Abstract
Slaughterhouse solid waste is one of the sources of greenhouse gas (GHG) today. Crop residue decomposition or incineration has a great impact on global warming. Therefore, it is urgent to study the possibility of better environmentally friendly approaches to solid waste management and its safe disposal. The digestion of this type of solid waste in a decomposing process from organic content allows the recovery of valuable resources (such as biogas) and the use of the digestate in various fertilizer industries. In this study, two substrates were studied to determine their biomethane (BMP) potential in anaerobic digestion. The substrates were fermented and digested anaerobically and biogas production was measured. Methane yield of the slaughterhouse substrates had a lower methane yield between 232.2 and 250.8 mL/gVS and 53.6 to 57.9% biodegradability. Harvest substrates produce between 167.1 and 274.9 mL/gVS with a biodegradability of 39.1 to 64.3%. Co-digestion of both substrates at a ratio of IS 1:2 (RR:WS 3:1) generated a higher yield 289.1 ml/gVS and 66.9%. biodegradability of A kinetic analysis was carried out using Gompertz models, transfer and logistic function for methane production biodegradation.
1. Introduction
Anaerobic digestion is characterized by being a reaction where biogas is produced from biodegradable matter under anaerobic conditions. It has been widely used as a method to provide energy, especially in Asian countries, such as: China, India and African countries [1]. As a beneficial method for the environment it is the best option for economic development and improvement of the quality of life in developing countries, anaerobic digestion is a suitable method to generate renewable energy [2]. European countries and the United States have developed a technology for the anaerobic treatment of wastewater and the production of biogas [3], this is possible with the use of modern reactors that enhance the treatment of a variety of waste [4]. This technology is capable of handling various organic residues, such as: cattle manure, biomass agricultural residues (crop residues) [5], residues from dairy industries, municipal residues (vegetable waste) [6], treatment of primary sludge and wastewater [7]. The nature of the substrate determines the possibility of generating biogas with a high percentage of methane, for this reason, numerous studies devote their attention to studying biomethanization potential of a variety of substrates and identifying their performance and energy recovery [8,9,10,11,12].
Biodegradable matter, such as livestock excrement, food plant residues or crop residues are potential feedstocks for generating methane in mono- and co-digestion. There have been precedents for producing biogas from cabbage waste and cattle manure, the study determined that biogas yield was 389.47 cm3/gVS with 61% methane composition [13]. Cocoa shells have also been used in co-digestion with cow dung on a laboratory scale to obtain biogas with 174 L/kgVS average methane yield [14]. In another study, codigested guinea pig droppings were treated with amaranth crop residues and wheat straw [11].
Agricultural fiber source, such as wheat straw, are promising lignocellulosic feedstocks for biofuel as biogas production. Nearly 733 million tons hectares of wheat are harvested annually worldwide [15]. Hence, millions of tons of wheat straw are mainly used for soil fertility, livestock feed, and incineration for energy production. In general, wheat straw contains 11–26% lignin, 32–45% cellulose, and 20–45% hemicelluloses [16]. Lignin is recalcitrant to biodegradation under anaerobic conditions unless the structure is modified. Wet oxidation followed by steam explosion (wet explosion) was previously found to make significant changes to the lignin structure allowing biodegradation under anaerobic conditions. Wet exploded lignin pretreated with 2% NaOH showing the highest lignin degradation (41.8%) as well as the highest methane potential 157.3 ± 9.9 mL/g VS [17]. In vitro degradation of wheat straw lignin has been reported with inoculation of cow rumen microorganisms. The dominant hydrogenotrophic methanogens (methanomassiliicoccus, Methanobrevibacter, Methanosphaera and Methanoculleus) in the cow rumen and the abundance of lignocellulolytic enzymes secreted by the amount of diverse rumen bacteria and fungi contributed to the continuous degradation of lignocellulosic waste for three months, resulting in 96 to 97% cellulose and hemicellulose decomposition and 42% lignin decomposition [18].
In livestock, currently one third of global anthropogenic methane emissions originate from ruminant cattle [19]. Enteric methane (CH4) is produced by methanogenic archaea found mainly in the rumen, where they convert the hydrogen (H2) and carbon dioxide (CO2) produced from fermentation by a complex community of ciliate protozoa, bacteria, and anaerobic fungi to CH4 [20]. Ruminants have a diverse microbiota in their rumen including bacteria, fungi, archaea, protozoa, and viruses. These microorganisms can degrade the plant cell wall and fibrous substances converted into absorbable compounds such as proteins and volatile fatty acids [21]. Ref. [22] Reported that the relative abundance of archaea of the Methanobacteriaceae family was 96.42% the Methanomassiliicoccaceae family was 3.53% in the rumen extracted from sheep. Another study carried out in the rumen of cattle reported that Firmicutes, Fibrobacteres, Euryarchaeota, Bacteroidetes and Proteobacteria were the predominant bacterial groups, with Firmicutes showing the highest abundance [23]: although the variability of each family is also influenced by cattle diet [24]. When cattle are slaughtered and the undigested remains are exposed to the environment, a fraction of the rumen microorganisms still remain active, methanogenic archaea has been found, generating methane under oxic conditions [25].
In view of this, slaughterhouse waste is a potential load of microbial communities available, a relevant candidate for co-digestion practices with other microbial community deficiency waste and hight lignin content. For this reason, the objective of this research is to carry out the anaerobic co-digestion of rumen residues with wheat straw in a potential biochemical methane assay and to define optimal parameters assisting in the selection of the most appropriate fermentation treatment by suggesting a potential pilot test.
2. Materials and Methods
2.1. Origin of Substrates and Inoculum
All experimental procedures were performed following the guidelines of [13,14]. This study was performed in the Stated Bolivar University, Guaranda—Ecuador. The main substrate (ruminal residue) was collected from the Municipal Slaughtering Center of the city of Guaranda, located at Latitude: 1°35′52.062″ S Longitude: 78°59′48.28″ O at an altitude of 2765 masl. This residue represents the matter in the process of digestion that was interrupted when the cattle were slaughtered. Daily slaughtering generates a large amount of this residue, a potential source of energy. Such samples were collected in polyethylene bags obtaining significant samples. Subsequently, they were stored in the laboratory at 6 C for 72 h before being added to the biodigesters.
The inoculum used in all the tests comes from the urban wastewater treatment station (WWTP) in the city of San Miguel de Ibarra (Ecuador) extracted from the anaerobic digester primary sludge that worked in mesophilic conditions (temperature between 35 and 37 °C approximately.
2.2. Characterization of Raw Materials and Biogas
The materials were characterized by proximal analysis and elemental analysis. Total substrate solids (ST) volatile solids percentage (VS) with respect to total solids and ashes were determined by the methodology proposed by UNE-EN 18134-1: 2016, UNE-EN 18134-2: 2017, UNE-EN ISO 18134-3, 2016, UNE-EN ISO 18123: 2016 standards [26,27,28] and [15].
Similarly, for the proximal analysis of the inoculum, whose composition was mostly liquid, a more proper wastewater methodology proposed by the American Public Health Association (APHA) [16] sections 2540A-2540G was used determining TS, VS and ashes.
The elementary analysis from which substrates N, C, O, H, S percentages and C/N ratio and inoculum are obtained were determined by VARIOUS MACRO CUBE elemental analyzer (Agilent technologies, Santa Clara, CA, USA), following the guidelines proposed by the standard UNE ISO16948 15104 [26]. The pH was defined at room temperature using a HACH HQ 40D digital multimeter meter potentiometer (HACH, Manchester, UK).
Biogas production was calculated from the pressure exerted by biogas inside the biodigester. The pressure was measured daily by the manometer (Delta OHM HD 2124.2, GHM GROUP, Via Marconi 5, ITALY) equipped with a sensor (Delta TP 704 with a capacity of 100 bar). After the daily pressure measurement, the biogas accumulated in the biodigester upper space was completely released causing a reduction in the pressure exerted by the biodigester close to atmospheric pressure. After releasing the biogas, the pressure in the biodigester head space was again measured as an initial condition for the next day measurement. Biogas components (CH4, H2S, CO2 and O2) were defined with the Geotech BIOGAS GA-5000 analyzer (QED, London, UK). The biogas estimate was evaluated daily from each biodigester by daily extraction of all the generated biogas.
2.3. Experimental Methodology
Experiments were carried out following BMP protocols [14]. Glass bottles of 311 mL with an effective 186 mL volume hermetically sealed with stoppers and gas control opening valves used as experimental units. The tests were performed using f 18 g VS/L concentration substrate and Inoculum/Substrate ratios of 2:1, 1:1, 1:2 based on VS; considered typical values for the BMP test [8]. The experiments were conducted within the mesophilic temperature range, at 38 °C ± 1. The experimental units were shaken manually twice a day during the experiment. The treatments were evaluated by triplicates and 9 control bottles without substrate were also included to correct the methane production from the inoculum as the experiments were carried out for 45 days. Biogas production was measured daily.
Experimental biogas yield was calculated from the pressure difference generated each day in the digesters [17]. Starting from the ideal gas equation, the volume of biogas generated at 38 °C was estimated by the equation:
ΔP: Absolute pressure difference (KPa), Vf: free volume, C: Molar gas volume at standard pressure and temperature (22.41 L/mol), R: ideal gas constant (8.314 L kPa/K/mol) and Tw: working temperature (mesophilic 38 °C) 311.15 K.
Theoretical performance calculation, biodegradability and synergistic effect index.
Theoretical maximum methane yield (TMY, mL/g-VS) was calculated from the elemental composition and substrate ash contents using the Buswell’s formula [18] and Chen’s formula [19], as shown in Equations (2) and (3), respectively.
Biodegradability (BD) was calculated from the highest cumulative methane yield (experimental methane yield, EMY) and TMY, as shown in Equation (3):
The synergistic effect index (SEI) for the anaerobic co-digestion proposed in this study was calculated as shown in Equation (4).
In Equation (4), EMYco is the EMY of co-digestion. EMY1 and EMY2 are mono-digestions EMYs of R and WS, respectively. X1 and X2 are the VS fractions of R and WS in the co-digestion, respectively.
2.4. Kinetic Modeling
The modified Gompertz model Equation (5), widely applied in simulating and predicting anaerobic digestion performance [20,21,22], was used in this study.
In Equation (5), B represents the simulated cumulative methane yield (mL/g-VS); B0 refers to the simulated maximum cumulative methane yield (mL/g-VS); Vmax means the maximum methane production rate (mL/g-VS/day); e is equal to 2.718; tlag stands for the lag phase time (day); t means digestion time (day).
Transfer function model Equation (6) predicts maximum methane production based only on accumulated methane production over time analyzing anaerobic digestion process as a system receiving inputs and generating outputs [15,23]
The Logistic function Equation (7) fits the global shape of the biogas production kinetics: it has an initial exponential increase and a final stabilization at a maximum production level. This model assumes that gas production rate is proportional to the amount of gas already produced, maximum production rate and maximum biogas production capacity. This model has been used for anaerobic fermentation and to estimate methane production in landfill leachate [24]. In this case, a modified version of the logistic function was used [25].
B (mL gVS−1) is the biogas produced at time t (d), B0 (mL gVS−1) is the maximum biogas production, Vmax (mL gVS−1 d−1) maximum biogas production rate and tlag (d) lag time.
3. Results and Discussion
3.1. Characterization of Substrates and Inoculums
Table 1 shows that ruminal residue volatile solids and wheat straw is 70.7% and 74.79% respectively, indicating that both substrates have a moderate volatile solids content equivalent to a normal organic matter content available for anaerobic digestion while both substrates ash low percentages are (12.81% and 8.45% respectively), justifying low volatile solids content of these substrates and a high amount of minerals. Likewise, the analyzed inoculum has 58.49% SV and 55.59% ash confirming organic matter high content availability since there are viable and non-viable microorganisms, as well as organic matter remains accompanied by the medium analyzed.

Table 1.
Characterization of substrates and inoculums.
Substrate C/N ratio is 100.99 and 28.93 rumen residue and wheat straw, respectively, these values could generate a wide range of C/N ratios available in anaerobic co-digestion for both substrates, compared each single mono- digestion substrate. On the one hand, the rumen residue contributes to a large amount of degradable carbon to the medium, due to its high C/N ratio. While wheat straw supplies nitrogen to the system due to its low C/N ratio while ruminal residue C/N ratio as well as its high organic matter content, are properties generating advantages when mixed with wheat straw—substrate with plenty of available nitrogen. In short, the co-digestion of both substrates could ameliorate the balance of nutrients in the digester increasing the amount of degradable organic matter, leading to an increase in methane production. An appropriate C/N ratio can accelerate the rate of substrate metabolism in fermentation. Some studies have shown that a C/N ratio of about 25:1 results in the highest methane synthesis output [26,27].
3.2. Cumulative Production of Biogas and Methane
Figure 1 shows the similarity in biogas generation at 40 days of digestion (Figure 1a,b) when the I:S rate is 1:2 and 2:1. Note that the order in which the substrate and co-substrate rate generate biogas is RR:WS (3:1, 2:2, 1:3, 4:0 and 0:4) for both proportions. There is an inversely proportional relationship of substrate and co-substrate (RR:WS), higher RR and lower WS, the greater the biogas generated implying that RR is easily biodegraded. It is well known that rumen has high cellulose-degrading efficiency thus its bacteria may be involved the AD degradation process. Methanogens active in the rumen may contribute to methanogenesis in the biogas production process [28]. Indeed, it can be observed that biodegradation starts immediately. When the amount of inoculum used is 2 times the amount of substrate (I:S—2:1), it can be observed that during RR and WS co-digestion, the biogas generated improves as the RR fraction added to the digester increases up to 50% with respect to WS, Figure 1c whereas RR to WS at 2:2 rate biogas yield was 493.247 mL/g-VS, being higher than RR (421.726 mL/g-VS) and WS (477.754 mL/g-VS) in monodigestion. Something similar occurs with the experiment I:S—1:1, Figure 1a RR to WS at 3:1 rate biogas yield was 427.787 mL/g-VS, slightly lower than WS (460.891 mL/g-VS) and higher than RR (365.335 mL/g-VS) in monodigestion. When the inoculum to substrate rate was 1:2, Figure 1b, the highest biogas production occurred. Additionally, RR to WS at 3:1 rate, biogas yield was 502.903 mL/g-VS, higher than RR (375.441 mL/g-VS) and WS (329.639 mL/g-VS) in monodigestion. As the amount of inoculum decreases in the digesters with respect to the substrate, the cumulative biogas yield (CBY) decreases for the RR:WS treatments at 0:4 to 2:2 rates. However, RR to WS at 3:1 and 4:0 rates, the amount of accumulated biogas increases from 421.726 mL/g-VS to 502.903 mL/g-VS, the same occurs with 4:0 rate indicating that adding a low percentage of inoculum and high percentage RR benefits biogas production.
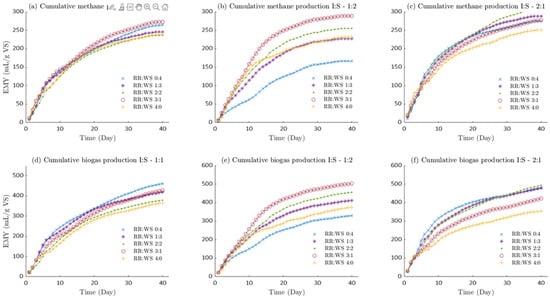
Figure 1.
Biogas and methane daily production. NOTE: inoculum to substrate ratio I:S (2:1, 1:1, 1:2); Wheat Straw (WS) to Rumen residue (RR) ratio (0:4, 1:3, 2:2, 3:1, 4:0); (a–c) shows the cumulative yield of methane after 40 days of digestion; (d–f) shows the cumulative yield of biogas at 40 days of digestion for different ratios of I:S.
Something similar occurs with methane generated (CMY), the order in which the substrate and co-substrate rate generate methane is RR:WS (3:1, 2:2, 4:0, 1:3 and 0:4). Maximum production can be noted in different inoculum fractions. A maximum of 306.205 mLCH4/g-VS (RE:TR-2:2), 272.836 mLCH4/g-VS and 289.078 mLCH4/g-VS were identified at I:S rate of 2:1, 1:1 and 1:2, respectively. An optimal methane range generated could be identified, corresponding ruminal residue fractions from 50 to 75% at any inoculum concentration. [27,29] Reported that animal gastrointestinal tract provides an ideal ecosystem for anaerobic microorganisms thriving for a great diversity of methanogens enriching methane production.
3.3. Effect of pH, C/N Rate, Biodegradability and Synergic Effect Index on Accumulated Methane Yield
Table 2 shows pH variation average occurred during co-digestion at 40 days. It can be observed that the initial pH rises as the amount of RR increases in the digesters regardless of the amount of inoculum present, indicating the favorable buffering capacity of RR; a slight drop in pH occurs at the end of the experiment, however, negligible enough to be considered less than optimal level. In the three experimental cases, digesters pH is maintained within 6.8 to 7.8 optimum range [30]. Ref. [31] Reported the reactor pH influences both the AD process and efficiency in the digestion process. Methanogens perform their roles more effectively between pH ranges of 6.5–8.2, with an optimum 7.0 pH. Although as previously reported, optimum pH ranges for the obtaining of the highest biogas and methane yield in AD are 6.5–7.5, pH range is generally wide for biogas production plants, and pH optimal value varies with the type of substrate and digestion process [32] which there could have been no inhibition due to VGA accumulation causing such decrease. The reason that the pH is similar in the three experiments is because the organic loading rate (OLR = 18 g-VS/L) is the same and does not cause a significant drop in pH. Table 2 indicates that the optimal initial pH maximizing CMY is 7.34, a lower amount of inoculum caused the pH to rise, leading the digester to a medium that tends to reach (7.76) alkalinity affecting CMY. Indeed, pH variation can be due to some influential digestion parameters, such as VFA, bicarbonate concentration, system alkalinity and by CO2 fraction produced during the process [31]. The initial and final pH levels belonging to the experiment I:S—2:1 is adequate to increase CMY.

Table 2.
Effect of pH, C/N rate, biodegradability and synergic effect index on methane production.
There is a range of C/N rates for which the CMY is the maximum. Table 2 presents C/N rate fluctuation occurred during RR and WS co-digestion in different fractions, on CMY. It is noticeable that RME reaches a 306.21, 272.84 and 289.08 mL/g-SV maximum when C/N is 23.26, 38.15 and 38.15 respectively. Adding more inoculum (I:S—2:1) benefits CMY as expected and C/N rates lower than 23.26 provide a higher methane concentration at 40 days digestion possibly due to C/N innocuous low rate (7.46) generating an additional medium with an optimal nutrient balance, thus higher methane concentration. When the amount of inoculum drops to 50% (I:S—1:1) or lower concentrations (I:S—1:2), Table 2 reveals that (16.65–38.15) C/N rate is optimal for RR and WS co-digestion. Similar results were reported by [23] with a C/N ratio (26–27.5). Therefore, the tendency is to decrease the amount of inoculum and maintain C/N rate at 38.15 maximizing CMY so the AD process is more stable when C/N ratio oscillates between 20 and 30, however, for AcoD the existing carbon of the easily degradable part, and the carbon not specifically affected by microorganisms must be considered [33].
With the help of the empirical formulas of the substrates, theoretical methane yield (TMY) in monodigestion and co-digestion is estimated. Regardless of the amount of inoculum used, TMY is the same for each case. A high synergistic effect index (SEI) corresponds to a high biodegradability percentage. SEI and BD for RE co-digestion and TR are detailed in Table 2 with a range of SEI from −8.08% to 33.86%. The highest SEI percentage occurs in RR to WS co-digestion at 3:1 (33.86%), 2:2 (16.48%) and 3:1 ratio (11.10%) for I:S ratios 1:2, 2:1 and 1:1, respectively. This is due to the adjustment of C/N ratios during the AcoD and 24.20–33.86) synergistic effect. (These values are higher than those reported by [23], whose SEI was (14.34–29.70). The negative values indicate that RR to WS ratio of 1:3 and 2:2, one of the substrates has a higher percentage of lignin (it is not easily degraded) and as a consequence they present very low BD values. Table 2 shows that BD of WS ranges from 39.05% to 64.25%, RR ranges from 53.59% to 57.88% in monodigestion and at different fractions of inoculum; a higher load of inoculum increases BD but not the SEI. Higher SEI percentages occurred at lower inoculum concentrations (I:S—1:2) and higher concentrations of RR with respect to WS (RR:WS 3:1); furthermore, BD in this experiment was slightly less than I:S—2:1 and had similar cumulative methane concentrations. RR and WS mixtures in ratios 1:3–3:1 could increase the variety of nutritional components and subsequently promote several microorganisms’ growth involved in methane production, [19] reported a relative 78% abundance in Methanospirillum as the most dominant archaea in the co-digestion process, the remaining belong to other archaeal species. Negative SEI percentages in I:S-1:1 experiment indicate that equal amounts of inoculum and substrates do not benefit SEI as substrates could be producing methane at the expense of organic matter present in the inoculum itself and not from the substrate as such.
3.4. Evaluation of the Different Kinetic Models
A nonlinear least-square regression analysis was performed using MATLAB software to fit the nonlinear equations (Gompertz, transfer and logistic) to the average specific cumulative methane production curves with respect to time generated from triplicate BMP assays. A kinetic parameters summary obtained from three models is shown in Table 3. Methane production experimental data was compared to the equivalent parameters Bo (Equations (5)–(7)) obtained from Gompertz equation (GE), Transfer equation (TE) and Logistic function (LF) respectively. It can be observed that the difference between experimental and adjusted data decreased as I:S rate decreased, particularly in TE model whereas higher differences were found for all treatments observing that TE overestimated 27.53% experimental value while GE and LF models did it in 6.13%. The results could be explained on the basis that smaller amount of inoculum added to the reactor required higher adaptation time of the methanogenic bacteria, reflected in the higher lag value obtained of most treatments in the I:S 1:2 experiment.

Table 3.
Methane and biogas kinetic parameters for different models.
For the evaluation of the models, two statistics have been used (Table 3); (a) adjustment R2 and (b) coefficient determination root mean -square error (RMSE). In the table, R2 GM and LF highest values were recorded IS 1:2 experiment, their highest R2 values being between 0.996 and 0.997. Similar results obtained by [15,34] Remaining experiments present values of R2 lower than 0.996 for the same fit. According to Table 3, TM is not useful to estimate experiments parameters, since they are much higher than those estimated by GM and LF. It is noticeable that the inoculum fraction in each experiment influences the final parameter estimate for instance, as inoculum fraction increases in the reactor, a greater methane production occurs and less latency time which in most of cases is zero or close. This is evidenced in I:S latency periods experiments (2:1, 1:1 and 1:2). In GM and LF adjustment, lag phase short periods indicate organic compounds high bioavailability within the substrates [25], Therefore, matching results were consistent with the experiment.
Vmax follows a parabolic pattern for the three settings, particularly GM in three inoculum and substrate concentrations, starting with 16.687 mLCH4/gVSd for I:S 2:1—RR:WS 0:4 experiment as the amount of inoculum in the experiment decreases reactor, values drop to 6.708 mLCH4/gVSd for the I:S 1:2-RR:WS 0:4 then an increase of 16.683 mLCH4/gVSd for I:S 1:2—RR:WS 3:1 takes place. It is notorious that there is no crucial difference with respect to the inoculum concentration to obtain a high methane generation rate. However, there is a difference with respect to substrate and co-substrate concentration since a lower inoculum concentration is required in the reactors to reflect substrate and co-substrate degradation, then it is suggested that experiment I:S 1:2—RR:WS 3:1 as the most representative.
In general, there is a CMY deviation between the measurements and the estimated less than 9.29% as low deviations between measurements and estimated cumulative methane indicate that these models accurately predict reactors behavior [15,35] reported 10%deviation in their research since RR and WS have low protein, fat content, and higher carbohydrate content. Carbohydrate conversion is fast (within a few days) while protein and fat conversion could take several weeks [21].
4. Discussion
Wheat straw monodigestion reveals inoculum lignin degradative capacity as the highest accumulated methane yield was 274.9 mL/gVS when the inoculum concentration was 2 times substrate concentration. At the same substrate concentration, ruminal monodigestion residue presents a maximum yield 250.8 mL/gVS. This lower yield compared to the monodigestion of wheat straw could be due to an antagonistic effect and metabolite competition among microorganisms present in the inoculum and rumen [27].
When the inoculum concentration is 2 times substrate concentration, 50% RR and 50% WS co-digestion presented a maximum yield of 296.6 mL/gVS, in this case a synergism (12.9%) is appreciated between inoculum and rumen microorganisms implying of 68.9% co-digested substrates degradability. In practice on a pilot scale, it is necessary to use a smaller fraction of inoculum, due to its availability. For this reason, co-digestion assays with an I:S fraction of 1:1 and percentages of 75% RR together with 25% WS leading to 11.1% substrate synergism and 63.2%, degradation generating 272.8 mL/gVS methane. Under these conditions, a lower yield is observed with respect to the inoculum concentration of 2 times substrate concentration. However, a measure that further reduces inoculum fraction (I:S—1:2) seems to have greater metabolite availability used by rumen microbiota, 289.1 mL/gVS accumulated methane yield is appreciated when the RR concentration is 75% with respect to wheat straw. Under these conditions the synergistic effect reached 33.9% with a 66.9% biodegradability.
In short, inoculum on the co-digested substrates influence is greater when a smaller fraction used and a higher concentration of ruminal residues, under these conditions we can underestimate that there is no competition for available substrates among inoculum microorganisms and those in the rumen.
5. Conclusions
In this study, RR with WS methane production anaerobic co-digestion was investigated. Results suggested RR and WS anaerobic co-digestion at a f 3:1 ratio in I:S concentrations 1:1 and 1:2 could be a viable alternative to producing methane in the future, because the co-digestion process not only could promote methane production, but also reduce amounts of agricultural waste slaughter plants waste. Specifically, RR and WS in a 3:1 ratio (I:S 1:2—RR:WS) co-digestion showed a SEI of 33.86% producing 289.08 mL/g-VS methane as 11.10% SEI was observed for RR and WS in a 3:1 ratio (I:S 1:1—RR:WS), co-digestion and a similar methane yield 272.84 mL/g-VS was achieved, in both experiments and with smaller amounts of inoculum similar productions were obtained, this could be due to codigestis diversity and microbial richness.
Experiments revealed that C/N ratio (16.65–38.15) is optimal for RR and WS co-digestion. Therefore, the tendency is to decrease the amount of inoculum maintaining the C/N ratio at 38.15 maximizing CMA indicating that the optimal pH initial used to maximize CMY is 7.34, a smaller amount of inoculum caused the pH to rise, leading the digester to a medium leaning towards alkalinity (7.76) affecting CMY. The highest R2 values were recorded for GM and LF in the IS 1:2 experiment achieving the highest R2 values between 0.996 and 0.997 whereas the remaining experiments present R2 values under 0.996 for the same setting.
Based on the results obtained, a pilot scale implementation with the foregoing optimized parameters could provide in greater detail the degradation process not only applying wheat straw, but also lignin subtrate content equal or lower than this substrate. We can suggest the use of ruminal residue as a source of microorganisms to replace the use of an inoculum, however, a detailed study is needed for further evidence.
Author Contributions
Conceptualization, O.M.Q. and D.P.H.; methodology, O.M.Q. and D.P.H.; validation, O.M.Q. and D.P.H.; formal analysis, O.M.Q.; investigation, O.M.Q. and D.P.H.; writing original draft preparation, D.P.H.; writing review and editing, O.M.Q.; visualization, O.M.Q. and D.P.H.; supervision, O.M.Q.; project administration, O.M.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Boe, K. Online Monitoring and Control of the Biogas Process. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2006. [Google Scholar]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, T.G.; Adelard, L. Improving biogas quality and methane yield via co-digestion of agricultural and urban biomass wastes. Waste Manag. 2016, 54, 118–125. [Google Scholar] [CrossRef]
- Aguilar, M.C.; Wang, Y.D.; Roskilly, T.; Pathare, P.B.; Lamidi, R.O. Biogas from anaerobic co-digestion of food waste and primary sludge for cogeneration of power and heat. Energy Procedia 2017, 142, 70–76. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Acosta, N.; De Vrieze, J.; Sandoval, V.; Sinche, D.; Wierinck, I.; Rabaey, K. Cocoa residues as viable biomass for renewable energy production through anaerobic digestion. Bioresour. Technol. 2018, 265, 568–572. [Google Scholar] [CrossRef]
- Gaibor-Chávez, J.; Niño-Ruiz, Z.; Velázquez-Martí, B.; Lucio-Quintana, A. Viability of Biogas Production and Determination of Bacterial Kinetics in Anaerobic Co-digestion of Cabbage Waste and Livestock Manure. Waste Biomass Valorization 2018, 10, 2129–2137. [Google Scholar] [CrossRef]
- Tufaner, F.; Avşar, Y. Effects of co-substrate on biogas production from cattle manure: A review. Int. J. Environ. Sci. Technol. 2016, 13, 2303–2312. [Google Scholar] [CrossRef]
- Valenti, F.; Zhong, Y.; Sun, M.; Porto, S.M.C.; Toscano, A.; Dale, B.E.; Sibilla, F.; Liao, W. Anaerobic co-digestion of multiple agricultural residues to enhance biogas production in southern Italy. Waste Manag. 2018, 78, 151–157. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Quelal, W.O.; Velázquez-Martí, B.; Gaibor-Chávez, J.; Niño-Ruiz, Z. Biochemical potential of methane (BMP) of camelid waste and the Andean region agricultural crops. Renew. Energy 2021, 168, 406–415. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater. Standard Methods for the Examination of Water and Wastewater; APHA (American Public Health Association): Washington, DC, USA, 2012; p. 1496. Available online: https://www.apha.org/ (accessed on 22 July 2023).
- Valero, D.; Montes, J.A.; Rico, J.L.; Rico, C. Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests. Waste Manag. 2016, 48, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Buswell, M.; Muellepi, H.F. Mechanis of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Li, W.; Siddhu, M.A.H.; Amin, F.R.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Methane production through anaerobic co-digestion of sheep dung and waste paper. Energy Convers. Manag. 2018, 156, 279–287. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, F.; Yu, J.; Cai, Y.; Luo, X.; Cui, Z.; Hu, Y.; Wang, X. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef]
- Pommier, X.L.S.; Chenu, D.; Quintard, M. A Logistic Model for the Prediction of the Influence of Water on the Solid Waste Methanization in Landfill. Biotechnol. Bioeng. 2007, 97, 473–481. [Google Scholar] [CrossRef]
- Ware, A.; Power, N. Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew. Energy 2017, 104, 50–59. [Google Scholar] [CrossRef]
- Almomani, F. Prediction of biogas production from chemically treated co-digested agricultural waste using artificial neural network. Fuel 2020, 280, 118573. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Z.; Xu, Y.; Shi, Q.; Ma, Y.; Aung, M.; Cheng, Y. Interactions between Anaerobic Fungi and Methanogens in the Rumen and Their Biotechnological Potential in Biogas Production from Lignocellulosic Materials. Microorganisms 2021, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Pope, P.B.; Eijsink, V.G.H.; Schnürer, A. Characterization of microbial community structure during continuous anaerobic digestion of straw and cow manure. Microb. Biotechnol. 2015, 8, 815–827. [Google Scholar] [CrossRef]
- Karekar, S.; Stefanini, R. Homo-Acetogens: Their Metabolism and Competitive Relationship with Hydrogenotrophic Methanogens. Microorganisms 2022, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Sumantri, I.; Kusnadi, P.; Handoyo, I.G.R.; Kumoro, A.C. Biodigestion of Mixed Substrates of Cow Manure-Delignified Spent Coffee Ground (DSCG) using Microorganism Enhancer for Biogas Production and Its Kinetic Study. Environ. Res. Eng. Manag. 2022, 78, 96–109. [Google Scholar] [CrossRef]
- Hahn, M.J.; Figueroa, L.A. Pilot scale application of anaerobic baffled reactor for biologically enhanced primary treatment of raw municipal wastewater. Water Res. 2015, 87, 494–502. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Kinetic study on the effect of temperature on biogas production using a lab scale batch reactor. Ecotoxicol. Environ. Saf. 2015, 121, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Borja, R.; Martín, M.A.; Martín, A.; de la Rubia, M.A.; Rincón, B. Influence of inoculum-substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: Process stability and kinetic evaluation. Chem. Eng. J. 2009, 149, 70–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).