Structural Characterization with Laser Scanning Microscopy and an Analysis of Volatile Components Using GC-MS in Vanilla Pods Coated with Edible Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Coating and Curing Process
2.2. The Surface and Internal Structure of Vanilla Pods Observed Using Laser Scanning Microscopy
2.3. Ethanol Extraction and Sample Preparation
2.4. Gas Chromatography-Mass Spectrometry (GC-MS)
2.5. Odor Description and Flavor Description Determination
2.6. The Quantitative Analysis of Volatile Compounds in Ethanol Extract of Vanilla Pods
2.7. The Odor Threshold and Odor Activity Value (OAV) of the Potent Aromatic Compounds of the Ethanol Extract of Vanilla Pods
2.7.1. Odor Thresholds of Compounds
2.7.2. The Odor Activity Value (OAV) of Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Surface and Internal Tissue Changes of Vanilla Pods
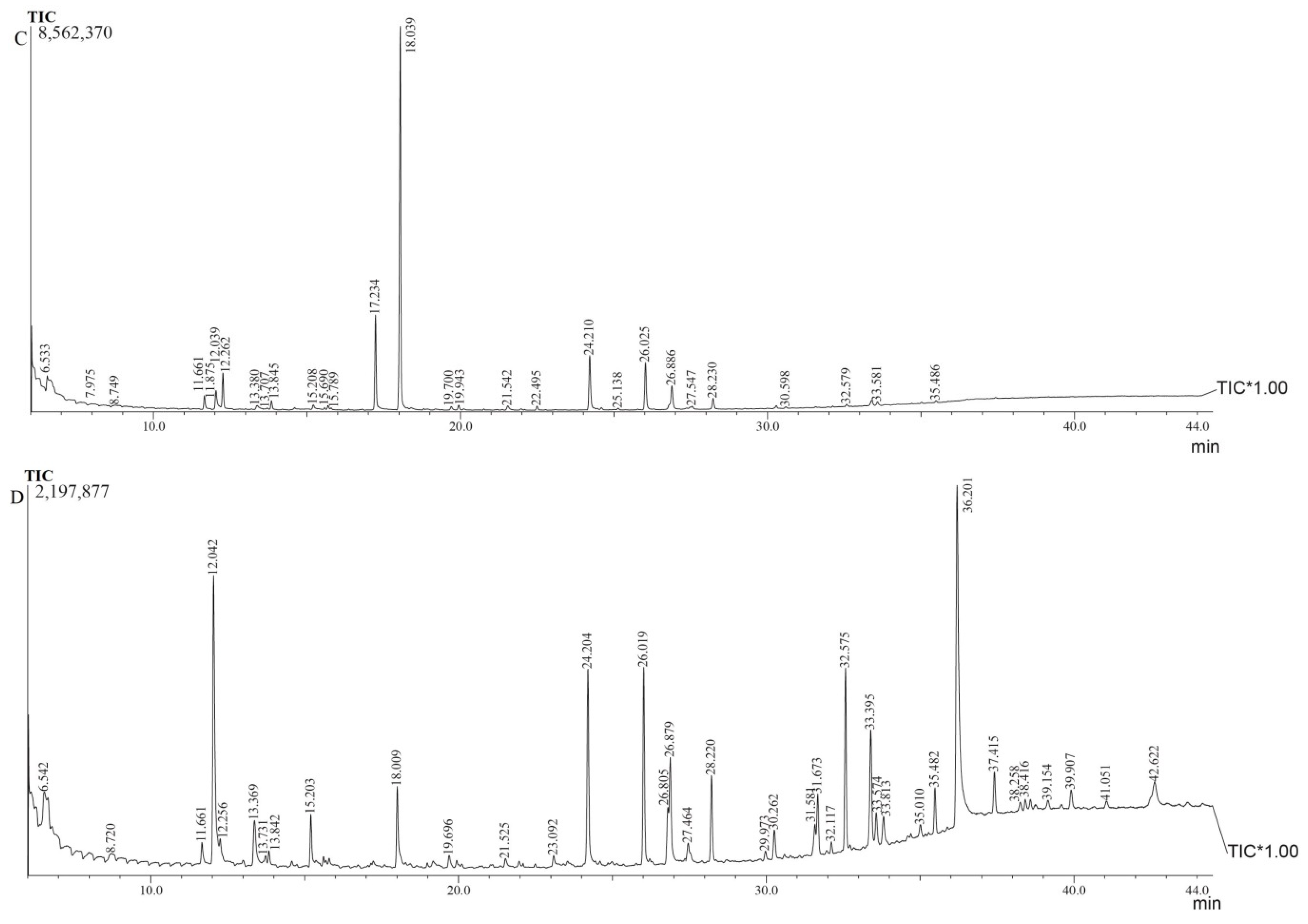
3.2. Gas Chromatographic Profiles
3.3. Analysis of Volatile Components in Ethanol Extracts
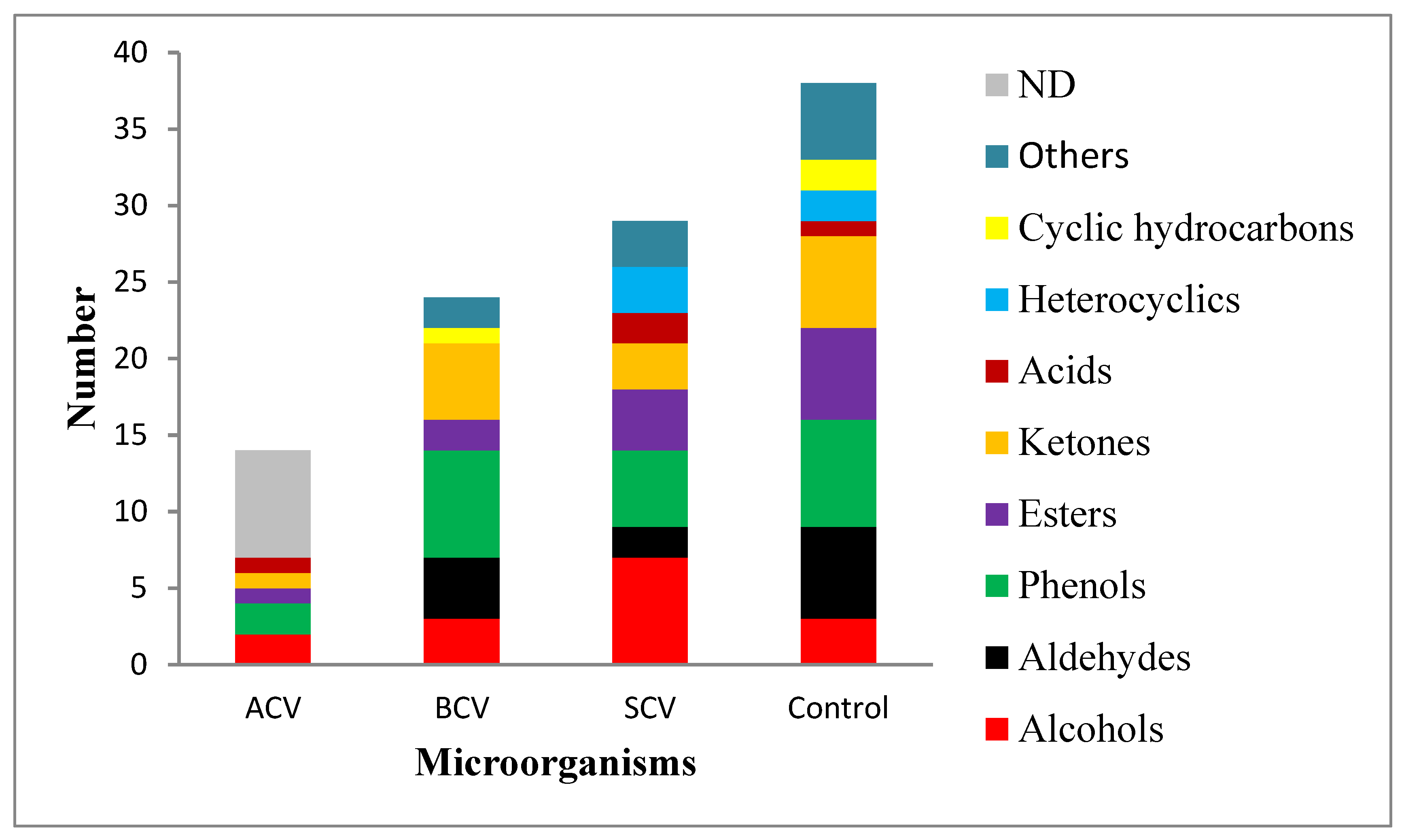
3.4. Numbers of Categories of Volatile Compounds in the Ethanol Extracts of Cured Whole Vanilla Pods
3.5. Odor/Flavor Description and Potential Odor/Flavor Contributors
3.5.1. Odor/Flavor Description
3.5.2. Potential Odor/Aroma Contributors
3.6. Odor Activity Value (OAV) for Potential Aromatic Compounds
3.7. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilde, A.S.; Frandsen, H.L.; Fromberg, A.; Smedsgaard, J.; Greule, M. Isotopic characterization of vanillin ex glucose by GC-IRMS-New challenge for natural vanilla flavour authentication? Food Control. 2019, 106, 106735. [Google Scholar]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J.Á. Vanilla (Vanilla planifolia Andr.), its residues and other industrial by-products for recovering high value flavor molecules: A review. J. Appl. Res. Med. Aromat. Plants 2017, 6, 1–9. [Google Scholar]
- Havkin-Frenkel, D.; French, J.C.; Graft, N.M.; Pak, F.; Frenkel, C.; Joel, D. Interrelation of curing and botany in vanilla (Vanilla planifolia) bean. Acta Hortic. 2004, 629, 93–102. [Google Scholar] [CrossRef]
- Wongsheree, T.; Wongs-Aree, C.; Srilaong, V.; Jitareerat, P. Vanilla Cultivation and Curing in Thailand; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2013. [Google Scholar]
- Sharp, M.D.; Kocaoglu-Vurma, N.A.; Langford, V.; Rodriguez-Saona, L.E.; Harper, W.J. Rapid discrimination and characterization of vanilla bean extracts by attenuated total reflection infrared spectroscopy and selected Ion flow tube mass spectrometry. J. Food Sci. 2012, 77, 284–292. [Google Scholar]
- Ramachandra Rao, S.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar]
- Khoyratty, S.; Kodja, H.; Verpoorte, R. Vanilla flavor production methods: A review. Ind. Crops Prod. 2018, 125, 433–442. [Google Scholar]
- Esen, A. ß-Glucosidases Overview. In ß-Glucosidases; American Chemical Society: Washington, DC, USA, 1993; Volume 533, pp. 1–14. [Google Scholar]
- Khoyratty, S.; Verpoorte, R.; Kodja, H. Vanillin: Biosynthesis, biotechnology, and bioproduction. In Orchids Phytochemistry, Biology and Horticulture: Fundamentals and Applications; Mérillon, J.M., Kodja, H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 341–358. [Google Scholar]
- Khoyratty, S.; Dupont, J.; Lacoste, S.; Palama, T.L.; Choi, Y.H.; Kim, H.K.; Payet, B.; Grisoni, M.; Fouillaud, M.; Verpoorte, R.; et al. Fungal endophytes of Vanilla planifolia across Réunion Island: Isolation, distribution and biotransformation. BMC Plant Biol. 2015, 15, 142. [Google Scholar]
- Che, H.; Yu, J.; Sun, J.; Lu, K.; Xie, W. Bacterial composition changes and volatile compounds during the fermentation of shrimp paste: Dynamic changes of microbial communities and flavor composition. Food Biosci. 2021, 43, 101169. [Google Scholar]
- Abd-Aziz, S.; Jenol, M.A.; Ramle, I.K. Biovanillin from oil palm biomass. In Biorefinery of Oil Producing Plants for Value-Added Products; Abd-Aziz, S., Gozan, M., Ibrahim, M.F., Phang, L.Y., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 493–514. [Google Scholar]
- Chen, P.; Yan, L.; Wu, Z.; Li, S.; Bai, Z.; Yan, X.; Wang, N.; Liang, N.; Li, H. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Sci. Rep. 2016, 6, 20400. [Google Scholar]
- Mehmood, T.; Ahmed, S.; Waseem, R.; Saeed, S.; Ahmed, W.; Irfan, M.; Ullah, A. Valorization of fruit peels into biovanillin and statistical optimization of process using enterobacter hormaechei through solid-state fermentation. Fermentation 2022, 8, 40–54. [Google Scholar]
- Yoshida, A.; Takenaka, Y.; Tamaki, H.; Frébort, I.; Adachi, O.; Kumagai, H. Vanillin formation by mcrobial amine oxidases from vanillylamine. J. Ferment. Bioeng. 1997, 84, 603–605. [Google Scholar]
- Rejani, C.T.; Radhakrishnan, S. Microbial conversion of vanillin from ferulic acid extracted from raw coir pith. Nat. Prod. Res. 2020, 36, 901–908. [Google Scholar]
- Vandamme, E.J. Bioflavours and fragrances via fungi and their enzymes. Fungal Divers. 2003, 13, 153–166. [Google Scholar]
- Yeh, C.H.; Chen, K.Y.; Chou, C.Y.; Liao, H.Y.; Chen, H.C. New insights on volatile components of Vanilla planifolia cultivated in Taiwan. Molecules 2021, 26, 3608. [Google Scholar] [PubMed]
- Odoux, E.; Brillouet, J.M. Anatomy, histochemistry and biochemistry of glucovanillin, oleoresin and mucilage accumulation sites in green mature vanilla pod (Vanilla planifolia; Orchidaceae): A comprehensive and critical reexamination. Fruits 2009, 64, 221–241. [Google Scholar]
- The Good Scents Company (TGSC). Information System. Available online: http://www.thegoodscentscompany.com/index.html (accessed on 1 December 2022).
- MFood Flavor Innovation (MFFi). Available online: https://mffi.sjtu.edu.cn/ (accessed on 16 July 2023).
- The LRI & Odour Database—Odour Data. Available online: http://www.odour.org.uk/odour/index.html (accessed on 16 July 2023).
- ISO 5565-1:1999; Vanilla [Vanilla fragrans (Salisbury) Ames]—Part 1: Specification. International Organization for Standardization (ISO): Geneva, Switzerland, 1999. Available online: https://www.iso.org/standard/22116.html (accessed on 16 July 2023).
- Gurnani, N.; Kapoor, N.; Mehta, D.; Gupta, M.; Mehta, B. Characterization of chemical groups and identification of novel volatile constituents in organic solvent extracts of cured Indian vanilla beans by GC-MS. Middle East J. Sci. Res. 2014, 22, 769–776. [Google Scholar]
- Silva, A.P.; Gunata, Z.; Lepoutre, J.P.; Odoux, E. New insight on the genesis and fate of odor-active compounds in vanilla beans (Vanilla planifolia G. Jackson) during traditional curing. Food Res. Int. 2011, 44, 2930–2937. [Google Scholar]
- Gu, F.; Chen, Y.; Fang, Y.; Wu, G.; Tan, L. Contribution of Bacillus isolates to the flavor profiles of vanilla beans assessed through aroma analysis and chemometrics. Molecules 2015, 20, 18422–18436. [Google Scholar]
- Chen, Y.; Gu, F.; Li, J.; He, S.; Xu, F.; Fang, Y. Involvement of colonizing Bacillus isolates in glucovanillin hydrolysis during the curing of Vanilla planifolia Andrews. Appl. Environ. Microbiol. 2015, 81, 4947–4954. [Google Scholar]
- Zhang, Y.; Mo, L.; Chen, F.; Lu, M.; Dong, W.; Wang, Q.; Xu, F.; Gu, F. Optimized production of vanillin from green vanilla pods by enzyme-assisted extraction combined with pre-freezing and thawing. Molecules 2014, 19, 2181–2198. [Google Scholar]
- Yeh, C.H.; Chou, C.Y.; Wu, C.S.; Chu, L.P.; Huang, W.J.; Chen, H.C. Effects of different extraction methods on vanilla aroma. Molecules 2022, 27, 4593. [Google Scholar]
- Havkin-Frenkel, D.; Belanger, F.C. Handbook of Vanilla Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–488. [Google Scholar]
- Pérez-Silva, A.; Odoux, E.; Brat, P.; Ribeyre, F.; Rodriguez-Jimenes, G.; Robles-Olvera, V.; García-Alvarado, M.; Günata, Z. GC–MS and GC–olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chem. 2006, 99, 728–735. [Google Scholar]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar]
- Dignum, M.J.W.; van der Heijden, R.; Kerler, J.; Winkel, C.; Verpoorte, R. Identification of glucosides in green beans of Vanilla planifolia Andrews and kinetics of vanilla β-glucosidase. Food Chem. 2004, 85, 199–205. [Google Scholar]
- Kim, S.J.; Kim, J.W.; Lee, Y.G.; Park, Y.C.; Seo, J.H. Metabolic engineering of Saccharomyces cerevisiae for 2, 3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 2241–2250. [Google Scholar]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [PubMed]
- Álvarez, A.; Gutiérrez, A.; Ramírez, C.; Cuenca, F.; Bolívar, G. Aroma compounds produced by liquid fermentation with Saccharomyces cerevisiae and Zygosaccharomyces rouxii from castor oil through cell permeabilization. Biocatal. Agric. Biotechnol. 2022, 39, 102243. [Google Scholar]
- Feng, J.; Zhan, X.B.; Zheng, Z.Y.; Wang, D.; Zhang, L.M.; Lin, C.C. New model for flavour quality evaluation of soy sauce. Czech J. Food Sci. 2013, 31, 292–305. [Google Scholar]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar]
- Draelos, Z.D. The combination of 2% 4-hydroxyanisole (mequinol) and 0.01% tretinoin effectively improves the appearance of solar lentigines in ethnic groups. J. Cosmet. Dermatol. 2006, 5, 239–244. [Google Scholar] [PubMed]
- Cadwallader, K. 18—Measuring cheese flavor. In Improving the Flavour of Cheese; Weimer, B.C., Ed.; Woodhead Publishing: Sawston, UK, 2007; pp. 401–417. [Google Scholar]
- Methven, L. 16—Techniques in sensory analysis of flavour. In Flavour Development, Dnalysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 353–368. [Google Scholar]
- San, J.F.; Cacho, J.; Ferreira, V.; Escudero, A. Chapter 21—Differences in chemical composition of croma among red wines of different price category. In Flavour Science; Ferreira, V., Lopez, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 117–121. [Google Scholar]
- Ferreira, V. 1—Volatile aroma compounds and wine sensory attributes. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 3–28. [Google Scholar]
- van Schijndel, J.; Molendijk, D.; van Beurden, K.; Canalle, L.A.; Noël, T.; Meuldijk, J. Preparation of bio-based styrene alternatives and their free radical polymerization. Eur. Polym. J. 2020, 125, 109534. [Google Scholar]
- Li, R.; Tian, S.; Guo, C.; Li, l.; Liang, M.; Zhang, J. Sensory-guided analysis and recombination of characteristic flavor compoundsin Vanilla planifolia Andrews. Food Sci. 2023, 44, 217–223. (In Chinese) [Google Scholar]
- Wang, B.; Xu, H.A.; XU, J.; Qu, M.; Zhang, Y.; Huang, Q.; Xiao, L. Analysis of characteristic volatile aroma components in Inner Mongolia dried Allium chrysanthum. Sci. Technol. Food Ind. 2022, 43, 296–304. (In Chinese) [Google Scholar] [CrossRef]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic fungi: Their physiology and enzymes. Microbiol. Mol. Biol. Rev. 2000, 80, 461–488. [Google Scholar]
- Gupta, P.; Samant, K.; Sahu, A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012, 2012, 578925. [Google Scholar] [CrossRef]



| Compounds | ACV a | BCV b | SCV c | Control | |
|---|---|---|---|---|---|
| 1 | Hexanal | 1.82 | 3.49 | ||
| 2 | 1,2-Ethanediamine, N,N′-dimethyl- | 0.85 | 0.56 | ||
| 3 | 2-Butanone, 3-hydroxy- | 1.81 | 0.74 | ||
| 4 | 2-Propanone, 1-hydroxy- | 3.26 | 13.56 | ||
| 5 | Propanoic acid, 2-hydroxy-, methyl ester | 4.18 | 0.28 | ||
| 6 | Pentane, 2-bromo- | 1.08 | 2.6 | ||
| 7 | 1-Hydroxy-2-butanone | 0.5 | |||
| 8 | Acetic acid, hydroxy-, ethyl ester | 1.04 | 0.43 | ||
| 9 | Pentanoic acid, 3-methyl-2-oxo-, methyl ester | 0.67 | 1.68 | ||
| 10 | 2,3-Butanediol | 66.47 | 46.1 | 3.25 | |
| 11 | Butyrolactone <gamma-> | 0.68 | 0.53 | 0.57 | |
| 12 | 2(5H)-Furanone, 3-methyl- | 0.72 | 0.31 | ||
| 13 | Cyclopentane, butyl- | 0.33 | |||
| 14 | Mequinol | 7.96 | 7.11 | ||
| 15 | Creosol | 4.34 | 6.59 | 6.54 | |
| 16 | Levoglucosenone | 1.87 | |||
| 17 | Phenol | 4.46 | 4.61 | ||
| 18 | Benzaldehyde, 4-methoxy- | 0.85 | |||
| 19 | Phenol, 3-methyl- | 1.55 | 2.99 | ||
| 20 | 2-Hydroxy-gamma-butyrolactone | 0.33 | |||
| 21 | 2-Methoxy-4-vinylphenol | 1.12 | |||
| 22 | 2,3-Anhydro-d-galactosan | 1.33 | |||
| 23 | 2,3-Anhydro-d-mannosan | 2.32 | |||
| 24 | Phenol, 2-methoxy-4-(methoxymethyl)- | 0.36 | |||
| 25 | Methyl-(2-hydoxy-3-ethoxy-benzyl)ether | 0.29 | 5.76 | ||
| 26 | Vanillin methyl ether | 4.24 | 4.82 | ||
| 27 | 1,4:3,6-Dianhydro-.alpha.-d-glucopyranose | 0.65 | 1.37 | ||
| 28 | 2(1H)-Pyridinone | 1.46 | |||
| 29 | 3-Propylglutaric acid | 0.46 | |||
| 30 | Phenol, 4-(ethoxymethyl)- | 0.25 | 1.6 | ||
| 31 | Vanillin | 30.22 | 20.97 | ||
| 32 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | 1.56 | |||
| 33 | 4-Hydroxy-2-methoxybenaldehyde | 0.33 | |||
| 34 | Ethyl homovanillate | 0.44 | |||
| 35 | 3,4-Anhydro-d-galactosan | 0.35 | |||
| 36 | Benzene, 1,1′-[1,2-ethanediylbis(oxy)]bis [2-methoxy- | 0.86 | |||
| 37 | Benzenemethanol, alpha.-(1-methylethyl)-, (R)- | 0.28 | |||
| 38 | Benzaldehyde, 4-hydroxy- | 2 | |||
| 39 | Acetoin | 2.67 | 1.43 | ||
| 40 | Methyl L-lactate | 2.86 | |||
| 41 | Lactic acid | 7.69 | 11.03 | ||
| 42 | Guaiacol | 0.44 | 10 | ||
| 43 | Phenyl alcohol | 0.65 | 4.96 | 0.15 | |
| 44 | sec-Butylamine | 0.5 | |||
| 45 | Hydroxyacetone | 25.25 | |||
| 46 | Pentene <3-hydroxy-> | 1 | |||
| 47 | Acetyl isovaleryl | 1.23 | |||
| 48 | 1-Acetoxy-2-propanone | 0.29 | |||
| 49 | Furfuryl alcohol | 2.34 | |||
| 50 | Corylon | 0.3 | |||
| 51 | Cresol <para-> | 0.81 | 2.64 | ||
| 52 | Allyl alcohol | 0.61 | |||
| 53 | Guaiacol <4-vinyl-> | 2.08 | |||
| 54 | Vanillyl butyl ether | 1.07 | |||
| 55 | Ferrolure isomer I | 1.28 | |||
| 56 | Eugenol | 1.36 | |||
| 57 | Guaiacol <4-propyl-> | 0.56 | |||
| 58 | Benzaldehyde <para-hydroxy-> | 1.32 | |||
| 59 | Phytol acetate <(E)-> | 0.42 | |||
| 60 | Pentanal, 3-methyl- | 3.06 | |||
| 61 | dl-Alanyl-l-alanine | 0.95 | |||
| 62 | n-Hexylmethylamine | 0.21 | |||
| 63 | 3-Pentanol | 0.26 | |||
| 64 | Oxiranemethanol, (R)- | 0.5 | |||
| 65 | Furfural | 0.18 | |||
| 66 | 2-Furanmethano | 0.59 | |||
| 67 | 1H-Pyrrole, 2,5-dihydro- | 0.48 | |||
| 68 | 2-Pyrrolidinone | 0.39 | |||
| 69 | Nicotinyl Alcohol | 0.19 |
| Compounds | Odor Description a | Flavor Description a |
|---|---|---|
| Alcohols | ||
| 2,3-Butanediol | Fruity, creamy, buttery | |
| Phenyl alcohol | Phenolic | |
| Furfuryl alcohol | Sweet, caramel, bread, coffee | Sweet, caramel-like/at 50.00 ppm |
| Allyl alcohol | Pungent, mustard | |
| 3-Pentanol | Sweet, herbal, oily, nutty | |
| 2-Furanmethanol | Sweet, caramel, bread, coffee | Sweet, caramel-like, bready, coffee |
| Aldehydes | ||
| Hexanal | Fresh, fruity | Apple, citrus, orange with a fresh, lingering aftertaste |
| Benzaldehyde,4-methoxy- | Sweet, powdery, hawthorn, balsam | Creamy, vanilla, spicy with a marshmallow flavor |
| Vanillin | Sweet, vanilla, creamy | Vanilla, sweet, creamy, spicy, milky |
| Benzaldehyde, 4-hydroxy- | Sweet, nutty, almond, balsam, woody | Creamy, nutty with vanilla and honey nuances |
| Furfural | Sweet, woody, almond, fragrant, baked, bread | Sweet, woody, bready, nutty, caramel-like/at 30.00 ppm. |
| Vanillin methyl ether | Sweet, woody, vanilla | Sweet, creamy, vanilla/at 50.00 ppm. |
| Phenols | ||
| Creosol | Sweet, candy, spice, eugenol, vanilla | Vanilla, spice, eugenol, woody |
| Phenol | Phenolic | |
| 2-Methoxy-4-vinylphenol | Sweet, spicy, clove, woody, powdery | Spicy, powdery, clove, woody, balsamic, amber |
| Guaiacol | Spice, vanilla, woody | Woody, phenolic, bacon, savory, smoky, medicinal |
| Cresol <para-> | Narcissus, mimosa | Phenolic |
| Guaiacol <4-vinyl-> | Sweet, spicy, clove, woody | Spicy, clove, woody |
| Guaiacol <4-propyl-> | Clove, spicy, sweet, allspice | Spicy, clove, allspice, peppery |
| Mequinol | Phenolic | |
| Vanillyl butyl ether | Vanilla, fruity | |
| Esters | ||
| Butyrolactone <gamma-> | Creamy, caramel | Milky with fruity peach-like afternotes/at 75.00 ppm |
| Phytol acetate <(E)-> | Mild, floral, fruity, orchid, balsamic | Nutty, waxy, woody, oily, creamy, nut, flesh |
| Ketones | ||
| 1-Hydroxy-2-butanone | Sweet, coffee, malt, butterscotch | Toasted grain notes/at 30.00 ppm. |
| Acetyl isovaleryl | Sweet, fruity, creamy | Fruity, creamy with a pineapple nuance/at 50.00 ppm. |
| Acetoin | Sweet, buttery, creamy, dairy, milky | Creamy, dairy, sweet, milky, buttery |
| Hydroxyacetone | Pungent, sweet, caramel-like | Sweet, burnt |
| 1-Acetoxy-2-propanone | Fruity, buttery, dairy, nutty | |
| Corylon | Caramel, maple syrup | Caramel-like |
| Acids | ||
| Lactic acid | Odorless | Sour, acid |
| Compound | Threshold (ppm) | OAV | Odor Type | |||
|---|---|---|---|---|---|---|
| ACV | BCV | SCV | Control | |||
| 2-Methoxy-4-vinylphenol | 0.003 a | - | - | - | 2,740,450 | spicy |
| 2,3-Butanediol | 100 b | 2509 | - | 3266 | 238 | creamy |
| Furfural | 9.562 b | - | - | 137 | - | bready |
| Cresol <para-> | 0.0039 b | 781,235 | 8,101,401 | - | - | phenolic |
| 3-Pentanol | 0.12 c | - | - | 15,400 | - | herbal |
| Acetoin | 0.8 c | 12,603 | 21,371 | - | - | buttery |
| Hexanal | 4.5 c | - | 4828 | - | 5682 | green |
| Vanillin | 0.35 d | - | 1,032,425 | - | 438,659 | vanilla |
| Benzaldehyde, 4-hydroxy- | 146.67 d | - | - | - | 100 | woody |
| Guaiacol | 5.53 d | 298 | 21,615 | - | - | smoky |
| Guaiacol <4-vinyl-> | 1.74 d | - | 3855 | - | - | spicy |
| Phenol | 20.01 d | - | - | 1580 | 1686 | phenolic |
| Furfuryl alcohol | 409.18 d | - | 60 | - | - | bready |
| Lactic acid | 899.35 d | 32 | - | 87 | - | odorless |
| Butyrolactone <gamma-> | 0.284 e | - | 28,737 | 13,320 | 14,779 | creamy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-E.; Lin, Y.-S.; Lo, H.-C.; Hsu, T.-H. Structural Characterization with Laser Scanning Microscopy and an Analysis of Volatile Components Using GC-MS in Vanilla Pods Coated with Edible Microorganisms. Fermentation 2023, 9, 724. https://doi.org/10.3390/fermentation9080724
Chen C-E, Lin Y-S, Lo H-C, Hsu T-H. Structural Characterization with Laser Scanning Microscopy and an Analysis of Volatile Components Using GC-MS in Vanilla Pods Coated with Edible Microorganisms. Fermentation. 2023; 9(8):724. https://doi.org/10.3390/fermentation9080724
Chicago/Turabian StyleChen, Chun-Erh, Yun-Sheng Lin, Hui-Chen Lo, and Tai-Hao Hsu. 2023. "Structural Characterization with Laser Scanning Microscopy and an Analysis of Volatile Components Using GC-MS in Vanilla Pods Coated with Edible Microorganisms" Fermentation 9, no. 8: 724. https://doi.org/10.3390/fermentation9080724
APA StyleChen, C.-E., Lin, Y.-S., Lo, H.-C., & Hsu, T.-H. (2023). Structural Characterization with Laser Scanning Microscopy and an Analysis of Volatile Components Using GC-MS in Vanilla Pods Coated with Edible Microorganisms. Fermentation, 9(8), 724. https://doi.org/10.3390/fermentation9080724







