Abstract
Aspartic acid, or “aspartate,” is a non-essential, four carbon amino acid produced and used by the body in two enantiomeric forms: L-aspartic acid and D-aspartic acid. The L-configuration of amino acids is the dominant form used in protein synthesis; thus, L-aspartic acid is by far the more common configuration. However, D-aspartic acid is one of only two known D-amino acids biosynthesized by eukaryotes. While L-aspartic acid is used in protein biosynthesis and neurotransmission, D-aspartic acid is associated with neurogenesis and the endocrine system. Aspartic acid production and use has been growing in recent years. The purpose of this article is to discuss various perspectives on aspartic acid, including its industrial utility, global markets, production and manufacturing, optimization, challenges, and future outlook. As such, this review will provide a thorough background on this key biochemical.
1. Industrial Utility
In addition to its biofunctionality, aspartic acid has wide application in the food, beverage, pharmaceutical, cosmetic, and agricultural industries [1]. L-aspartic acid is used as a nutritional supplement in both functional foods and beverages, but its primary use is in combination with the amino acid phenylalanine which together make aspartame, an artificial sweetener [2]. Aspartic acid is also used to bolster immune function and as a natural combatant to depression [1]. Its ability to aid in energy production, fatigue resistance, RNA and DNA synthesis, and liver detoxification give it broad clinical use [1]. Additionally, it is used as an intermediary substrate in the manufacture of pharmaceuticals and organic chemicals, serving as the building block molecule for active pharmaceutical ingredients [1]. Aspartic acid’s utility stretches further upon consideration of its derivatives including acetyl aspartic acid, used as an active ingredient in anti-aging cosmetics that target wrinkling, skin lifting, and loss of firmness [3]. It is also used to produce polyaspartic acid, a fertilizer synergist which increases both nitrogen absorption and crop yields [4]. Polyaspartic acid hydrogels are a type of biodegradable superabsorbent polymer which have exceptional water-holding abilities and are used in the production of many modern amenities including diapers, feminine products, and engineered tissue [5]. The range and depth of aspartic acid’s applicability, in particular the L-configuration, has placed it on the Department of Energy’s Top Value Added Chemicals from Biomass list [2].
2. Global Markets
The global aspartic acid market is a highly fractionated market meaning it consists of several small company players rather than large conglomerates, yet it is growing with significant potential for industrial relevance [6]. According to a 2015 report by Grand View Research, the global aspartic acid market is projected to reach $101 million with a market demand of 60.6 kilotons by 2022 which represents a compound annual growth rate of 5.6% [6]. As of 2014, the baseline year of said report, polyaspartic acid represented 22.6% of the total aspartic acid market volume making it the largest market segment, seconded by aspartame [6]. Both aspartic acid derivatives are anticipated to increase in demand as polyaspartic biodegradable polymers replace polyacrylic acid in agriculture, water treatment, and the petrochemical industries and as food and beverage trends shift towards added sugar labeling and health-conscious, convenience foods [6]. Of all aspartic acid market sectors, the medical sector is projected to grow the most as is attributed to the American healthcare system, which is housed in the largest regional market, accounting for 39.0% of total aspartic acid volume as of 2014 [6]. Internationally increased demand for aspartic acid is also expected to increase in the form of greater aspartame demand for carbonated beverages in Asia Pacific [6].
3. Production and Manufacturing
There are three main methods to produce aspartic acid: protein extraction, chemical synthesis, and enzymatic conversion [2]. The hydrolysis of protein for extraction methods produces an abundance of amino acids from which the L-aspartic acid must be separated. Chemical synthesis requires high temperature and pressure and results in a racemic mixture, producing both L- and D-isomers thereby requiring the additional processing steps of optical resolution and racemization to achieve the preferred L-isomer [1]. Thus, enzymatic conversion is the currently favored route of production. The enzymatic conversion process exists in two forms: simple enzyme-substrate interaction (hereafter referred to as “enzymatic conversion”) or whole-cell enzymatic conversion, i.e., fermentation. Table 1 summarizes various economic and technical aspects of the production of aspartic acid.
Stereospecific, industrial production of L-aspartic acid currently utilizes a one-step reaction of fumaric acid, in the presence of high concentrations of ammonia, to L-aspartic acid via L- aspartate ammonia-lyase, an enzyme also referred to as “L-aspartase” (Figure 1) [7]. L-aspartase can be purified and immobilized in a gel matrix for continuous production or overexpressed in bacterial cells bound to polyurethane carriers [7]. Production via immobilized enzymatic conversion or bacterial fermentation utilizes the same enzyme and substrate; however, enzymatic production is favored for its high product concentration, productivity, minimal byproducts, and the ease of downstream processing [2]. Yet, fermentative production, albeit less productive, has been around since the 1950s when research and development into the production of aspartic acid began [8].
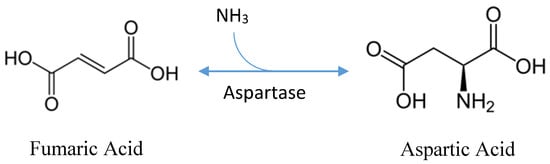
Figure 1.
Chemical mechanism of aspartic acid production (based, in part, on [8]).

Table 1.
Summary of Aspartic Acid Supply Chain, Economic and Technological Considerations for Aspartic Acid Production.
Table 1.
Summary of Aspartic Acid Supply Chain, Economic and Technological Considerations for Aspartic Acid Production.
| Category | Summary |
|---|---|
| Industrial importance and potential of biochemical | Aspartic acid is used in the food, beverage, pharmaceutical, cosmetic, and agricultural industries. The global aspartic acid market is projected to reach $101 million with a market demand of 60.6 kilotons by 2022 representing a compound annual growth rate of 5.6% [6]. |
| Industrial uses for biochemical | Aspartic acid is used in the production of: nutritional (amino acid) supplements; artificial sweetener (aspartame); polyaspartic acid hydrogels; and acetyl aspartic acid, the active ingredient in anti-aging cosmetics. |
| Substrates used for the production of biochemical | primary substrate: fumaric acid [7] cofactor: ammonia [7] enzyme: L-aspartate ammonia-lyase [7] |
| Microorganisms used for fermentation | Primary industrial species:E. coli and Cornybacterium glutamacium [9] Exploratory species [1]: Pseudomonas aeroginosa Pseudomonas fluorescens Candida hydrocarbofumarica Bacillus stearothermophilus Bacillus subtilis |
| What enzymes are needed to break down the substrate for fermentation | Fumaric acid used in aspartic acid production does not need to be broken down, rather, it is fermentatively produced from glucose or chemically produced from maleic anhydride [6]. |
| Fermentation conditions used: pH, substrate loading, temperatures, times, maximum yield, maximum fermentation rates | pH is initialized to 7.0 [10] substrate concentration: 1:1 or 1:2 ammonia to fumaric acid [10] time 2 to 10 days [10] temperature 27–40 °C [10] yield 77–95% (w/w of fumaric acid) depending on bacterial strain and fermentation conditions [10] |
| Separation equipment, conditions, efficiencies | batch fermentation: separation via anion exchange column and crystallization [10] continuous fermentation: separation via isoelectric point precipitation and crystallization [10] |
| Total energy used to produce this chemical | Data not currently published. |
| Estimated costs to produce this chemical | Cost as well as upstream and downstream raw materials and equipment analysis available in the global L-aspartic acid market report provided by Market Watch (2019), at https://www.researchreportsworld.com/purchase/14314090 (accessed on 21 February 2021) |
| Current aspartic acid manufacturers | The following companies are the top industrial producers of aspartic acid [11]; the corresponding links, when applicable, are to each respective company’s product information page. Ajinomoto Group https://www.ajiaminoacids.com/product/l-aspartic-acid (accessed on 21 February 2021) Evonik https://healthcare.evonik.com/product/health-care/en/products/pharmaceutical-amino-acids/REXIM/pages/parenteralnutrition.aspx?xd_co_f=M2Q2OWQ5N2ItYTZkOC00ZWZjLThjNmUtODFiYjQ3YmYwM2I2 (accessed on 21 February 2021) KYOWA http://www.kyowahakko-bio.co.jp/english/products/aminoacids/l_aspartic_acid/ (accessed on 21 February 2021) Jinghai Amino Acid http://en.chinaaminoacid.com/products/L-AsparticAcid.shtml (accessed on 21 February 2021) JIRONG PHARM Not currently available OR product catalogue not in English Siwei Amino AcidEnglish product description not available Zhangjiagangxingyu Technology http://www.zjgxykj.com/template/p13e.html (accessed on 21 February 2021) Hubei Bafeng Pharmaceutical Company page not accessible in English |
| Potential market segments, sales, etc. | The aspartic acid market is segmented into six market categories: Feed Supplements, Medicine, Polyaspartic Acid, Aspartame, L-Alanine, and “Others” [6]. The report summary states that polyaspartic acid represents 22.6% of the total market volume in 2014. Market volumes and revenue values available upon report purchase [11]. |
| Primary economic setbacks and challenges | Fermentative production competes economically with petroleum-derived production. Economic setbacks of aspartic acid include high fumaric substrate cost and the low yields currently achieved by switching to cheaper sugar-based feedstocks [10]. Crystallization utilized in downstream processing separations can be expensive and time-consuming [1]. |
| Technological setbacks and challenges | The fermentative production of aspartic acid from glucose or sugar-based feedstocks, both much cheaper and more available substrates than fumarate, currently generate much poorer yields, i.e., 95% versus 29% [8]. Thus, the main technological setback to more economical aspartic acid production is the ability to directly ferment sugar to L-aspartic acid. |
| Side products, byproducts, waste products and associated cost | Organisms whose genomes also code fumarase (e.g., C. glutamicum, E. coli) produce malic acid from fumarate as a byproduct in effect utilizing substrate and decreasing aspartic acid yield. Without heat treatments Tajima et al. (2015) lost 25% of the fumaric acid substrate to malic acid production which translates to significant yield losses [7]. |
| Downstream processing operations | L-aspartic acid can be separated from the culture broth or eluate in batch systems via ion exchange resins utilizing an anion exchange column followed by crystallization of the eluate [1]. Continuous systems can extract the L-aspartic acid via isoelectric point precipitation (adjust broth pH to 2.8) followed by crystallization [10]. |
| New technologies, strains, equipment developments | Membrane reactor systems (MRS), as they are currently being developed, utilize growth-arrested cells eliminating the need for cell or enzyme immobilization [8]. The MRS system employed by Yukawa et al. (2009) overcomes the low mass transfer rates and low volumetric productivity issues associated with immobilization systems and simplifies the overall production process, allowing for easier separation of cells from the reaction mixture and generating high yield and productivity during long periods of operation [8]. Genetic modification of the metabolic pathways and feedback regulators within E. coli and C. glutamicum, the two major strains involved in industrial amino acid synthesis, are the next steps in improving L-aspartic acid production via the development of new, high-producing strains. |
While several species of bacteria including select Pseudomonas, Bacillus, and Proteus have been identified as producers of aspartic acid, E. coli and Cornybacterium glutamacium are nearly exclusively used by industry [1,9]. Fumaric acid is the primary substrate in L-aspartic acid production; however, maleate, a less expensive feedstock, can be used in a two-step reaction which uses maleate isomerase to convert maleate to fumaric acid which is then converted via L-aspartase to L-aspartic acid in the presence of ammonium ions [8].
The basic fermentation process, as developed and patented in the 1960s, utilizes a sugar-free medium wherein fumaric acid is the sole source of carbon subsequently minimizing the production of unwanted byproducts [10]. Ammonia, while required for catalysis, also serves as the nitrogen source and is formulated at a 1:1 or 1:2 fumaric acid-ammonia ratio [10]. Prior to inoculation, the broth pH is initialized to 7.0 and left unregulated as it will naturally increase to a 8.4–9.6 range in the initial stages of the fermentation, allowing for the production of acid [10].
Fermentation can be conducted with or without agitation for 2 to 10 days at 27–40 °C [10]. The L-aspartic acid will be extracellularly secreted and accumulate in the culture broth [10]. Several methods of downstream processing are available to separate L-aspartic acid from the culture broth or eluate. In the case of batch fermentation, ion exchange resins can be used to separate and purify the L-aspartic acid on an anion exchange column followed by crystallization of the eluate (Figure 2). For continuous fermentation, L-aspartic acid can be separated by adjusting the broth to 90 °C and a pH of 2.8 with sulfuric acid [10]. Adjusting the pH to 2.8, i.e., the isoelectric point, will cause L-aspartic acid to precipitate out of solution where it is then subjected to a two hour incubation period at 15 °C to induce protein crystallization [10]. Under these conditions, 95% of the theoretical yield of L-aspartic acid was achieved by Masahiro et al. (1965) which aligns with the 77–95% yield range achieved similar fermentation processes utilizing various bacterial strains [1,10]. Table 2 provides commonly used L-aspartic acid fermentation parameters.
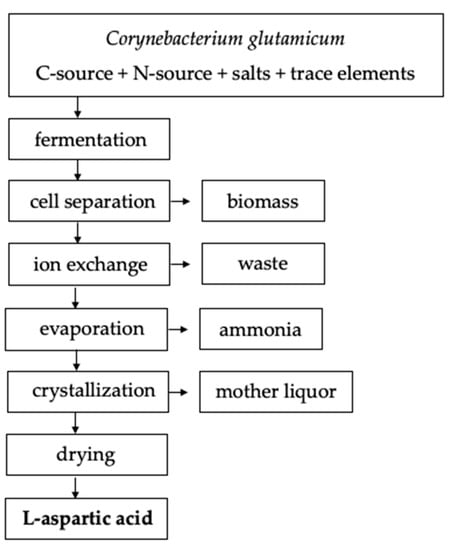
Figure 2.
Example amino acid fermentation and downstream process flowchart adapted from Leuchtenberger et al. (2005) [12].

Table 2.
Specific Production Parameters for the Fermentative Synthesis of Aspartic Acid.
4. Optimization
Traditional fermentation setups have since been adapted and optimized to increase productivity and process efficiency. Most notable is the development of continuous systems such as the one employed by Tosa et al. (1973) using immobilized E. coli on a cell column. Tosa et al. found production to be optimal at pH 8.5, 50 °C, and a maximized media flow rate of space velocity, i.e., the ratio of volumetric flow rate to reactor volume, of 0.8 [16]. Further process improvement included fortification of the fermentation medium with divalent cations (Mg+2, Mg+2, Ca+2) as was found to protect cells from heat inactivation subsequently increasing the stability of the column [16].
More recently, membrane reactor systems (MRS) are being developed, the use of growth-arrested cells eliminating the need for immobilization (Figure 3) [8]. The MRS system employed by Yukawa et al. (2009) overcomes the low mass transfer rates and low volumetric productivity issues associated with immobilization systems and simplifies the overall process, allowing for easier separation of cells from the reaction mixture and generating high yield and productivity during long periods of operation [8]. While industrial L-aspartic acid production began with immobilized enzymes or cells, it has progressed to include both fermentative and membrane reactor systems [8].
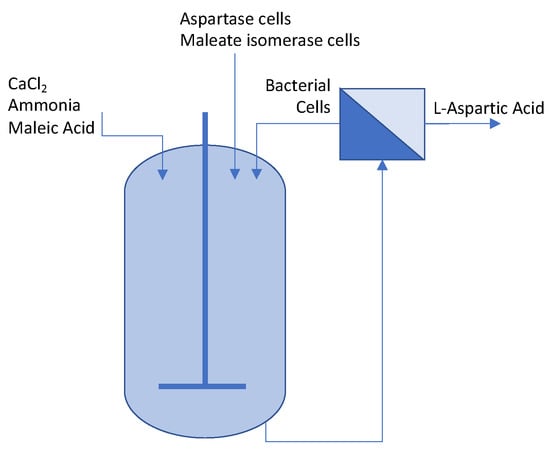
Figure 3.
Example membrane reactor system for continuous production of aspartic acid (adapted from [8]).
5. Challenges
According to the U.S. Department of Energy, the major challenges confronting the development of biomass-generated L-aspartic acid as a major chemical building block for the synthesis of multiple high-value biochemicals and materials include: the development of an economically comparable, direct fermentation process from sugar to L-aspartic acid or reduction in the cost of fumaric acid for current production methods [2].
Fermentable sugars are the target substrate for L-aspartic acid production due to their abundance and low cost but are not yet realizable due to the low yields currently achieved [8]. Production of L-aspartic acid from fumaric acid is a one-step reaction and is currently preferred for its higher yields, achieving over 95% yield as compared to 29% from fermentable sugars [8]. While the market price and operational expenses of L-aspartic acid production and its substrates are not free-access, the cost reduction in transitioning from a fumaric acid substrate to glucose or other sugar from biomass represents a significant—nearly 4.5 times less expensive—cost saving as seen by comparing the 2020 retail price of PharmaGrade fumaric acid to USP grade dextrose [17]. However, such cost savings cannot be capitalized upon until improvements in the yield of sugar-based L-aspartic acid fermentation are achieved.
The fermentative synthesis of L-aspartic acid from glucose occurs via the direct conversion of oxaloacetate or fumarate, two interconvertible intermediates within the citric acid cycle, into L-aspartic acid [18]. The maximum theoretical yield based on sugar reducing equivalents from either aforementioned intermediate is 2 mol L-aspartic acid per 1 mol glucose [18]. Direct fermentation of glucose to L-aspartic acid is rarely reported within the literature and when so, the associated yields are low even for known aspartic acid producing strains, e.g., Brevibacterium flavum and Corynebacterium glutamicum were reported to produce 0.30 mol and 1.02 mol L-aspartic acid per mol glucose, respectively [18]. Work involving metabolic engineering to maximize L-aspartic acid yield on glucose, in particular within industrially relevant strains such as E. coli, is severely lacking.
Recent work by Piao et al. (2019) has demonstrated that cost-effective fermentation of L-aspartic acid from biomass is possible through the use of metabolically engineered E. coli and as such is the first work to demonstrate metabolic optimization achieving substantive yields of L-aspartic acid production from glucose [18]. Piao et al. were able to generate a final titer of 33.1 g/L for a 21 h fed-batch fermentation which correlates to a yield of 1.01 mol/mol glucose, i.e., approximately 50% of the maximum theoretical yield [18]. While successful proof-of-concept of an efficient route for the production of L-aspartic acid within E. coli, this groundbreaking work elicits the need for further studies that address two current challenges in the production of maximal stoichiometric yields of L-aspartic acid.
First, L-aspartic acid is an intermediate for a wide array of downstream metabolic reactions and the precursor molecule to over ten different metabolic pathways thereby making it difficult to accumulate large quantities of L-aspartic acid mid-metabolism [18]. Further studies need address this bottleneck through metabolic redesign that favors L-aspartic acid accumulation or the development of fermentation strategies that target production of the final L-aspartic acid derivative generated via irreversible reactions. The second challenge is to identify the key to maximum theoretical yield of L-aspartic acid via fermentative synthesis which is currently not known due to the function of its precursor molecule, oxaloacetate, as a critical molecule within several carbon metabolic pathways including glycolysis and gluconeogenesis. This missing key to comprehensive metabolic design strategy makes identification of key bottlenecks in L-aspartic acid accumulation complex and as yet, unelucidated [18]. In addition to designing an optimized metabolic pathway, optimization of a fermentation strategy, growth media, and operational parameters to support the growth and physiological metabolism of E. coli genetically modified for L-aspartic acid production needs to be developed [18].
Until such improvements are met, reducing the production cost of fumaric acid is the most economical alternative. Like aspartic acid synthesis, fumaric acid is metabolically produced from oxaloacetate which is converted to malic acid by malate dehydrogenase and then to fumaric acid via fumarase [19]. Several microbial species naturally synthesize fumaric acid, but Rhizopus, a type of saprophytic fungi, are considered the best producer for industrial scalability [19]. Engel et al. (2008) investigated fermentative fumaric acid production from glucose as compared to petroleum-based chemical synthesis. While the fermentative process yields are lower than chemical synthesis (85% w/w versus 112% w/w) and the cost effectiveness of fermentation heavily dependent on the price of both oil and glucose, the fermentation pathway shows economic potential especially considering the additional process efficiencies achievable with metabolic engineering [20]. Thus, critical developments in the production of fumaric acid particularly in regard to strain improvement, fungal morphological control, cheaper substrate utilization, as well as upstream and downstream yield and process efficiencies [19] will have a trickle-down effect upon the production of aspartic acid.
6. Summary and Future Outlook
The development of bio-based chemicals and processes represents an invaluable opportunity in the protection of natural resources, reduced reliance on limited fossil fuels, and byproduct optimization. Aspartic acid plays a role in the development of resource sufficiency as a biodegradable superabsorbent polymer, a natural source of protein, and as a renewable pharmaceutical, nutritional, and cosmetic compound. While continuous columns and membrane reactor systems have advanced aspartic acid production from where it began in the 1950s, future developments taking advantage of modern genetic modification and engineering can direct its future as a bio-based building block to meet increasing market demand. In order to be competitive against petrochemically derived aspartic acid, bio-based production calls for improvements in direct fermentative production, i.e., development of a one-step glucose to aspartic acid process; reducing the cost of fumaric acid; increasing sugar-based fermentation yields.
Author Contributions
Conceptualization, H.A. and K.A.R.; data collection and synthesis, H.A.; writing—original draft preparation, H.A.; writing—review and editing, K.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank two anonymous reviewers and the editors for helpful comments which ultimately led to an improved final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, P.; Nika, B.; Mangala, D. Production of Aspartic Acid—A Short Review. Int. J. Eng. Trends Technol. 2017, 45, 254–257. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Mavon, A. Acetyl Aspartic Acid, a Novel Active Ingredient, Demonstrates Potential to Improve Signs of Skin Ageing: From Consumer Need to Clinical Proof. Int. J. Cosmet. Sci. 2015, 37, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, A.M.; Koskan, L.P.; Strom, D.J.; Meah, A.R.Y. Kingenta. Polyaspartic Acid. US Patent US5593947A, 14 January 1997. Available online: http://en.kingenta.com/Goods/good_show/cid/30/sid/30.html (accessed on 14 February 2020).
- Meng, H.; Zhang, X.; Chen, Q.; Wei, J.; Wang, Y.; Dong, A.; Yang, H.; Tan, T.; Cao, H. Preparation of Poly(Aspartic Acid) Superabsorbent Hydrogels by Solvent-free Processes. J. Polym. Eng. 2015, 35, 647–655. [Google Scholar] [CrossRef]
- Global Aspartic Acid Market by Application (Feed Supplements, Medicine, Polyaspartic Acid, Aspartame, L-Alanine) Expected to Reach USD 101.0 Million by 2022: Grand View Research, Inc. Available online: https://www.grandviewresearch.com/press-release/global-aspartic-acid-market (accessed on 14 February 2020).
- Tajima, T.; Hamada, M.; Nakashimada, Y.; Kato, J. Efficient Aspartic Acid Production by a Psychrophile-based Simple Biocatalyst. J. Ind. Microbiol. Biotechnol. 2015, 42, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, H.; Ookino, S.; Inui, M. L-Aspartic Acid, Production Processes. In Encyclopedia of Industrial Biotechnology; Wiley: Hoboken, NJ, USA, 2010; pp. 1–3. [Google Scholar]
- Li, Y.; Wei, H.; Wang, T.; Xu, Q.; Zhang, C.; Fan, X.; Ma, Q.; Chen, N.; Xie, X. Current Status on Metabolic Engineering for the Production of l-aspartate Family Amino Acids and Derivatives. Bioresour. Technol. 2017, 245, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Masahiro, T.; Yasoji, M.; Shinji, O.; Moriyoshi, I.; Tetsuo, H. Process for Producing l-aspartic Acid. U.S. Patent US2953499A, 1965.
- L-aspartic Acid Market 2019 Global Industry Demand, Recent Trends, Size and Share Estimation by 2025 with Top Players—ResearchReportsWorld.com. Available online: https://www.marketwatch.com/press-release/l-aspartic-acid-market-2019-global-industry-demand-recent-.trends-size-and-are-estimation-by-2025-with-top-players---researchreportsworldcom-2019-06-18 (accessed on 18 June 2019).
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological Production of Amino Acids and Derivatives: Current Status and Prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chibata, I.; Tosa, T.; Sato, T. Continuous Production of L-aspartic Acid. Appl. Biochem. Biotechnol. 1986, 13, 231–240. [Google Scholar] [CrossRef]
- Szymańska, G.; Sobierajski, B.; Chmiel, A. Immobilized Cells of Recombinant Escherichia coli Strain for Continuous Production of L-aspartic Acid. Pol. J. Microbiol. 2011, 60, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Papierz, M.; Gadomska, G.; Sobierajski, B.; Chmiel, A. Selection and Activation of Escherichia coli Strains for L-aspartic Acid Biosynthesis. Pol. J. Microbiol. 2007, 56, 71. [Google Scholar] [PubMed]
- Tosa, T.; Sato, T.; Mori, T.; Chibata, I. Basic Studies for Continuous Production of L-aspartic Acid by Immobilized Escherichia coli Cells. Appl. Environ. Microbiol. 1974, 27, 886–889. [Google Scholar] [CrossRef]
- Millipore Sigma. Microbiological Culture Media. Available online: https://www.sigmaaldrich.com/united-states.html (accessed on 14 February 2020).
- Piao, X.; Wang, L.; Lin, B.; Chen, H.; Liu, W.; Tao, Y. Metabolic Engineering of Escherichia coli for Production of L-aspartate and Its Derivative β-alanine with High Stoichiometric Yield. Metab. Eng. 2019, 54, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, S.; Huang, H.; Wen, J. Key Technologies for the Industrial Production of Fumaric Acid by Fermentation. Biotechnol. Adv. 2012, 30, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.C.R.; Straathof, A.J.J.; Zijlmans, T.W.; Van Gulik, W.M.; Van Der Wielen, L.A.M. Fumaric Acid Production by Fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).