Abstract
Milk and sodium alginate beads (SA) as encapsulation materials can improve the viability of Lacticaseibacillus acidophilus LAC5. The present study focused on interactive structural optimization of milk and SA-based beads for improved survival of L. acidophilus LAC5 in cheddar cheese. L. acidophilus was microencapsulated using varying concentrations of milk and SA, e.g., T0 (Milk/SA 0:0), T1 (Milk/SA 1/1:1), T2 (Milk/SA 1/2:1), T3 (Milk/SA 1/1:1.5), T4 (Milk/SA1/2:1.5), T5 (Milk/SA 1/1:2.0) and T6 (Milk/SA 1/2:2.0). Free and encapsulated L. acidophilus were compared for their survival in gastroenteric conditions. Structural and spectral analysis was performed using scanning electron microscope (SEM) and Fourier transform infrared spectrometry (FTIR). The free and encapsulated probiotics were incorporated into cheddar cheese. Organic acids were quantified using HPLC. The combination of SA and milk significantly (p < 0.05) improved the survival of L. acidophilus as compared to free cells. The increase in polymer concentration improved the structure of beads and the survival of probiotics. However, the release profile of beads decreased with the increase in polymer concentration. FTIR showed the presence of milk and SA in the beads. Better storage stability (108 CFU/mL) was observed for T6 in all the treatments as compared to free cells. The addition of encapsulated cells improved the sensory characteristics of cheese. This may help the local food industry to utilize native probiotic strains to be incorporated into probiotic foods with improved bio-accessibility.
1. Introduction
Milk is a rich source of protein, fat, vitamins, and minerals. Milk protein consists of caseins and whey proteins. Caseins are intrinsically unstructured proteins that precipitate at an acidic pH. Casein micelles are highly hydrated colloidal particles. The casein micelles allow milk to be supersaturated in calcium phosphate due to their capacity to bind divalent and multivalent ions. Whey proteins are globular proteins, including β-lactoglobulin and α-lactalbumin. The structure of β-lactoglobulin has several binding sites for hydrophobic ligands such as fatty acids and vitamins [1]. Due to its structural and functional diversity, it can be used as an encapsulation material for different bioactive materials. Milk proteins have excellent interfacial agents used to form and stabilize emulsions. They can form covalent or electrostatic complexes with bioactive molecules to entrap them through the formation of gels. Silva et al. [2] explored the potential of milk fat as an encapsulating material for L. acidophilus. The combination of lecithin and milk fat provided better protection for probiotics. Milk proteins and fats can self-assemble to form supra-structures that allow the encapsulation and transport of a diversity of small molecules [3].
Probiotics are live microorganisms that, on adequate administration, can confer health benefits on the host [4]. Probiotics help to lower cholesterol levels, gastrointestinal tract (GIT) infections, and the chance of inflammatory bowel disease. They maintain the microbiota’s equilibrium in the bowel and help to improve the host’s immune system [5]. Probiotics are reported to treat constipation and diarrhea and improve microbial balance. The selection of probiotic microorganisms is critical due to their different probiotic capabilities. The issues related to health and the beneficial effects of probiotics are causing an increasing consumer demand for probiotics. Probiotic survival is crucial at the time and site of action [6]. Azam et al. [7] reported that low pH conditions in the stomach and bile salt in the small intestine could reduce the viability of probiotics. The recommended amount (107–109 CFU/mL) is reduced due to harsh conditions [8]. The viability of probiotics should be maintained during their production, processing, and utilization [8].
Microencapsulation protects probiotics by providing a shield against harsh conditions in gastrointestinal conditions. It helps to increase the survival rate of probiotics in the gastrointestinal tract. The major challenges faced during encapsulation are the structural stability of encapsulation materials, the selection of proper strain, and their effects on the sensory profile of the food matrix [1]. Different types of coating materials are used for the microencapsulation of probiotics. Sodium alginate is a widely used encapsulation material due to its better intermolecular bonding. It is a polysaccharide with good gelling ability. Its three-dimensional structure provides a good shape with divalent calcium and magnesium ions. However, Azam et al. [9] reported the loss of viability of probiotics in alginate beads due to the presence of pores. The release mechanism was inefficient due to bioactive materials’ slow and uncontrolled release. Incorporating different polymers may provide better bead structure to improve probiotics’ viability.
An effective encapsulation matrix can be developed using a combination of wall materials. Proteins as encapsulating materials have a better nutritional and functional profile than carbohydrate and fat-based materials. Whey protein is an effective encapsulating material for the encapsulation of probiotics. Whey protein produces a thermodynamically stable bead structure by forming an intermolecular bond with alginate. The whey proteins can help to improve the structural deformities in alginate beads. Whey proteins have gelation, emulsifying, and film-forming properties. The characteristics of beads can be influenced by encapsulation material and core-to-wall ratio [10].
Cheese is widely used due to its nutritional and sensory characteristics. Cheese accounts for approximately 30% of the total business of dairy products. It is rich in vitamins (vitamin A and B12), minerals (phosphorus and zinc), and proteins [11]. The addition of probiotics can enhance the nutritional status of cheese. It may improve the textural and sensory properties of cheese. However, few studies suggest that adding probiotics can affect dairy products’ sensory characteristics [7,8]. The proposed study was designed to develop and utilize dairy-based encapsulation matrices for improved structure and survival of L. acidophilus under GIT conditions. The bio-accessibility of free and encapsulated probiotics was evaluated through cheddar cheese.
2. Materials and Methods
2.1. Materials
Cow milk (fat 4.14%, protein 3.58%, lactose 4.96%, ash 0.72%, and SNF 9.25%) was purchased from the local market. Sodium alginate, whey protein, pepsin, rennet, and simulated intestinal fluid were obtained from Sigma Aldrich (St. Louis, MO, USA). MRS agar (De Man Rogosa and Sharpe), MRS broth, and all other analytical grade chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Inoculum Preparation
L. acidophilus LAC5 was (previously characterized for its probiotic potential in the Laboratory of Food Microbiology, National Institute of Food Science and Technology, University of Agriculture Faisalabad, Faisalabad, Punjab, Pakistan). The strain was revitalized before final use using MRS broth. The final concentration was maintained (1010 CFU/mL) [12].
2.3. Preparation of Alginate-Milk Microspheres
Milk and sodium alginate (low viscosity, Sigma Aldrich) were sterilized for 15 min at 121 °C. Alginate-milk microspheres were made using an Encapsulator (Buchi B-390, Switzerland) through extrusion into 100 mM CaCl2 while gently stirring (100 rpm). The microspheres were prepared using a 0.45 mm nozzle and solidified in a 100 mM CaCl2 solution for 30 min. The microspheres (in wet condition) were then washed with distilled water, filtered, and sealed in sterile conical tubes. Different microsphere formulations were prepared and further studied for specific characteristics (Table 1).

Table 1.
Treatment plan for alginate-milk microspheres.
2.4. Microencapsulation of L. acidophilus in Alginate-Milk Microspheres
A mixture containing lactobacilli (1010 log CFU/mL) with sodium alginate (2.5%) and milk was passed through the encapsulator (Buchi 390, London, UK) into a 100 mM CaCl2 solution while stirring at 100 rpm. The beads were prepared using a 0.45 mm nozzle and solidified in a 100 mM CaCl2 solution for 30 min. The beads were washed with distilled water, filtered, and sealed in sterile conical tubes [10].
2.5. Encapsulation Yield and Diameter of Microspheres
The encapsulation yield of L. acidophilus was calculated by following the method described by Liao et al. [13].
Encapsulation yield (%) = (quantity of lactobacilli released from the broken microspheres/quantity of lactobacilli initially taken to prepare the microspheres) × 100
The size of microspheres was calculated using an optical microscope. For each formulation, 30 microspheres were randomly picked and examined following the method described by Shi et al. [14].
2.6. Structural Analysis of Beads
The structural analysis of beads was carried out following the protocol of Azam et al. [7]. Triplicate samples of treatments’ primary, intermediate, and final concentrations were selected for analysis using scanning electron microscopy (SU1510, Hitachi, Tokyo, Japan). The freeze-dried beads were coated with gold to take surface and cross-sectional images (Vacuum 9.75 × 10−5 Torr, 15 kV accelerating voltage, magnification ≥ × 1000).
2.7. Spectral Analysis of Beads
The spectral analysis of beads was carried out using a Fourier transform infrared spectrometer (FTIR) (Thermo Nicolet 6700, Thermo Electron Corp., Waltham, MA, USA). The beads with encapsulated bacteria (1 g) were mixed with potassium bromide (100 mg of KBr). Potassium bromide helps to get high-quality beam splitting. The process was completed at a wave number of 400 to 4000 cm−1 at a 4 cm−1 resolution. A total of 144 spectra were collected for 9 samples, with 16 scans per sample. The spectral speed was set at 1 cms−1. The collected spectra were pre-treated for baseline correction using Unscrambler software (Camo, Ltd., Oslo, Norway) [9].
2.8. Determination of Encapsulated and Free L. acidophilus LAC5
L. acidophilus LAC5 (0.50 g) was suspended in the 4.5 mL of sodium citrate (50 mM) on a shaker at a pH of 7.5 till released at room temperature. Discharged lactobacilli were serially diluted 10 times in the saline solution. After the incubation (37 °C for 24 h), colonies of the lactobacilli were counted in aerobic environments. Serial dilutions of the free lactobacilli were made 10 times using a saline solution. MRS agar was utilized for plating aliquots of 100 µL [14].
2.9. Stability of the Encapsulated and Free L. acidophilus LAC5 at Different pH
The pH stability of the encapsulated and free lactobacilli was determined as described by Afzaal et al. [8]. Milk-alginate microspheres containing lactobacilli were in tubes containing simulated gastric fluid (SGF) (4.5 mL) at pH 2.0 and 2.5. The plates were incubated at 37 °C for 10–120 min. After a specific time, the samples were subjected to analysis. Serial dilutions of the free lactobacilli were made 10 times using a saline solution. MRS agar was utilized for plating aliquots of 100 µL. Microspheres that contained lactobacilli were recovered from the SGF. These microspheres were dissolved in a 4.5 mL sodium citrate solution (50 mM) at pH 7.5. Discharged lactobacilli were serially diluted 10 times in the saline solution. MRS agar was utilized for plating aliquots of 100 µL.
2.10. Tolerance of Encapsulated and Free L. acidophilus LAC5 against Bile Salts
The stability of the free and encapsulated L. acidophilus was analyzed in the bile salts. Free lactobacilli suspensions of 0.5 mL were present in the tube containing bile salts solution (4.5 mL) at 1% w/v and incubated at 37 °C for 2 h. Encapsulated and free lactobacilli were accumulated at different time intervals. Serial dilutions of the free lactobacilli were made 10 times using saline solution. MRS agar was utilized for plating aliquots of 100 µL. After the breakage of microspheres in the solution of sodium citrate, a viable count was determined for lactobacilli [15].
2.11. Stability of Encapsulated and Free L. acidophilus LAC5 during Storage
The stability of the encapsulated and free L. acidophilus (The lyophilized free cells and encapsulated beads were packed in zip-lock polyethylene bags) during storage was analyzed at 4 °C for 30 days. The samples were collected for viability measurement after 1, 3, 5, 7, 14, 21, and 28 storage days. Serial dilutions of the free lactobacilli were made 10 times using a saline solution. MRS agar was utilized for plating aliquots of 100 µL. These microspheres were dissolved in the 4.5 mL sodium citrate solution (50 mM) at pH 7.5. Discharged lactobacilli were serially diluted 10 times in the saline solution. MRS agar was utilized for plating aliquots of 100 µL [15].
2.12. Release of the Encapsulated L. acidophilus LAC5 from Simulated Intestinal Fluid
The release study of the encapsulated L. acidophilus from beads was conducted using milk-alginate microspheres in the SIF at pH 6.8. Encapsulated L. acidophilus were resuspended in 5 mL of sterile PBS (0.1%) before use. Milk-alginate microspheres (5 g), which contained L. acidophilus, were poured into the conical tubes which contained pre-warmed SIF (4.5 mL). Incubated at 37 °C and centrifuged at 100 rpm for 5 min. At the predetermined time (0, 20, 40, 60, 80,100, and 120 min), aliquots of 100 µL were collected and instantly examined for the number of discharged lactobacilli. A fresh medium of the same volume was added to replace the volume of the withdrawn sample. The collective quantity of the released rate is plotted against the time. Viable counts of L. acidophilus were determined [1].
Rate of release (RR%) = Vr/Va × 100
Vr = Viable cells released from beads in SIF; Va = Viable cells added into beads in SIF.
2.13. Cheddar Cheese Preparation
Fresh cow milk was procured from a local farm (University of Agriculture, Faisalabad, Punjab, Pakistan) and stored in sterilized glass bottles in a refrigerator at 4 °C. Rennet (Sigma Aldrich) and starter culture were obtained from the Microbiology lab, University of Agriculture, Faisalabad, Punjab, Pakistan. Cheddar cheese was produced in two-liter milk batches (06 variants of cheese were prepared using the treatment plan). The frozen probiotic culture was melted at room temperature. This culture was added to the milk to get 107 CFU/mL. The starter culture was inoculated in the milk at 107 CFU/mL. Microencapsulated beads, starter culture (0.75 g), and rennet (0.5 mL) were also added. The curd was cut using a steel mesh and cooked. The whey drainage and cheddaring process were performed. The curd was cut, salted, incubated (25 °C for 24 h), and ripened for 4 weeks. The cheese samples were collected on production days 1, 7, 14, and 21 days. These samples were subjected to further analysis [16].
2.14. HPLC Analysis of the Organic Acids in Cheddar Cheese
The production of lactic and acetic acids in cheese was determined using HPLC [17]. Grated samples of the cheese (5 g) were mixed with 0.009 N sulfuric acid (25 mL) and 70 mL of nitric acid (15.5 N). The mixture was homogenized at room temperature. After staying in the water bath (50 °C for 1 h), centrifugation (4000 rpm for 20 min) of the obtained slurry was performed. The soluble fraction situated between fat and casein was more centrifuged. The obtained supernatant was filtered using filters of 0.22 Millipore. About 1mL aliquot of each sample was stored at −20 °C in the HPLC vials until analyzed. HPLC analysis of the reverse phase was performed using the HPLC system furnished with the C18 column. Phosphate buffer (25 mM) at pH 2.5 was utilized as the mobile phase. Precisely 10 mL of the prepared sample was injected into HPLC. The flow rate was kept at 1.5 mL per minute, and a UV detector was utilized to detect 200 nm [18].
2.15. Viable Cell Count (VCC) of Cheddar Cheese
The viable cell count (VCC) of L. acidophilus in cheese samples was determined using the pour plate method [19]. Ten grams of the sample were added to the 90 mL solution of sodium citrate (20 g/L at 45 °C). The incubation (25 °C for 15 min) and homogenization (27,000 rpm for 30 s) were performed to release the bacteria in the microbeads. Suitable dilutions from the cheese with beads/free cells were added to the sodium citrate solution. After pouring inoculation on MRS agar plates, the plates were incubated at (37 °C for 48 h) in an anaerobic environment [20].
2.16. Sensory Evaluation of Cheddar Cheese
The sensory evaluation of control and encapsulated cheddar cheese samples was performed using the Hedonic scale for crumbliness, stickiness, firmness, slice-ability, taste, flavor and general acceptability. The trained panelists were more than twenty years old and liked cheddar cheese. To evaluate samples, the panel comprises 62 members (43 males and 19 females, aged 20 to 45). The samples were randomly coded, and panelists were asked to rate the samples. Spring water was used as a palate cleanser. The sensory evaluation was performed organoleptically using fluorescent white light and marked the samples based on their organoleptic characteristics with a Hedonic scale (1–5, 1 = poor, 5 = excellent) [10].
2.17. Statistical Analysis
Statistical analysis of the data was performed according to [21]. The data were subjected to a two-factor factorial with a completely randomized design (CRD) for statistical analysis using Statistics 8.1. An analysis of variance (ANOVA) was used with a p < 0.05 significance level. The mean values of triplicates were expressed with their standard deviation.
3. Results and Discussion
3.1. Encapsulation of L. acidophilus in Alginate-Milk Microspheres
L. acidophilus was encapsulated with different concentrations of alginate and milk. The mean size of alginate milk microspheres produced by a nozzle size of 0.45 mm was between 709 ± 1.08 and 879 ± 1.71 µm (Table 2). The concentration of polymer could cause variation in microsphere size. The size of microspheres increased with the increase in total polymer concentration. The alginate-milk microsphere size increased from 709 ± 1.08 to 879 ± 1.71 µm for T1 to T6 (Table 2). Alginate concentration affects the size and shape of microspheres. A higher concentration of alginate led to the superior shape of microspheres, such as in T4 to T6. On the other hand, microspheres with less alginate concentration produced not only small sizes but also soft and tail-like structures with a lack of proper sphere shape, for example, in T1. The results indicated that the alginate contribution is more in shape than milk, but alginate microspheres are not stable in an acidic environment. Milk proteins induce specific physicochemical and structural properties. These are gelation properties, molecular abilities, gel swelling, biodegradability, and biocompatibility.

Table 2.
Size (µm) and yield (%) of alginate-milk microspheres.
Similar results were reported by Ahangaran et al. [22], who observed that by increasing the polymer concentration, the wall thickness of microspheres increased, resulting in greater protection. Due to increased wall thickness, the overall size of microspheres also increases along with their smooth texture. Prasanna and Charalampopoulos [23] described that encapsulation of L. acidophilus and Bifidobacterium bifidum in simple alginate beads was not proven to be effective in 3 to 4% bile salt. The combination of alginate with proteins as coating material produces more protection in foods and the gastrointestinal tract due to matrix formation [24].
The extrusion method is efficient for higher encapsulation yield and smaller microsphere sizes. The average microsphere size is a significant property for efficiency and firmness. The size of microspheres directly correlates with protection and a negative correlation with sensory attributes. Encapsulation yield increased with the increase of total polymer concentration. The microbial suspension used for encapsulation was 10 log CFU/mL. The alginate-milk concentration has a direct relationship to yield (Table 2). The maximum yield was observed in T6 (92.4 ± 1.21%), while the minimum was obtained in T1 (60.7 ± 1.06%). There are two major factors that affect the yield, i.e., encapsulation technique and microbial type. Sipailiene and Petraityte [25] reported that the low encapsulation yield is due to the sensitivity of microorganisms and heat treatment during encapsulation. High encapsulation yield can be obtained due to the natural resistance of bacteria and encapsulation conditions [23]. Encapsulation yield is a significant factor in checking the encapsulation process efficiency and material wall characteristics [26].
3.2. Determination of Free and Encapsulated L. acidophilus Viable Cells
The cell suspension in a free form used for encapsulation was 10 log CFU/mL. The cells encapsulated in the form of microcapsules ranged from 9.95 to 9.98 log CFU/g for various treatments. As a result, a high encapsulation yield in alginate-milk microcapsules was attained in the present study. There was not found a significant difference between the yield of encapsulation and the concentration of total polymer. Many researchers have reported that the polymer concentration could affect the encapsulation yield. Zanjani et al. [27] observed that the increase of starch with alginate in the formulation of microcapsules could improve encapsulation efficiency for L. casei. Rodrigues et al. [28] reported that the yield of microcapsules for L. casei ranged from 50.9% to 80%, with variation in the total polymer concentration.
3.3. Structural Analysis of Beads
Images of wet and dry beads were taken using a digital camera (Figure 1). The sodium alginate beads were spherical in shape and glossy appearance. The increasing concentration of milk in sodium alginate beads resulted in elongated beads. SEM micrographs showed that the increase in milk concentration significantly reduced the pores of sodium alginate (Figure 2). Sodium alginate beads were spherical in shape with intermolecular spaces. The addition of milk reduced the intermolecular pores. However, the milk-based beads were elongated but had a smooth structure. Shriveled and elongated beads may be due to the effect of freeze-drying. Freeze-drying can affect the spherical shape of beads [9]. Sodium alginate plays an important role in coating the materials. Though, sodium alginate is not successful in protection due to the pores. The combination of sodium alginate and other materials, such as starch, proteins, and carbohydrates, produces splendid results [28]. Milk, along with all its constituents, exhibits good properties when used with alginate in the formulation as a coating material. Lactose in milk is a growth booster for immobilized probiotics in the gastrointestinal tract. Milk proteins, both casein and whey, when fragmented in the intestine, produce several other beneficial by-products. Proteins supply essential amino acids for humans and bacteria and act as co-factor for many enzymatic activities. Azam et al. [7] studied the composite encapsulation of the beads and found that the composite beads were better in structure and protection. Wiessel et al. [29] have reported the ability of L. acidophilus to be alive in the alginate-xanthan gum-inulin matrix in carrot juice. The main advantage of encapsulation is the higher survival rate in high bile and low pH conditions [30].

Figure 1.
Dry and wet images of encapsulated beads with varying concentrations of sodium alginate and milk (A, B, C, D, E, F indicated wet images; a, b, c, d, e, f indicated dry images).
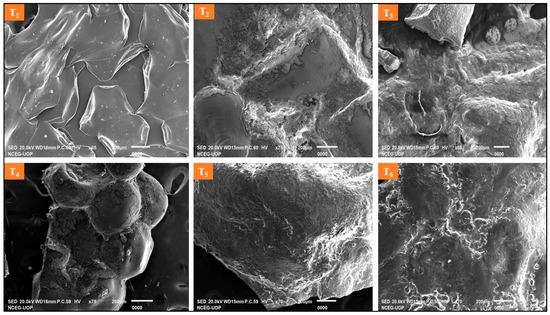
Figure 2.
Encapsulated beads with varying concentration of sodium alginate and milk (T1: SA 1%, Alginate/Milk ratio 1/1; T2: SA 1%, Alginate/Milk ratio 1/2; T3: SA 1.5%, Alginate/Milk ratio 1/1; T4: SA 1.5%, Alginate/Milk ratio ½; T5: SA 2%, Alginate/Milk ratio 1/1; T6: SA 2%, Alginate/Milk ratio 1/2).
3.4. FTIR Spectral Analysis of Beads
The FTIR spectral analysis was performed for varying concentrations of sodium alginate and milk-based beads to identify functional groups. The spectra of different treatments (T1, T2, T3, T4, T5, and T6) are given in Figure 3. Sodium alginate spectra showed aliphatic groups bands C–H (2900–2700 cm−1) and OH stretching vibrations (3600–3000 cm−1). The symmetric and asymmetric stretching of COO− corresponded to 1415 and 1592 cm−1, respectively. These bands were distinctive among alginate and its conjugated products. Pyranosyle ring and functional groups of C–O, and C–C–H deformations were found at 1021 and 817 cm−1. The spectra of milk showed absorption bands at 1442, 1630, and 3430 cm−1. The presence of O-H, C-H, and C=C functional groups indicated the presence of milk in beads. These regions were present in all the treatments, indicating the presence of sodium alginate and milk. However, the intensity is directly proportional to the concentration of the component. Azam et al. [9] indicated the presence of sodium alginate in beads with their spectral fingerprints. Sodium alginate and its conjugated molecules showed aliphatic groups bands C–H (2900–2700 cm−1), and OH stretching vibrations (3600–3000 cm−1).
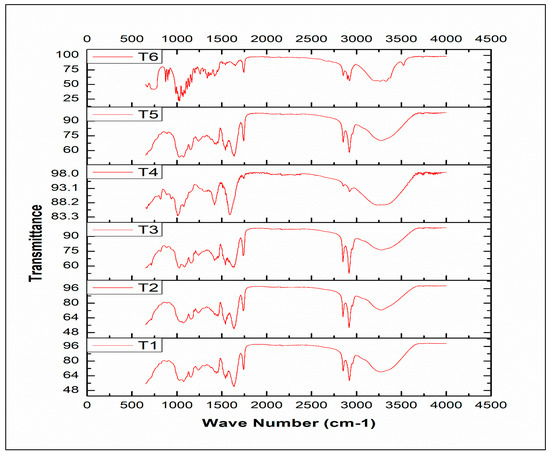
Figure 3.
Spectral analysis of encapsulated beads with varying concentrations of sodium alginate and milk (T1: SA 1%, Alginate/Milk ratio 1/1; T2: SA 1%, Alginate/Milk ratio 1/2; T3: SA 1.5%, Alginate/Milk ratio 1/1; T4: SA 1.5%, Alginate/Milk ratio ½; T5: SA 2%, Alginate/Milk ratio 1/1; T6: SA 2%, Alginate/Milk ratio 1/2).
The symmetric and asymmetric stretching of COO- were present at 1415 and 1592 cm−1. Due to the interaction of different functional groups, spectral overlapping can be observed in spectral results. The alginate monomers can bind calcium ions during encapsulation. Atia et al. [31] used inulin and sodium alginate-based encapsulation material for probiotics. The hydroxyl and aliphatic groups were present at 1200–900 and 3500–300 cm−1 wavenumber, respectively. The functional groups of sodium alginate were present at 3500–2700 cm−1.
3.5. Low pH Stability of Free and Encapsulated L. acidophilus
Encapsulation of L. acidophilus in alginate-milk microcapsules significantly improved the survival of L. acidophilus in SGF (Figure 4A,B). In SGF (pH 2.5), the viability of L. acidophilus encapsulated in microcapsules was maintained after 120 min of incubation. In SGF (pH 2.0), the viability of encapsulated L. acidophilus could be maintained for 30 min. After that, the viability of encapsulated L. acidophilus was observed to decrease to 8 logs CFU/mL (T6 8.43 ± 0.1 log CFU/mL) after 120 min of exposure time (Figure 3). The analysis of variance for viability in SGF of free and encapsulated L. acidophilus indicated significant results with bead formulations, storage time and for their interaction. The observed excellent pH resistance of encapsulated L. acidophilus can be attributed to the buffering capacity of milk utilized in the formulation of microcapsules.
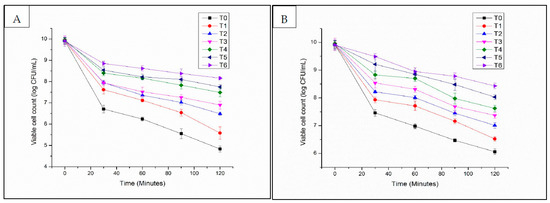
Figure 4.
Low pH stability of encapsulated L. acidophilus (log10 CFU/mL) ((A): pH 2.0; (B): pH 2.5) (T1: SA 1%, Alginate/Milk ratio 1/1; T2: SA 1%, Alginate/Milk ratio 1/2; T3: SA 1.5%, Alginate/Milk ratio 1/1; T4: SA 1.5%, Alginate/Milk ratio ½; T5: SA 2%, Alginate/Milk ratio 1/1; T6: SA 2%, Alginate/Milk ratio 1/2).
Ji et al. [32] mentioned that the whey proteins contributed to the high viability of bifidobacteria encapsulated in alginate–pectin–whey protein microcapsules when exposed to SGF (pH 2.5). Chen and Hang [33] demonstrated the survival rate of Lactobacillus paracasei spp. paracasei F19 and Bifidobacterium lactis Bb 12 encapsulated in beads having casein in the formulation can be increased with exposure to SGF (pH 2.5). The present results also exhibited that a higher concentration of milk contributed to the improved protection of probiotics. Viable counts of encapsulated L. acidophilus were observed to reduce to 5.5 log CFU/mL microcapsules and 7 log CFU/mL microcapsules after 120 min of incubation time for T1 and T3, respectively. The reason could be attributed to a denser hydrogel network due to a higher milk concentration, thereby reducing the acid diffusion rate into the microcapsules. Rather et al. [15] reported that the resistance of L. casei NCDC-298 was encapsulated in various concentrations (2%, 3%, and 4%) of alginate. The results showed that the increased pH stability was directly proportional to the alginate concentrations without any adverse effect on the release of the entrapped cells at colonic pH.
3.6. Bile Salt Solution Tolerance of Free and Encapsulated L. acidophilus
Free L. acidophilus was lost in bile salt solution after exposure for 1 h, probably due to the damage caused by bile salts to cell wall integrity (Figure 5). Maximum viability was observed in T6 (8.92 ± 0.13 CFU/mL). The viability increased with the increase in polysaccharides and milk protein concentration. The analysis of variance for viability in bile salt solution of free and encapsulated L. acidophilus indicated significant results with bead formulations, storage time, and their interaction. The researchers mentioned that probiotics are sensitive to the bile salt solution. Lin et al. [34] reported a five log decrease in viable cell numbers of Bifidobacterium adolescentis after 12 h of incubation time in a 2% bile salt solution and at 37 °C. Shori [35] observed that B. adolescentis decreased their numbers after a 2 h incubation time of about 2 log CFU/mL, in 0.5% bile salt at 37 °C. However, the alginate-milk microcapsules can offer protection against bile salts as compared to free cells. This may be attributed to the alginate-milk matrix trapping structure that offered more resistance to the adverse effects of bile salts. In the present study, improved bile salt solution tolerance was demonstrated by encapsulated cells as compared to free cells. It was impossible to compare the present study’s results with others because other scientists used variable concentrations of bile salts. Halim et al. [36] reported that encapsulated probiotics could improve survival compared to free probiotic cells in bile salt solution concentrations of 1–3%.
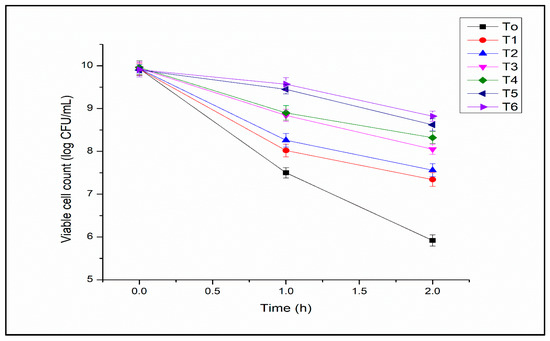
Figure 5.
Bile salts tolerance of encapsulated L. acidophilus (log10 CFU/mL) (T1: SA 1%, Alginate/Milk ratio 1/1; T2: SA 1%, Alginate/Milk ratio 1/2; T3: SA 1.5%, Alginate/Milk ratio 1/1; T4: SA 1.5%, Alginate/Milk ratio ½; T5: SA 2%, Alginate/Milk ratio 1/1; T6: SA 2%, Alginate/Milk ratio 1/2).
3.7. Storage Stability of Free and Encapsulated L. acidophilus
The storage stability of free and encapsulated L. acidophilus at 4 °C is given in Figure 6. The L. acidophilus encapsulated in alginate–milk microcapsules presented good viability. However, L. acidophilus cells in free form showed poor stability during storage. The analysis of variance for the viability of free and encapsulated L. acidophilus indicated significant results with bead formulations, storage time, and their interaction. The viability of L. acidophilus in free form was reduced from 10 to 2.3 log CFU/mL (T0) after storage for 28 days (Figure 6). Microencapsulation with optimized concentration of sodium alginate and milk can help to improve the viability of probiotics. Feng et al. [37] demonstrated that the blending of polymers for the encapsulation of probiotics has a potential approach to improving the stability of probiotics during storage. The protective role of utilizing whey proteins as encapsulation material was assessed during storage by de Araújo Etchepare et al. [38], who reported more than 9 log CFU/g microcapsules viability after 180 days of storage. Chávarri et al. [39] evaluated that the microcapsules coated with chitosan possessed better protection for probiotics cells than other microcapsules during the storage period. This may be attributed to the microcapsules coated with a denser membrane formed due to chitosan. In the present study, the denser membrane formed of alginate-milk polymers might provide excellent protection to probiotics during storage.
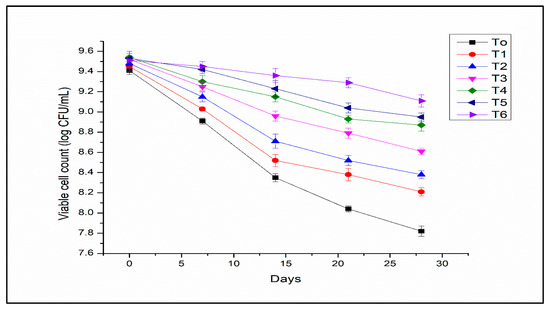
Figure 6.
Storage stability of encapsulated L. acidophilus (log10 CFU/mL) at 4 °C (T0 free cells; T1: SA 1%, Alginate/Milk ratio 1/1; T2: SA 1%, Alginate/Milk ratio 1/2; T3: SA 1.5%, Alginate/Milk ratio 1/1; T4: SA 1.5%, Alginate/Milk ratio ½; T5: SA 2%, Alginate/Milk ratio 1/1; T6: SA 2%, Alginate/Milk ratio 1/2).
3.8. Release Study of Encapsulated L. acidophilus
In order to confer health benefits on the human body, L. acidophilus should be released from encapsulation in SIF. The release rate of microencapsulated L. acidophilus from microcapsules decreased significantly with increasing alginate concentration in the alginate-milk ratio due to the denser polymer membrane. The releasing properties of microencapsulated L. acidophilus in SIF have been presented in Figure 7. All the microencapsulated L. acidophilus were released from microcapsules in 120 min. However, T1 showed rapid release compared to other treatments and in T6 slow release was observed. The probiotics were released from the beads as because of swelling that caused erosion of the network composed of alginate and milk in SIF. The composition and concentration of polymers affected the release profile and the acid protection of microencapsulated L. acidophilus. Generally, the higher concentrations of polymers increased the survival of L. acidophilus in an acidic medium while reducing the release rate. However, Dimitrellou et al. [40] observed that the release properties of microencapsulated probiotics were not significantly affected by an increase in the concentration of alginate.
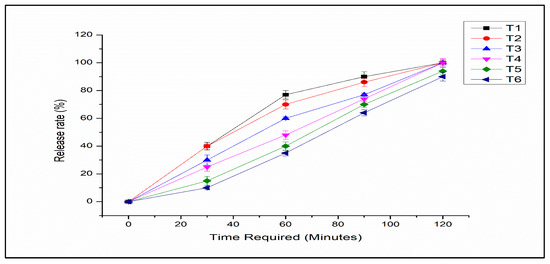
Figure 7.
Release of encapsulated L. acidophilus (log CFU/g) from microcapsules in simulated intestine fluid (T1: SA 1%, Alginate/Milk ratio 1/1; T2: SA 1%, Alginate/Milk ratio 1/2; T3: SA 1.5%, Alginate/Milk ratio 1/1; T4: SA 1.5%, Alginate/Milk ratio ½; T5: SA 2%, Alginate/Milk ratio 1/1; T6: SA 2%, Alginate/Milk ratio 1/2).
3.9. Organic Acids in Cheddar Cheese
Organic acids play an important role in contributing to the flavor and aroma [41] of various varieties of cheese. Lactic acid bacteria ferment sugar producing lactic acid as the major product. The analysis of the variance of lactic acid and acetic acid content for probiotic cheddar cheese illustrated that lactic acid and acetic acid content increased significantly with varying concentrations of encapsulation materials. Maximum lactic acid and acetic acid production was observed in T4 (39.28 ± 6.3 mg g−1) and (1.02 ± 0.04 mg g−1) (Table 3). The lowest lactic and acetic acid production was observed in T0 (34.24 ± 5.2 mg/g) and T1 (0.82 ± 0.04 mg/g). Lipolysis and normal bacterial metabolism produce organic acids (short-chain and water-soluble) in cheese. Therefore, lactic acid was the most abundant organic acid observed in probiotic cheddar cheese. The value increased with the increase in milk concentration. The quantitative evaluation of organic acids can monitor bacterial growth and metabolism. The microbial metabolic activity in cheese was monitored by evaluating the metabolic products, i.e., lactic acid and acetic acid. Lactobacillus spp. uses the Leloir pathway to convert galactose to glucose-6-P, which is metabolized to DL-lactate through the Embden-Meyerhof-Parnas pathway (EM pathway) [42]. Acetic acid may be produced from lactose, citrate, and amino acids [43]. This increase might be attributed to the higher concentration of free amino acids in cheeses produced by adding adjuncts to probiotics. These amino acids might have contributed to the formation of acetic acid as precursors. Zheng et al. [44] reported that the degradation (oxidative deamination of decarboxylation) of amino acids (alanine and serine) could play a potential role in the production of acetic acid.

Table 3.
Organic acids in probiotic cheddar cheese.
Acetic acid was found in all cheese treatments at levels that kept on enhancing with an increase in milk concentration for microencapsulation. Significant effects were observed due to the milk/alginate ratio used to encapsulate probiotics in the cheese. The increasing milk concentration for encapsulation demonstrated a higher amount of acetic acid in cheese. Stefanovic et al. [45] observed that L. casei could produce acetic acid as the end product of their metabolism.
3.10. Viable Cell Count (VCC)
The results for the survival of L. acidophilus after 21 days of storage at 4 °C are presented in Table 4. The data demonstrated that the probiotics remained stable at 4 °C during storage. A mean reduction of only 0.15 Log/CFU was observed after 21 days of storage at 4 °C. A statistically nonsignificant effect of treatments (alginate/milk ratio) was observed on viability loss. The appropriate freezing matrix and a low storage temperature can be accredited for the minimal loss in viability.

Table 4.
Viable cell count (log10 CFU/g) of L. acidophilus in probiotic cheddar cheese.
Milk as a freezing matrix for lactic cultures has long been demonstrated as an excellent material [46], and the results from the current study exhibited that L. acidophilus coincides with this observation. Moreover, the low storage temperature of 4 °C was recommended earlier for lactic cultures [47]. However, storing frozen starters and probiotics at temperatures lower than −40 °C is desirable, and commercial freezers at −20 °C are inappropriate for this typical storage. It has been proposed that viable probiotic organisms should be present at at least 107 log CFU/g of a product to confer health benefits on the host [48]. All probiotics containing cheese developed in the present study thus satisfied the criteria of a probiotic food product.
3.11. pH of Probiotic Cheddar Cheese
The pH of probiotic cheddar cheese decreased significantly with treatments and storage. The rise in acidity could be attributed to lactic acid production by lactic acid bacteria during lactose fermentation. The analysis of variance of pH for probiotic cheddar cheese illustrated that pH decreased significantly with varying concentrations of encapsulating material and storage days. Their interaction had significant effect on the pH. The pH of cheese prepared with various treatments was significantly lower than that of the control. As the level of milk increased in the various treatments of cheese, the pH gradually decreased (Table 5). Maximum pH reduction was observed in T4 (3.96 ± 0.05), and minimum in T0 (4.20 ± 0.14). The reason can be the metabolic activity of lactic culture and/or high fermentable sugar concentration in various cheese treatments. The whole and robust metabolism of lactose and /or its monosaccharides in cheese curd is vital for producing high-quality cheese. Subsequently, the existence of a fermentable carbohydrate might result in the development of undesirable secondary flora during the ripening period of cheese. The acidity depends chiefly on the quantity and type of starter, pre-acidification, duration, and temperature of acid development [49].

Table 5.
pH of probiotic cheddar cheese.
3.12. Sensory Evaluation of Probiotic Cheddar Cheese
The sensory characteristics of probiotic cheddar cheese have been presented in Table 6. The analysis of variance for means of taste and flavor of probiotic cheese demonstrated significant variations with increasing concentration of encapsulation material and ripening days. Their interaction significantly affected the taste of probiotic cheddar cheese. However, the prepared cheddar cheese was not significantly different from that of the control treatment (T0) with respect to crumbliness, stickiness, firmness, slice-ability, and general acceptability. Incorporating microencapsulated probiotic L. acidophilus in the cheese did not cause any defects. A maximum score was observed for taste in T6 (4.13 ± 0.07), and a minimum score was observed for T0 (2.64 ± 0.07). The maximum score for flavor was observed in T5 (4.05 ± 0.06), and the minimum score was observed for T0 (2.75 ± 0.04). A maximum score was observed for crumbliness in T2 (3.58 ± 0.03) and a maximum score for stickiness was observed for T4 (3.51 ± 0.11). T2 was observed to be the firmest cheese among all the treatments (3.56 ± 0.08). Generally, consumers choose cheeses based on sensory characteristics [50]. Higher moisture retention may result in a tough and rubbery texture, thereby improving the body, texture, and functional properties of cheese [51].

Table 6.
Sensory characteristics of probiotic cheddar cheese. Hedonic scale (1–5, 1 = poor, 5 = excellent).
The cheese flavor results from lipolysis and proteolysis by starter cultures/non-starter lactic acid bacteria, and these microbes play a significant role in the flavor, body, and texture of finished cheese.
4. Conclusions
The results demonstrated that encapsulated L. acidophilus could be utilized to produce probiotic cheddar cheese. The encapsulated probiotics maintained viability and stability under low pH, storage, and bile salt conditions. Therefore, whole milk can be combined with alginate to protect the probiotics from harsh conditions like low pH, high bile concentration, elevated temperature, and targeted delivery of core material. This may help the local food industry utilize native probiotic strains to be incorporated into probiotic foods with improved bio-accessibility. Adding milk with alginate as an encapsulant may open the doors for more natural materials to be used for encapsulation with a green image. The outcomes of the present study provided an economical encapsulating material and an optimized formulation to improve their viability. Furthermore, the increased viability of encapsulated probiotics (L. acidophilus) will positively impact the probiotic’s food products.
Author Contributions
Conceptualization, M.S. and M.B. methodology, M.S. and M.A.; software, M.A., A.A. and M.H.; validation, A.F., M.B. and M.A.; formal analysis, M.B. and H.A.; investigation, H.S.K., M.H., A.F. and H.K.W.; resources, H.S.K., M.A., A.A. and A.F.; data curation, H.S.K., A.A., T.A., H.A., H.K.W. and M.B.; writing—original draft, M.H. and A.F.; writing—review & editing, M.S., M.A., M.B., H.A., H.K.W., H.S.K. and A.A.; visualization, M.A., M.B., A.F. and A.A.; supervision, M.S.; project administration, M.H., M.A., T.A., H.K.W. and A.F.; funding acquisition, M.S., M.H., M.B., M.A., T.A. and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the University of Agriculture, Faisalabad and Bahauddin Zakariya University, Multan-Pakistan for providing facilities for the research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yasmin, H.M.; Zahoor, T.; Sagheer, A.; Nadeem, M.; Khaliq, A.; Iqbal, R.; Ahsan, S.; Ahmad, Z. Assessment of antagonistic activity of free and encapsulated Bifidobacterium bifidum against Salmonella. J. Food Saf. 2018, 38, e12546. [Google Scholar] [CrossRef]
- Silva, K.K.D.P.; de Souza Queirós, M.; Ribeiro, A.P.B.; Gigante, M.L. Modified milk fat as encapsulating material for the probiotic microorganism Lactobacillus acidophilus LA3. Int. Dairy J. 2022, 125, 105237. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk proteins as encapsulation devices and delivery vehicles: Applications and trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Molecul. Nutri. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Nunes, G.L.; de Araújo Etchepare, M.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; de Moraes Flores, É.M.; da Silva, C.D.B.; de Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT-Food Sci. Technol. 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, A.; Zahra, S.; Hassan, Z.U.; Kubra, K.T.; Azam, M.; Zahid, H.F. Phytochemical Composition and Pharmacological Potential of Lemongrass (Cymbopogon) and Impact on Gut Microbiota. AppliedChem 2022, 2, 229–246. [Google Scholar] [CrossRef]
- Azam, M.; Saeed, M.; Yasmin, I.; Afzaal, M.; Ahmed, S.; Khan, W.A.; Iqbal, M.W.; Hussain, H.T.; Asif, M. Microencapsulation and invitro characterization of Bifidobacterium animalis for improved survival. J. Food Meas. Charact. 2021, 15, 2591–2600. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Arshad, M.S.; Khan, M.A.; Saeed, M.; Maan, A.A.; Khan, M.K.; Ismail, Z.; Ahmed, A.; et al. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in ice cream. Food Sci. Nutr. 2020, 8, 1649–1656. [Google Scholar] [CrossRef]
- Azam, M.; Saeed, M.; Pasha, I.; Shahid, M. A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Biosci. 2020, 37, 100679. [Google Scholar] [CrossRef]
- Yasmin, I.; Saeed, M.; Pasha, I.; Zia, M.A. Development of whey protein concentrate-pectin-alginate based delivery system to improve survival of B. longum BL-05 in simulated gastrointestinal conditions. Probiotics Antimicrob. Proteins 2019, 11, 413–426. [Google Scholar] [CrossRef]
- Velciov, A.B.; Popescu, S.; Cozma, A.; Lalescu, D.; David, I. Statistical evaluation of nutritional characteristics for several cheese types. Int. Multidiscip. Sci. Geo Conf. SGEM 2018, 18, 441–447. [Google Scholar]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Liao, N.; Luo, B.; Gao, J.; Li, X.; Zhao, Z.; Zhang, Y.; Ni, Y.; Tian, F. Oligosaccharides as co-encapsulating agents: Effect on oral Lactobacillus fermentum survival in a simulated gastrointestinal tract. Biotechnol. Lett. 2019, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.Q.; Zhou, J.; Walker, J.; Li, L.; Hong, J.K.; Olsen, K.F.; Tang, J.; Ackermann, R.; Wang, Y.; Qin, B.; et al. Microencapsulation of luteinizing hormone-releasing hormone agonist in poly (lactic-co-glycolic acid) microspheres by spray-drying. J. Control. Release 2019, 321, 756–772. [Google Scholar] [CrossRef]
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of double alginate microencapsulation on in vitro digestibility and thermal tolerance of Lactobacillus plantarum NCDC201 and L. casei NCDC297. LWT-Food Sci. Technol. 2017, 83, 50–58. [Google Scholar] [CrossRef]
- Soodam, K.; Ong, L.; Powell, I.B.; Kentish, S.E.; Gras, S.L. Effect of elevated temperature on the microstructure of full fat Cheddar cheese during ripening. Food Struct. 2017, 14, 8–16. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, alpha-glucosidase inhibition activities, in silico molecular docking and pharmacokinetics study of phenolic compounds from native australian fruits and spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Legione, A.R.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. Probiotic yoghurt enriched with mango peel powder: Biotransformation of phenolics and modulation of metabolomic outputs after in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 8560. [Google Scholar] [CrossRef]
- Upadhyay, P.; Joshi, H. Viability of Lactobacillus fermentum CM36 and Lactobacillus rhamnosus CW40 in skimmed milk during refrigeration. J. Camel Pract. Res. 2020, 27, 77–79. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material encapsulation in poly (methyl methacrylate) shell: A review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef]
- Pradeep Prasanna, P.H.; Charalampopoulos, D. Encapsulation in an alginate–goats’ milk–inulin matrix improves survival of probiotic Bifidobacterium in simulated gastrointestinal conditions and goats’ milk yoghurt. Int. J. Dairy Technol. 2019, 72, 132–141. [Google Scholar] [CrossRef]
- Guimaraes, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible films and coatings as carriers of living microorganisms: A new strategy towards biopreservation and healthier foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef] [PubMed]
- Šipailienė, A.; Petraitytė, S. Encapsulation of probiotics: Proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Prob. Antimicrob. Proteins 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Zanjani, M.A.K.; Ehsani, M.R.; Ghiassi Tarzi, B.; Sharifan, A. Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L-lysine coatings in ice cream. J. Food Process. Preserv. 2018, 42, e13318. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.; Barbosa-Dekker, A.M.; Garcia, S. Effect of natural polymers on the survival of Lactobacillus casei encapsulated in alginate microspheres. J. Microencapsul. 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Wiessel, A.L. Shelf-Stable Fermented Dairy Products and Methods of Making Same. Google Patents WO2014170716A1, 23 October 2014. [Google Scholar]
- Praepanitchai, O.A.; Noomhorm, A.; Anal, A.K. Survival and behavior of encapsulated probiotics (Lactobacillus plantarum) in calcium-alginate-soy protein isolate-based hydrogel beads in different processing conditions (pH and temperature) and in pasteurized mango juice. BioMed Res. Int. 2019, 2019, 9768152. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fliss, I.; Beyssac, E.; Garrait, G.; Subirade, M. A prebiotic matrix for encapsulation of probiotics: Physicochemical and microbiological study. J. Microencapsul. 2016, 33, 89–101. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending viability of Bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Chen, W.; Hang, F. Lactic acid bacteria starter. In Bioengineering and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 93–143. [Google Scholar]
- Lin, T.C.; Chen, B.Y.; Chen, C.Y.; Chen, Y.S.; Wu, H. Comparative analysis of spray-drying microencapsulation of Bifidobacterium adolescentis and Lactobacillus acidophilus cultivated in different growth media. J. Food Process Eng. 2019, 42, e13258. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Halim, M.; Mustafa, N.A.M.; Othman, M.; Wasoh, H.; Kapri, M.R.; Ariff, A.B. Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT-Food Sci. Technol. 2017, 81, 210–216. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.M.; Wu, R.Q.; Wei, Y.S.; Zong, M.H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef]
- de Araújo Etchepare, M.; Nunes, G.L.; Nicoloso, B.R.; Barin, J.S.; Flores, E.M.M.; de Oliveira Mello, R.; de Menezes, C.R. Improvement of the viability of encapsulated probiotics using whey proteins. LWT-Food Sci. Technol. 2020, 117, 108601. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT-Food Sci. Technol. 2019, 116, 108501. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A comparative study for nutritional and phytochemical profiling of coffea arabica (c. Arabica) from different origins and their antioxidant potential and molecular docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef]
- Zuljan, F.A.; Mortera, P.; Alarcón, S.H.; Blancato, V.S.; Espariz, M.; Magni, C. Lactic acid bacteria decarboxylation reactions in cheese. Int. Dairy J. 2016, 62, 53–62. [Google Scholar] [CrossRef]
- Tekin, A.; Güler, Z. Glycolysis, lipolysis and proteolysis in raw sheep milk Tulum cheese during production and ripening: Effect of ripening materials. Food Chem. 2019, 286, 160–169. [Google Scholar] [CrossRef]
- Zheng, X.; Li, K.; Shi, X.; Ni, Y.; Li, B.; Zhuge, B. Potential characterization of yeasts isolated from Kazak artisanal cheese to produce flavoring compounds. Microbiol. Open 2018, 7, e00533. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, E.; Kilcawley, K.N.; Rea, M.C.; Fitzgerald, G.F.; McAuliffe, O. Genetic, enzymatic and metabolite profiling of the Lactobacillus casei group reveals strain biodiversity and potential applications for flavour diversification. J. Appl. Microbiol. 2017, 122, 1245–1261. [Google Scholar] [CrossRef]
- Gomand, F.; Borges, F.; Burgain, J.; Guerin, J.; Revol-Junelles, A.M.; Gaiani, C. Food matrix design for effective lactic acid bacteria delivery. Ann. Rev. Food Sci. Technol. 2019, 10, 285–310. [Google Scholar] [CrossRef]
- Dantas, A.B.; Jesus, V.F.; Silva, R.; Almada, C.N.; Esmerino, E.A.; Cappato, L.P.; Silva, M.C.; Raices, R.S.; Cavalcanti, R.N.; Carvalho, C.C.; et al. Manufacture of probiotic Minas Frescal cheese with Lactobacillus casei Zhang. J. Dairy Sci. 2016, 99, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Evivie, S.E.; Huo, G.C.; Igene, J.O.; Bian, X. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutri. Res. 2017, 61, 1318034. [Google Scholar] [CrossRef] [PubMed]
- Rama, G.R.; Kuhn, D.; Beux, S.; Maciel, M.J.; de Souza, C.F.V. Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 2019, 98, 25–37. [Google Scholar] [CrossRef]
- Drake, M.A.; Delahunty, C.M. Sensory character of cheese and its evaluation. In Cheese; Academic Press: Cambridge, MA, USA, 2017; pp. 517–545. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; Volume 1, p. 271. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).