Abstract
Value-added utilization of pineapple waste is very import for the food industry and environmental protection. In this study, whey protein (2.6%, w/w) was added to pineapple waste in order to make up for the protein deficiency of the raw material and give the final products better flavor characteristics. Autochthonous Lactococcus lactis LA5 and Hanseniaspora opuntiae SA2 were used for the co-inoculation of pineapple by-products; during fermentation, the metabolite profiling and microbial community dynamics were investigated. Results showed that the contents of organic acids, total FAAs, total phenolic compounds and flavonoids significantly increased with fermentation, and 152 kinds of peptides were identified in the final products. Relevant analyses demonstrated that dominant strains including Lactococcus lactis, Hanseniaspora and Saccharomyces not only significantly promoted the accumulation of organic acids, total phenols and other active substances, but also inhibited the growth of pathogenic bacteria and further influenced the fermentation process of pineapple waste.
1. Introduction
Pineapple (Ananas comosus L.) is one of the most popular tropical fruits in the world; it is high in organic acids, antioxidants, vitamins and minerals as well as rich in aromatic flavor and sweet taste [1]. Pineapple is widely cultivated in many countries around the world. China is one of the top ten pineapple-producing countries in the world [2]. Guangdong is the largest pineapple-producing province in China, with its planting area and total output ranking first, accounting for about 60 percent of the country’s planting area [2]. Pineapple can be consumed as fresh fruit or processed pineapple products, mainly canned pineapple and pineapple juice [3]. It can produce a large number of by-products during industrial processing, mainly pineapple peels and cores, and pineapple cores account for 9.4–20% (w/w) of the weight of pineapples [3]. These residues could be a serious source of environmental pollution and energy waste.
Sustainable approaches to transforming pineapple waste into a variety of valuable bio-based products can effectively avoid environmental pollution. Microbial fermentation is considered a good method to convert food processing by-products into value-added end-products. Microbial fermentation has a long history in food processing, it can improve the nutritional properties and extend the shelf life of food. Meanwhile, during fermentation processes, probiotics can also produce many beneficial components such as polyphenols, vitamins and organic acids [4]. Recently, many advantages were found for using of autochthonous starter cultures in fruit fermentation [5,6,7]. Additionally, it was demonstrated that the starter of mixed strains was better than that of single strains [5]. However, there are rare reports on the fermentation of pineapple by-products using endophytic microorganisms.
Pineapple processing by-products are high in cellulose, hemicellulose and other kinds of carbohydrates which can be suitable substrates for microorganisms, but they are low in protein. Improving the nutritional value of pineapple processing by-products, especially the protein content, is conducive for fermentation; it can make end-products more comprehensive and balance nutrients.
Researchers have proposed the possibility of adding whey protein as a nitrogen source in the fermentation process [8]. Whey protein is considered one of the most nutritious by-products during dairy processing, particularly in the production of cheese. In addition to providing a nitrogen source for the fermentation system, whey protein also produces lots of bioactive peptides during fermentation, and these peptides have various health benefits such as anti-hypertensive properties, antioxidant effects and immunomodulatory and antimicrobial effects [9]. Additionally, whey protein could also give the final fermented products better flavor characteristics. However, the production of value-added products from pineapple waste combined with whey protein using autochthonous starter cultures has not been studied yet.
Therefore, in the current study, autochthonous lactic acid bacteria (Lactococcus lactis LA5) and yeast (Hanseniaspora opuntiae SA2) isolated from pineapple cores were used to ferment a mixture of pineapple processing by-products and whey protein. We aimed to (1) investigate the changes in metabolites including organic acids, peptides, free amino acids and total phenolic compounds during the fermentation of pineapple waste–whey protein, (2) explore the evolution of microbial community structure during the fermentation process, and (3) evaluate the relationship between the core functional microbiota and typical metabolites.
2. Materials and Methods
2.1. Chemicals
Whey protein was purchased from Yuan Ye Bio-Technology (purity: 98%, Shanghai, China). MRS and YPD broth were purchased from Solarbio Science (Beijing, China). Organic acid standards (lactic acid, citric acid, malic acid, oxalic acid, tartaric acid, succinic acid and ascorbic acid) were purchased from Aladdin (Shanghai, China). Amino acid standards were purchased from Wako (Wako-Shi, Japan). HPLC-grade methanol was purchased from Sigma-Aldrich (Milan, Italy). All other reagents were analytical grade and purchased from Aladdin (Shanghai, China).
2.2. Microorganisms
The lactic acid bacteria (Lactococcus lactis LA5) and yeast (Hanseniaspora opuntiae SA2) were used as potential starter cultures for fermentation (Supplementary Materials Table S1). These two strains were endophytic microorganisms isolated from pineapple cores in our previous experiment, and the procedures were followed as per the methods described by Mariam et al. [10] and Peng et al. [11]. Microorganisms were stored in 30% glycerol tubes at −80 °C. The second transfer of culture strains was reactivated in the following steps: lactic acid bacteria were cultured in MRS broth at 37 °C for 24 h, yeasts were cultured in YPD broth at 30 °C for 24 h, and then they were stored in a refrigerator at 4 °C for subsequent experiments.
2.3. Sample Preparation and Collection
The fresh pineapples used in this study were purchased from Zhanjiang, Guangdong Province. The pineapples were cleaned; after removing peel and flesh, the pineapple cores were collected. The pineapple cores were pulped using a high-speed blender and mixed with distilled water at a mass ratio of 6:4 (homogenate:water), then whey protein (2.6%, w/w) was added, and the pH of the system was adjusted to 5.0. Afterward, the mixture of substrates was sterilized in a water bath at 65 °C for 30 min. The starter culture strains were simultaneously inoculated at a volume ratio of 6:1 (lactic acid bacteria:yeast) into an incubator, of which the inoculation ratio of lactic acid bacteria was 1.6% (w/w), and the concentration of both lactic acid bacteria and yeast was 1 × 106 CFU/mL. Then, the mixtures were incubated at 37.5 °C for 26 h in the dark. The fermentation broth was collected at 0, 8, 14 and 26 h; at each fermentation time, the samples were freeze dried for 48 h to obtain the powdery products, and then stored at −80 °C for subsequent experiments.
2.4. Determination of pH and Titratable Acidity (TA)
An amount of 5 mL of distilled water was added to freeze-dried samples from different fermentation stages; after mixing well for 1 min, the mixtures were centrifuged at 10,000 r/min for 10 min to obtain the supernatant for determining pH and TA. The pH was measured with a calibrated pH meter. The value of TA was determined by the indicator titration method [7] and expressed as percentage (w/w) of lactic acid.
2.5. Determination of Organic Acid Content
Organic acid content determination was carried out as previously described with some slight modifications [12]. Samples were analyzed by HPLC equipment (1260 Infinity II, Agilent, Palo Alto, CA, USA) fitted with an Athena C18-WP column (4.6 × 250 mm, 5 µm particle size). The injection volume was set to 20 µL; the mobile phase (1% methanol and 99% 0.01 mol/L KH2PO4 buffer, pH 2.6) was set up at a flow rate of 0.6 mL/min. The detection wavelength of the UV-DAD detector was set at 210 nm. Samples were measured three times and expressed as mg/g (w/w).
2.6. Measurement of Total Phenolic Compounds and Flavonoids
The total phenolic compounds and flavonoids were determined using a colorimetric method [13]. One-gram samples were mixed thoroughly with 5 mL of distilled water, then sonicated for 10 min. For total phenolic compound analysis, the supernatant was obtained after centrifugation. Then, 0.05 mL of supernatant was mixed with 0.5 mL of Folin’s phenol reagent and 1.5 mL of saturated Na2CO3 solution; after that, the mixture was incubated for 1 h at room temperature protected from light, and then UV absorbance was analyzed at 700 nm. Total phenolic content was expressed as mg of gallic acid (GAE) per gram of the sample. For flavonoid analysis, the supernatant was mixed with 5% NaNO2 and 10% Al(NO3)3 and reacted for 10 min, then 10% NaOH solution was added and the UV absorbance was measured at 510 nm. The flavonoid content in samples was calculated by the rutin standard curve. All experiments were repeated three times.
2.7. Measurement of Peptides and Free Amino Acids (FAAs)
Peptides in the samples were collected based on the method described before [14]. The identification of peptides was performed by RPLC–MS equipment (Q Exactive Plus, Thermo, MA, USA) equipped with a C18 column (75 × 250 mm, 1.9 µm particle size). Chromatographic conditions: the injection volume was set to 3 µL, and the mobile phase (A: 0.1% aqueous formic acid solution; B: 80% acetonitrile and 0.1% formic acid) was set up at a flow rate of 200 nL/min and the temperature of the column for determination was 25 °C. Mass spectrometry conditions: MS scan range (m/z) was 350–1700; resolution was 70,000; maximum ion injection time was 100 ms; MS/MS ion injection time was 75 ms; scan range was 200–2000; fragmentation mode was higher energy collisional dissociation (HCD); fragmentation energy (NCE) was 27; we excluded 1-valence and higher-than-7 valence ions; the dynamic exclusion time was 60 s; we operated under the data-dependent acquisition mode (DDA); and the parent ions were selected for tandem scanning for one MS1 profile. All samples were subjected to three replicate experiments.
Determination of FAAs was slightly modified according to the method of Lin et al. (2018) [15]. The samples were determined using a fully automated amino acid analyzer fitted (LA8080, Hitachi, Tokyo, Japan) with a cation exchange column and a UV detector (570 and 440 nm 1260 Infinity II, Agilent, Palo Alto, CA, USA). The total content of FAAs was the sum of 16 kinds of amino acid, including Asp, Glu, Ser, Gly, Thr, Ala, Pro, Lys, His, Tyr, Val, Met, Ile, Leu, Phe and Cys.
2.8. Microbiological Diversity
Total genome DNA from samples was extracted. DNA concentration was monitored by a Qubit® dsDNA HS Assay Kit (GENEWIZ, Inc., South Plainfield, NJ, USA). For bacteria, 20–30 ng of DNA was used to generate amplicons that covered V3 and V4 hypervariable regions of the 16s rRNA gene of bacteria. The forward primer was the sequence ‘CCTACGGRRBGCASCAGKVRVGAAT’ and the reverse primer was the sequence ‘GGACTACNVGGGTWTCTAATCC’; for fungi, 20–50 ng of DNA was used to generate amplicons that covered the ITS rRNA gene of fungal organisms. The forward primer sequence was ‘GTGAATCATCGARTC’ and the reverse primer sequence was ‘TCCTCCGCTTATTGAT’. The concentration was detected by a microplate reader (Tecan, Infinite 200 Pro, Canton of Zurich, Switzerland) and the fragment size was detected by 1.5% agarose gel electrophoresis, which expected at 600 (16 s) and 400 (ITS) bp. Next-generation sequencing was conducted on an Illumina Miseq/Novaseq Platform (Illumina, San Diego, CA, USA) at Genewiz, Inc. (GENEWIZ, Inc., South Plainfield, NJ, USA). Automated cluster generation and 250/300 paired-end sequencing with dual reads were performed according to the manufacturer’s instructions. Double-end sequencing of positive and negative reads involved the first of the two joining together; the results contained in the sequence of N retain the sequence length, which was larger than the 200 bp sequence. After quality filtering and purification of chimeric sequences, for the resulting sequence for OTU clustering, we conducted VSEARCH clustering (1.9.6) (sequence similarity was set to 97%), using Silva (version 138) as a 16 s rRNA reference database. Then, we used the RDP classifier (Ribosomal Database Program) based on the Bayesian algorithm of OTU species taxonomy to analyze representative sequences and—under different species classification level statistics—the microbial community compositions of each sample [16].
2.9. Data Analysis
All analyses were performed with three replicates and the results of the experiments were expressed as means ± SD. SPSS Statistics software, one-way analysis of variance (ANOVA) tests and Tukey’s post-hoc test were used to determine differences between samples. Marked differences between two groups were indicated by different letters and determined at the p < 0.05 alpha level. The Circos plots were drawn in the R program using Circize and applied Gephi to visualize the network of interactions between microbial communities and metabolites.
3. Results and Discussion
3.1. Changes in the Values of pH and TA during Fermentation
During fermentation, pH and TA are key factors influencing microbial growth and metabolite accumulation [12]. It is reported that low pH and high TA values could inhibit the growth of spoilage bacteria [6]. Table 1 shows that the pH of the fermentation system was 5.01 at the beginning of fermentation (0 h) and decreased as fermentation time increased. At the later fermentation stage, the pH significantly decreased to 3.97. During fermentation, the TA value significantly increased from 0.63 to 1.11; this trend was consistent with the results reported by Andeta and others found during Ensete ventricosum fermentation [17]. In the current study, the changes in pH and TA may mainly be due to acid production by lactic acid bacteria during the fermentation process.

Table 1.
Changes in pH value, TA and metabolites during pineapple waste–whey protein fermentation.
3.2. Changes in Metabolites
3.2.1. Organic Acids
Organic acids are the main flavor attributes for fermentation products, and their composition and content are important factors influencing the sourness of end-products [18]. Additionally, organic acids are widely used worldwide because of their ability to decrease intestinal pH and improve intestinal microflora [4]. Table 1 shows the changes in the seven major organic acids. The content of malic and citric acids gradually decreased as fermentation time increased. Malic acid decreased significantly by 63.5%; these data were consistent with the results of Li and others obtained during the fermentation of blueberry juice [7]. The decrease in Malic acid can be explained by the malic acid being readily available to most microorganisms [19], especially the yeast cytosolic MDH (malate dehydrogenase) and NADP+ (triphosphopyridine nucleotide) which could synergistically convert malic acid into a usable carbon source and lactic acid [20]. Additionally, the lactic acid bacteria may convert malic acid into lactic acid, which is consistent with the phenomenon reported by Fahimi, N et al. [21]. Citric acid also decreased by 28.9%, which may be due to lactic acid bacteria metabolizing citric acid to produce lactic acid, diacetyl or other substances and, thus, making flavor changes [22]. The contents of tartaric acid, oxalic acid and ascorbic acid gradually increased as fermentation time increased; these results were consistent with findings in pickled radish fermentation [23]. As shown in Table 1, lactic acid and succinic acid were the main organic acids in fermentation products. Lactic acid is usually produced due to lactic acid bacteria fermentation [4]. Succinic acid is an important metabolite of yeast. Bechthold et al. (2008) found that, during fermentation, yeast could utilize glucose, fructose and other sugars as carbon sources and produce succinic acid as the main product [24]. So, the accumulation of these two dominant organic acids could be mainly attributed to the action of microorganisms.
3.2.2. Total Phenolic Compounds and Flavonoids
Phenolic compounds including flavonoids and phenolic acids are secondary metabolites widely found in various fruits [25]. They can play an antioxidant role by providing hydrogen atoms to free radicals and chelating metal ions; they also have the functions of lowering blood pressure, lowering blood lipid, resisting osteoporosis, preventing arteriosclerosis, etc. [26]. As shown in Table 1, the total content of phenolic compounds increased with fermentation. A similar trend was found during the fermentation of pineapple juice [6], sea buckthorn–apple juice [27] and blueberry juice [7]. Amorim, Piccoli and Duarte (2018) found that, compared to unfermented samples, the total phenolic compounds decreased in fermented pineapple juice prepared with Saccharomyces cerevisiae var. boulardii inoculation; however, it increased with M. caribbica inoculation [6]. In this study, the increase in total phenolic compounds may be due to cell wall degradation by microbial fermentation and promoted phenolic compound release [26]. Additionally, the degradation of macromolecules such as sugars and proteins by microorganisms allows free phenols to bind them, leading to an increase in total phenolic compounds [28]. Researchers demonstrated another possibility for this phenomenon: they considered that the organic acids produced during the fermentation process prevented the degradation of phenolics and, thus, led to an increase in total phenolic compounds [29]. At the end of fermentation stage (26 h), the content of phenolic compounds was 0.83 mg GAE/g. This value was much higher (0.24 mg GAE/g) than cantaloupe samples fermented by lactic acid bacteria for 12 d. It is believed that the main phenolic compounds in pineapple are flavonoids [28]; therefore, we detected changes in flavonoids during pineapple waste fermentation. As shown in Table 1, the content of flavonoids significantly increased during the fermentation process, and the content of flavonoids in the final products was 5.44 mg/g, which was higher than sea buckthorn–apple juice fermented with different lactic acid bacteria strains for 72 h [27]. The differences in total phenolic compounds and flavonoids of various fermented plant products may be attributed to the substrate, fermentation conditions and microorganisms.
3.2.3. Peptides and Total FFAs
In this study, whey protein was added to pineapple by-products in order to give the fermented products balanced nutrition values and better flavor characteristics. Additionally, during the fermentation process, protein could be degraded into small molecules such as peptides and amino acids by endogenous and exogenous enzymes produced by microorganisms, which can give the product functional features. Figure 1 shows the changes in peptides at different stages of fermentation. The number of peptides showed a trend of first increasing and then decreasing throughout the fermentation process. The number of peptides in the pineapple by-products was only 4 at the beginning of fermentation (0 h) and significantly increased to 262 after 8 h. This may be due to microbial growth and metabolism, which resulted in proteins breaking down into peptides. The number of peptides significantly decreased to 152 at the later stage of fermentation (26 h), which could be attributed to the further degradation of peptides by microorganisms that leads to produce large amounts of FAAs. These results were consistent with the findings of total FAAs. As shown in Table 1, the total amount of FAAs significantly increased with fermentation time (p < 0.05).
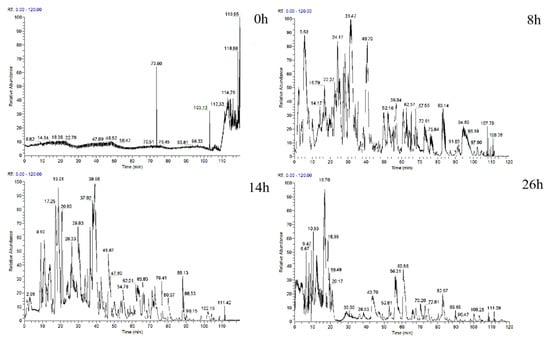
Figure 1.
Primary mass spectrometry of peptides in samples of different fermentation times.
In the samples from different fermentation stages, a total of 609 peptides were identified belonging to 55 protein entities, which corresponded to 46 unique protein annotations (Table 2), mainly whey proteins (alpha-lactalbumin, beta-lactoglobulin and immunoglobulins) as well as a small fraction of caseins (beta- and kappa-casein) and other proteins such as progestagen-associated endometrial protein, osteopontin, transthyretin, fatty acid-binding protein 3 and some gene products were also found. Beta-lactoglobulin is the main component of whey protein, and it is also the main allergen in milk. Results showed that beta-lactoglobulin was degraded into peptides and amino acids by microorganisms during the fermentation process; this suggested that fermentation of whey protein could reduce its allergenicity. Wroblewska and others also indicated that the immunoreactivity of whey protein and casein was significantly reduced after lactic acid bacteria fermentation [30]. In this study, some peptides sourced from casein were found. This phenomenon could be explained by the presence of impurities in the whey protein. Undoubtedly, a large number of peptides identified in final fermented products could improve the functionality of fermented pineapple waste.

Table 2.
Identified peptides in samples from different fermentation times.
Next, we looked for peptides with potential biological activity by searching the Biopep database (Table 3). Based on the findings of peptide datasets, we identified five peptides that exactly matched the primary sequence stored in the Biopep database. Two peptides belong to beta-lactoglobulin and three peptides belong to beta-casein. Three of the five peptides are described as having antihypertensive activity, one having antibacterial activity and one having antihypertensive, immune and cellular regulatory activity. All five of the identified bioactive peptides were present in products fermented for 26 h, and two fragments were identified in both 8 h and 14 h fermentation samples, while no bioactive peptide was identified in unfermented samples (0 h). In this study, among the 604 peptides identified, the function of most peptides was not clear, but there is no doubt that these peptides can not only improve the digestibility of whey protein, but may also have various functions, such as antioxidant, antibacterial, antihypertensive, etc., which need further research.

Table 3.
Identified peptides that exactly matched in sequence and length to entries in the Biopep database.
3.3. Changes in Microbial Communities during Fermentation
3.3.1. Bacterial Communities
High-quality sequences were classified at the genus and species level to investigate the structure and dynamic succession of bacterial communities during pineapple by-product fermentation. At the genus level, a total of 36 bacterial genera were identified (Figure 2A). The dominant bacterial taxa at the initiation of fermentation (0 h) were Streptococcus, Serratia, Enterobacter and Pantoea, accounting for 67.14% of the total bacterial population. The presence of these conditional pathogenic bacteria may be due to the pasteurization (65 °C, 30 min) of raw materials, resulting in incomplete sterilization. At the beginning of fermentation (0 h), Streptococcus (25.31%), Serratia (17.71%), Pantoea (15.82%), Enterobacter (8.30%), Rahnella1 (6.47%), Lactobacillus (3.99%), Lactococcus (1.70%) and Leuconostoc (1.09%) were unevenly distributed. However, most of the initially dominant bacterial genus decreased rapidly after 8 h of fermentation, and Lactococcus and Weissella increased rapidly. At the post-fermentation stage (26 h), Lactococcus (81.18%) was the dominant microorganism in the fermented pineapple by-products, followed by Weissella (6.02%) and Klebsiella (5.34%), while the relative abundance of other bacterial genera was low (0.01 to 4.00%). As described before, Lactococcus is widely found in naturally fermented dairy products, and it produces organic acids, antimicrobial peptides and other antibacterial substances that inhibit most bacteria growth [31]. Lactococcus also plays a crucial role in the formation of the aroma, texture and acidity of the final fermented product [31]. Weissella is widely found in plant-based fermented foods; it not only provides a unique flavor, but also reduces biogenic amines during food fermentation [32]. Therefore, as the dominant flora, these two genera can enhance the safety of the final product to a large extent and give the product special flavor.
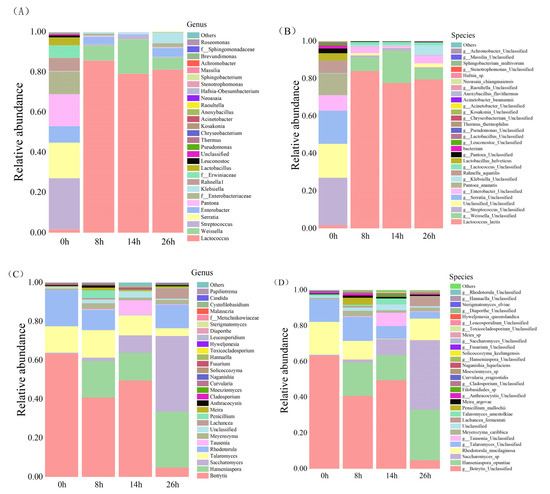
Figure 2.
Relative abundance of bacterial (A) bacterial genus; (B) bacterial species and fungal (C) fungal genus; (D) fungal species populations of fermented products at different fermentation stages.
At the species level, a total of 39 bacterial species were identified (Figure 2B). At the beginning of fermentation (0 h), Streptococcus Unclassified, Pantoea_ananatis, Rahnella_aquatilis, Serratia Unclassified and Enterobacter Unclassified were the dominant species, accounting for about 69.6% of the total number of bacteria. At the end of fermentation (26 h), they accounted for only 4.81%. This phenomenon is probably due to the increase in organic acids and the decrease in pH during the fermentation process, which inhibited their growth [33]. Lactococcus lactis accounted for only 1.66% at the beginning of fermentation (0 h) and 79.59% at the end of fermentation (26 h), indicating that it could play a vital function in the fermentation of pineapple by-products. Lactococcus lactis has been used for centuries during fermented food processing, especially cheese, sauerkraut and fruit; therefore, it is generally considered safe by the Food and Drug Administration (FDA). In this study, the inoculated strain Lactococcus lactis was the autochthonous bacteria of pineapple, and the results of a fermentation performance test showed that it had good growth properties, strong resistance and enzyme production capacity (data not shown). During pineapple waste fermentation, it became the dominant bacteria after 8 h of fermentation, which provided good evidence for the superiority of endophytic bacteria in fruit fermentation.
3.3.2. Fungal Communities
In addition to bacteria, fungi also play an important role in the fermentation process [34]. Figure 2C showed that a total of 103 fungal genera were identified. Botrytis (63.26%), Talaromyces (13.61%) and Rhodotorula (18.49%) were the predominant fungal genera at the beginning of fermentation (0 h). Botrytis is described as a common pathogen in plants with a wide range of hosts, a long incubation period and a tendency to mutate, and it is the main cause of grey mold in plants [35]. Talaromyces and Rhodotorula are a group of saprophytic fungi that are widely distributed in nature and human habitats; they are quite closely related to plants, animals and humans [36]. Based on Figure 2C, compared to unfermented samples, the levels of fungal genera in 26 h fermentation samples were significantly different in abundance. At the end of fermentation (26 h), the abundance of Botrytis and Talaromyces significantly decreased to 4.76% and 3.72%. However, Hanseniaspora and Saccharomyces, which were the two most important genera in the fermentation process, showed a significant increase in abundance. At the end of fermentation (26 h), the proportions of Hanseniaspora and Saccharomyces were 28.68% and 39.05%, respectively. The reduced abundance of Botrytis and Talaromyces may be related to the rapid growth of Hanseniaspora and Saccharomyces. Wang and others found that rapid colonization of plant surfaces by yeasts could inhibit the growth of pathogenic bacteria on plant surfaces [34]. As stated before, Saccharomyces is the most commonly used microorganism in fermentation and contributes to aromatic compound accumulation [37]. Hanseniaspora is also the main non-enological yeast used in the production of wine, and researchers found that, during fermentation, Hanseniaspora could inhibit the growth of other microorganisms and produced aromatic flavors [35].
At the species level (Figure 2D), Hanseniaspora_opuntiae and Saccharomyces_sp. only accounted for 0.01% and 0.06% of the total fungi at the beginning of fermentation (0 h). In this study, Hanseniaspora_opuntiae was inoculated to the fermentation system as a co-cultured strain; it became the dominant species during the middle stage of fermentation (8 and 14 h), and increased from 0.01% to 14.12%. Ferreira-Saab et al. found that Hanseniaspora_opuntiae could inhibit the growth of Botrytis cinerea and Corynespora cassiicola by releasing a compound of its own, which could be responsible for the reduction in Botrytis in the late fermentation stage [38]. At the end of fermentation, Saccharomyces sp. increased from 0.06% to 38.84% and became one of the dominant species in the fermented pineapple by-products. Saccharomyces sp. is the most commonly used microorganism in the brewing industry and contains bioactive and nutritional compounds including protein, polysaccharides, fiber and vitamins [38]. Therefore, during pineapple waste fermentation, the action of Saccharomyces sp. could improve the quality and safety of fermented products.
3.4. Correlation between Microorganisms and Metabolites
The relationship between the core microbial community and the physicochemical properties and metabolites of fermented pineapple by-products was calculated using Spearman correlation and visualized by Gephi. As shown in Figure 3, there was a significant correlation between the core microbial community (correlation coefficients, ΙRΙ > 0.6; p < 0.05) and the physicochemical properties and metabolites. Lactococcus was the main bacterial genus identified in fermented pineapple wastes. It has a long history of being used extensively in the fermentation of food products [39]. In this study, Lactococcus was highly correlated with the contents of various metabolites. In particular, Lactococcus was positively correlated with five kinds of organic acids (oxalic acid, tartaric acid, ascorbic acid, lactic acid and succinic acid) and total FAAs; Lactococcus also contributed to the increase in total phenolic compounds and flavonoids. This is consistent with the findings of Huang and others, who demonstrated that, during fermentation, lactic acid bacteria could break down larger proteins into smaller polypeptide fragments and further into free amino acids, resulting in the production of a variety of organic acids. These metabolites could provide a bright sour flavor to fermented foods through high levels of lactic acid [39]. Previous reports indicated that Weissella could play an important role in the synthesis of esters, organic acids and short-chain fatty acids in foods and had antibacterial properties [12]. In this study, Weissella also showed a high correlation with physicochemical indicators and played important role in the fermentation process; these data were in agreement with the findings of Liang, Zhang, Wu, Liu and Zhang (2016) [40]. During fermentation, Streptococcus, Serratia, Enterobacter, Pantoea and Erwiniaceae showed a negative correlation with active substances such as organic acids and total FAAs. These pathogenic bacteria, which were in dominant abundance at the beginning of the fermentation, were significantly inhibited due to the accumulation of active substances such as organic acids and total phenols and the combined effects of other probiotic bacteria. These data were consistent with the study conducted on fermented seaweed wastes by Salgado, Munoz, Blanco and Lienqueo (2021) [41]. In terms of fungi, Hanseniaspora, Saccharomyces and Lachancea were positively correlated with most of the organic acids (oxalic acid, tartaric acid, ascorbic acid, lactic acid and succinic acid) and total FAAs. They also contributed to the accumulation of total phenolic compounds and flavonoids. van Wyk et al. (2022) found that addition of Hanseniaspora reduced the malic acid content of wine, resulting in better taste and aroma [42]. Escribano-Viana et al. (2022) used Lachancea to prevent the growth of pathogenic bacteria during wine fermentation; these results were consistent with the findings of the present study [43]. However, as shown in Figure 3, Clostridium botulinum, Tara and Rhodotorula were negatively correlated, probably due to the growth inhibition occurring among them. From Figure 3, it is shown that Lactococcus, Weissella, Hansenella and Saccharomyces were the main producers of active substances such as organic acids and FAAs during the fermentation of pineapple by-products. As for physicochemical properties, Lactococcus and Hanseniaspora were highly positively correlated with TA, which also led to an increase in acidity in the fermentation system. The results coincided with previous studies which demonstrated that Lactococcus could lower pH in the traditional manufacturing of cheese [44], and Hanseniaspora significantly increased acidity and aroma production in wine making [45]. In this study, the proportion of Lactococcus was positively correlated with the number of peptides, suggesting that it plays a dominant role in the breakdown of proteins in the fermentation process.
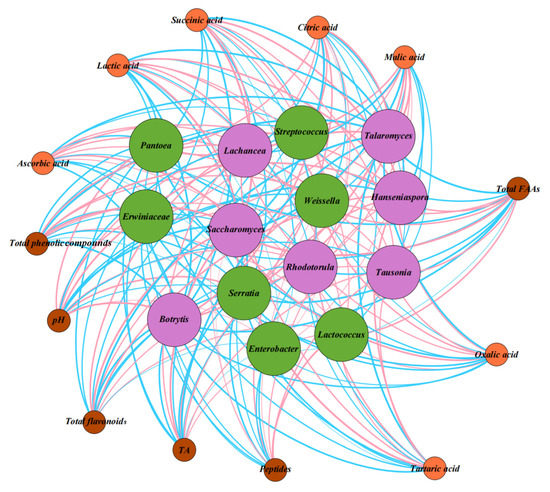
Figure 3.
Correlation between microbial genera, organic acids, total free amino acids and physicochemical properties. Note: the red line connecting the circles indicates a positive correlation, while the blue line indicates a negative correlation between the microbiota and the indicator.
The microbiological composition of fermented products also correlates strongly with other important qualities. We have determined the aroma, flavors and functional activity of the fermented product, and it has good organoleptic properties. The fermented product was also subjected to in vitro digestion simulations and in vitro fermentation experiments, and we found that the fermented product has a beneficial effect on the improvement of human intestinal flora. These organoleptic and functional properties are closely linked to the microorganisms in the fermented product. However, due to the space limitation of the article, we did not show this part.
4. Conclusions
Results showed that the pH value decreased, and the TA value increased with prolonged fermentation time. Total phenolic compounds and flavonoids also accumulated as fermentation time was prolonged. Organic acids and total FAAs significantly increased compared to unfermented samples. Above data suggest that the fermentation of pineapple waste–whey protein using co-inoculation of lactic acid bacteria and yeast could promote the accumulation of functional metabolites. Over 26 h of fermentation, a total of 609 kinds of peptides were identified, and biologically active peptides were mainly found at the end of fermentation (26 h). These data indicated that adding whey protein could improve the functionality of fermented products. Correlation analysis showed that 11 kinds of microbial species (at the genus level) were highly correlated with the quality attributes of fermented pineapple by-products (p < 0.05, R > 0.6). Lactococcus, Weissella, Hanseniaspora and Saccharomyces were the main microbial genera involved in the fermentation of pineapple by-products. The results of this study demonstrated that metabolite changes were highly associated with microbial community succession during the fermentation of pineapple waste–whey protein. The findings of this study could contribute to the high-value utilization of pineapple by-products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9020079/s1, Table S1: The enzyme-producing capacity of LA5 and SA2.
Author Contributions
Conceptualization, S.X., W.H. and J.W.; methodology, J.L., S.X. and W.H.; validation, J.L., S.X., Y.C. and B.W.; formal analysis, W.H. and S.X.; investigation, J.L.; resources, S.X. and J.W.; data curation, J.L., S.X. and Y.C.; writing—original draft preparation, J.L.; writing—review and editing, S.X., W.H. and J.W.; supervision, W.H. and J.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Dongguan Institute of Science and Technology High Level Talent Research Start Project (GC300501-139), the Project of Educational Commission of Guangdong Province of China (2021KTSCX132) and the National Natural Science Foundation of China (31901682).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pino, J.A. Odour-active compounds in pineapple (Ananas comosus [L.] Merril cv. Red Spanish). Int. J. Food Sci. Technol. 2013, 48, 564–570. [Google Scholar] [CrossRef]
- Li, D.; Jing, M.; Dai, X.; Chen, Z.; Ma, C.; Chen, J. Current status of pineapple breeding, industrial development, and genetics in China. Euphytica 2022, 218, 85. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef]
- Yao, D.; Xu, L.; Wang, C.Y. Diversity of the microbial community and antioxidant activity during fermentation of red raspberry Enzymes. Food Sci. Nutr. 2021, 9, 99–110. [Google Scholar] [CrossRef]
- Di Cagno, R.; Cardinali, G.; Minervini, G.; Antonielli, L.; Rizzello, C.G.; Ricciuti, P.; Gobbetti, M. Taxonomic structure of the yeasts and lactic acid bacteria microbiota of pineapple (Ananas comosus L. Merr.) and use of autochthonous starters for minimally processing. Food Microbiol. 2010, 27, 381–389. [Google Scholar] [CrossRef]
- Amorim, J.C.; Piccoli, R.H.; Duarte, W.F. Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages. Food Res. Int. 2018, 107, 518–527. [Google Scholar] [CrossRef]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef]
- Wen-Qiong, W.; Jie-Long, Z.; Qian, Y.; Ji-Yang, Z.; Mao-Lin, L.; Rui-Xia, G.; Yujun, H. Structural and compositional changes of whey protein and blueberry juice fermented using Lactobacillus plantarum or Lactobacillus casei during fermentation. RSC Adv. 2021, 11, 26291–26302. [Google Scholar] [CrossRef]
- Khalifa, I.; Peng, J.; Jia, Y.; Li, J.; Zhu, W.; Yu-Juan, X.; Li, C. Anti-glycation and anti-hardening effects of microencapsulated mulberry polyphenols in high-protein-sugar ball models through binding with some glycation sites of whey proteins. Int. J. Biol. Macromol. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Mohd Taha, M.D.; Mohd Jaini, M.F.; Saidi, N.B.; Abdul Rahim, R.; Md Shah, U.K.; Mohd Hashim, A. Biological control of Erwinia mallotivora, the causal agent of papaya dieback disease by indigenous seed-borne endophytic lactic acid bacteria consortium. PLoS ONE 2019, 14, e224431. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, Y.; Tang, L.J.; Li, X.X.; Xiao, Y.W.; Bin Zhang, Z.; Yan, R.M.; Yang, H.L.; Chang, J.; Zhu, B.; et al. Yeasts from Nanfeng mandarin plants: Occurrence, diversity and capability to produce indole-3-acetic acid. Biotechnol. Biotechnol. Equip. 2018, 32, 1496–1506. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Hazra, S.K.; Chakraborty, R. The impact of raw and differently dried pineapple (Ananas comosus) fortification on the vitamins, organic acid and carotene profile of dairy rasgulla (sweetened cheese ball). Heliyon 2020, 6, e5233. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Koh, E. Changes in the antioxidant capacity and phenolic compounds of maesil extract during one-year fermentation. Food Sci. Biotechnol. 2017, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Gallego, M.; Escudero, E.; Reig, M.; Toldra, F. Small peptides hydrolysis in dry-cured meats. Int. J. Food Microbiol. 2015, 212, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, X.; Fang, J.; Lu, Y.; Liu, P.; Xing, Y.; Wang, Q.; Che, Z.; He, Q. Flavor Compounds in Pixian Broad-Bean Paste: Non-Volatile Organic Acids and Amino Acids. Molecules 2018, 23, 1299. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, Z.; Zhang, X.; Xi, G.; Zhao, Z.; Lai, M.; Zhao, M. Microbial community and metabolic function analysis of cigar tobacco leaves during fermentation. MicrobiologyOpen 2021, 10, e1171. [Google Scholar] [CrossRef]
- Andeta, A.F.; Vandeweyer, D.; Woldesenbet, F.; Eshetu, F.; Hailemicael, A.; Woldeyes, F.; Crauwels, S.; Lievens, B.; Ceusters, J.; Vancampenhout, K.; et al. Fermentation of enset (Ensete ventricosum) in the Gamo highlands of Ethiopia: Physicochemical and microbial community dynamics. Food Microbiol. 2018, 73, 342–350. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Z.; Hao, Y.; Zhang, L.; Ren, Y.; Zhang, Y.; Chen, Z.; Mandlaa. Correlation between bacterial communities and organic acids in the fermentation stage of traditional Chinese sour porridge. Int. J. Food Prop. 2020, 23, 1430–1440. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef]
- Moriyama, S.; Nishio, K.; Mizushima, T. Structure of glyoxysomal malate dehydrogenase (MDH3) from Saccharomyces cerevisiae. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 617–624. [Google Scholar] [CrossRef]
- Fahimi, N.; Brandam, C.; Taillandier, P. A mathematical model of the link between growth and L-malic acid consumption for five strains of Oenococcus oeni. World J. Microbiol. Biotechnol. 2014, 30, 3163–3172. [Google Scholar] [CrossRef]
- García-Quintáns, N.; Repizo, G.; Martín, M.; Magni, C.; López, P. Activation of the Diacetyl/Acetoin Pathway in Lactococcus lactis subsp.lactis bv. diacetylactis CRL264 by Acidic Growth. Appl. Environ. Microb. 2008, 74, 1988–1996. [Google Scholar] [CrossRef]
- Chen, A.; Luo, W.; Peng, Y.; Niu, K.; Liu, X.; Shen, G.; Zhang, Z.; Wan, H.; Luo, Q.; Li, S. Quality and microbial flora changes of radish paocai during multiple fermentation rounds. Food Control 2019, 106, 106733. [Google Scholar] [CrossRef]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic Acid: A New Platform Chemical for Biobased Polymers from Renewable Resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of Physicochemical Properties, Functional Compounds and Antioxidant Capacity during Spontaneous Fermentation of Lycium ruthenicum Murr. (Qinghai–Tibet Plateau) Natural Vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef]
- Ruiz-Garcia, Y.; Beres, C.; Chavez, D.; Pereira, D.; Santiago, M.; Godoy, R.D.; Gomes, F.D.; Antoniassi, R.; Tonon, R.V.; Cabral, L. In vitro digestion and colonic fermentation of an Alicante Bouschet (Vitis vinifera L.) skin extract. LWT-Food Sci. Technol. 2022, 157, 113083. [Google Scholar] [CrossRef]
- Sun, Y.; Chou, C.; Yu, R. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem. 2009, 115, 912–917. [Google Scholar] [CrossRef]
- Wroblewska, B.; Markiewicz, L.H.; Szyc, A.M.; Dietrich, M.A.; Szymkiewicz, A.; Fotschki, J. Lactobacillus casei LcY decreases milk protein immunoreactivity of fermented buttermilk but also contains IgE-reactive proteins. Food Res. Int. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Li, W.; Ren, M.; Duo, L.; Li, J.; Wang, S.; Sun, Y.; Li, M.; Ren, W.; Hou, Q.; Yu, J.; et al. Fermentation Characteristics of Lactococcus lactis subsp. lactis Isolated from Naturally Fermented Dairy Products and Screening of Potential Starter Isolates. Front. Microbiol. 2020, 11, 1794. [Google Scholar] [CrossRef]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef]
- Sobrino-Lopez, A.; Martin-Belloso, O. Use of nisin and other bacteriocins for preservation of dairy products. Int. Dairy J. 2008, 18, 329–343. [Google Scholar] [CrossRef]
- Wang, X.; Glawe, D.A.; Kramer, E.; Weller, D.; Okubara, P.A. Biological Control of Botrytis cinerea: Interactions with Native Vineyard Yeasts from Washington State. Phytopathology 2018, 108, 691–701. [Google Scholar] [CrossRef]
- Feng, C.; Du, X.; Wee, J. Microbial and Chemical Analysis of Non-Saccharomyces Yeasts from Chambourcin Hybrid Grapes for Potential Use in Winemaking. Fermentation 2021, 7, 15. [Google Scholar] [CrossRef]
- Belloch, C.; Villa-Carvajal, M.; Alvarez-Rodriguez, M.L.; Coque, J. Rhodotorula subericola sp nov., an anamorphic basidiomycetous yeast species isolated from bark of Quercus suber (cork oak). Int. J. Syst. Evol. Microbiol. 2007, 57, 1668–1671. [Google Scholar] [CrossRef]
- Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A.; Ruiz-Montañez, G.; Ortiz-Basurto, R.I.; Luna-Solano, G.; Calderón-Santoyo, M. Saccharomyces cerevisiae Mixed Culture of Blackberry (Rubus ulmifolius L.) Juice: Synergism in the Aroma Compounds Production. Sci. World J. 2014, 2014, 163–174. [Google Scholar] [CrossRef]
- Ferreira-Saab, M.; Formey, D.; Torres, M.; Aragón, W.; Padilla, E.A.; Tromas, A.; Sohlenkamp, C.; Schwan-Estrada, K.R.F.; Serrano, M. Compounds Released by the Biocontrol Yeast Hanseniaspora opuntiae Protect Plants Against Corynespora cassiicola and Botrytis cinerea. Front. Microbiol. 2018, 9, 1596. [Google Scholar] [CrossRef]
- Huang, X.; Yu, S.; Han, B.; Chen, J. Bacterial community succession and metabolite changes during sufu fermentation. LWT 2018, 97, 537–545. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, A.; Wu, Z.; Liu, C.; Zhang, W. Characterization of Microbial Community during the Fermentation of Chinese Homemade paocai, a Traditional Fermented Vegetable Food. Food Sci. Technol. Res. 2016, 22, 467–475. [Google Scholar] [CrossRef]
- Salgado, C.L.; Munoz, R.; Blanco, A.; Lienqueo, M.E. Valorization and upgrading of the nutritional value of seaweed and seaweed waste using the marine fungi Paradendryphiella salina to produce mycoprotein. Algal Res. 2021, 53, 102135. [Google Scholar] [CrossRef]
- Van Wyk, N.; Scansani, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pretorius, I.S.; Rauhut, D.; von Wallbrunn, C. The Use of Hanseniaspora occidentalis in a Sequential Must Inoculation to Reduce the Malic Acid Content of Wine. Appl. Sci. 2022, 12, 6919. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Gonzalez-Arenzana, L.; Garijo, P.; Fernandez, L.; Lopez, R.; Santamaria, P.; Gutierrez, A.R. Bioprotective Effect of a Torulaspora delbrueckii/Lachancea thermotolerans-Mixed Inoculum in Red Winemaking. Fermentation 2022, 8, 337. [Google Scholar] [CrossRef]
- Feng, M.F.; Lv, Y.; Li, T.T.; Li, X.M.; Liu, J.Y.; Chen, X.L.; Zhang, Y.; Chen, X.; Wang, A.X. Postharvest Treatments with Three Yeast Strains and Their Combinations to Control Botrytis cinerea of Snap Beans. Foods 2021, 10, 2736. [Google Scholar] [CrossRef]
- Valera, M.J.; Olivera, V.; Boido, E.; Dellacassa, E.; Carrau, F. Wine Aroma Characterization of the Two Main Fermentation Yeast Species of the Apiculate Genus Hanseniaspora. Fermentation 2021, 7, 162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).