Abstract
In this study, Limosilactobacillus fermentum PTCC 1638 and Lacticaseibacillus rhamnosus PTCC 1637 were used alone and in combination to ferment quinoa seeds, and the effect of fermentation (37 °C; 24 h) on the pH, total phenols, tocopherols, vitamin C, antioxidant activity, and enzymes inhibition (α-amylase and α-glucosidase; antidiabetic effect) was investigated. The results showed that with the increase in the fermentation time, the bacterial population, total phenols, antioxidant activity, and enzymes inhibition increased, which showed the greatest increase for the co-culture of L. rhamnosus and L. fermentum compared to the pure culture of each strain. Due to the increase in the fermentation time, the tocopherol isomers (α, β, γ, and δ), vitamin C, and pH decreased, and the largest decrease was related to the co-culture of the strains, followed by L. rhamnosus and L. fermentum. The results of this study showed that the co-culture and pure culture of bacteria can have different effects on the physicochemical properties and bioactive compounds of quinoa seeds.
1. Introduction
Today, the increasing consumer awareness of healthy diets and changing eating habits due to the fact of urbanization has led to the creation of large food markets for the production of new functional foods with a beneficial effect on health [1,2]. In this regard, there has been a great interest in using pseudocereals such as quinoa to formulate new foods. Quinoa is a grain-like food crop that belongs to the family Amaranthaceae. The botanical name of this seed is Chenopodium quinoa Willd., although it is also known as Inca rice or vegetable caviar [3]. Currently, quinoa seed is grown in Peru, Bolivia, Chile, Argentina, and Colombia. However, efforts are being made to introduce this valuable seed to Africa, North America, Europe, and Asia [4]. Due to the fact of its high nutritional value, it is called “the mother of all edible grains”. Quinoa is a good source of vitamins (C, E, B6, B2, and B1), minerals (calcium, iron, phosphorus, zinc, etc.), dietary fibers, essential fatty acids (Omega-3-6-9), proteins, and other nutritious compounds [5]. It has recently been reported that the consumption of one serving of this seed (approximately 40 g) meets a notable portion of the recommended daily allowance (RDA) for minerals, essential amino acids, mainly vitamins, and essential nutrients [6]. The various biological properties of quinoa seed have been reported, including antioxidant, antiviral, antifungal, anti-inflammatory, anticancer, hypoglycemic, hypocholesterolemic, diuretic, and antithrombotic activities [6,7]. In addition, this crop is naturally gluten-free and has recently been suggested as an alternative to common grains (e.g., barley and wheat) for people with celiac disease. Celiac disease, which is also called gluten-sensitive enteropathy or celiac sprue, is known as a gluten intolerance disease and is one of the inherited immunodeficiency disorders. People who are allergic to gluten in food are prohibited from consuming gluten-containing products and face a major problem in choosing food [3].
The fermentation process is considered one of the most effective methods to enhance the functional and nutritional properties of food products [8]. Typically, the fermentation process improves nutritional quality, increases bioactive compounds, increases antioxidant activity, and changes chemical compounds. The fermentation process improves the nutrient content of foods through the biosynthesis of vitamins, amino acids, and essential proteins and increases the bioavailability of micronutrients by destroying antinutritional factors [9]. The health-promoting impacts of lactic acid bacteria (LAB) have attracted the attention of various researchers in the food and pharmaceutical industries [10]. LAB are considered fastidious microorganisms, since they need nucleic acid bases, free amino acids, and vitamins to grow. In addition, these bacteria have special proteolytic systems that allow the transport of peptides and further degradations by different intracellular peptidases [11]. The fermentation of grains by LAB can lead to an increase in the release of organic acids as well as the production of amino acids and small peptides [12]. The peptides produced during the fermentation process can have properties, including antioxidant, antimicrobial, and antihypertensive properties [5]. The health promotions of quinoa seed as a result of fermentation by fermenting microorganisms (e.g., Bifidobacterium species, Rhizopus oligosporus, Lactobacillus rossiae, and Lactiplantibacillus plantarum) have been indicated [4,13,14,15]. Among these studies, quinoa seed fermentation has mostly been performed with pure culture, and there is little information about the use of mixed cultures for quinoa fermentation and its health-promoting attributes. In this sense, the aim of the present study was to investigate the impact of fermentation by the mixed cultures of two Lactobacillus species (L. fermentum and L. rhamnosus) on the total phenols, tocopherols, antioxidant, ⍺-glucosidase, and ⍺-amylase inhibitions activities of quinoa seed.
2. Materials and Methods
2.1. Preparation of the Strain
Limosilactobacillus fermentum PTCC 1638 and Lacticaseibacillus rhamnosus PTCC 1637 were bought from Iranian Research Organization for Science and Technology, Tehran, Iran. The strains were reactivated in the MRS broth (Oxoid, UK) at 37 °C for 20–22 h, and the cell pellets were harvested using centrifugation (Hettich, Germany) at 3500× g for 15 min at 4 °C.
2.2. Fermentation Process
Quinoa seeds (maturity stage) were purchased from a shop in Shiraz, Fars province, Iran. After cleaning, the seeds were milled and then sieved (0.5 mm). A mixture of quinoa flour and sterile distilled water (1/5 (w/v)) was poured into 250 mL Erlenmeyer flasks and then autoclaved (121 °C; 15 min). After cooling to 37 °C, L. fermentum and L. rhamnosus were added to the samples in pure or mixed form (L. fermentum + L. rhamnosus), and the fermentation process was allowed to take place for 24 h at 37 °C. The inoculums were ~5.2–5.4 log CFU/mL for the pure and mixed fermentation. At the defined times during the fermentation process, the pH and microbial cells enumeration of the flour suspension were determined, and to measure the other parameters, approximately 100 mL of the suspension was dried at 55 °C and stored in plastic bags in a desiccator at 4 °C until the experiments.
2.3. Microbial Cells Enumeration
MRS agar (Oxoid, UK) was utilized to count the viable cells and peptone water (HiMedia, India) was applied to obtain the appropriate dilutions. The colonies were counted after incubation of the plates at 37 °C for 72 h.
2.4. pH Determination
The pH of the samples was measured with a pH meter (EZDO, Taiwan) [16].
2.5. Preparation of the Extracts
The fermented quinoa flour samples (3 g) were mixed with 30 mL of 80% ethanol and then shaken for 25 min at 200 rpm using a shaker (Jeiotech, Korea). After, centrifugations (4500× g; 20 min) were performed, and the supernatants were collected. This process was repeated again. Finally, all supernatants were filtered through a 0.22 µm PVDF filter and kept at −18 °C for further analysis.
2.6. Total Phenolic Content
For the determination of the total phenolics of the extract, 0.5 mL of the extract was added to 2.5 mL of Folin–Ciocalteu reagent (10%). After 4 min, 2 mL of Na2CO3 (7.5% w/v) was mixed with the mixture and subsequently placed in the dark for 60 min. As a final point, the absorbance was determined at 765 nm. The results are expressed as the gallic acid equivalent (GAE) per 100 g on a dry weight basis (dw) [16].
2.7. HPLC Analysis of the Tocopherols
The content of tocopherols in the samples was measured with a high-performance liquid chromatography (HPLC) (Waters, Alliance system, USA) with a Spherisorb column (250 mm; 4 mm i.d., Waters, USA) packed with silica and a fluorescence detector operating at λexc = 292 nm and λem = 330 nm. Hexane–isopropanol (98.5:0.5, v/v) was utilized as the mobile phase, with a flow rate of 1 mL/min [17].
2.8. HPLC Analysis of Vitamin C
The vitamin C of the flour extract was determined using an HPLC (Knauer, Azura, Germany) with a C18 column (15 cm × 4.6 mm, pore size 5 µm). The mobile phase was aqueous metaphosphoric acid (0.8%, v/v), with a flow rate of 1 mL/min. The detection of L-ascorbic acid was performed at λ = 245 nm [18].
2.9. DPPH Radical Scavenging Activity
The DPPH solution (0.1 mmol/L) was obtained by means of methanol, and 200 μL of the solution was mixed with 100 μL of the sample. Afterward, the mixture was incubated at 37 °C for 30 min, and the absorbance was read at 517 nm [19].
2.10. α-Amylase Inhibition
The flour extract (50 μL) was mixed with α-amylase (50 μL) and kept at an ambient temperature for 10 min. After, the soluble starch solution (50 μL; 1%) was added and after keeping at room temperature for 10 min, 100 μL dinitrosalicylic acid color reagent was added to the mixture. After dilution with distilled water, the absorbance was read at 540 nm [20].
2.11. α-Glucosidase Inhibition
The flour extract sample (50 μL) was mixed with α-glucosidase (1 U/mL; 100 μL) and kept at 37 °C for 10 min. Afterward, 50 μL p-nitrophenyl-α-glucopyranoside (5 mmol/L) was added and kept at 37 °C for 5 min. Finally, the absorbance was measured at 405 nm [21].
2.12. Statistical Analysis
The results were carried out in triplicate, and the data are presented as the mean ± SD. Analysis of variance (ANOVA) and comparison of treatments means (Duncan post hoc test, p < 0.05) were carried out using the SPSS package program (v. 20.0).
3. Results and Discussion
3.1. Microbial Growth Analysis and pH
The alterations in the viable cell counts and pH of the quinoa seeds over 24 h of fermentation at 37 °C are presented in Figure 1. According to Figure 1A, the numbers of bacteria significantly increased (p < 0.05) in all treatments with the elapse of the fermentation time. Among the treatment groups, the microbial population in the co-culture group was significantly higher (p < 0.05) than that of the monoculture groups. In addition, the data showed that the growth of L. fermentum was greater than L. rhamnosus. After 24 h of fermentation, the initial number of cells (5.2–5.4 log CFU/mL) increased to 7.7 log CFU/mL in the L. rhamnosus pure culture, 8.1 log CFU/mL in the L. fermentum pure culture, and 8.9 log CFU/mL in the binary co-culture of L. fermentum and L. rhamnosus. The achieved data revealed that the mixed co-culture stimulated the growth of Lactobacillus species. These results concur with our previous study [10] in which mixed cultures indicated higher viable cells compared to single cultures of the L. plantarum strains. It has been observed that in mixed cultures, metabolic interactions can stimulate microbial growth in which a compound produced by one organism may later be metabolized by another. Moreover, the production of specific compounds such as vitamins by other organisms may cause growth stimulation [22]. It has been shown that Leuconostoc strains metabolize and diminish the acetaldehyde secreted by strains of Lactococcus [23]. In contrast, growth inhibition may occur in mixed cultures due to the fact of competition for nutrients [24].
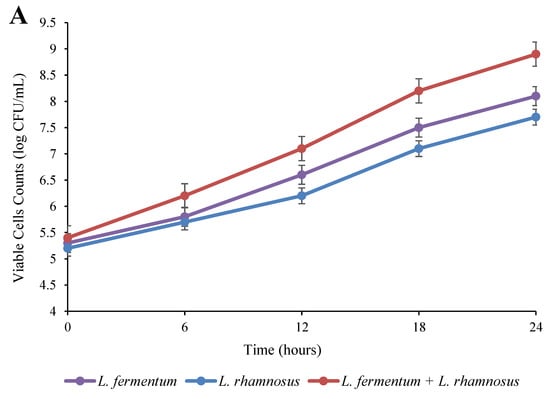
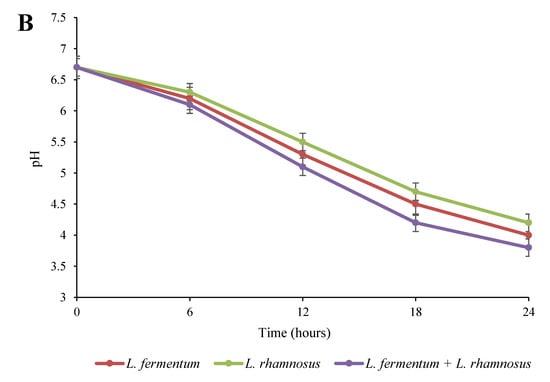
Figure 1.
Impact of fermentation on the viable cell count (A) and pH (B) in quinoa seed fermented by pure and binary mixed cultures of two Lactobacillus species. The error bars reveal the standard deviation of each measurement in quinoa seed over 24 h of fermentation. L. rhamnosus: Lacticaseibacillus rhamnosus monoculture; L. fermentum: Limosilactobacillus fermentum monoculture; L. rhamnosus + L. fermentum: binary co-cultures of L. rhamnosus and L. fermentum.
According to Figure 1B, the pH values notably (p < 0.05) decreased in all fermented seed groups over 24 h of fermentation. In addition, the mixed culture resulted in a greater decrease in the pH value compared to the monoculture groups. These findings are consistent with the results shown in Figure 1A regarding the growth enhancement. The decrease in the pH of the fermented quinoa was probably due to the production of organic acids from the fermentation of carbohydrates by Lactobacillus strains [13]. Along this line, Akpinar-Bayizit et al. [25] reported that one of the most important reasons for pH reductions during the fermentation process is the activity of bacteria and the production of acids (especially lactic acid). Similarly, lower pH values have been reported in mixed cultures of yeast and L. reuteri compared to a pure culture of L. reuteri during the fermentation of cereals [22].
3.2. Bioactive Compounds
The alterations of the bioactive compounds, including vitamin C, total phenols, and tocopherols, in the quinoa samples over 24 h of fermentation are depicted in Figure 2, Figure 3 and Figure 4. As can be seen in Figure 2, the amount of total phenols in all of the fermented samples showed an increasing trend and reached its highest level after 24 h of fermentation. The highest content of total phenols was observed in the co-culture group, followed by the L. fermentum pure culture and L. rhamnosus pure culture groups. The initial amount of total phenols (56.3 mg/100 g dw) increased to 81.9 mg/100 g dw in the binary mixture, 77.3 mg/100 g dw in the L. fermentum monoculture, and 70.5 mg/100 g dw in the L. rhamnosus monoculture at the end of the fermentation period. Phenolic compounds are one of the most significant compounds in plants that are able to remove free radicals; therefore, these compounds are of great interest due to the fact of their antioxidant properties [26]. Cereals and pseudocereals are good sources of phenolic compounds, such as derivatives of cinnamic and benzoic acids, quinines, chalcones, anthocyanidins, flavanones, chalcones, amino phenolic compounds, and flavonols [27]. Studies have shown that acidification throughout fermentation causes the hydrolysis of the glycosylated form of phenolic compounds (bound phenolics) and the formation of free phenolics [26]. Moreover, enzymes from fermenting microorganisms (including β-glucosidase, reductases, decarboxylases, hydrolases, and esterases) play a significant role in these complex reactions [28]. Rizzello et al. [15] demonstrated that esterases produced by Bifidobacteria species led to the hydrolysis of the complex form of phenolic compounds, thereby increasing the amount of free ones. It is also reported that Lactobacillus species synthesize some bioactive compounds identified as phenolic compounds [29]. A similar increase in total phenols was reported in wheat, rye, and barley during fermentation by L. rhamnosus [30].
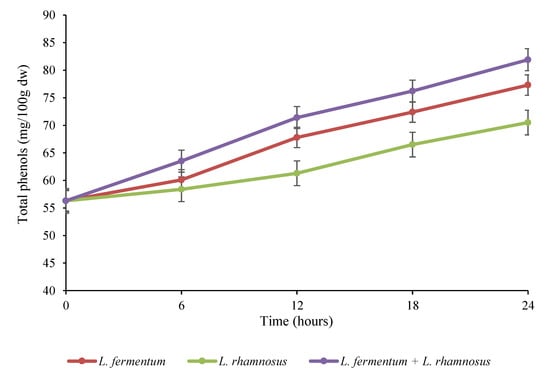
Figure 2.
Variations in the total phenols (mg/100 g dw) in quinoa seed fermented by pure and binary mixed cultures of two Lactobacillus species. The error bars reveal the standard deviation of total phenols in quinoa seed over 24 h of fermentation. L. rhamnosus: Lacticaseibacillus rhamnosus monoculture; L. fermentum: Limosilactobacillus fermentum monoculture; L. rhamnosus + L. fermentum: binary co-cultures of L. rhamnosus and L. fermentum.
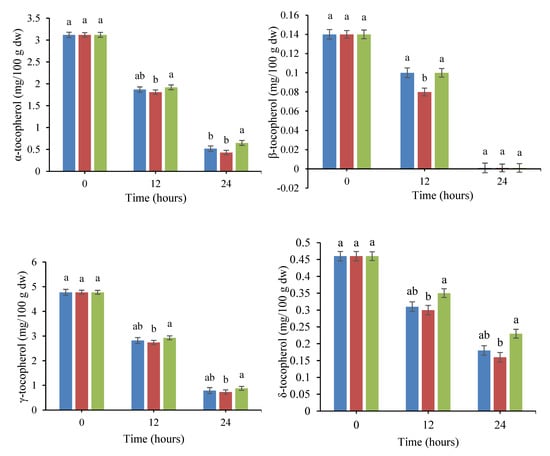
Figure 3.
Changes in the levels of tocopherol isomers in quinoa seed fermented by pure and binary mixed cultures of two Lactobacillus species. The error bars reveal the standard deviation of each measurement in quinoa seed over 24 h of fermentation. The values with the same lowercase letters are not significantly different within the same fermentation hour (p > 0.05). Red column: Lacticaseibacillus rhamnosus monoculture; blue column: Limosilactobacillus fermentum monoculture; green column: binary co-cultures of L. rhamnosus and L. fermentum.
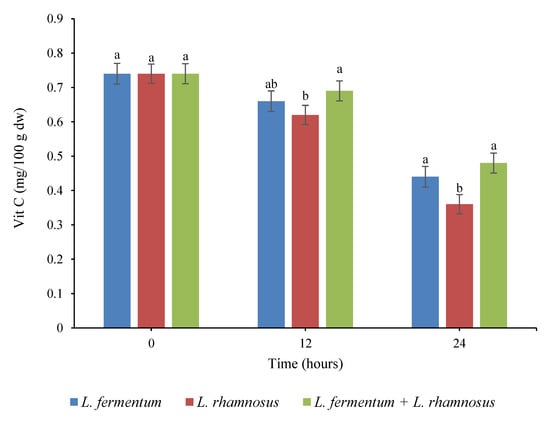
Figure 4.
Changes in the vitamin C (mg/100 g dw) content in quinoa seed fermented by pure and binary mixed cultures of two Lactobacillus species. The error bars reveal the standard deviation of vitamin C in quinoa seed over 24 h of fermentation. The values with the same lowercase letters are not significantly different within the same fermentation hour (p > 0.05). Red column: Lacticaseibacillus rhamnosus monoculture; blue column: Limosilactobacillus fermentum monoculture; green column: binary co-cultures of L. rhamnosus and L. fermentum.
The impact of fermentation on the levels of tocopherol isomers in treated quinoa seeds are shown in Figure 3. According to the data, fermentation caused a significant decrease (p < 0.05) in the content of all tocopherol isomers with the elapse of the fermentation time. The highest and lowest retentions of tocopherols were observed in the mixed culture (L. fermentum and L. rhamnosus) and the single culture of L. rhamnosus, respectively. The unfermented quinoa grains contained 3.12, 0.14, 4.77, and 0.46 mg/100 g dw of α-tocopherol, β-tocopherol, γ-tocopherol, and δ-tocopherol, respectively. After 24 h of incubation, the amount of α-tocopherol, γ-tocopherol, and δ-tocopherol decreased to 0.65, 0.88, and 0.23 mg/100 g dw and 0.43, 0.73, and 0.16 mg/100 g dw in the mixed culture fermentation and L. rhamnosus monoculture, respectively. In addition, β-tocopherol was completely lost in all treatments at the end of the fermentation process. Researchers have attributed tocopherol reduction during fermentation to the temperature of the incubation and the presence of dissolved oxygen in the fermentation medium. The main reason for this reduction is the oxidation process, since tocopherols are sensitive to dissolved oxygen as a result of agitation in the incubation medium. Our findings are in good agreement with the results of Hubert et al. [31], who indicated a decline in the tocopherol content of soybean germ during fermentation by lactobacilli. They stated that the oxidation process takes place in an oxygen-enriched medium in which substrates are exposed to heat or ultraviolet (UV) light. Similar results were reported for a reduction in tocopherol isomers in Lupinus albus L. var. Multolupa, quinoa, and chickpea seeds during fermentation [32,33,34].
The alterations in the vitamin C content of quinoa seeds during fermentation over 24 h were also investigated. With respect to Figure 4, the amount of vitamin C decreased significantly (p < 0.05) throughout the fermentation time, and the greatest vitamin C loss was observed in the pure culture of L. rhamnosus. The fermentation of the quinoa seeds resulted in a 40.54%, 51.35%, and 35.14% loss of vitamin C in the L. fermentum monoculture, L. rhamnosus monoculture, and binary co-culture treatments, respectively. Vitamin C is an essential vitamin but a very unstable molecule, and it is easily decomposed by several factors, including light, alkaline pH, high temperature, oxygen, heavy metals, UV rays, and storage time [35]. The loss of vitamin C during fermentation has been attributed to factors including the oxidation process and ascorbate oxidase enzyme produced by fermenting microorganisms [36,37]. As a result of the oxidation process, ascorbic acid is converted into dehydroascorbic acid, which then quickly decomposes into 2,3-diketogulonic acid, with no vitamin C activity [38]. In addition, Urbienė and Mitkutė [39] demonstrated that the increase in the number of LAB during fermentation did not have a notable impact on the alterations of vitamin C and considered the oxidation process to be the main cause of vitamin C loss throughout milk fermentation. It was also reported that pH strongly affected the degradation of vitamin C, so that vitamin C was most stable at pH 3.5 and least stable at pH 5.5 [38]. These achievements are consistent with the results of Sangija et al. [40], who showed a substantial decrease in the vitamin C content of African nightshade during controlled and spontaneous fermentation by Leuconostoc mesenteroides and L. plantarum. Similarly, different researchers detected vitamin C loss after the fermentation of lupini beans, cowpea, and pigeon pea [34,41,42].
3.3. DPPH Free Radical Scavenging Activity
The antioxidant activity of the fermented quinoa samples (based on DPPH free radical scavenging) was evaluated, and the results are shown in Figure 5. The DPPH radical scavenging activities of fermented seeds markedly increased (p < 0.05) as the fermentation continued. The fermentation treatments carried out with the monoculture of L. fermentum, the monoculture of L. rhamnosus, and a binary combination of the mentioned bacteria increased the initial radical scavenging activity (41.2%) to 66.3%, 60.7%, and 72.4%, respectively, after 24 h of incubation. According to the data, the scavenging impact of the co-culture treatment was greater (p < 0.05) than the pure cultures. Several authors have revealed that fermentation enhances the DPPH radical scavenging ability of okra seeds, white cabbage, and legumes [37,43,44]. The bioactive components in fermented food products play an important role in reducing the effect of reactive oxygen species (ROS), including peroxyl (ROO●), hydroxyl (●OH), and superoxide (●O2−, ●OOH) radicals produced by cells under oxidative stress [28]. The ROS substances are involved in various pathogenic and physiological processes, such as apoptosis, cell proliferation, cell signaling transduction, and other neurological disorders [13]. According to recent studies, protein-derived peptides and phenolics have been shown to be bioactive compounds in pseudocereals and cereals that have the ability to neutralize free radicals [27]. On the other hand, the increase in antioxidant activity during fermentation may be due to the release of isoflavoneaglycones through the catalytic action of β-glucosidase, as well as the intracellular antioxidants of fermenting microorganisms [45]. Ayyash et al. [13] attributed the high antioxidant activity in quinoa grains fermented by Bifidobacterium species to bioactive peptides and free amino acids generated as a result of microbial protease activity. In addition, Watanabe et al. [46] identified peptides, free amino acids, and isoflavoneaglycones as responsible for the antioxidant capacities of fermented soybean (tempeh). Previous studies reported the amount of lactic acid produced and the growth of fermenting microorganisms as the main factors in the difference in the rate of free radical scavenging ability during the fermentation process. In other words, the higher the amount of lactic acid produced and the higher the survival of microorganisms, the greater the decomposition of the cell wall and, finally, more phenolic compounds are released, thus increasing the antiradical activity [47]. Therefore, higher microbial growth in the binary combination treatment led to a lower pH, more total phenols and, finally, more antioxidant activity compared to single culture treatments. Moreover, it has been found that the increment in antioxidant capacity may be affected by various factors, including pH, microorganism species, solvent, temperature, fermentation time, aerobic conditions, type of food, and water content [48].
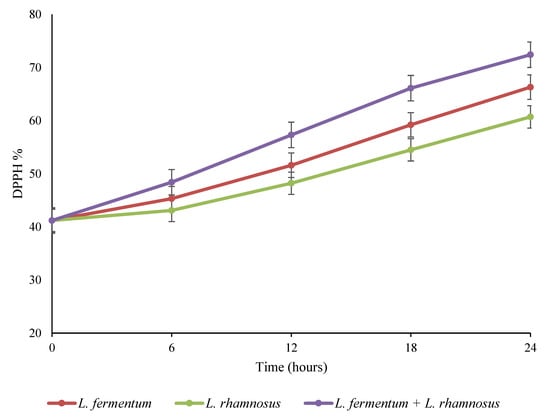
Figure 5.
Changes in the antioxidant activity of quinoa seed fermented by pure and binary mixed cultures of two Lactobacillus species. The error bars reveal the standard deviation of DPPH in quinoa seed over 24 h of fermentation. L. rhamnosus: Lacticaseibacillus rhamnosus monoculture; L. fermentum: Limosilactobacillus fermentum monoculture; L. rhamnosus + L. fermentum: binary co-cultures of L. rhamnosus and L. fermentum.
3.4. Enzyme Inhibition Ability
The effect of fermentation on the inhibition rate of α-glucosidase and α-amylase in quinoa seeds was investigated and shown in Figure 6. As can be seen, the fermentation had a notable impact on the inhibition of α-glucosidase and α-amylase, where the inhibition percentage of both enzymes increased (p < 0.05) over 24 h of fermentation. According to Figure 6A,B, the highest and lowest inhibition rates of α-glucosidase and α-amylase were observed in the binary co-culture and L. rhamnosus monoculture treatments, respectively. The initial rate of α-glucosidase (29.4%) and α-amylase (37.4%) reached 50.7% and 53.7% in the L. rhamnosus pure culture, 56.8% and 57.4% in the L. fermentum pure culture, and 62.3% and 63.3% in the binary co-culture of L. fermentum and L. rhamnosus, respectively, at the end of the fermentation period. Inhibiting the activity of hydrolyzing enzymes, such as α-glucosidase and α-amylase, is regarded as an effective strategy for managing and controlling postprandial hyperglycemia in diabetic patients by decreasing the hydrolysis rate of dietary polysaccharides [49,50]. Recently, researchers have indicated that some photochemical substances, such as polyphenols, terpenes, and flavonoids, possess inhibitory activity against digestive enzymes [51]. There is limited information on the impact of grain fermentation on the inhibition of α-glucosidase and α-amylase. Ayyash et al. [13] reported an increased α-glucosidase inhibition rate in fermented grains, including wheat, quinoa, and lupin, while they observed increased α-amylase inhibitory activity only in fermented lupin. They attributed the inhibition of the mentioned enzymes to bioactive peptides, especially small peptides, which are produced by endogenous proteolytic enzymes secreted by Bifidobacterium species. Zhang et al. [20] stated that the increase in total phenols and the biotransformation of active substances by LAB were the main reasons for the inhibition of digestive enzymes in fermented blueberry juice. In addition, the antihyperglycemic impact of fermented bitter melon juice has been attributed to aglycones and other phytochemical compounds [52].
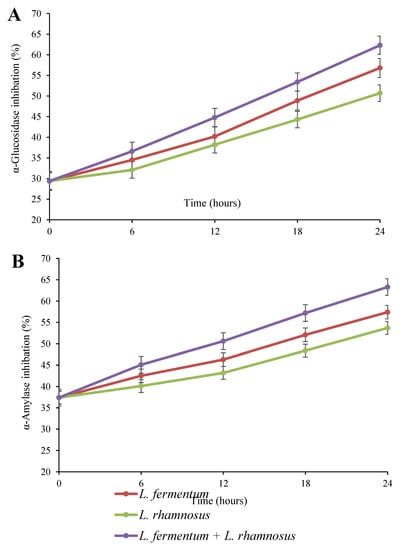
Figure 6.
α-Glucosidase (A) and α-amylase (B) inhibitions of quinoa seed fermented by pure and binary mixed cultures of two Lactobacillus species. The error bars reveal the standard deviation of each measurement in quinoa seed over 24 h of fermentation. L. rhamnosus: Lacticaseibacillus rhamnosus monoculture; L. fermentum: Limosilactobacillus fermentum monoculture; L. rhamnosus + L. fermentum: binary co-cultures of L. rhamnosus and L. fermentum.
4. Conclusions
In this study, the effect of pure and mixed L. rhamnosus and L. fermentum fermentation on the physicochemical properties and bioactive compounds of quinoa seeds was investigated. By increasing the fermentation time, mixed strains compared to pure cultures increased the enzyme inhibition, phenolic compounds, and antioxidant activity. However, vitamin C and tocopherols decreased with the increasing fermentation time, especially in the samples that were fermented with the mixed strains. Based on the results of this study, pure cultures and mixed cultures can have different behaviors in terms of the effect on bioactive compounds during the fermentation process. As a result, the use of pure or mixed cultures can be chosen based on the purpose of the fermentation process. In future research, other factors affecting fermentation, such as temperature and aeration, can be investigated.
Author Contributions
Conceptualization, S.M.B.H. and D.J.; methodology, S.M.B.H. and D.J.; formal analysis, S.M.B.H.; investigation, S.M.B.H.; resources, S.M.B.H. and D.J.; data curation, S.M.B.H. and D.J.; writing—original draft preparation, S.M.B.H.; visualization, S.M.B.H. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The research data are not shared.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saadi, S.; Saari, N.; Anwar, F.; Hamid, A.A.; Ghazali, H.M. Recent Advances in Food Biopeptides: Production, Biological Functionalities and Therapeutic Applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [PubMed]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of Potentially Probiotic Beverages Using Single and Mixed Cereal Substrates Fermented with Lactic Acid Bacteria Cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Wang, M. The Functional Ingredients of Quinoa (Chenopodium quinoa) and Physiological Effects of Consuming Quinoa: A Review. Food Front. 2021, 2, 329–356. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Duliński, R.; Stodolak, B.; Mickowska, B.; Wikiera, A. Prolonged Tempe-Type Fermentation in Order to Improve Bioactive Potential and Nutritional Parameters of Quinoa Seeds. J. Cereal Sci. 2016, 71, 116–121. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, Functional, and Antinutritional Aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and Biological Value of Quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D.; Jouki, M. Improving Bioactive Properties of Peach Juice Using Lactobacillus Strains Fermentation: Antagonistic and Anti-Adhesion Effects, Anti-Inflammatory and Antioxidant Properties, and Maillard Reaction Inhibition. Food Chem. 2021, 365, 130501. [Google Scholar] [CrossRef]
- Magala, M.; Kohajdova, Z.; Karovičová, J.; Greifova, M.; Hojerova, J. Application of Lactic Acid Bacteria for Production of Fermented Beverages Based on Rice Flour. Czech J. Food Sci. 2015, 33, 458–463. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of Bergamot Juice with Lactobacillus plantarum Strains in Pure and Mixed Fermentations: Chemical Composition, Antioxidant Activity and Sensorial Properties. LWT 2020, 131, 109803. [Google Scholar] [CrossRef]
- Hebert, E.M.; Raya, R.R.; De Giori, G.S. Nutritional Requirements and Nitrogen-Dependent Regulation of Proteinase Activity of Lactobacillus helveticus CRL 1062. Appl. Environ. Microbiol. 2000, 66, 5316–5321. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Johnson, S.K.; Liu, S.-Q.; Al-Mheiri, A.; Abushelaibi, A. Cytotoxicity, Antihypertensive, Antidiabetic and Antioxidant Activities of Solid-State Fermented Lupin, Quinoa and Wheat by Bifidobacterium Species: In-Vitro Investigations. LWT 2018, 95, 295–302. [Google Scholar] [CrossRef]
- Dallagnol, A.M.; Pescuma, M.; De Valdez, G.F.; Rollán, G. Fermentation of Quinoa and Wheat Slurries by Lactobacillus plantarum CRL 778: Proteolytic Activity. Appl. Microbiol. Biotechnol. 2013, 97, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of Sourdough Made with Quinoa (Chenopodium quinoa) Flour and Autochthonous Selected Lactic Acid Bacteria for Enhancing the Nutritional, Textural and Sensory Features of White Bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Michiels, J.; Yousefabad, S.H.A.; Hosseini, M. Kolkhoung (Pistacia khinjuk) Kernel Oil Quality Is Affected by Different Parameters in Pulsed Ultrasound-Assisted Solvent Extraction. Ind. Crops Prod. 2015, 70, 28–33. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Roohi, R.; Mahmoudi, M.R.; Granato, D. Modeling Inactivation of Listeria monocytogenes, Shigella sonnei, Byssochlamysfulva and Saccharomyces cerevisiae and Ascorbic Acid and β-Carotene Degradation Kinetics in Tangerine Juice by Pulsed-Thermosonication. LWT 2019, 111, 612–621. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Antioxidant Activity and γ-Aminobutyric Acid (GABA) Producing Ability of Probiotic Lactobacillus plantarum DM5 Isolated from Marcha of Sikkim. LWT-Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of Functional Characteristics of Blueberry Juice Fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates Type 2 Diabetes in Mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
- Kedia, G.; Wang, R.; Patel, H.; Pandiella, S.S. Use of Mixed Cultures for the Fermentation of Cereal-Based Substrates with Potential Probiotic Properties. Process Biochem. 2007, 42, 65–70. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Marshall, V.M.E. Microbiology and Technology of Fermented Milks. In Microbiology and Biochemistry of Cheese and Fermented Milk; Springer: Berlin/Heidelberg, Germany, 1997; pp. 57–152. [Google Scholar]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of Chemical Composition and Sensorial Properties of Ciders Fermented with Different Non-Saccharomyces Yeasts in Pure and Mixed Fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]
- Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Ozcan, T. Determination of Boza’s Organic Acid Composition as It Is Affected by Raw Material and Fermentation. Int. J. Food Prop. 2010, 13, 648–656. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V. Impact of Fermentation on Phenolic Compounds and Antioxidant Capacity of Quinoa. Fermentation 2021, 7, 20. [Google Scholar] [CrossRef]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant Properties of Diverse Cereal Grains: A Review on in Vitro and in Vivo Studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant Phenolics and Their Microbial Production by Submerged and Solid State Fermentation Process: A Review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of Cereals and Cereal Components in Functional Foods: A Review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of Some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of Fermentation on the Phytochemical Composition and Antioxidant Properties of Soy Germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Frias, J.; Zielinski, H.; Muñoz, R.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Evaluation of Bioprocesses to Improve the Antioxidant Properties of Chickpeas. LWT-Food Sci. Technol. 2009, 42, 885–892. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of Germination and Fermentation on the Antioxidant Vitamin Content and Antioxidant Capacity of Lupinus albus L. Var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Grzelakowska, A.; Cieślewicz, J.; Łudzińska, M. The Dynamics of Vitamin C Content in Fresh and Processed Cucumber (Cucumis sativus L.). Chem. -Didact. -Ecol. -Metrol. 2013, 18, 97–102. [Google Scholar]
- Adetuyi, F.O.; Osagie, A.U.; Adekunle, A.T. Antioxidant Degradation in Six Indigenous Okra Abelmoschus esculentus (L) Moench Varieties during Storage in Nigeria. J. Food Technol. 2008, 6, 227–230. [Google Scholar]
- Herbig, A.-L.; Renard, C.M.G.C. Factors That Impact the Stability of Vitamin C at Intermediate Temperatures in a Food Matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef]
- Urbienė, S.-A.; Mitkutė, D. Changes of Vitamin C during Milk Fermentation. Milchwissenschaft 2007, 62, 130–132. [Google Scholar]
- Sangija, F.; Martin, H.; Matemu, A. Effect of Lactic Acid Fermentation on the Nutritional Quality and Consumer Acceptability of African Nightshade. Food Sci. Nutr. 2022, 10, 3128–3142. [Google Scholar] [CrossRef]
- Torres, A.; Frías, J.; Granito, M.; Vidal-Valverde, C. Fermented Pigeon Pea (Cajanus cajan) Ingredients in Pasta Products. J. Agric. Food Chem. 2006, 54, 6685–6691. [Google Scholar] [CrossRef]
- Doblado, R.; Frias, J.; Muñoz, R.; Vidal-Valverde, C. Fermentation of Vigna sinensis Var. Carilla Flours by Natural Microflora and Lactobacillus Species. J. Food Prot. 2003, 66, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Kusznierewicz, B.; Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. The Effect of Heating and Fermenting on Antioxidant Properties of White Cabbage. Food Chem. 2008, 108, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademiluyi, A.O.; Akindahunsi, A.A. Changes in Polyphenols Distribution and Antioxidant Activity during Fermentation of Some Underutilized Legumes. Food Sci. Technol. Int. 2009, 15, 41–46. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yu, R.-C.; Chou, C.-C. Antioxidative Activities of Soymilk Fermented with Lactic Acid Bacteria and Bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Fujimoto, K.; Aoki, H. Antioxidant Activities of the Water-Soluble Fraction in Tempeh-like Fermented Soybean (GABA-Tempeh). Int. J. Food Sci. Nutr. 2007, 58, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Shobharani, P.; Nanishankar, V.H.; Halami, P.M.; Sachindra, N.M. Antioxidant and Anticoagulant Activity of Polyphenol and Polysaccharides from Fermented Sargassum Sp. Int. J. Biol. Macromol. 2014, 65, 542–548. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory Effect of Polyphenol-Rich Extracts of Jute Leaf (Corchorus olitorius) on Key Enzyme Linked to Type 2 Diabetes (α-Amylase and α-Glucosidase) and Hypertension (Angiotensin I Converting) in Vitro. J. Funct. Foods 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D.; Soto, E.R.; Barba, F.J. Ultrasound-Assisted Lactic Acid Fermentation of Bakraei (Citrus reticulata Cv. Bakraei) Juice: Physicochemical and Bioactive Properties. Fermentation 2023, 9, 37. [Google Scholar] [CrossRef]
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and Hepatoprotective Activity of Fermented Fruit Juice of Morinda citrifolia (Noni) in Diabetic Rats. Evid. Based Complement. Altern. Med. 2010, 2011, 875293. [Google Scholar]
- Mazlan, F.A.; Annuar, M.S.M.; Sharifuddin, Y. Biotransformation of Momordica charantia Fresh Juice by Lactobacillus plantarum BET003 and Its Putative Anti-Diabetic Potential. PeerJ 2015, 3, e1376. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).