Abstract
Generation of state-of-the-art highly productive cabbage genotypes (Brassica oleracea convar. capitata (L.) Alef.) with improved agronomic traits is attainable using modern biotechnological approaches. However, capitata cabbage is relatively recalcitrant to de novo shoot organogenesis from callus tissue, especially with loss of somatic cell totipotency during genetic transformation. An effective and rapid protocol for in vitro indirect shoot organogenesis from hypocotyl and cotyledon explants derived from 6-day-old aseptic donor seedlings of Russian cabbage genotypes (the DH line as well as cvs. Podarok and Parus) has been developed. In order to obtain standardized donor explants, aseptic cabbage seeds were germinated under dim light conditions (30–40 µmol m−2 s−1) with a 16 h light/8 h dark photoperiod. Multiple indirect shoot organogenesis (1.47–4.93 shoots per explant) from both cotyledonary leaves and hypocotyl segments with a frequency of 55.2–89.1% was achieved through 45 days of culture on the 0.7% agar-solidified (w/v) Murashige and Skoog (MS) basal medium containing 2 mg/L 6-benzylaminopurine (6-BAP), 0.02 mg/L 1-naphthalene acetic acid (NAA), and 5 mg/L AgNO3. The regenerants were successfully rooted on an MS basal medium (69.2%) without plant growth regulators (PGRs), as well as supplemented with 0.5 mg/L NAA (86.8%). Subsequently, in vitro rooted cabbage plantlets were adapted to soil conditions with an efficiency of 85%. This rapid protocol, allowing for the performance of a full cycle from in vitro seed germination to growing adapted plantlets under ex vitro conditions over 95 days, can be successfully applied to induce an indirect shoot formation in various cabbage genotypes, and it is recommended to produce transgenic plants with improved quality traits and productivity.
1. Introduction
Cabbage (Brassica oleracea convar. capitata (L.) Alef.) is one of the key species in industrial vegetable production worldwide. According to the data of the Food and Agriculture Organization (FAO) of the United Nations, the land area under this crop in 2023 amounted to approximately 1.36 million hectares, and its total production amounted to 73.8 million tons at an average yield of 31.3 t/ha. China and India are the largest cabbage producers in the world (over 62% of the total world yield), and Russia is the third on this list (~2.6 million tons or 3.5% of the world’s total yield, grown in an area of 70.3 thousand hectares) [1].
Successfully growing cabbage in a particular ecological and geographic zone requires a range of cultivars and F1 hybrids with high adaptation potential in the abiotic and biotic conditions of the corresponding region. The most relevant for achieving the potential cabbage yield is an increase in the resistance to several fungal (Alternaria brassicicola, Botrytis cinerea, Pythium spp., and Plasmodiophora brassicae) and bacterial (Xanthomonas campestris pv. campestris and Erwinia carotovora subsp. carotovora) phytopathogens [2,3,4] as well as to abiotic stresses [4,5]. Generation of state-of-the-art, highly productive genotypes with resistance to stressful conditions of different etiologies is attainable using the traditional molecular breeding approaches [5,6,7], modern biotechnological applications [6,8,9], and genetic engineering methods [10,11,12,13], including genome editing [14]. A recent study has shown that the optimized CRISPR/Cas9 technology used to knock out the BoBPM6 and BoDMR6 genes provides genome-edited cabbage plants with broad-spectrum disease resistance, including Fusarium wilt, black rot, and clubroot [14]. However, a wide application of the state-of-the-art biotechnology and genetic engineering methods is limited by the low in vitro regeneration and transformation potentials of cabbage cells. Gerszberg, in a review [15] on the tissue culture and genetic transformation of cabbage, infers that the cabbage is, in general, recalcitrant to shoot regeneration and genetic transformation. This problem is most relevant in that the morphogenetic potential of this species is built up using genetic engineering. In particular, expression of the fusion of the GRF5–GIF1–GRF5 gene, coding plant-specific growth-regulating factor (GRF), and its GRF-interacting factor was shown to significantly (by 55.2%) increase the average regeneration rate of cabbage explants by 55.2%, demonstrating its potential in promoting the Agrobacterium-mediated transformation [14].
The in vitro shoot regeneration of cabbage is performed via somatic organogenesis and embryogenesis. Somatic embryogenesis is induced to obtain haploid plants from isolated anthers [16,17] and microspore [18,19] cultures. Cotyledonary leaves and hypocotyl segments obtained from juvenile aseptic cabbage seedlings are most frequently used as the explants for the induction of in vitro somatic shoot organogenesis [20,21,22,23,24]. In addition, indirect shoot regeneration was also successfully achieved using a protoplast culture [25,26,27], leaf and petiole explants [28], stem segments [29,30], roots [30], lateral buds [31], and immature embryos [32].
Callus formation and subsequent shoot organogenesis were induced mainly using the Murashige and Skoog (MS) basal medium [33] supplemented with different types and amounts of plant growth regulators (PGRs). Most frequently, 6-benzylaminopurine (6-BAP) and 1-naphthalene acetic acid (NAA) in a wide range of concentrations (0.1–4.0 mg/L) are used as cytokinin and auxin components, respectively [15,20,21,22,34]. Thidiazuron (TDZ) [24,28], zeatin [34], or kinetin [32] are less frequently applied instead of 6-BAP. The alternative sources of auxins are 2,4-D [32], 3-indolyl butyric acid [29,31], and 3-indolyl acetic acid (IAA) [34]. In general, the selection of PGR type and its concentration essentially depend on the genotype and explant source of B. oleracea L. convar. capitata (L.) Alef.
Thus, the development of a highly efficient protocol for induced shoot organogenesis in the hypocotyl and cotyledon explants that are the most appropriate for genetic transformation of a wide range of cabbage genotypes is a highly relevant task in the generation of transgenic and genome-edited plants with improved agronomic traits. In this study, we used the models of cotyledon and hypocotyl explants of the agronomic B. oleracea L. convar. capitata (L.) Alef. genotypes of Russian breeding to develop a highly efficient protocol for induced in vitro indirect shoot organogenesis.
2. Materials and Methods
2.1. Plant Material
Three current industrial genotypes of B. oleracea var. capitata (L.) Alef. of Russia breeding (the DH line, cvs. Parus and Podarok) were used as the plant material. The doubled haploid (DH) Nag1-8 breading line was obtained from an early-maturing non-commercialized F1 hybrid at the Limited Company “Breeding Station after N.N. Timofeev” (Moscow, Russia). The late-maturing commercial cvs. Podarok and Parus were obtained by conventional breeding at the Federal Scientific Center for Vegetable Growing (FSCVG) (Odintsovo, Russia) in 1961 and 2004, respectively. Cabbage seeds of the DH line, as well as cvs. Parus and Podarok were kindly provided by Monakhos G.F. (Limited Company “Breeding Station after N.N. Timofeev”, Moscow, Russian Federation) and Bondareva L.L. (FSCVG, Odintsovo, Russia), respectively.
2.2. Generation of Aseptic Donor Explants
Aseptic seedlings were produced in vitro from the seeds after treating their surface with sterilizing solutions. First, the seeds were rinsed with tap water for 10 min to further sterilize their surface using the following schemes: (i) sterilization with 12.5% water solution (v/v) of a fresh commercial bleach Belizna (3–5% sodium hypochlorite (NaOCl)) (Spektr, Saint Petersburg, Russia) for 20 min at a room temperature, and (ii) combined sterilization with 70% ethanol solution for 2 min and subsequent treatment with 15% water solution (v/v) of a fresh commercial bleach Belizna (3–5% NaOCl) (Spektr, Saint Petersburg, Russia) containing 0.1% Tween 20 as a non-ionic surfactant to improve the surface wettability for 15 min at a room temperature. The sterilized seeds were then three times washed with autoclaved distilled water and transferred to culture vessels containing the basal culture medium with mineral components and vitamins (MS medium; product ID: M519, PhytoTech Labs, Lenexa, KS, USA) supplemented with 3% sucrose and 0.7% agar (product ID: P1001, Duchefa Biochemie, Haarlem, The Netherlands), pH 5.7–5.8. The culture medium was autoclaved for 20 min at 121 °C and at a pressure of 1.1 atm. The donor seedlings were cultivated under WLR-351H (Sanyo, Japan) climate chamber conditions at a temperature of 24/22 °C (day/night) using both darkness and dim light (30–40 µmol m−2 s−1) with a 16 h light/8 h dark photoperiod.
The efficiency of the sterilization technique was assessed according to the germination rate (the ratio of germinated seeds to the total number of sterilized ones, %) and the number of aseptic seedlings (the ratio of the number of aseptic seedlings to the total number of sterilized seeds, %) after 6 days of culture. Each treatment involved 20 seeds and was performed in triplicate (n = 60). The initial germination rate of each cabbage genotype was preliminarily assessed by germination of non-sterile seeds in Petri dishes on paper disks moistened with water.
2.3. Induction of Callus Formation and Somatic Organogenesis
Morphogenesis was induced by culturing the cotyledonary explants, including the petioles with a length of 2–3 mm, and the hypocotyl segments from their middle parts with a length of about 10 mm, obtained from 6-day-old aseptic seedlings. The intact explants were cultured on an MS basal medium that was supplemented with 5 mg/L AgNO3 as an inhibitor of ethylene biosynthesis, as well as with 6-BAP and NAA as sources of cytokinin and auxin, respectively. The following PGR concentrations were used: 2.0 mg/L 6-BAP and 0.1 NAA (MS1); 2.0 mg/L 6-BAP and 0.05 mg/L NAA (MS2); 2.0 mg/L 6-BAP and 0.02 mg/L NAA (MS3); and 3.0 mg/L 6-BAP and 0.1 mg/L NAA (MS4). The stock solutions of PGRs and AgNO3 were sterilized by filtering through nitrocellulose filters with a pore diameter of 0.22 μm (Jet Biofil, Guangzhou, China) and added after autoclaving to the culture medium that was cooled to 45 °C. The hypocotyl and cotyledon explants were placed horizontally with the abaxial side on the surface of culture medium to cultivate in a WLR-351H (Sanyo, Japan) climate chamber at a temperature of 24/22 °C (day/night) using 70–80 µmol m−2 s−1 light intensity with a 16 h light/8 h dark photoperiod. Subculture on a fresh MS1–MS4 media was performed every 15 days. Each treatment procedure was performed in triplicate, involving 10 explants for each (n = 30).
The efficiency of morphogenesis was assessed according to the following characteristics: callus formation rate (after 30 days of culture), rate of shoot/root organogenesis, and mean number of shoots/roots per explant (after 45 days of culture). Callus formation rate (%) was determined as the ratio of the explant number that formed callus tissue to their total number (n = 30). The rate of shoot and root organogenesis (%) was determined as the ratio of the number of explants that developed shoots or roots to the total number of explants (n = 30). The mean number of shoots per explant was determined as the ratio of the total regenerant number to the total number of explants.
2.4. Rooting of In Vitro Regenerated Shoots and Their Adaptation to Soil Conditions
The cabbage shoots regenerated on MS3 culture medium from the callus tissue of each genotype were separated and transferred for rooting to the MS medium free of PGRs (RIM1) and supplemented with 0.5 mg/L NAA (RIM2). The efficiency of rhizogenesis (%) was assessed after 30 days of culture as the ratio of regenerants that developed roots to the total number of regenerants. Each treatment procedure was performed in triplicate, each set involving 10 regenerants (n = 30). The in vitro rooted regenerants were further adapted to soil conditions. For this purpose, the roots were thoroughly washed from the culture medium with tap water to subsequently transfer the regenerants to plastic pots filled with sterilized garden soil under culture room conditions (22 °C; humidity, 60–70%, and illumination, 150 µmol m−2 s−1).
2.5. Statistical Processing of Experimental Data
The experimental data were processed at 5% significance level (α = 0.05) using ANOVA and Duncan’s multiple range tests with AGROS software (version 2.11, Moscow, Russia) and standard MS Excel software packages. The percentage values (rates of callus induction, shoot/root organogenesis, and root formation) were arcsin transformed prior to the ANOVA test. The mean values and standard error of the mean (SEM) are shown.
3. Results
3.1. Selection of Seed Sterilization Method and Culture Conditions for Generation of Aseptic Cabbage Seedlings and Standardized Explants
Initially, we assessed two surface sterilization techniques for cabbage seeds, aiming to obtain the maximum number of standardized explants, namely, using NaOCl as a sterilizing agent (method I) and NaOCl in combination with the pretreatment using C2H5OH (method II). The goal was to prevent the inhibition of the initial seed germination rate, determined prior to sterilization. We showed that the initial seed germination rate on day 6 after the beginning of the experiment varied from 75.0% (the DH line and cv. Parus) to 83.3% (cv. Podarok) and did not differ in the studied genotypes (Figure 1A).
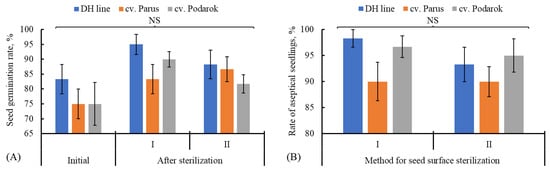
Figure 1.
Effect of the methods for surface seed sterilization (method I: 12.5% water solution (v/v) of a fresh commercial bleach Belizna for 20 min, and method II: 70% C2H5OH for 2 min, followed by the 15% water solution (v/v) of a fresh commercial bleach Belizna supplemented with 0.1% Tween 20, 12 min) on (A) seed germination rate and (B) number of aseptic seedlings in different cabbage genotypes. Mean values for three biological replicates (n = 60) are shown; SEM, vertical lines. NS—non-significant differences between variants at α = 0.05 according to the ANOVA test.
As is evident from Figure 1A, the seed germination efficiency did not decrease or even insignificantly increased after the treatment with sterilizing agents using method I or II. The number of aseptic seedlings varied from 81.7 to 95% depending on the cabbage genotype and sterilization method (Figure 1B). Any statistically significant differences in the number of aseptic seedlings depending on the used sterilization method of the cabbage genotype were unobservable.
Subsequently, we optimized the illumination conditions for the generation of cabbage seedlings with standardized explants. As we observed, cultivation of the seedlings of all studied genotypes in the dark or dim light provides the explants of different qualities. Etiolated cabbage seedlings of all genotypes had pale yellow cotyledons with evident signs of chlorosis and elongated (over 40 mm) thin hypocotyls and were easily injured by tools during manipulations (Figure S1A). In the case of dim light, seedlings formed light-green cotyledons and the hypocotyls of expected size, which could not be injured by tools during the subsequent manipulations (Figure S1B). In general, we intentionally use dim light for cultivating seedlings to obtain the standardized aseptic explants of cotyledons and hypocotyls.
3.2. Callus Formation Efficiency
Both types of explants cultured on the media with different PGR compositions increased in size in the middle of the first passage. By the end of the first passage, a dense light-green callus tissue appeared on a section of the cotyledon petioles (Figure 2A) and at both ends of hypocotyls, only in part of the explants (Figure 2B).
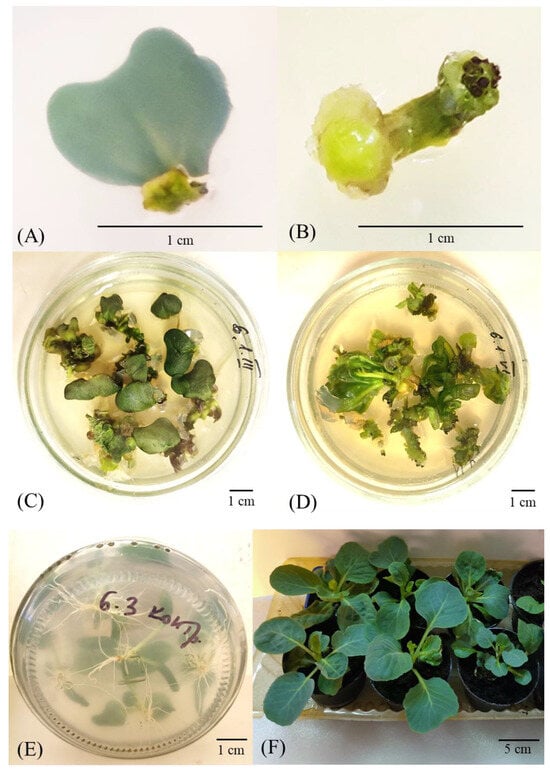
Figure 2.
Different stages of cabbage indirect in vitro somatic organogenesis. Formation of callus tissue on the (A) cotyledon and (B) hypocotyl explants of cv. Parus after 15-day culture on MS3 medium. Mass-scale indirect shoot organogenesis of (C) cotyledon and (D) hypocotyl explants of cv. Podarok after 45-day culture on MS3 and MS4 media, respectively. (E) In vitro rooting of regenerants on the PGR-free MS medium and (F) their successful adaptation to soil conditions. Scale bars: 1 cm (A–E) and 5 cm (F). The rate of callus formation was assessed on day 30 of culture. Three-way ANOVA test demonstrated pronounced differences at a 5% significance level in the callus formation rate between both different genotypes and explant types, as well as the studied variants of culture media (Table S1). Additionally, the differences emerged to be statistically significant for the interaction of factors such as culture medium × genotype, genotype × explant, and all three factors.
In general, note that the cotyledon and hypocotyl explants of the studied genotypes had a high ability for callus formation when cultured on all used media (MS1–MS4) at a rate of 60.1 to 100% (Table 1).

Table 1.
Effect of cabbage genotype, explant type, and culture medium composition on callus formation rate.
Cotyledonary explants of the DH line displayed a significantly higher callus formation rate (98.3%) as compared with the hypocotyl segments (85.8%) on average over all culture medium variants versus the other studied cultivars, for which these differences were unobservable. However, any statistically significant differences in the callus formation ability of cotyledon (89.2%) and hypocotyl explants (88.5%) from any studied genotypes on all culture medium variants were not recorded. Analogous results were also observed for the composition of culture medium on average over all genotypes and types of explants (82.5–93.1%). Explants of the DH line (92.1%) and cv. Parus (95.6%) displayed a significantly higher callus formation rate as compared with cv. Podarok (78.9%) on average over all culture medium variants (Table 1).
3.3. Efficiency of Indirect Somatic Shoot Organogenesis
The callus tissue formed on the explants increased in size; the induction of in vitro somatic organogenesis was triggered on days 20–27, approximately. The shoot formation from the callus developed on hypocotyl explants started approximately 7 days earlier as compared with the callus formed on cotyledon explants (Figure 2C,D).
Similarly to the callusogenesis, the three-way ANOVA test demonstrated significant differences (5% significance level) in the rate of indirect somatic organogenesis between both different genotypes and types of explants and the studied culture medium variants (Table S2). Moreover, the differences emerged to be statistically significant for the interaction of the factors culture medium × genotype and genotype × explant, as well as all three factors.
As shown, the rate of indirect somatic shoot organogenesis from cotyledon and hypocotyl explants of the studied genotypes cultured on MS1–MS4 media varied in a wide range from 13.0 to 100% (Table 2). The cotyledon explants of the DH line displayed a significantly higher rate of shoot somatic organogenesis (71.1%) as compared with the hypocotyl explants (51.9%) on average over all culture medium variants. On the contrary, the segments of cvs. Parus and Podarok hypocotyls displayed a higher capability of shoot somatic organogenesis on average over all culture medium variants. Nonetheless, any statistically significant differences in the rate of shoot somatic organogenesis on average over the studied genotypes between the explants of cotyledons and hypocotyls were unobservable (55.5 and 61.1%, respectively).

Table 2.
Effect of cabbage genotype, explant type, and culture medium composition on the rate of somatic shoot organogenesis.
On average, over all types of explants and culture medium variants, the studied genotypes significantly differed in their capability of somatic shoot organogenesis, ranking in the following order: cv. Parus (78.0%) > DH line (61.5%) > cv. Podarok (36.3%). Note that MS3 medium emerged as the best variant, guaranteeing, on average, over all studied genotypes, the highest rate of somatic shoot organogenesis for both types of explants (the rate of organogenesis for cotyledons and hypocotyls amounted to 73.4 and 72.6%, respectively). In general, the maximum rate of shoot organogenesis was recorded on MS3 medium with the lowest NAA content.
Another important characteristic determining the regeneration potential is the mean number of shoots per explant (Figure 3). We demonstrated that the studied cabbage genotypes differ in this characteristic.
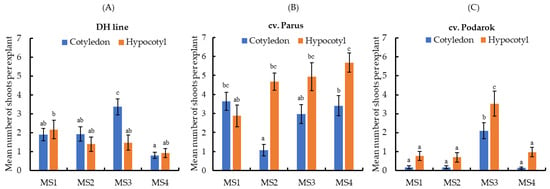
Figure 3.
Effects of the culture medium composition and explant type on the mean number of shoots per explant developed in vitro by (A) DH line, (B) cv. Parus, and (C) cv. Podarok. Mean values are shown; vertical lines, SEM. Treatments followed by at least one letter do not differ in a statistically significant manner at α = 0.05 according to Duncan’s multiple range test.
In the context of mass-scale indirect shoot regeneration for both types of explants, cv. Parus is the most preferable because it is able to generate shoots at a high rate in a wide range of PGRs (Figure 3B). For the studied cultivars, the mean number of shoots regenerated from hypocotyl segments cultured on MS2 and MS4 media (cv. Parus), as well as MS3 medium (cv. Podarok), was significantly higher than compared with cotyledons (Figure 3B,C). On the contrary, a characteristic of the DH line was the formation of large shoot numbers per cotyledon explant only when cultured on MS3 medium (Figure 3A).
3.4. Efficiency of Indirect Somatic Root Organogenesis
An indirect organogenesis, not only of shoots, but also of roots (Figure 2C,D), at a rate of 1.2 to 100% was observed during the culture of cotyledon and hypocotyl explants from all studied genotypes on almost all culture medium variants (Table 3).

Table 3.
Effect of cabbage genotype, explant type, and culture medium composition on the rate of somatic root organogenesis.
The hypocotyl segments of cv. Parus cultured on MS2 and MS3 induction media were the only exception, being completely unable to generate roots. As shown, the rate of root regeneration from cotyledon explants (52.9%) was higher in a statistically significant manner as compared with the hypocotyl segments (20.6%). However, any statistically significant differences in this characteristic between cabbage genotypes were unobservable (30.3 to 43.6%). On average, over all genotypes and types of explants, the culture medium variants with a high auxin concentration (MS1, MS2, and MS4) also displayed a significantly higher rate of indirect somatic root organogenesis. The maximum rate of indirect somatic root organogenesis (67.9%) was observable on MS4 medium with the highest 6-BAP (3 mg/L) and NAA (0.1 mg/L) concentrations. Note also that MS3 culture medium, providing the maximum rate of somatic shoot organogenesis (Table 2) and their mean number per explant (Figure 3), displayed the lowest (10.2%) capability of root morphogenesis (Table 3).
3.5. In Vitro Rooting of Cabbage Regenerants and Plantlets Adaptation to Soil Conditions
Both variants of RIM were successfully applied for rooting the shoots regenerated on MS3 culture medium from cotyledon- and hypocotyl-derived callus tissue (Figure 2E). The rooting efficiency of regenerants cultured on RIM1 and RIM2 media averaged over all studied cabbage genotypes amounted to 69.2 and 86.8%, respectively. The two-way ANOVA test did not show any significant differences in the root formation rate by the regenerants of different cabbage genotypes cultured on both RIM1 and RIM2 media (Table S3). Most of the in vitro plantlets (85%) with a well-developed root system were successfully acclimated to soil conditions (Figure 2F).
4. Discussion
The efficiency of morphogenetic responses in in vitro plant cell and tissue cultures depends on the very first stage, namely, the generation of standardized aseptic explants. It is necessary to obtain plant material free of both surface and inner bacterial and fungal infections because the products of microbial metabolism have a toxic and detrimental effect on plant tissue. The juvenile hypocotyl and cotyledon segments of 3–10-day-old seedlings are mainly used to induce cabbage morphogenesis because they display a high capability of somatic shoot organogenesis [15,21,22,23,24,35,36,37,38]. In this process, sodium hypochlorite solution at a concentration of 3–15% with different exposures is mainly used as a sterilizing agent; this solution is also applicable in combination with a short pretreatment with 70 or 96% ethanol [24,35,36,38,39,40]. Less common techniques are also used; in particular, soaking of seeds in 1.66% dichloroisocyanuric acid solution [25] or 0.1% HgCl2 solution [22]. In our study, both sterilization techniques with NaOCl were effective in generating aseptic seedlings of all studied cabbage genotypes at a rate of 90–98.3% (Figure 1B). Note that the basic seed germination rate of all genotypes did not decrease after sterilization (Figure 1A). Nonetheless, the aseptic sterilization method I (12.5% water solution (v/v) of a fresh commercial bleach Belizna, 20 min) is preferable because only one sterilizing agent is used, making the procedure simpler.
Some researchers propose cultivating seedlings under different illumination conditions; for example, in the dark [35] or using dim light [36] to obtain standardized cotyledon and hypocotyl explants. As is known, the availability of water and different culture medium components, including PGRs, is higher for etiolated cells as compared with the light-grown cultivated cells because of increased cell wall permeability, especially at the cuticle–cell wall interface [41]. We have shown that the cultivation of aseptic seeds using dim light to generate standardized donor explants is intentional because etiolated seedlings form chlorotic and fragile cotyledonary leaves and considerably elongated, thin hypocotyls that are easily injured by tools during manipulations (Figure S1).
It is necessary to supplement basal culture medium with PGRs to induce indirect in vitro somatic shoot organogenesis in cabbage. However, note that the cotyledon and hypocotyl explants derived from juvenile seedlings display a high endogenous hormone concentration. In particular, Gerszberg et al. [21] reported that the shoot regeneration rate of the cotyledon and hypocotyl explants excised from 10-day-old seedlings of eight B. oleracea var. capitata cultivars cultured on MS medium without PGRs varied from 1.1 to 22.2%. The induction medium containing cytokinin alone (0.5–4.0 mg/L for 6-BAP) [20,22,24] or in combination with low concentrations of auxins (0.088–0.1 mg/L NAA or IAA) [21,22,24,28,37] is prevalently used for culture. TDZ [24,28], zeatin [34], or kinetin [32] are used much less frequently. So far, any universal protocols for in vitro induced cabbage morphogenesis applicable to both explant types and a wide range of genotypes are absent; correspondingly, the optimal PGR concentration is individually selected, taking into account the specific physiological and genetic features.
In our study, morphogenetic responses from hypocotyl and cotyledonary explants of Russian cabbage genotypes were induced using four variants of the MS basal culture medium supplemented with 2 or 3 mg/L 6-BAP in combination with 0.02–0.1 mg/L NAA. This PGR composition was selected based on the relevant published data [20,22,24,28,38] and our own preliminary studies. In particular, the use of zeatin and TDZ as the cytokinin source emerged to be ineffective in inducing the indirect somatic shoot organogenesis for the Russian cabbage genotypes (unpublished data). Additionally, all tested culture media contained 5 mg/L AgNO3 as an inhibitor of ethylene biosynthesis [42] along with PGRs. AgNO3 modulates organogenesis and improves the quality of regenerants for the Brassica genus [43,44,45,46]. Moreover, this substance at a concentration of 3.5 or 5 mg/L is recommended for Agrobacterium-mediated transformation of some Brassica sp. [36,47].
Munshi et al. [22] demonstrated that 6-BAP as a cytokinin source is the most preferable for callus induction on cotyledon and hypocotyl explants. However, indirect shoot regeneration was only achieved from cotyledons when cultured on the MS medium supplemented with 2.0 mg/L 6-BAP and 0.1 mg/L NAA [22]. Similar concentrations of 6-BAP and NAA were most effective for shoot regeneration from 10-day-old hypocotyl explants for five of the eight studied cabbage cultivars, which are mainly cultured in Poland [21]. In this study, the regeneration rate and mean number of regenerants per explant for the eight studied genotypes varied from 22.2 to 61.1% and from 1.05 to 7.10 shoots, respectively. According to the authors, hypocotyl explants are more suitable for callus induction and shoot organogenesis as compared with cotyledon explants for all tested cultivars [21]. In our experiment, MS1 culture medium with the same PGR compositions (2.0 mg/L 6-BAP and 0.1 mg/L NAA) provided a comparable rate of somatic shoot organogenesis from both cotyledon (13.0–86.9%) and hypocotyl (33.2–66.7%) explants (Table 2). Depending on the cabbage genotype, the number of shoots per explant varies from 0.1 to 3.9, as well as from 0.5 to 4.0 for cotyledons and hypocotyl segments, respectively (Figure 2). However, the maximum frequency of somatic shoot organogenesis for all tested Russian genotypes was achieved from cotyledon (61.8–80.6%) and hypocotyl (55.2–89.1%) explants cultured on MS3 culture medium with a 5-fold reduced NAA content (0.02 mg/L) (Table 3). Additionally, the mean number of shoots regenerated on MS3 medium was either the maximum for cotyledons of the DH line (3.37) and both explant types of cv. Podarok (2.10 and 3.53 for cotyledonary and hypocotyl explants, respectively) or comparable to the other best variants, as in the case of both explant types for cv. Parus and the DH line hypocotyl segments (1.47–4.93) (Figure 2).
Similar PGR composition (2.0 mg/L 6-BAP and 0.02 mg/L NAA) without AgNO3 was also recommended for maximum shoot regeneration from hypocotyls and cotyledons of twelve Chinese cauliflower and broccoli inbred lines at a rate of 52.4–90.1%. Subsequently, independent transgenic cabbage lines were produced by Agrobacterium-mediated transformation using the induction medium mentioned above. The authors declared that achieving regeneration efficiency of more than 50% is considered effective in experiments on genetic transformation of cabbage [38]. According to our estimates, the MS3 culture medium induces shoot organogenesis from both explant types of Russian cabbage genotypes with an efficiency of more than 55.2% (Table 2), and it can also be recommended for subsequent genetic transformation.
Ravanfar et al. [39] previously established that indirect shoot formation on induction medium from hypocotyl segments developed faster as compared with cotyledonary leaves. A similar morphogenetic response was also observed in our investigation. However, rapid shoot regeneration from hypocotyl explants during Agrobacterium-mediated transformation can lead to false-positive transformants (escapes), even during long-term cultivation on the medium with a high concentration of selective agent [36].
The regenerants formed on MS3 induction medium from cotyledon- and hypocotyl-derived callus tissues successfully developed roots on both the PGR-free medium (RIM1) and RIM2, containing 0.5 mg/L NAA, at a rate of 69.2 and 86.8%, respectively. The cabbage plantlets with well-developed root systems were efficiently acclimated to soil conditions at a rate of 85%. The efficiencies of rhizogenesis and adaptation to ex vitro conditions are comparable to the earlier published data of other researchers [21,22,24,28].
Thus, we have developed a highly efficient protocol for multiple in vitro somatic shoot organogenesis using juvenile cotyledon and hypocotyl explants, allowing for performance of a full cycle from commencing the in vitro seed germination to growing adapted plantlets under ex vitro conditions over 95 days using the model of Russian cabbage genotypes (Figure 4).
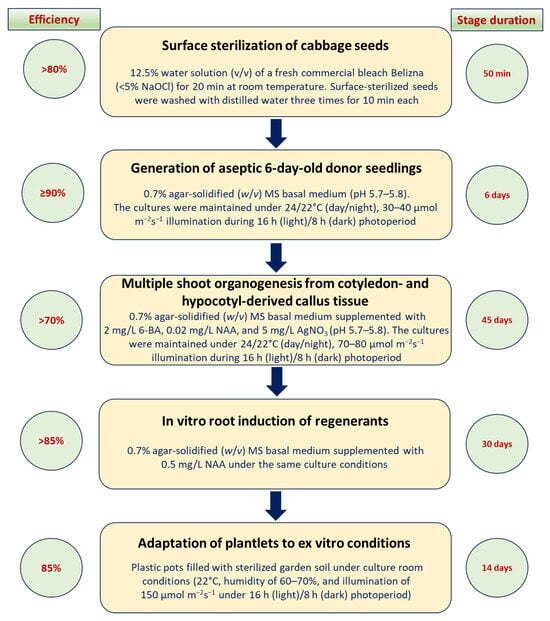
Figure 4.
Highly efficient protocol for multiple in vitro indirect somatic shoot organogenesis from juvenile cotyledon and hypocotyl explants of three Russian cabbage genotypes.
5. Conclusions
A highly efficient and rapid protocol for in vitro indirect shoot organogenesis from the hypocotyl and cotyledon explants derived from 6-day-old aseptic donor seedlings of Russian cabbage (B. oleracea L. convar. capitata (L.) Alef.) genotypes (cvs. Parus and Podarok, as well as the DH line) has been developed. Multiple indirect shoot organogenesis at a rate of 73.0% was achieved through 45 days of culture of juvenile hypocotyl and cotyledon explants derived from 6-day-old seedlings on an MS basal medium supplemented with 2 mg/L 6-BAP, 0.02 mg/L NAA, and 5 mg/L AgNO3. This protocol can be recommended for inducing efficient shoot formation in different cabbage genotypes and producing transgenic plants with improved agronomic traits.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11101246/s1, Figure S1: Development of cabbage seedlings (by the example of cv. Parus) depending on the illumination conditions on day 6 of culture: (A) seedlings cultivated in the dark and (B) seedlings cultivated under dim light conditions (30–40 µmol m−2 s−1); Table S1: Statistical significance of the effect of culture medium, genotype, and explant type on the callus formation rate in cabbage (three-way ANOVA test); Table S2: Statistical significance of the effect of culture medium, genotype, and explant type on the shoot regeneration rate in cabbage (three-way ANOVA test); Table S3: Statistical significance of the effect of cabbage genotype and culture medium composition on the root formation rate (two-way ANOVA test).
Author Contributions
Conceptualization, M.R.K. and R.A.K.; methodology, M.R.K. and N.V.V.; software, M.R.K.; conducting the experiment, N.V.V.; formal analysis, M.R.K., N.V.V., and R.A.K.; investigation and visualization, M.R.K., N.V.V., and R.A.K.; data curation, M.R.K. and R.A.K.; writing—original draft preparation, M.R.K. and R.A.K.; writing—review and editing, M.R.K.; supervision, M.R.K. and R.A.K.; project administration, R.A.K.; funding acquisition, M.R.K., N.V.V., and R.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was supported by the Russian Ministry of Science and Higher Education, project No. 075-15-2023-582. The article processing charge was funded by the authors.
Data Availability Statement
The experimental data obtained and analyzed during estimation are included in this article.
Acknowledgments
We are grateful to Monakhos G.F. and Bondareva L.L. for providing seeds of the cabbage genotype.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FAO | Food and Agriculture Organization of the United Nations |
| GRF | Growth-regulating factor |
| MS | Murashige and Skoog basal medium |
| PGRs | Plant growth regulators |
| 6-BAP | 6-benzylaminopurine |
| IAA | 3-indolyl acetic acid |
| TDZ | Thidiazuron (1-phenyl-3-(1,2,3-thidiazol-5-yl)urea) |
| NAA | 1-naphthalene acetic acid |
| SEM | Standard error of the mean |
References
- Food and Agriculture Organization (FAO). World’s Cabbage Production. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 15 June 2025).
- Lu, L.; Monakhos, S.G.; Lim, Y.P.; Yi, S.Y. Early Defense Mechanisms of Brassica oleracea in Response to Attack by Xanthomonas campestris pv. campestris. Plants 2021, 10, 2705. [Google Scholar] [CrossRef]
- Das Laha, S.; Kundu, A.; Podder, S. Impact of Biotic Stresses on the Brassicaceae Family and Opportunities for Crop Improvement by Exploiting Genotyping Traits. Planta 2024, 259, 97. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, N.K.; Meena, R.C.; Kumar, S.; Kumar, P.; Chand, K. Cabbage Breeding Tools for Biotic and Abiotic Resistance. Rom. J. Hortic. 2024, 5, 23–32. [Google Scholar] [CrossRef]
- Parkash, C.; Kumar, S.; Thakur, N.; Singh, S.; Sharma, B.B. Cabbage: Breeding and Genomics. Veg. Sci. 2023, 50, 231–243. [Google Scholar] [CrossRef]
- Jabeen, A.; Mir, J.I.; Malik, G.; Yasmeen, S.; Ganie, S.A.; Rasool, R.; Hakeem, K.R. Biotechnological Interventions of Improvement in Cabbage (Brassica oleracea var. capitata L.). Sci. Hortic. 2024, 329, 112966. [Google Scholar] [CrossRef]
- Bursakov, S.A.; Karlov, G.I.; Kharchenko, P.N. Marker Breeding of White Cabbage. RUDN J. Agron. Anim. Ind. 2024, 19, 578–591. [Google Scholar]
- Pivovarov, V.F.; Bondareva, L.L.; Shmykova, N.A.; Shumilina, D.V.; Mineikina, A.I. New Generation Hybrids of White Cabbage (Brassica oleracea L. convar. capitata var. alba DC) Based on Doubled Haploids. Sel’skokhozyaistvennaya Biol. 2017, 52, 143–151. [Google Scholar]
- Thakur, P.; Kumari, N.; Kumar, A.; Sharma, P.; Chadha, S. Recent Advances in Development and Utilization of Double Haploids (DHs) in Economically Important Vegetable Crops. Plant Cell Tissue Organ Cult. 2023, 156, 15. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yoon, I.S.; Suh, S.C.; Kim, H.I. Enhanced Disease Resistance in Transgenic Cabbage and Tobacco Expressing a Glucose Oxidase Gene from Aspergillus niger. Plant Cell Rep. 2002, 20, 857–863. [Google Scholar] [CrossRef]
- Bhattacharya, R.C.; Maheswari, M.; Dineshkumar, V.; Kirti, P.B.; Bhat, S.R.; Chopra, V.L. Transformation of Brassica oleracea var. capitata with Bacterial betA Gene Enhances Tolerance to Salt Stress. Sci. Hort. 2004, 100, 215–227. [Google Scholar]
- Rafat, A.; Abd Aziz, M.; Abd Rashid, A.; Abdullah, S.N.A.; Kamaladini, H.; Sirchi, M.T.; Javadi, M. Optimization of Agrobacterium tumefaciens-mediated Transformation and Shoot Regeneration after Co-cultivation of Cabbage (Brassica oleracea subsp. capitata) cv. KY Cross with AtHSP101 gene. Sci. Hortic. 2010, 124, 1–8. [Google Scholar]
- Xing, Y.; Raza, M.A.; He, Y.; Song, J.; Song, J. BoPRR9, a Pseudo-Response Regulator Protein from Cabbage, Plays a Negative Regulatory Role in the Response to Cold Stress. Environ. Exp. Bot. 2024, 224, 105801. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Ma, H.; Ji, J.; Wang, Y.; Zhuang, M.; Yang, L.; Fang, Z.; Li, J.; et al. Generation of Novel bpm6 and dmr6 Mutants with Broad-Spectrum Resistance Using a Modified CRISPR/Cas9 System in Brassica oleracea. J. Integr. Plant Biol. 2025, 67, 1214–1216. [Google Scholar] [CrossRef]
- Gerszberg, A. Tissue Culture and Genetic Transformation of Cabbage (Brassica oleracea var. capitata): An Overview. Planta 2018, 248, 1037–1048. [Google Scholar]
- Kirakosyan, R.; Kalashnikova, E. Morphogenesis of Anthers Isolated from Cabbage (Brassica oleracea L.) in vitro. KnE Life Sci. 2022, 7, 385–393. [Google Scholar] [CrossRef]
- Mineykina, A.; Bondareva, L.; Soldatenko, A.; Domblides, E. Androgenesis of Red Cabbage in Isolated Microspore Culture In Vitro. Plants 2021, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kozar, E.V.; Kozar, E.G.; Domblides, E.A. Effect of the Method of Microspore Isolation on the Efficiency of Isolated Microspore Culture In Vitro for Brassicaceae Family. Horticulturae 2022, 8, 864. [Google Scholar] [CrossRef]
- Kozar, E.V.; Domblides, E.A. Protocol for Obtaining Doubled Haploids in Isolated Microspore Culture in vitro for Poorly Responsive Genotypes of Brassicaceae Family. Biol. Methods Protoc. 2024, 9, bpae091. [Google Scholar] [CrossRef] [PubMed]
- Doğru, S.M.; Balkaya, A.; Kurtar, E.S. In vitro Micropropagation of Maintainer White Head Cabbage Lines Using Cotyledon and Hypocotyl Explants. Black Sea J. Agric. 2022, 5, 180–188. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T. In vitro Regeneration of Eight Cultivars of Brassica oleracea var. capitata. In Vitro Cell. Dev. Biol. Plant 2015, 25, 80–87. [Google Scholar] [CrossRef]
- Munshi, M.K.; Roy, P.K.; Kabir, M.H.; Ahmed, G. In vitro Regeneration of Cabbage (Brassica oleracea L. var. capitata) through Hypocotyl and Cotyledon Culture. Plant Tissue Cult. Biotech. 2007, 17, 131–136. [Google Scholar]
- Hasan, A.M.; ElKaaby, E.A.; AL-Jumaily, R.M.K. In Vitro Effects of Different Combinations of Phytohormones on Callus Induction from Different Explants of Cabbage Brassica oleracea var. capitata L. Seedlings. Iraq. J. Sci. 2021, 62, 3476–3486. [Google Scholar] [CrossRef]
- Gambhir, G.; Kumar, P.; Srivastava, D. High Frequency Regeneration of Plants from Cotyledon and Hypocotyl Cultures in Brassica oleracea cv. Pride of India. Biotechnol. Rep. 2017, 15, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stajič, E. Improvements in Protoplast Isolation Protocol and Regeneration of Different Cabbage (Brassica oleracea var. capitata L.) Cultivars. Plants 2023, 12, 3074. [Google Scholar] [PubMed]
- Stelmach-Wityk, K.; Szymonik, K.; Grzebelus, E.; Kiełkowska, A. Development of an Optimized Protocol for Protoplast-to-plant Regeneration of Selected Varieties of Brassica oleracea L. BMC Plant Biol. 2024, 24, 1279. [Google Scholar] [CrossRef] [PubMed]
- Kiełkowska, A.; Brąszewska, A. Demethylating Drugs Alter Protoplast Development, Regeneration, and the Genome Stability of Protoplast-derived Regenerants of Cabbage. BMC Plant Biol. 2025, 25, 463. [Google Scholar] [CrossRef]
- Gambhir, G.; Srivastava, D.K. Thidiazuron Induces High Frequency Shoot Regeneration in Leaf and Petiole Explants of Cabbage (Brassica oleracea L. var. capitata). J. Biotechnol. Biomater. 2015, 5, 172. [Google Scholar]
- Dănăilă-Guidea, S.; Rosu, A.; Ionica, M.; Visan, L.; Dobrinoiu, R. Caulogenesis Approaches Applied in vitro Micropropagation Varieties—Cabeza Negra 2, Arena and Red Amager—Of Brassica oleracea var. capitata rubra Form. Curr. Trend Nat. Sci. 2012, 1, 75–79. [Google Scholar]
- Daud, N.; Hasbullah, N.; Azis, N.; Rasad, F.; Amin, M.; Lassim, M. In vitro Regeneration of Brassica oleracea var. capitata trough Steams, Roots, Leaves and Petioles Cultures. In Proceedings of the International Conference on Agricultural, Ecological and Medical Sciences (AEMS-2015), Dubai, United Arab Emirates, 23–24 December 2015; pp. 7–8. [Google Scholar]
- Pavlović, S.; Adžić, S.; Cvikić, D.; Zdravković, J.; Zdravković, M. In vitro Culture as a Part of Brassica oleracea var. capitata L. Breeding. Genetika 2012, 44, 611–618. [Google Scholar]
- Pavlović, S.; Vinterhalter, B.; Zdravković-Korać, S.; Zdravković, J.; Cvikić, D.; Mitić, N. Recurrent Somatic Embryogenesis and Plant Regeneration from Immature Zygotic Embryos of Cabbage (Brassica oleracea var. capitata) and Cauliflower (Brassica oleracea var. botrytis). Plant Cell Tissue Organ. Cult. 2013, 113, 397–406. [Google Scholar]
- Murasnige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Qamar, Z.; Jahangir, G.Z.; Nasir, I.A.; Husnain, T. In-vitro Production of Cabbage and Cauliflower. Adv. Life Sci. 2014, 1, 112–118. [Google Scholar]
- Sparrow, P.A.C.; Irwin, J.A.; Goldsack, C.M.; Østergaard, L. Brassica Transformation: Commercial Application and Powerful Research Tool. Transgenic Plant J. 2007, 1, 330–339. [Google Scholar]
- Bhalla, P.L.; Singh, M.B. Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat. Protoc. 2008, 3, 181–189. [Google Scholar] [CrossRef]
- Zontikova, S.A.; Sharafova, O.F.; Polyakov, A.V. Genetic Transformation of White Head Cabbage with the Purpose of Increasing of Resistance to Phytopathogens. Potato Veg. 2009, 7, 24–25. (in Russian). [Google Scholar]
- Sheng, X.; Yu, H.; Wang, J.; Shen, Y.; Gu, H. Establishment of a Stable, Effective and Universal Genetic Transformation Technique in the Diverse Species of Brassica oleracea. Front. Plant Sci. 2022, 3, 1021669. [Google Scholar] [CrossRef]
- Ravanfar, S.A.; Orbovic, V.; Moradpour, M.; Abdul Aziz, M.; Karan, R.; Wallace, S.; Parajuli, S. Improvement of Tissue Culture, Genetic Transformation, and Applications of Biotechnology to Brassica. Biotech. Genet. Eng. Rev. 2017, 33, 1–25. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.L.; Guo, Y.D. High-frequency Embryogenesis, Regeneration of Broccoli (Brassica oleracea var. italica) and Analysis of Genetic Stability by RAPD. Sci. Hortic. 2007, 111, 203–208. [Google Scholar]
- Jacq, A.; Pernot, C.; Martinez, Y.; Domergue, F.; Payré, B.; Jamet, E.; Burlat, V.; Pacquit, V. The Arabidopsis Lipid Transfer Protein 2 (AtLTP2) is Involved in Cuticle-cell Wall Interface Integrity and in Etiolated Hypocotyl Permeability. Front. Plant Sci. 2017, 8, 263. [Google Scholar] [CrossRef]
- Neves, M.; Correia, S.; Cavaleiro, C.; Canhoto, J. Modulation of Organogenesis and Somatic Embryogenesis by Ethylene: An Overview. Plants 2021, 10, 1208. [Google Scholar] [CrossRef]
- Paladi, R.K.; Rai, A.N.; Penna, S. Silver Nitrate Modulates Organogenesis in Brassica juncea (L.) through Differential Antioxidant Defense and Hormonal Gene Expression. Sci. Hortic. 2017, 226, 261–267. [Google Scholar] [CrossRef]
- Assou, J.; Wamhoff, D.; Rutzen, L.; Winkelmann, T. Optimization of in vitro Adventitious Shoot Regeneration in Brassica juncea L. of Different Origins for Application in Genetic Transformation and Genome Editing. Europ. J. Hortic. Sci. 2024, 89, 1–16. [Google Scholar] [CrossRef]
- Al Ramadan, R.; Karas, M.; Ranušová, P.; Moravčíková, J. Effect of Silver Nitrate on in vitro Regeneration and Antioxidant Responses of Oilseed Rape Cultivars (Brassica napus L.). J. Microbiol. Biotech. Food Sci. 2021, 10, e4494. [Google Scholar]
- Cristea, T.O.; Leonte, C.; Brezeanu, C.; Brezeanu, M.; Ambarus, S.; Calin, M.; Prisecaru, M. Effect of AgNO3 on Androgenesis of Brassica oleracea L. Anthers Cultivated in vitro. Afr. J. Biotechnol. 2012, 11, 13788–13795. [Google Scholar] [CrossRef]
- Swamy, H.M.; Nagesha, S.N.; Navale, P.M.; Gowda, T.K.S.; Asokan, R.; Mahmood, R. Genetic Transformation of Cabbage (Brassica oleracea var. capitata) with Synthetic cry1F Gene to Impart Resistant to Diamondback Moth (Plutella xylostella). J. Appl. Hortic. 2013, 15, 3–9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).