera L.). Sci. Hortic. 2021, 283, 121–134. [Google Scholar] [CrossRef]
- Bogs, J.; Jaffé, F.; Takos, A.; Walker, A.; Robinson, S. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007, 143, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Koyama, K.; Numata, M.; Nakajima, I.; Goto-Yamamoto, N.; Matsumura, H.; Tanaka, N. Functional characterization of a new grapevine MYB transcription factor and regulation of proanthocyanidin biosynthesis in grapes. J. Exp. Bot. 2014, 65, 4433–4449. [Google Scholar] [CrossRef]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, G.; Liu, L.; Zhang, Q.; Han, Z.; Chen, X.; Li, B. A R2R3-MYB Transcription Factor, VvMYBC2L2, Functions as a Transcriptional Repressor of Anthocyanin Biosynthesis in Grapevine (Vitis vinifera L.). Molecules 2018, 24, 92–107. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Ma, D.; Mellway, R.; Gesell, A.; Yoshida, K.; Walker, V.; Tran, L.; Stewart, D.; Reichelt, M.; Suvanto, J.; et al. Poplar MYB115 and MYB134 Transcription Factors Regulate Proanthocyanidin Synthesis and Structure. Plant Physiol. 2017, 174, 154–171. [Google Scholar] [CrossRef]
- Ma, D.; Reichelt, M.; Yoshida, K.; Gershenzon, J.; Constabel, C. Two R2R3-MYB proteins are broad repressors of flavonoid and phenylpropanoid metabolism in poplar. Plant J. Cell Mol. Biol. 2018, 96, 949–965. [Google Scholar] [CrossRef]
- Wan, S.; Li, C.; Ma, X.; Luo, K. PtrMYB57 contributes to the negative regulation of anthocyanin and proanthocyanidin biosynthesis in poplar. Plant Cell Rep. 2017, 36, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ma, D.; Constabel, C. The MYB182 protein down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiol. 2015, 167, 693–710. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, L.; Liu, S.; Zhou, L.; Wang, X.; Wang, W.; Cai, L.; Wu, X.; Chang, Y.; Wang, S. A repressor motif-containing poplar R3 MYB-like transcription factor regulates epidermal cell fate determination and anthocyanin biosynthesis in Arabidopsis. J. Plant Biol. 2016, 59, 525–535. [Google Scholar] [CrossRef]
- Verdier, J.; Zhao, J.; Torres-Jerez, I.; Ge, S.; Liu, C.; He, X.; Mysore, K.; Dixon, R.; Udvardi, M. MtPAR MYB transcription factor acts as an on switch for proanthocyanidin biosynthesis in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2012, 109, 1766–1771. [Google Scholar] [CrossRef]
- Li, P.; Chen, B.; Zhang, G.; Chen, L.; Dong, Q.; Wen, J.; Mysore, K.; Zhao, J. Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol. 2016, 210, 905–921. [Google Scholar] [CrossRef]
- Liu, C.; Jun, J.; Dixon, R. MYB5 and MYB14 Play Pivotal Roles in Seed Coat Polymer Biosynthesis in Medicago truncatula. Plant Physiol. 2014, 165, 1424–1439. [Google Scholar] [CrossRef]
- Naik, J.; Rajput, R.; Pucker, B.; Stracke, R.; Pandey, A. The R2R3-MYB transcription factor MtMYB134 orchestrates flavonol biosynthesis in Medicago truncatula. Plant Mol. Biol. 2021, 106, 157–172. [Google Scholar] [CrossRef]
- Peel, G.; Pang, Y.; Modolo, L.; Dixon, R. The LAP1 MYB transcription factor orchestrates anthocyanidin biosynthesis and glycosylation in Medicago. Plant J. Cell Mol. Biol. 2009, 59, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Liu, C.; Xiao, X.; Dixon, R. The Transcriptional Repressor MYB2 Regulates Both Spatial and Temporal Patterns of Proanthocyandin and Anthocyanin Pigmentation in Medicago truncatula. Plant Cell 2015, 27, 2860–2879. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Rao, X.; Li, Y.; Jun, J.; Dixon, R. Dissecting the transcriptional regulation of proanthocyanidin and anthocyanin biosynthesis in soybean (Glycine max). Plant Biotechnol. J. 2021, 19, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Han, T.; Xun, H.; Zeng, X.; Li, P.; Li, Y.; Wang, Y.; Shao, Y.; Cheng, X.; Feng, X.; et al. MYB transcription factors GmMYBA2 and GmMYBR function in a feedback loop to control pigmentation of seed coat in soybean. J. Exp. Bot. 2021, 72, 4401–4418. [Google Scholar] [CrossRef]
- Martínez-Rivas, F.; Blanco-Portales, R.; Serratosa, M.; Ric-Varas, P.; Guerrero-Sánchez, V.; Medina-Puche, L.; Moyano, L.; Mercado, J.; Alseekh, S.; Caballero, J.; et al. FaMYB123 interacts with FabHLH3 to regulate the late steps of anthocyanin and flavonol biosynthesis during ripening. Plant J. Cell Mol. Biol. 2023, 114, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y. A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria× ananassa). Plant Biotechnol. J. 2023, 15, 83–98. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.; de Vos, R.C.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-b HLH-WD 40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Cumplido-Laso, G.; Amil-Ruiz, F.; Hoffmann, T.; Ring, L.; Rodríguez-Franco, A.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; Blanco-Portales, R. MYB10 plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria× ananassa fruits. J. Exp. Bot. 2014, 65, 401–417. [Google Scholar] [CrossRef]
- Aharoni, A.; De Vos, C.; Wein, M.; Sun, Z.; Greco, R.; Kroon, A.; Mol, J.; O’Connell, A. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. Cell Mol. Biol. 2001, 28, 319–332. [Google Scholar] [CrossRef]
- He, G.; Zhang, R.; Jiang, S.; Wang, H.; Ming, F. The MYB transcription factor RcMYB1 plays a central role in rose anthocyanin biosynthesis. Hortic. Res. 2023, 10, 80–94. [Google Scholar] [CrossRef]
- Jiao, T.; Huang, Y.; Wu, Y.; Jiang, T.; Li, T.; Liu, Y.; Liu, Y.; Han, Y.; Liu, Y.; Jiang, X.; et al. Functional diversity of subgroup 5 R2R3-MYBs promoting proanthocyanidin biosynthesis and their key residues and motifs in tea plant. Hortic. Res. 2023, 10, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wang, L.; Zhang, Y.; Ruan, L.; Li, H.; Wu, L.; Xu, L.; Zhang, C.; Zhou, X.; Cheng, H.; et al. A coupled role for CsMYB75 and CsGSTF1 in anthocyanin hyperaccumulation in purple tea. Plant J. Cell Mol. Biol. 2019, 97, 825–840. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Han, Y.; Wei, G.; Korban, S. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013, 13, 176–186. [Google Scholar] [CrossRef]
- An, X.; Tian, Y.; Chen, K.; Liu, X.; Liu, D.; Xie, X.; Cheng, C.; Cong, P.; Hao, Y. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Jiang, S.; Zhang, Z.; Lu, N.; Qiu, H.; Qu, C.; Wang, Y.; Wu, S.; Chen, X. MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant J. Cell Mol. Biol. 2017, 90, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Jiang, H.; Mao, Z.; Wang, N.; Jiang, S.; Xu, H.; Yang, G.; Zhang, Z.; Chen, X. The R2R3-MYB transcription factor MdMYB24-like is involved in methyl jasmonate-induced anthocyanin biosynthesis in apple. Plant Physiol. Biochem. 2019, 139, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Yu, L.; Jiang, H.; Guo, Z.; Xu, H.; Jiang, S.; Fang, H.; Zhang, J.; Su, M.; et al. MdWRKY11 Participates in Anthocyanin Accumulation in Red-Fleshed Apples by Affecting MYB Transcription Factors and the Photoresponse Factor MdHY5. J. Agric. Food Chem. 2019, 67, 8783–8793. [Google Scholar] [CrossRef] [PubMed]
- Chagné, D.; Lin-Wang, K.; Espley, R.; Volz, R.; How, N.; Rouse, S.; Brendolise, C.; Carlisle, C.; Kumar, S.; De Silva, N.; et al. An ancient duplication of apple MYB transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol. 2013, 161, 225–239. [Google Scholar] [CrossRef]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W.; et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. Cell Mol. Biol. 2018, 96, 39–55. [Google Scholar] [CrossRef]
- Xu, H.; Wang, N.; Liu, J.; Qu, C.; Wang, Y.; Jiang, S.; Lu, N.; Wang, D.; Zhang, Z.; Chen, X. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes. Plant Mol. Biol. 2017, 94, 149–165. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, K.; Qi, Y.; Lv, G.; Ren, X.; Liu, Z.; Ma, F. Transcriptional regulation of anthocyanin synthesis by MYB-bHLH-WDR complexes in kiwifruit (Actinidia chinensis). J. Agric. Food Chem. 2021, 69, 3677–3691. [Google Scholar] [CrossRef]
- Wang, L.; Tang, W.; Hu, Y.; Zhang, Y.; Sun, J.; Guo, X.; Lu, H.; Yang, Y.; Fang, C.; Niu, X.; et al. A MYB/bHLH complex regulates tissue-specific anthocyanin biosynthesis in the inner pericarp of red-centered kiwifruit Actinidia chinensis cv. Hongyang. Plant J. Cell Mol. Biol. 2019, 99, 359–378. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22–38. [Google Scholar] [CrossRef]
- Cao, X.; Qiu, Z.; Wang, X.; Van Giang, T.; Liu, X.; Wang, J.; Wang, X.; Gao, J.; Guo, Y.; Du, Y.; et al. A putative R3 MYB repressor is the candidate gene underlying atroviolacium, a locus for anthocyanin pigmentation in tomato fruit. J. Exp. Bot. 2017, 68, 5745–5758. [Google Scholar] [CrossRef]
- Li, J.; Luan, Q.; Han, J.; Zhang, C.; Liu, M.; Ren, Z. CsMYB60 directly and indirectly activates structural genes to promote the biosynthesis of flavonols and proanthocyanidins in cucumber. Hortic. Res. 2020, 7, 103–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Ma, H.; Duan, X.; Gao, S.; Zhou, X.; Cheng, Y. The R2R3-MYB gene PsMYB58 positively regulates anthocyanin biosynthesis in tree peony flowers. Plant Physiol. Biochem. 2021, 164, 279–288. [Google Scholar] [CrossRef]
- Duan, A.; Deng, Y.; Tan, S.; Xu, Z.; Xiong, A. A MYB activator, DcMYB11c, regulates carrot anthocyanins accumulation in petiole but not taproot. Plant Cell Environ. 2023, 46, 2794–2809. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Q.; Feng, K.; Yu, X.; Xiong, A. DcMYB113, a root-specific R2R3-MYB, conditions anthocyanin biosynthesis and modification in carrot. Plant Biotechnol. J. 2020, 18, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Katayama-Ikegami, A.; Yonemori, K. Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci. Hortic. 2011, 130, 373–380. [Google Scholar] [CrossRef]
- Akagi, T.; Ikegami, A.; Tsujimoto, T.; Kobayashi, S.; Sato, A.; Kono, A.; Yonemori, K. DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2009, 151, 2028–2045. [Google Scholar] [CrossRef]
- Zhou, H.; Lin-Wang, K.; Liao, L.; Gu, C.; Lu, Z.; Allan, A.; Han, Y. Peach MYB7 activates transcription of the proanthocyanidin pathway gene encoding leucoanthocyanidin reductase, but not anthocyanidin reductase. Front. Plant Sci. 2015, 6, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xie, L.; Ma, Y.; Ren, C.; Xing, M.; Fu, Z.; Wu, X.; Yin, X.; Xu, C.; Li, X. PpMYB15 and PpMYBF1 transcription factors are involved in regulating flavonol biosynthesis in peach fruit. J. Agric. Food Chem. 2018, 67, 644–652. [Google Scholar] [CrossRef]
- Rahim, M.; Busatto, N.; Trainotti, L. Regulation of anthocyanin biosynthesis in peach fruits. Planta 2014, 240, 913–929. [Google Scholar] [CrossRef]
- Schwinn, K.; Venail, J.; Shang, Y.; Mackay, S.; Alm, V.; Butelli, E.; Oyama, R.; Bailey, P.; Davies, K.; Martin, C. A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. Plant Cell 2006, 18, 831–851. [Google Scholar] [CrossRef] [PubMed]
- Moyano, E.; Martínez-Garcia, J.F.; Martin, C. Apparent redundancy in myb gene function provides gearing for the control of flavonoid biosynthesis in antirrhinum flowers. Plant Cell 1996, 8, 1519–1532. [Google Scholar] [PubMed]
- Tamagnone, L.; Merida, A.; Parr, A.; Mackay, S.; Culianez-Macia, F.; Roberts, K.; Martin, C. The AmMYB308 and AmMYB330 transcription factors from antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 1998, 10, 135–154. [Google Scholar] [CrossRef]
- Quattrocchio, F.; Wing, J.; van der Woude, K.; Souer, E.; de Vetten, N.; Mol, J.; Koes, R. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell 1999, 11, 1433–1444. [Google Scholar] [CrossRef][Green Version]
- Quattrocchio, F.; Verweij, W.; Kroon, A.; Spelt, C.; Mol, J.; Koes, R. PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell 2006, 18, 1274–1291. [Google Scholar] [CrossRef]
- Albert, N.; Lewis, D.; Zhang, H.; Schwinn, K.; Jameson, P.; Davies, K. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. Cell Mol. Biol. 2011, 65, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.; Davies, K.; Lewis, D.; Zhang, H.; Montefiori, M.; Brendolise, C.; Boase, M.; Ngo, H.; Jameson, P.; Schwinn, K. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, T.; Pan, Q.; Anupol, N.; Chen, H.; Shi, J.; Liu, F.; Deqiang, D.; Wang, C.; Zhao, J.; et al. RrMYB5- and RrMYB10-regulated flavonoid biosynthesis plays a pivotal role in feedback loop responding to wounding and oxidation in Rosa rugosa. Plant Biotechnol. J. 2019, 17, 2078–2095. [Google Scholar] [CrossRef]
- Zou, K.; Wang, Y.; Zhao, M.; Zhao, L.; Xu, Z. Cloning and expression analysis of RrMYB113 gene related to anthocyanin biosynthesis in Rosa rugose. Am. J. Plant Sci. 2018, 9, 701–710. [Google Scholar] [CrossRef][Green Version]
- Zhai, R.; Zhao, Y.; Wu, M.; Yang, J.; Li, X.; Liu, H.; Wu, T.; Liang, F.; Yang, C.; Wang, Z.; et al. The MYB transcription factor PbMYB12b positively regulates flavonol biosynthesis in pear fruit. BMC Plant Biol. 2019, 19, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Zhao, S.; Xu, H.; Allan, A.; Zhang, X.; Fan, L.; Chen, L.; Su, J.; Shu, Q.; Li, K. The interaction of MYB, bHLH and WD40 transcription factors in red pear (Pyrus pyrifolia) peel. Plant Mol. Biol. 2021, 106, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Ming, M.; Allan, A.; Gu, C.; Li, L.; Wu, X.; Wang, R.; Chang, Y.; Qi, K.; Zhang, S.; et al. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. Cell Mol. Biol. 2017, 92, 437–451. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).