Abstract
Yoghurt is a delectable fermented milk product suitable for all ages. Chromium (Cr), a trace mineral is found in two forms: trivalent and hexavalent. Recent studies have shown that the Cr (III), or chromium picolinate (Cri), is beneficial for carbohydrate metabolism. Thus, Cr supplements are used to treat diabetes and manage blood sugar. However, the effect of the incorporation of Cri on starter growth and the quality of yoghurt still needs to be determined. In this study, we aim to produce Cr (CrPi)-fortified yoghurt to fulfil the proposed recommended daily allowance (RDA) of Cr (35–50 µg/day for adults). Additionally, it might act as a nutraceutical for persons with special medical conditions, such as patients with obesity or type 2 diabetes mellitus disease. In this respect, the effect of different concentrations of CrPi, (1, 2, 5, 10, 20 ppm) chromium chloride [Cr (III)] (1, 2, 3, 4, 5 ppm), and potassium chromate [Cr (VI)] (1, 2 ppm) on the activity of yoghurt starter culture were investigated in vitro on de Man, Rogosa, and Sharpe (MRS) media. Compared to the control (without Cr), the obtained data revealed significant inhibition of the yoghurt starter culture by Cr (VI) at 2 ppm compared to Cr (III), which did not affect the bacterial growth up to 5 ppm and was comparable with CrPi [Cr (III)]. We also produced yoghurt supplemented with two doses of Cr (0.25 and 0.5 ppm). We did not observe any significant differences in the physicochemical, rheological, microbiological, and sensory properties of the Cr-fortified yoghurt and the control up to 2 weeks in cold storage. These results clearly indicate that CrPi (0.25 and 0.5 ppm) can be used to manufacture yoghurt with the RDA for intact Cr without affecting its quality.
1. Introduction
In its trivalent state, chromium (Cr) is a primary nutrient necessary for fat and sugar metabolism. In 2001, chromium was listed as an essential nutrient by the Food and Nutrition Board of the National Academies of Sciences, Engineering, and Medicine [1] based on its effect on insulin function. Chromodulin, which contains Cr, facilitates the functions of insulin in the body [2,3]; however, the European Food Safety Authority Panel [4] concluded that as there is insufficient data to determine that Cr is a necessary micronutrient and that it is inappropriate to establish its consumption recommendations. For adult males and females, the recommended daily allowances (RDA) of Cr are 35 and 25 µg, respectively, which can be increased to 45 µg during lactation [1,5]. Cr is generally included in multivitamin/mineral supplements at doses of 35–120 mcg. Additional Cr-only supplements typically deliver 200–500 mcg, or even up to 1000 mcg of Cr [5,6].
Between 0.4% and 2.5% of Cr (III) is estimated to be absorbed from food, depending on the chemical makeup of the food and the presence of other dietary ingredients. Reports have shown that the absorption efficiency of supplementary Cr (III) ranges from 0.1% to 5.2% depending on the Cr complex consumed [4]. As the Cr (VI) found in water and food is completely converted to Cr (III), the analytical results for the total Cr in food represent the Cr (III) content. This was also applicable to meals that included water [7]. Cr is found in many food types, including meat, grains, fruits, vegetables, nuts, spices, brewer’s yeast, beer, and wine. However, Cr level in these meals vary significantly based on the regional soil and water conditions, as well as the farming and industrial practices used to produce them [8,9]. Nearly all dairy products and high-sugar diets (containing sucrose and fructose) are poor in Cr [10]. Supplements contain several forms of Cr, including Cr picolinate, Cr nicotinate, Cr polynicotinate, Cr chloride, and Cr histidinate [5]. Trivalent Cr [Cr (III)], a highly stable form, is used in dietary supplements [11]. However, epidemiological studies have shown that workers exposed to Cr via inhalation are predisposed to lung cancer. Although workers who were exposed to Cr were in contact with both Cr (III) and Cr (VI) compounds, only Cr (VI) has been linked to cancer in animal studies, which led the U.S. Environmental Protection Agency (EPA) to declare that only Cr (VI) should be categorized as a human carcinogen [12,13]. As it is not yet known if Cr (III) compounds only can be carcinogenic [12,14], the EPA has categorized Cr (III) as Group D (not classifiable as a human carcinogen) [14]. Cr picolinate (CrPi), a trivalent Cr complex, is frequently used to treat people with carbohydrate metabolism disorders, such as insulin resistance and type 2 diabetes mellitus. It is more bioavailable and less toxic than Cr (VI) [15,16]. Interestingly, Cr absorption is deficient in the intestines, with less than 2.5% of ingested Cr being absorbed [4,17]. However, CrPi shows better absorption [3,18] and hence, is commonly found in dietary supplements. Picolinic acid is an amino acid metabolite produced in the liver, kidney, and pancreas of animals, which acts as a physiological carrier that transports zinc, copper, and chromium ions from the small intestine into the bloodstream [19,20]. Different studies used trivalent chromium (CrCl3) for dairy and non-dairy food fortification. Different levels of dietary chromium (Cr) supplements in casein or yoghurt-based diets were fed to genetically obese C57BL/6J-OB (ob/ob) mice. The trivalent Cr had a significant effect on preventing lipid accumulation in the liver of obese mice. The mean plasma glucose, insulin and insulin/glucose (I/G) ratio of those obese mice suggest that Cr supplementation might beneficially affect glucose tolerance and improve insulin sensitivity [21]. The hypoglycemic and antioxidative effects of chromium-fortified parboiled rice were investigated on diabetic rats [22,23]. The use of Cr+3 at a level of 1.55 mg kg−1 in a diet containing 30% fermented yellow corn meal and 42.79% total carbohydrate was also suggested for the best growth and feed efficiency of jelawat fish [24].
Due to the increased emphasis on functional foods in recent times [25,26,27,28,29,30,31,32,33,34,35], we aim to evaluate the effect of CrPi on the growth of yoghurt culture grown on de Man, Rogosa, and Sharpe (MRS) media and its application in manufacturing yoghurt.
2. Materials and Methods
2.1. Materials
Chromium chloride (CrCl3 · 6H2O) and potassium chromate (K2CrO4) were obtained from Sigma-Aldrich, St. Louis, MO, USA. Oxoid MRS broth medium was obtained from Thermo Fisher Scientific Inc. Waltham, MA, USA. Chromium picolinate (CrPi) was prepared according to the method described elsewhere [36]. Ultra-high temperature cow’s milk (3% fat and 8.25% SNF) was purchased from the local market in Kafrelsheikh city, Egypt. DVS yoghurt starter culture (YC-X11) consisting Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus was obtained from Chr. Hansen, Copenhagen, Denmark.
2.2. Manufacturing Methods
2.2.1. Preparation of Chromium Picolinate
Chromium picolinate (CrPi) was prepared based on a previous method [36]. Chromium (III) chloride (2.66 g) was dissolved in 25 mL of deionized water at 60 °C. Then, 3.69 g of picolinic acid was added and the solution was heated gently with continuous stirring until it turned purple and crystals started to form. The solution was kept at 4 °C overnight. The crystals were separated using filter paper and then air-dried at room temperature.
2.2.2. Growth of Starter Culture in Different Chromium Forms -Fortified MRS Media
The effect of CrCl3 · 6H2O, K2CrO4, and CrPi on the growth of yoghurt culture in MRS media was monitored by measuring its absorbance at 650 nm [37]. Acidity development was indicated by the reduction in the pH of the media (Crison, Spain). These parameters were monitored at two-hour intervals over a 10 h incubation period at 37 °C in a water bath.
2.2.3. Preparation of Yoghurt
The yoghurt was prepared using the method described by Tamime and Robinson (1999) [38]. The milk was inoculated with the DVS starter culture (0.03%) and divided into three parts. The first part was without CrPi (control), the second was fortified with 0.25 ppm Cr (CrPi) (T1), and the third was fortified with 0.5 ppm Cr (CrPi) (T2). All the treatments were incubated at 40 °C until they were completely coagulated, followed by cooling overnight at 5 ± 2 °C. The chemical, rheological, microbiological, and sensory properties of the yoghurt were evaluated when they were fresh and after 7 and 15 days in cold storage.
2.3. Analysis Method
2.3.1. Growth of Yoghurt Culture in CrPi-Fortified Milk
Culture growth was determined by measuring the pH reduction during fermentation at specified intervals until reached a pH of approximately 4.6 [35].
2.3.2. Chemo-Physical Analysis of Yoghurt
The titratable acidity was determined based on A.O.A.C (2000) [39] and recorded as (%) of lactic acid. The pH value was measured electrometrically using a pH meter (Crison pH meter, Spain). Acetaldehyde was detected according to Less and Jago (1969) [40]. The viscosity, expressed as centipoise (cp), was determined (after manually stirring the yoghurt gels) using a digital Brookfield viscometer (LVDV–E, Brookfield Eng. Lab., Middleboro, MA, USA) and spindle No. 63 at a speed of 50 rpm. Whey separation (wheying-off) was determined based on Lucey et al. (1998) [41]. The water holding capacity (WHC) of yoghurt was measured according to Isanga and Zhang, (2009) [42] using a Universal 32R centrifuge, the stirred yoghurt was centrifuged for 15 min at 8000× g and 4 °C. The WHC was calculated using the following formula:
where: W1 = weight of whey after centrifugation, W2 = yoghurt weight. All measurements were performed in triplicate.
WHC% = (1 − W1/W2) × 100
2.3.3. Microbiological Analysis
Total viable bacterial counts (TBC) were counted on a tryptone glucose extract agar medium using a previous method [43]. Lactobacillus bulgaricus growth was monitored using MRS agar based on a previous method [44]. As previously described, S. thermophilus were cultivated and enumerated on M17 media [45]. Potato dextrose agar was used to determine the yeast and mould counts [46].
2.3.4. Sensory Evaluation
The sensory properties of yoghurt were assessed by 15 staff members from the Kafrelsheikh University, Faculty of Agriculture according to the method given described [47]. The tested attributes were overall acceptability (10 points), firmness (10 points), smoothness (10 points), wheying-off (10 points), and acid flavour (10 points).
2.3.5. Statistical Analysis
Statistical analysis was conducted using the SPSS version 10.0 software [48]. Any significant differences between means were assessed using analysis of variance and Duncan’s test at the significance level of p = 0.05. The data were expressed as mean ± standard error (SE) of three replicates.
3. Results and Discussion
3.1. Growth of Yoghurt Starter in MRS Media Supplemented with Different Forms of Chromium
3.1.1. Effect of Chromium Chloride (CrCl3 · 6H2O)
Figure 1 illustrates the effect of CrCl3 [Cr (III)] on the activity of yoghurt culture in MRS media as estimated by a reduction in pH (A) and an increase in absorbance (B). We did not notice any significant difference in the activity of the starter culture compared to the control as the pH gradually decreased with a continuous increase in absorbance during the 10 h incubation period. These findings indicate that the starter culture bacteria were not affected by up to 5 ppm of CrCl3.
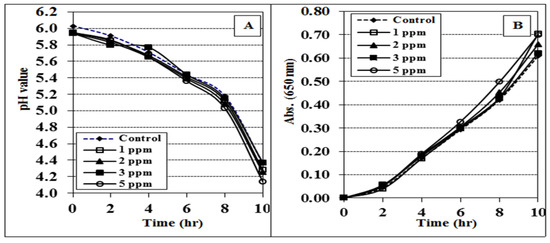
Figure 1.
Effect of chromium chloride (CrCl3) on the pH (A) and absorbance (B) progress of MRS broth media inoculated with yoghurt culture and incubated at 40 °C for 10 h. The data are shown as mean ± SE for three replicates.
3.1.2. Effect of Potassium Chromate (K2CrO4)
Figure 2 shows the effect of the hexavalent chromium (K2CrO4) on the growth of the yoghurt starter culture. As expected, the Cr (VI) at 2 ppm completely suppressed the activity of the yoghurt culture, as shown by pH reduction and absorbance development of the MRS media. Due to its high water solubility, mobility, and ease of reduction, Cr (VI) is 100 times more hazardous than Cr (III) in case of acute and chronic exposures [49,50].
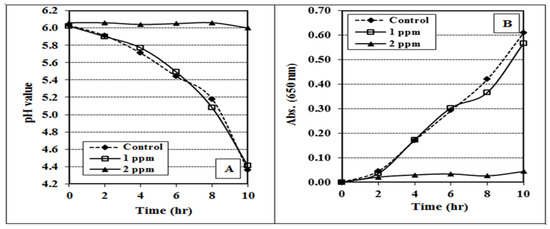
Figure 2.
Effect of potassium chromate (K2CrO4) on the pH (A) and absorbance (B) progress of MRS broth media inoculated with yoghurt culture and incubated at 40 °C for 10 h. The data are shown as mean ± SE for three replicates.
The effect of 1, 2, 5, 10, and 20 ppm of Cr (CrPi) on the growth of yoghurt starter culture in MRS media for 10 h was indicated by the absorbance and acidity development (pH) medium as shown in Figure 3A,B, respectively.
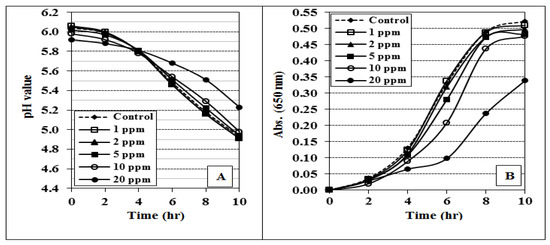
Figure 3.
Effect of chromium picolenate (CrPic) on the pH (A) and absorbance (B) progress of MRS broth media inoculated with yoghurt culture and incubated at 40 °C for 10 h. The data are shown as mean ± SE for three replicates.
We did not observe any significant effect on the bacterial growth in the media fortified with up to 5 ppm of Cr (CrPi) compared with the control. However, adding 10 or 20 ppm Cr into the media retarded the bacterial growth in the starter culture. Notably, the RDA of Cr does not exceed 35 µg. For manufacturing yoghurt, 0.5 ppm (0.5 µg/mL of milk) of CrPi is added to the milk, making its total amount in 100 mL 50 µg, fulfilling the RDA. Therefore, adding 0.5 ppm or less to the milk is not expected to negatively affect the growth of the starter bacterial culture while manufacturing yoghurt.
3.2. Growth of Yoghurt Culture in CrPi Fortified Milk
Figure 4 shows the reduction in pH during fermentation as an indicator of the yoghurt culture activity during the incubation of CrPi-fortified milk. It indicates that adding Cr (CrPic) at 0.25 µg (T1) or 0.50 µg (T2)/mL of milk did not affect the activity of the bacterial culture compared to the control. Thus, insignificant differences were observed in the measured pH values found among all treatments during fermentation till it reached pH 4.6 after 3.5 h.
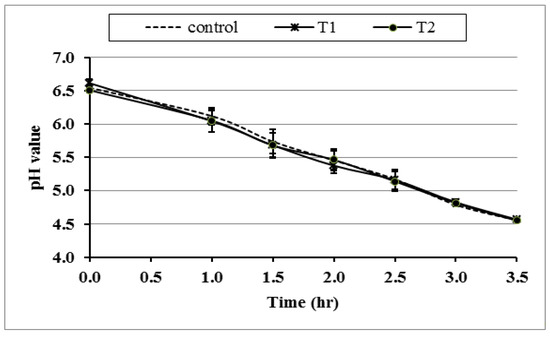
Figure 4.
Effect of different concentrations of CrPi on the pH of the media during yoghurt fermentation. Control: without Cr (CrPi), T1: with 0.25 ppm Cr (CrPi), T2: fortified with 0.5 ppm Cr (CrPi). The data are shown as mean ± SE for three replicates.
Figure 5 shows the effect of the storage period on the acidity development of CrPi-fortified yoghurt. The pH values (Figure 5A) did not differ significantly due to the applied treatments (T1 or T2), whether they were fresh or cold-stored for up to 2 weeks. Meanwhile, the pH values of all groups decreased significantly during storage. Consistent with these results (Figure 5B), all the tested fresh samples had almost the same acidity values (p > 0.05), while those stored in the cold for a week showed a slight increase (p > 0.05) in acidity. Whereas the acidity significantly increased by increasing the storage period of the yoghurt samples to 2 weeks without any significant differences among the treatments. Previous studies have also reported an increase in the acidity of the yoghurt during storage [51,52], which then decreases during the storage period [53].
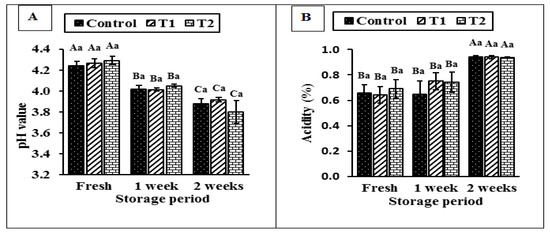
Figure 5.
Effect of different concentrations of chromium (CrPi) on the pH (A) and acidity (B) of yoghurt during storage. Control without Cr (CrPi), T1 with 0.25 ppm Cr (CrPi), T2 fortified with 0.5 ppm Cr (CrPi). Data are shown as mean ± SE for three replicates. Means with different small letters are significantly different between the treatments (p ≤ 0.05). Means with different capital letters are significantly different between the storage periods (p ≤ 0.05).
The data illustrated in Figure 6 represent the changes in the acetaldehyde content of yoghurt during storage after adding different concentrations of Cr (CrPi). While the addition of CrPi clearly did not affect the acetaldehyde content of fresh and stored samples, it sharply decreased (p ≤ 0.05) during the storage period and was at its lowest after 2 weeks. This is consistent with Hussein et al. (2011) [54], who stated that the acetaldehyde content of yoghurt decreased progressively during storage, possibly due to the conversion of acetaldehyde to other organic molecules, such as ethanol or diacetyl [55].
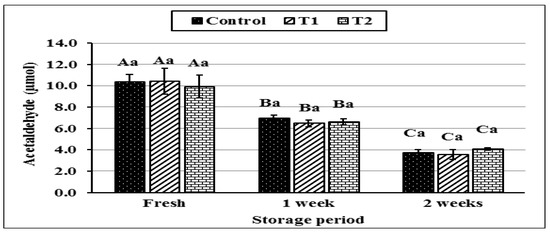
Figure 6.
Acetaldehyde of yoghurt during storage period as affected by adding different concentrations of chromium (CrPic). Control: without Cr (CrPi), T1: with 0.25 ppm Cr (CrPi), T2: fortified with 0.5 ppm Cr (CrPi). Data are mean ± SE for three replicates. Means with different small letters are significantly different between the treatments (p ≤ 0.05). Means with different capital letters significantly differ between the storage periods (p ≤ 0.05).
3.3. Physical Properties of Yoghurt
3.3.1. Yoghurt Viscosity
Figure 7 shows the viscosity of the yoghurt during cold storage. We observed comparable viscosity values among all the treated samples. This was expected as there was no difference in the chemical composition (such as dry matter and fat content) in all the treatments except for the added Cr, which did not affect the acidity development, namely the gel formation and firmness, which are the main factors affecting yoghurt viscosity [56].
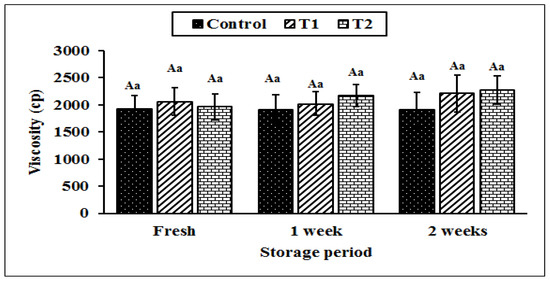
Figure 7.
The effect of different concentrations of chromium (CrPic) on the viscosity of yoghurt during storage. Control without Cr (CrPi), T1 with 0.25 ppm Cr (CrPi), T2 fortified with 0.5 ppm Cr (CrPi). Data are mean ± SE for three replicates. Means with different small letters are significantly different between the treatments (p ≤ 0.05). Means with different capital letters significantly differ between the storage periods (p ≤ 0.05).
Figure 8A,B show the WHC and wheying-off, respectively, of the yoghurt treated with or without Cr (CrPi). The results revealed no significant difference in the percentage of WHC and wheying-off of the fresh and cold-stored yoghurt in the treated samples. Moreover, the storage period had no significant effect on both properties.
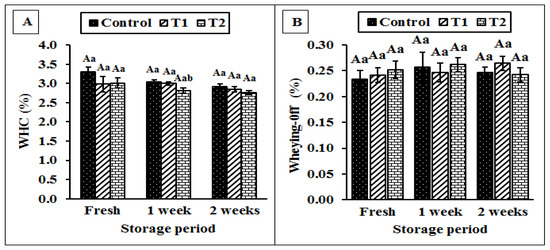
Figure 8.
Effect of adding different concentrations of chromium (CrPic) on the wheying-off (%) (A) and water holding capacity (%) (B) of yoghurt during storage. Control: without Cr (CrPi), T1: with 0.25 ppm Cr (CrPi), T2: fortified with 0.5 ppm Cr (CrPi). Data are shown as mean ± SE for three replicates. Means with different small letters are significantly different between the treatments (p ≤ 0.05). Means with different capital letters significantly differ between the storage periods (p ≤ 0.05).
3.3.2. Microbiological Analysis
To evaluate the effect of CrPi on the starter culture and microbial composition of the yoghurt, the colony forming units (CFUs)/g of S. thermophilus and L. bulgaricus, TBC, yeast, and mould counts were determined for all the treatments (Table 1). The results revealed no significant differences in the TBC of the fresh samples. However, increasing the storage period slightly increased the counts, although the difference was still insignificant.

Table 1.
The effect of adding different concentrations of chromium (CrPi) on the microbial composition of yoghurt during storage.
We found a similar trend in the Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus counts in the fresh and stored samples from all treatments, indicating no effect on the bacterial count. While the fresh samples were free of yeast and mould, their counts increased significantly after a week of cold storage and reached 3.72, 4.02, and 3.47 for control, T1, and T2, respectively, which remained stable till the end of the storage period. Çon et al. (1996) reported that the storage time did not significantly affect the total plate count of the yoghurt. Another previous study [57] reported that the refrigerated storage of yoghurt inhibited the growth of lactic acid bacteria; however, Ibrahim et al. (1989) [58] observed elevated yeast and mould counts, at approximately 3 log CFU/g, after 7 days of storage. Therefore, the in vitro effect of CrPi observed on the yoghurt starter culture using MRS media (Figure 1) is much lower than that seen in other in vitro experiments, possibly due to the amount of Cr used. Several studies reported that some lactic acid bacterial strains, such as Pediococcus acidilactici, Lactobacillus plantarum MF042018, Lactobacillus plantarum, and Lactobacillus rhamnosus can tolerate Cr [59,60,61].
3.3.3. Sensory Evaluation
Figure 9 shows the sensory properties of fresh (A) and after one (B) and two weeks (C) of cold storage. All the measured attributes were almost similar among all treatments without any deleterious effect during storage. As the concentration of CrPi added (0.25–0.5 ppm) to the milk for yoghurt production is extremely low, it did not affect the taste of the final product. Expectedly, we did not notice any abnormal or off-flavours in the CrPi-fortified yoghurt compared to the control. Unfortunately, no data are available regarding using CrPi as a supplement in yoghurt or other dairy products.
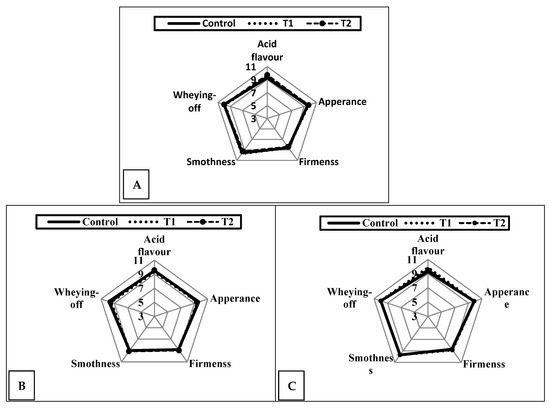
Figure 9.
Effect of chromium (CrPic) on the sensory properties of fortified yoghurt when fresh (A) and after cold storage for a week (B) and 2 weeks (C). Control: without Cr (CrPi), T1: with 0.25 ppm Cr (CrPi), T2: fortified with 0.5 ppm Cr (CrPi). Data are shown as the mean of three replicates.
4. Conclusions
The obtained results confirm that CrPi can be potentially used as a Cr source to fortify yoghurt at a dose of 0.25–0.5 ppm. At this concentration, CrPi did not affect the activity of the used culture and the chemo-physical, microbiological, and sensory properties of the treated samples, which were similar to the control (untreated sample). Therefore, Cr-fortified yoghurt might be used as an alternative to the commercially available pharmaceutical Cr supplements. This will facilitate the expansion of therapeutic nutrition either for special cases or cover the daily needs of healthy individuals.
Author Contributions
M.A.Z., E.G.B., and S.S. conceptualized the research idea and methodology design, supervised the work, performed data analysis, and interpreted the results. E.K.E., A.A.H., and A.A. were involved in the methodology, and they drafted, prepared, and revised the manuscript for publication. All the authors read and approved the final manuscript. The funding agents had no role in data collection and analysis, decision to publish, or manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Taif University Researchers Supporting Program (Project number: TURSP-2020/151), Taif University, Saudi Arabia for support.
Institutional Review Board Statement
This study was conducted with the approval of the ethical committee of the Faculty of Agriculture, Kafrelsheikh University and the Institutional Review Board Number KFS-2021/7.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Russell, R.; Beard, J.L.; Cousins, R.J.; Dunn, J.T.; Ferland, G.; Hambidge, K.; Yates, A.A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Institute of Medicine/Food and Nutrition Board, National Academy Press: Washington, DC, USA, 2001.
- Hua, Y.; Clark, S.; Ren, J.; Sreejayan, N. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 2012, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. The biochemistry of chromium. J. Nutr. 2000, 130, 715–718. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for chromium. EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef]
- NIH. Fact Sheet by the National Institutes of Health (NIH); Office of Dietary Supplements (ODS): Bethesda, MD, USA, 2022.
- Costello, R.B.; Dwyer, J.T.; Merkel, J.M. Chromium supplements in health and disease. In The Nutritional Biochemistry of Chromium (III); Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–249. [Google Scholar]
- EFSA. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011, 9, 34. [Google Scholar] [CrossRef]
- Ross, A.C.C.; Benjamin, H.; Cousins, R.J.; Tucker, K.L. Modern Nutrition in Health and Disease, 11th ed.; Wolters Kluwer Health Adis (ESP): London, UK, 2012; 1616p. [Google Scholar]
- Hamilton, E.M.; Young, S.D.; Bailey, E.H.; Watts, M.J. Chromium speciation in foodstuffs: A review. Food Chem. 2018, 250, 105–112. [Google Scholar] [CrossRef]
- Kozlovsky, A.S.; Moser, P.B.; Reiser, S.; Anderson, R.A. Effects of diets high in simple sugars on urinary chromium losses. Metabolism 1986, 35, 515–518. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles: Atlanta, GA, USA, 2012.
- ATSDR. Toxicological Profile for Chromium; U.S. Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 1998.
- USEPA. Integrated Risk Information System (IRIS) on Chromium VI.; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999.
- USEPA. Integrated Risk Information System (IRIS) on Chromium III.; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999.
- Suksomboon, N.; Poolsup, N.; Yuwanakorn, A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014, 39, 292–306. [Google Scholar] [CrossRef]
- Pala, R.; Sari, M.A.; Erten, F.; Er, B.; Tuzcu, M.; Orhan, C.; Deeh, P.B.D.; Sahin, N.; Cinar, V.; Komorowski, J.R. The effects of chromium picolinate on glucose and lipid metabolism in running rats. J. Trace Elem. Med. Biol. 2020, 58, 126434. [Google Scholar] [CrossRef]
- Vincent, J.B. Chromium. In Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.Y., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 457–465. [Google Scholar]
- Vincent, J.B. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 2003, 33, 213–230. [Google Scholar] [CrossRef]
- Fernandez-Pol, J.; Johnson, G.S. Selective toxicity induced by picolinic acid in simian virus 40-transformed cells in tissue culture. Cancer Res. 1977, 37, 4276–4279. [Google Scholar]
- Grant, R.; Coggan, S.; Smythe, G.A. The physiological action of picolinic acid in the human brain. Int. J. Tryptophan Res. 2009, 2, S2469. [Google Scholar] [CrossRef]
- Li, Y.-C. Chromium and Yogurt Effects on Glucose, Insulin and Hepatic Lipid In Obese Mice. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 1984. [Google Scholar]
- Leba, H.; Yulianto, W.A.; Pujimulyani, D. Hypoglycemic and Antioxidative Effects of Chromium, Magnesium, and Cinnamon Fortified Parboiled Rice on Diabetic Rats. J. Funct. Food Nutraceutical 2022, 4, 49–55. [Google Scholar] [CrossRef]
- Yulianto, W.A.; Suryani, C.L.; Susiati, A.; Luwihana, S. Evaluation of chromium fortified-parboiled rice coated with herbal extracts: Resistant starch and glycemic index. Int. Food Res. J. 2018, 25, 2608–2613. [Google Scholar]
- Yanto, H.; Junianto, J.; Rostika, R.; Andriani, Y.; Iskandar, I. Addition of Chromium (Cr+ 3) in the diets containing fermented yellow corn meal on jelawat, Leptobarbus hoevenii. Nusant. Biosci. 2017, 9, 214–219. [Google Scholar] [CrossRef]
- Swelam, S.; Zommara, M.A.; Abd El-Aziz, A.E.-A.M.; Elgammal, N.A.; Baty, R.S.; Elmahallawy, E.K. Insights into Chufa Milk Frozen Yoghurt as Cheap Functional Frozen Yoghurt with High Nutritional Value. Fermentation 2021, 7, 255. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Radwan, H.A.; Elmeligy, A.A.; Hafiz, A.A.; Albrakati, A.; Elmahallawy, E.K. Production of a Yogurt Drink Enriched with Golden Berry (Physalispubescens L.) Juice and Its Therapeutic Effect on Hepatitis in Rats. Fermentation 2022, 8, 112. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Alrashdi, B.M.; Ramadan, M.F.; Abd El-Sattar, E.S.; Siam, A.A.H.; Alblihed, M.A.; Elmahallawy, E.K. Effect of Fermented Camel Milk Containing Pumpkin Seed Milk on the Oxidative Stress Induced by Carbon Tetrachloride in Experimental Rats. Fermentation 2022, 8, 223. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; Alrashdi, B.M.; Hassan, M.A.A.; Alblihed, M.A.; Dahran, N.; Ali, F.A.Z.; Elmahallawy, E.K. Effects of Fermented Camel Milk Supplemented with Sidr Fruit (Ziziphus spina-christi L.) Pulp on Hyperglycemia in Streptozotocin-Induced Diabetic Rats. Fermentation 2022, 8, 269. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; El-Zahar, K.M.; Elmaadawy, A.A.; Hijazy, H.H.A.; Sitohy, M.Z.; Albrakati, A.; Elmahallawy, E.K. Remedial Action of Yoghurt Enriched with Watermelon Seed Milk on Renal Injured Hyperuricemic Rats. Fermentation 2022, 8, 41. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Elkot, W.F.; Ateteallah, A.H.; Al-Moalem, M.H.; Shahein, M.R.; Alblihed, M.A.; Abdo, W.; Elmahallawy, E.K. Functional, Physicochemical, Rheological, Microbiological, and Organoleptic Properties of Synbiotic Ice Cream Produced from Camel Milk Using Black Rice Powder and Lactobacillus acidophilus LA-5. Fermentation 2022, 8, 187. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Elkot, W.F.; Hijazy, H.H.A.; Kassab, R.B.; Alblihed, M.A.; Elmahallawy, E.K. The Impact of Date Syrup on the Physicochemical, Microbiological, and Sensory Properties, and Antioxidant Activity of Bio-Fermented Camel Milk. Fermentation 2022, 8, 192. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; Radwan, H.A.; Mohammed, N.S.; Aloraini, M.A.; Albezrah, N.K.A.; Alharbi, M.A.; Sayed, H.H.; Daoud, M.A.; Elmahallawy, E.K. Antimicrobial Activity of Some Plant Extracts and Their Applications in Homemade Tomato Paste and Pasteurized Cow Milk as Natural Preservatives. Fermentation 2022, 8, 428. [Google Scholar] [CrossRef]
- Shahein, M.R.; Elkot, W.F.; Albezrah, N.K.A.; Abdel-Hafez, L.J.M.; Alharbi, M.A.; Massoud, D.; Elmahallawy, E.K. Insights into the Microbiological and Physicochemical Properties of Bio-Frozen Yoghurt Made with Probiotic Strains in Combination with Jerusalem Artichoke Tubers Powder. Fermentation 2022, 8, 390. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.-S.H.; Babalghith, A.O.; Alrashdi, B.M.; Radwan, H.A.; Umair, M.; Abdalmegeed, D.; Mahfouz, H.; Dahran, N.; Cacciotti, I.; et al. Impact of Incorporating the Aqueous Extract of Hawthorn (C. oxyanatha) Leaves on Yogurt Properties and Its Therapeutic Effects against Oxidative Stress Induced by Carbon Tetrachloride in Rats. Fermentation 2022, 8, 200. [Google Scholar] [CrossRef]
- Press, R.I.; Geller, J.; Evans, G.W. The effect of chromium picolinate on serum cholesterol and apolipoprotein fractions in human subjects. West J. Med. 1990, 152, 41–45. [Google Scholar]
- Loualeche, H.; Bracquart, P.; Saulnier, F.; Desmazeaud, M.; Linden, G. Carbon dioxide effects on the growth and metabolites of morphological variants of Streptococcus thermophilus. J. Dairy Sci. 1993, 76, 3683–3689. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology, 2nd ed.; Woodhead Publishing Limited: London, UK, 1999. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Lees, G.J.; Jago, G.R. Methods for the estimation of acetaldehyde in cultured dairy products. Aust. J. Dairy Tech. 1969, 24, 181–183. [Google Scholar]
- Lucey, J.A.; Munro, P.A.; Singh, H. Whey separation in acid skim milk gels made with glucono-δ-lactone: Effects of heat treatment and gelation temperature. J. Texture Stud. 1998, 29, 413–426. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G. Production and evaluation of some physicochemical parameters of peanut milk yoghurt. LWT Food Sci. Tech. 2009, 42, 1132–1138. [Google Scholar] [CrossRef]
- Difco Laboratories. Difco Manual of Dehydrated Culture Media and Reagent for Microbiological Clinical Laboratory Procedures, 11th ed.; Difco Laboratories: Detroit, MI, USA, 1971. [Google Scholar]
- Kaszab, T.; Szigeti, F.; Bodor, Z.; Zaukuu, J.; Rashed, M.; Slavchev, A.; Kovacs, Z. Monitoring of probiotic and non probiotic Lactobacillus strains’ growth by different physico-chemical parameters. In Researched Risk Factors of Food Chain; Szent István Egyetemi Kiadó: Gödöllő, Hungary, 2018; pp. 61–66. [Google Scholar]
- Terzaghi, B.E.; Sandine, W.E. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 1975, 29, 807–813. [Google Scholar] [CrossRef]
- Frank, J.F.; Christen, G.L.; Bullerman, L.B. Tests for groups of microorganisms. In Standard Methods for the Examination of Dairy Products, 16th ed.; Marshall, R.T., Ed.; American Public Health Association (APHA): Washington, DC, USA, 1992; pp. 271–286. Available online: https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/ (accessed on 15 October 2022).
- Fernández-Garía, E.; McGregor, J.U.; Traylor, S. The addition of oat fiber and natural alternative sweeteners in the manufacture of plain yogurt. J. Dairy Sci. 1998, 81, 655–663. [Google Scholar] [CrossRef]
- SPSS. SPSS for Windows. Statistical Package for Social Studies Software (SPSS, 2016), version 24; IBM Corp.: Armonk, NY, USA, 2016. [Google Scholar]
- Liang, J.; Huang, X.; Yan, J.; Li, Y.; Zhao, Z.; Liu, Y.; Ye, J.; Wei, Y. A review of the formation of Cr (VI) via Cr (III) oxidation in soils and groundwater. Sci. Total Environ. 2021, 774, 145762. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Badran, S.; Dawood, I.; El-Assar, M. Evaluation of fermented camel milk prepared using yoghurt starter. In Proceedings of the 9th Egyptian conference for Dairy Science and Technology, International Agriculture Centre, Cairo, Egypt, 9–11 October 2004; pp. 9–11. [Google Scholar]
- Chramostova, J.; Mošnová, R.; Lisova, I.; Pešek, E.; Drbohlav, J.; Němečková, I. Influence of cultivation conditions on the growth of Lactobacillus acidophilus, Bifidobacterium sp., and Streptococcus thermophiles, and on the production of organic acids in fermented milks. Czech J. Food Sci. 2014, 32, 422–429. [Google Scholar] [CrossRef]
- Rafiq, L.; Zahoor, T.; Sagheer, A.; Khalid, N.; Rahman, U.; Liaqat, A. Augmenting yoghurt quality attributes through hydrocolloidal gums. Asian-Australas J. Anim. Sci. 2020, 33, 323. [Google Scholar] [CrossRef]
- Hussein, M.M.; Hassan, F.A.M.; Abdel Daym, H.H.; Salama, A.; Enab, A.K.; Abd El-Galil, A.A. Utilization of some plant polysaccharides for improving yoghurt consistency. Ann. Agri. Sci. 2011, 56, 97–103. [Google Scholar] [CrossRef]
- El-Loly, M.; El-Hofi, M. Effect of fortification with zinc and iron on the properties of buffaloes acidophilus milk. J. Agric. Sci. Mansoura Univ. 1999, 24, 5757–5767. [Google Scholar]
- Fetahagic, S.; Denin-Djurdjevic, D.J.; Jovanovic, T.S.; Macej, D.O. Influence of selected factors on the viscosity of set style yogurt and acid casein gel at constant speed of spindle rotation. J. Agri. Sci. 2004, 49, 233–250. [Google Scholar] [CrossRef][Green Version]
- Muhammad, B.F.; Abubakar, M.M.; Adegbola, T.A. Effect of period and condition of storage on properties of yoghurt produced from cow milk and soymilk materials. Res. J. Dairy Sci. 2009, 3, 18–24. [Google Scholar]
- Ibrahim, M.K.E.; EI-Batawy, M.A.; Girgis, E.S. Evaluation of yogurt on the Cairo market. Egyptian J. Dairy Sci. 1989, 17, 125–136. [Google Scholar]
- Lytras, G.; Lytras, C.; Argyropoulou, D.; Dimopoulos, N.; Malavetas, G.; Lyberatos, G. A novel two-phase bioreactor for microbial hexavalent chromium removal from wastewater. J. Hazard. Mater. 2017, 336, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the heavy metal bioremediation efficiency of the novel marine lactic acid bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadede, L.T.; Hasan, S.K.; Authman, S.H.; Nassri, K.S. Bioremediation ability of lactobacillus strains to some of heavy metals. Plant Arch. 2019, 19, 1705–1710. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).