Application of Lactic Acid Bacteria in Fermentation Processes to Obtain Tannases Using Agro-Industrial Wastes
Abstract
1. Introduction
2. Tannins Overview
2.1. Biological Properties of Tannins
2.1.1. Antioxidant Properties
2.1.2. Anticancer Properties
2.1.3. Cardioprotective Properties
2.1.4. Digestive, Carminative, and Astringent Properties
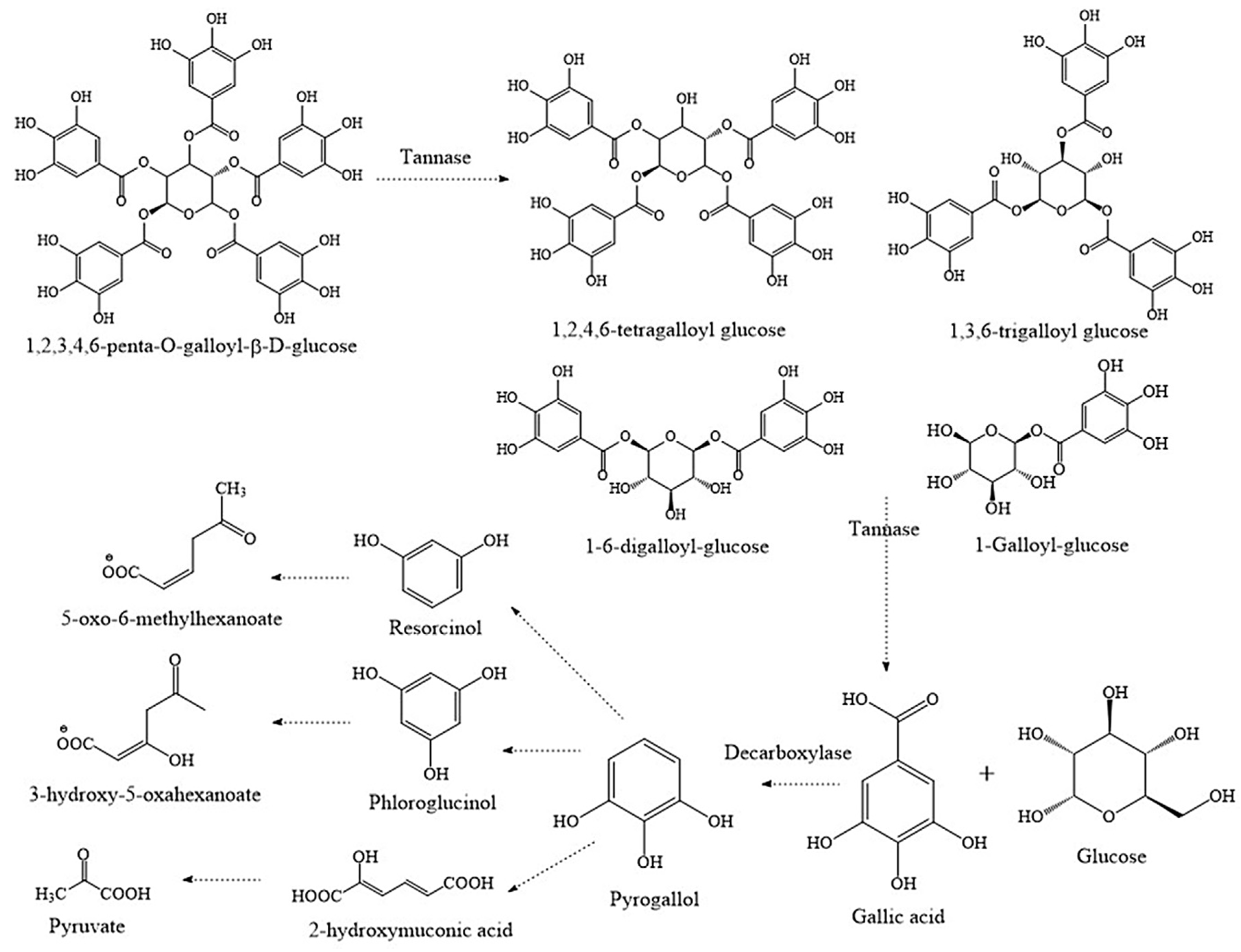
3. Biodegradation of Tannins
4. Production of Tannases with Submerged Fermentation (SF)
5. Production of Tannases Using Solid-State Fermentation
6. Microorganisms Used in Tannases Production
7. Lactic Acid Bacteria (LAB) That Produce Tannases
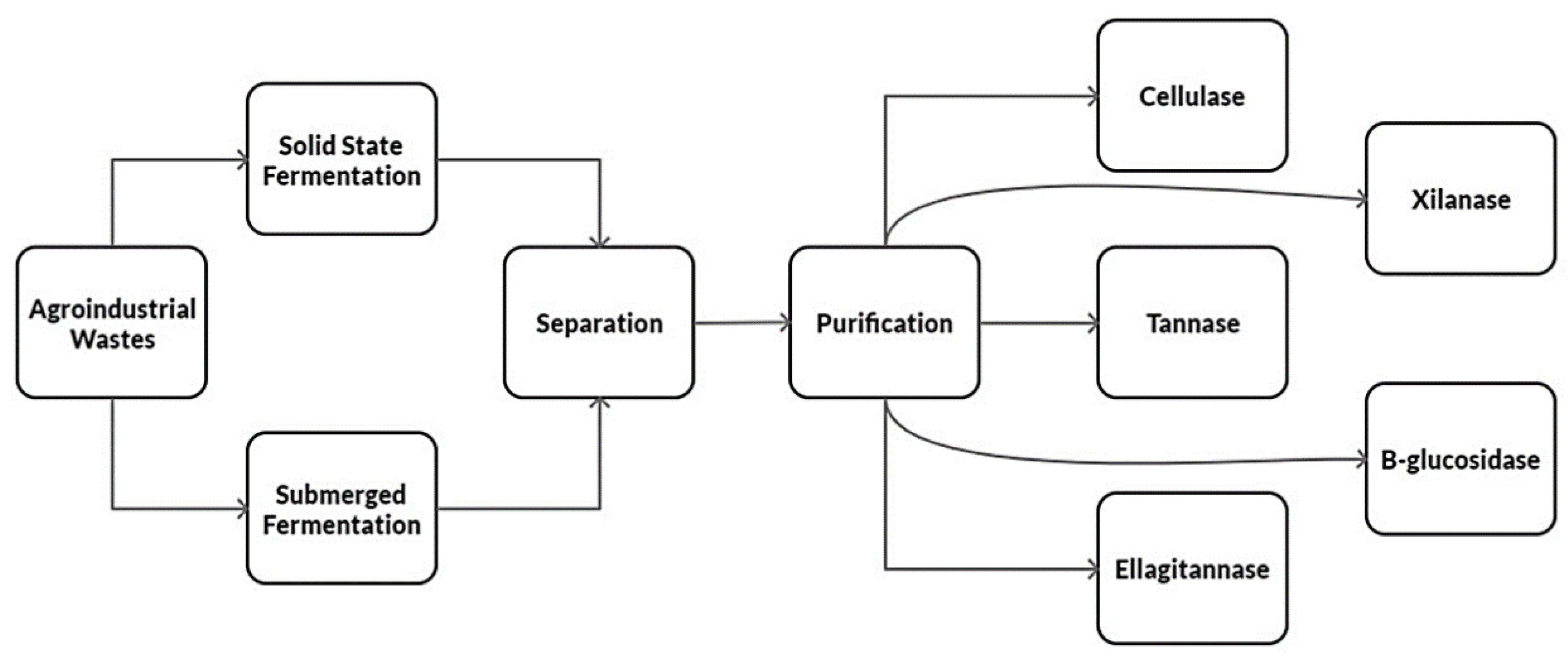
8. Agro-Industrial Wastes to Obtain Tannases
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. An Introduction to Fermentation Processes. In Principles of Fermentation Technology, 3rd ed.; Stanbury, P.F., Whitaker, A., Hall, S.J., Eds.; Elsevier: London, UK, 2011; pp. 1–20. [Google Scholar]
- Paulová, L.; Patáková, P.; Brányik, T. Advanced Fermentation Processes. In Engineering Aspects of Food Biotechnology, 1st ed.; Teixeira, J.A., Vicente, A.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 89–105. [Google Scholar]
- Suárez-Arango, C.; Nieto, I.J. Biotechnological cultivation of edible macro-fungi: An alternative in obtaining nutraceuticals. Rev. Iberoam. Mycol. 2012, 30, 1–8. [Google Scholar]
- Bailon-Neira, R.C. Fermentaciones Industriales. Master’s Thesis, National University of Callao, Bellavista, Peru, April 2012. [Google Scholar]
- Ren, B.; Wu, M.; Wang, Q.; Peng, X.; Wen, H.; McKinstry, W.J.; Chen, Q. Crystal Structure of Tannase from Lactobacillus plantarum. J. Mol. Biol. 2013, 425, 2737–2751. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Lin, P.; Tian, X.-H.; Yi, Y.-S.; Jiang, W.-S.; Zhou, Y.-J.; Cheng, W.-J. Luteolin-induced protection of H2O2-induced apoptosis in PC12 cells and the associated pathway. Mol. Med. Rep. 2015, 12, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, X.; Wen, H.; Wang, Q.; Chen, Q.; McKinstry, W.J.; Ren, B. Expression, purification, crystallization and preliminary X-ray analysis of tannase from Lactobacillus plantarum. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 456–459. [Google Scholar] [CrossRef]
- Velmurugan, B.; Singh, R.P.; Agarwal, R.; Agarwal, C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol. Carcinog. 2010, 49, 641–652. [Google Scholar] [CrossRef]
- Chávez-González, M.; Guyot, S.; Rodríguez-Herrera, R.; Prado-Barragán, A.; Aguilar, C.N. Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochem. 2014, 49, 541–546. [Google Scholar] [CrossRef]
- Rani, J.; Indrajeet; Rautela, A.; Kumar, S. Biovalorization of Winery Industry Waste to Produce Value-Added Products. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Rathinam, N.K., Sani, R.K., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 63–85. [Google Scholar]
- Rodríguez-Durán, L.V.; Valdivia-Urdiales, B.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Química y biotecnología de la tanasa. Acta Química Mex. 2010, 2, 1–10. [Google Scholar]
- Aguilar-Zárate, P.; Cruz-Hernández, M.A.; Montañez, J.C.; Belmares-Cerda, R.E.; Aguilar, C.N. Bacterial tannases: Production, properties and applications. Rev. Mex. Ing. Quim. 2014, 13, 63–74. [Google Scholar]
- Singh, S.K.; Patra, A. Evaluation of phenolic composition, antioxidant, anti-inflammatory and anticancer activities of Polygonatum verticillatum (L.). J. Integr. Med. 2018, 16, 273–282. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Queiroz, M.; Rodrigues, M.; Barros, A.I. Evaluation of the Phytochemistry and Biological Activity of Grape (Vitis vinifera L.) Stems: Toward a Sustainable Winery Industry. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Elsevier: London, UK, 2019; pp. 381–394. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Tyagi, A.; Raina, K.; Shrestha, S.P.; Miller, B.; Thompson, J.A.; Wempe, M.F.; Agarwal, R.; Agarwal, C. Procyanidin B2, 3,3¨ di –O- gallate, a biologically active constituent of grape seed extract, induces apoptosis in human prostate cancer cells via target in NK-kB, Stat3 and AP1 transcription factors. Nutr. Cancer 2014, 66, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Chen, P.-T.; Hong, Z.-S.; Cheng, C.-L.; Ng, I.-S.; Lo, Y.-C.; Nagarajan, D.; Chang, J.-S. Exploring fermentation strategies for enhanced lactic acid production with polyvinyl alcohol-immobilized Lactobacillus plantarum 23 using microalgae as feedstock. Bioresour. Technol. 2020, 308, 123266. [Google Scholar] [CrossRef]
- Bao, X.M.; Wu, C.F.; Lu, G.P. Atorvastatin inhibits homocysteine-induced oxidative stress and apoptosis in endothelial progenitor cells involving Nox4 and p38MAPK. Atherosclerosis 2010, 210, 114–121. [Google Scholar] [CrossRef]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jeon, D.Y.; Javaid, H.M.A.; Sahar, N.E.; Lee, H.-N.; Hong, S.-J.; Huh, J.Y.; Kim, Y.-M. Bio-transformation of green tea infusion with tannase and its improvement on adipocyte metabolism. Enzym. Microb. Technol. 2020, 135, 109496. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Revathi, S.; Rameshkumar, N.; Krishnan, M.; Kayalvizhi, N. Microbial tannase: Current perspectives and biotechnological advances. Biocatal. Agric. Biotechnol. 2016, 6, 168–175. [Google Scholar] [CrossRef]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Enzyme Production from Food Wastes Using a Biorefinery Concept. Waste Biomass Valoriz. 2014, 5, 903–917. [Google Scholar] [CrossRef]
- Sharma, K.P.; John, P.J. Purification and characterization of tannase and tannins from Enterobacter sp. Process Biochem. 2011, 46, 240–244. [Google Scholar] [CrossRef]
- Curiel, J.A.; Betancor, L.; Rivas, B.D.L.; Muñoz, R.; Guisán, J.M.; Fernandez-Lorente, G. Hydrolysis of Tannic Acid Catalyzed by Immobilized−Stabilized Derivatives of Tannase from Lactobacillus plantarum. J. Agric. Food Chem. 2010, 58, 6403–6409. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Beena, P. Tannase: Source, Biocatalytic Characteristics, and Bioprocesses for Production. In Marine Enzymes for Biocatalysis; Trincone, A., Ed.; Elsevier: London, UK, 2013; pp. 259–293. [Google Scholar]
- Jiménez-Martín, N. Metabolismo de Galotaninos en Bacterias Con Actividad Tanasa Presentes en El Tracto Gastrointestinal Humano. Ph.D. Thesis, Autonomous University of Madrid, Madrid, Spain, July 2014. [Google Scholar]
- Jiménez, N.; Esteban-Torres, M.; Mancheño, J.M.; Rivas, B.D.L.; Muñoz, R. Tannin Degradation by a Novel Tannase Enzyme Present in Some Lactobacillus plantarum Strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Arango, C. Utilización de la Fermentación Líquida de Lentinula Edodes (Shiitake), para la Producción de Metabolitos Secundarios Bioactivos y Evaluación de su Potencial Empleo en la Producción de un Alimento Funcional. Master’s Thesis, National University of Colombia, Bogota, Colombia, 2012. [Google Scholar]
- Coradi, G.V.; Da Visitação, V.L.; De Lima, E.A.; Saito, L.Y.T.; Palmieri, D.A.; Takita, M.A.; Neto, P.D.O.; Nascimento, V.M.G. Comparing submerged and solid-state fermentation of agro-industrial residues for the production and characterization of lipase. Ann. Microbiol. 2012, 63, 533–540. [Google Scholar] [CrossRef]
- Pintać, D.; Četojević-Simin, D.; Berežni, S.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef]
- Selwal, M.K.; Selwal, K.K. High-level tannase production by Penicillum atramentosum KM using agro residues under submerged fermentation. Ann. Microbiol. 2012, 62, 139–148. [Google Scholar] [CrossRef]
- Hemansi; Chakraborty, S.; Yadav, G.; Saini, J.K.; Kuhad, R.C. Comparative Study of Cellulase Production Using Submerged and Solid-State Fermentation. In New and Future Developments in Microbial Biotechnology and Bioengineering; Srivastava, N., Srivastava, M., Singh, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 99–113. [Google Scholar]
- Barragán, L.P.; Figueroa, J.; Durán, L.R.; González, C.A.; Hennigs, C. Fermentative Production Methods. In Biotransformation of Agricultural Waste and By-Products; Poltronieri, P., D’Urso, O.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 189–217. [Google Scholar]
- Fernandes, M.B.A.; Habu, S.; De Lima, M.A.; Soccol, V.T.; Soccol, C.R. Influence of drying methods over in vitro antitumoral effects of exopolysaccharides produced by Agaricus blazei LPB 03 on submerged fermentation. Bioprocess Biosyst. Eng. 2010, 34, 253–261. [Google Scholar] [CrossRef]
- Colla, L.M.; Primaz, A.L.; Benedetti, S.; Loss, R.A.; De Lima, M.; Reinehr, C.O.; Bertolin, T.E.; Costa, J.A.V. Surface response methodology for the optimization of lipase production under submerged fermentation by filamentous fungi. Braz. J. Microbiol. 2016, 47, 461–467. [Google Scholar] [CrossRef]
- Chegwin, C.A.; Nieto, R.I.J. Influence of culture medium in the production of secondary metabolites of edible fungus Pleurotus ostreatus cultivated by liquid state fermentation using grain flour as a carbon source. Rev. Mex. Micol. 2013, 37, 1–9. [Google Scholar]
- Kirsch, L.D.S.; De Macedo, A.J.P.; Teixeira, M.F.S. Production of mycelial biomass by the Amazonian edible mushroom Pleurotus albidus. Braz. J. Microbiol. 2016, 47, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Parras-Huertas, R.A. Review lactic acid bacteria: Functional role in the foods. Rev. Bio. Agro 2010, 8, 93–105. [Google Scholar]
- Lekshmi, R.; Nisha, S.A.; Kaaleeswaran, B.; Alfarhan, A. Pomegranate peel is a low-cost substrate for the production of tannase by Bacillus velezensis TA3 under solid state fermentation. J. King Saud. Univ. Sci. 2020, 32, 1831–1837. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef]
- Chebaibi, S.; Grandchamp, M.L.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of protein content and decrease of anti-nutritional factors in olive cake by solid-state fermentation: A way to valorize this industrial by-product in animal feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Chen, H. Comparative characterization of proteins secreted by Neurospora sitophilia in solid-state and submerged fermentation. J. Biosci. Bioeng. 2013, 116, 493–498. [Google Scholar] [CrossRef]
- Joshi, R.; Sharma, V.; Kuila, A. Fermentation Technology: Current Status and Future Prospects. In Principles and Applications of Fermentation Technology, 1st ed.; Kuila, A., Sharma, V., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–13. [Google Scholar]
- Mansor, A.; Ramli, M.; Rashid, N.A.; Samat, N.; Lani, M.; Sharifudin, S.; Raseetha, S. Evaluation of selected agri-industrial residues as potential substrates for enhanced tannase production via solid-state fermentation. Biocatal. Agric. Biotechnol. 2019, 20, 101216. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Mathivanan, K.; Rameshkumar, N.; Shyu, D.J.H.; Krishnan, M.; Kayalvizhi, N. Purification, structural characterization and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem. 2019, 77, 37–47. [Google Scholar] [CrossRef]
- Zhang, B.B.; Xing, H.B.; Jiang, B.J.; Chen, L.; Xu, G.R.; Jiang, Y.; Zhang, D.Y. Using millet as substrate for efficient production of monacolin K by solid-state fermentation of Monascus ruber. J. Biosci. Bioeng. 2018, 125, 333–338. [Google Scholar] [CrossRef]
- Julian, R.M.C.; Ramos-Sánchez, L.B.; Suárez-Rodríguez, Y. Fermentación en estado sólido (II) optimización de medios de cultivos. Tecnol. Química 2008, 28, 5–10. [Google Scholar]
- Ito, K.; Gomi, K.; Kariyama, M.; Miyake, T. Rapid enzyme production and mycelial growth in solid-state fermentation using the non-airflow box. J. Biosci. Bioeng. 2013, 116, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Gomi, K.; Kariyama, M.; Miyake, T. Change in enzyme production by gradually drying culture substrate during solid-state fermentation. J. Biosci. Bioeng. 2015, 119, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A.; Roberto, B.; Chen, Q.; Blumberg, J.B.; Chen, C. Tannase enhances the anti-inflammatory effect of grape pomace in Caco-2 cells treated with IL-1b. J. Funct. Foods 2016, 29, 69–76. [Google Scholar] [CrossRef]
- Ichikawa, K.; Shiono, Y.; Shintani, T.; Watanabe, A.; Kanzaki, H.; Gomi, K.; Koseki, T. Efficient production of recombinant tannase in Aspergillus oryzae using an improved glucoamylase gene promoter. J. Biosci. Bioeng. 2020, 129, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kanpiengjai, A.; Unban, K.; Nguyen, T.-H.; Haltrich, D.; Khanongnuch, C. Expression and biochemical characterization of a new alkaline tannase from Lactobacillus pentosus. Protein Expr. Purif. 2019, 157, 36–41. [Google Scholar] [CrossRef]
- Sharma, K. Tannin degradation by phytopathogen’s tannase: A Plant’s defense perspective. Biocatal. Agric. Biotechnol. 2019, 21, 101342. [Google Scholar] [CrossRef]
- López, T.; Prado-Barragán, A.; Nevárez-Moorillón, G.V.; Contreras, J.C.; Rodríguez, R.; Aguilar, C.N. Enhancement of antioxidant capacity of coffee pulp extracts by solid-state lactic fermentation. CYTA J. Food 2013, 11, 359–365. [Google Scholar] [CrossRef]
- Jurado-Gámez, H.; Ramírez, C.; Aguirre, D. Fermentation kinetics of Lactobacillus plantarum at a rich culture medium as a potential probiotic. Vet. Anim. Sci. 2013, 7, 37–53. [Google Scholar]
- Bamforth, C.W.; Cook, D.J. The Science Underpinning Food Fermentation. In Food, Fermentation, and Micro-Organisms, 2nd ed.; Bamforth, C.W., Cook, D.J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; pp. 5–42. [Google Scholar]
- Mayo, B.; Flórez, A.B. Lactic Acid Bacteria: Lactobacillus spp. Lactobacillus plantarum. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Zhang, Z.; Man, C.; Sun, L.; Yang, X.; Li, M.; Jiang, Y.; Zhang, W. Short communication: Complete genome sequence of Lactobacillus plantarum J26, a probiotic strain with immunomodulatory activity. J. Dairy Sci. 2019, 102, 10838–10844. [Google Scholar] [CrossRef]
- Pérez-Leonard, H.; Hernández-Monzón, A. Evaluation of substrates with aloe vera juice for the growth of Lactobacillus plantarum. RTQ 2015, 35, 156–166. [Google Scholar]
- Yao, J.; Guo, G.S.; Ren, G.H.; Liu, Y. Production, characterization and applications of tannase. J. Mol. Catal. B Enzym. 2014, 101, 137–147. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- DelCarlo, S.B.; Parada, R.; Schelegueda, L.I.; Vallejo, M.; Marguet, E.R.; Campos, C.A. From the isolation of bacteriocinogenic LAB strains to the application for fish paste biopreservation. LWT Food Sci. Technol. 2019, 110, 239–246. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Hongwittayakorn, P. Potential Probiotic Lactic Acid Bacteria of Human Origin Induce Antiproliferation of Colon Cancer Cells via Synergic Actions in Adhesion to Cancer Cells and Short-Chain Fatty Acid Bioproduction. Appl. Biochem. Biotechnol. 2013, 169, 511–525. [Google Scholar] [CrossRef]
- Albano, C.; Morandi, S.; Silvetti, T.; Casiraghi, M.; Manini, F.; Brasca, M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018, 101, 10807–10818. [Google Scholar] [CrossRef]
- Wang, Q.; Lillevang, S.K.; Rydtoft, S.M.; Xiao, H.; Fan, M.; Solem, C.; Liu, J.; Jensen, P.R. No more cleaning up—Efficient lactic acid bacteria cell catalysts as a cost-efficient alternative to purified lactase enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 6315–6323. [Google Scholar] [CrossRef]
- Gregirchak, N.; Stabnikova, O.; Stabnikov, V. Application of Lactic Acid Bacteria for Coating of Wheat Bread to Protect it from Microbial Spoilage. Plant Foods Hum. Nutr. 2020, 75, 223–229. [Google Scholar] [CrossRef]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdullah, N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J. Anim. Sci. Biotechnol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Bhoite, R.N.; Murthy, P.S. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod. Process. 2015, 94, 727–735. [Google Scholar] [CrossRef]
- Azzaz, H.; Kholif, A.; El Tawab, A.A.; Khattab, M.; Murad, H.; Olafadehan, O. A newly developed tannase enzyme from Aspergillus terreus versus commercial tannase in the diet of lactating Damascus goats fed diet containing pomegranate peel. Livest. Sci. 2020, 241, 104228. [Google Scholar] [CrossRef]
- Albuquerque, K.K.S.A.; Albuquerque, W.W.C.; Costa, R.M.P.B.; Batista, J.M.S.; Marques, D.A.V.; Bezerra, R.P.; Herculano, P.N.; Porto, A.L.F. Biotechnological potential of a novel tannase-acyl hydrolase from Aspergillus sydowii using waste coir residue: Aqueous two-phase system and chromatographic techniques. Biocatal. Agric. Biotechnol. 2020, 23, 101453. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Natarajan, K.; Rajendran, A. Evaluation and optimization of food-grade tannin acyl hydrolase production by a probiotic Lactobacillus plantarum strain in submerged and solid state fermentation. Food Bioprod. Process. 2012, 90, 780–792. [Google Scholar] [CrossRef]
- Selvaraj, S.; Natarajan, K.; Nowak, A.; Murty, V.R. Mathematical modeling and simulation of newly isolated Bacillus cereus M1GT for tannase production through semi-solid state fermentation with agriculture residue triphala. S. Afr. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Teles, A.S.; Chávez, D.W.; Oliveira, R.A.; Bon, E.P.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.; Tonon, R.V. Use of grape pomace for the production of hydrolytic enzymes by solid-state fermentation and recovery of its bioactive compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef]
| Submerged Fermentation | |||
|---|---|---|---|
| Advantages | Reference | Disadvantages | Reference |
| Easier and the product is easy to recover | [32] | The difficulty for the passage of oxygen in the liquid medium | [33,34] |
| Sterilization facilitates the process and its control | [35] | Detrimental to fungal growth | [36,37] |
| Efficiency in preventing the growth of other microorganisms | [38] | ||
| Homogeneity in culture media and control in temperature and pH | [39,40] | ||
| Better control of physicochemical parameters, more biomass growth in less time | [41] | ||
| Ease in determining biomass by simple filtration or centrifugation | [33] | ||
| It is used for bioremediation of effluents in industries | [42] | ||
| Bacteria, yeasts, and fungi can be used depending on the objective | [37] | ||
| It requires less investment, less energy, and a simple means of fermentation. Better condition of bacterial control | [26] | ||
| It is used on an industrial scale, nutrients, and oxygen dissolve easily in the medium and disperse throughout the bioreactor, so heat and mass increase efficiency | [36] | ||
| Incubation time decrease, better control in the process | [43] | ||
| Have a better performance, reduces costs and is sustainable, making it beneficial for the environment and the economy of production | [44] | ||
| Solid-State Fermentation | |||
| Effective to produce enzymes | [45] | Low O2 and CO2 transfer ratio, it is difficult to monitor, control, and scale. There is no uniformity in culture | [32] |
| Effective to produce bioactive compounds | [46] | Slower microorganism’s growth | [3] |
| Lower demand for water and energy, easy aeration in the medium | [33] | It is less efficient for the growth of microorganisms that require high water content | [47] |
| Most used for agro-industrial waste. Economical and superior enzymatic performance | [48] | ||
| There is less waste of water, simplicity | [46] | ||
| They are much more efficient fermentations; their products are stable and can be easily recovered | [36] | ||
| Microorganism | Reference |
|---|---|
| Aspergillus niger | [6] |
| Enterococcus faecalis | [55] |
| Aspergillus ficuum | [56] |
| Achromobacter | |
| Corynebacterium spp. and Klebsiella pneumoniae | [49] |
| Azotobacter Lactiplantibacillus paraplantarum | [57] |
| Aspergillus oryzae | [55] |
| Lactiplantibacillus paraplantarum Fusarium Trichoderma | [56] |
| Enterobacter cloacae | [49] |
| Microorganism | Isolation Source | Application | Reference |
|---|---|---|---|
| Limosilactobacillus reuteri | Whole wheat sourdough | Antifungal activity against Aspergillus niger | [65] |
| Lactobacillus delbrueckii subsp. lactis NRRL B-633, Lactobacillus delbrueckii subsp. cremoris NRRL B-634, Pediococcus acidilactici NRRL B-1116, P. pentosaceus NRRL B-14009, Leuconostoc mesenteroides subsp. mesenteroides NRRL B-1118, Latilactobacillus sakei subsp. sakei NRRL B1917, Limosilactobacillus fermentum NRRL B-1932, Limosilactobacillus reuteri NRRL B-14171, Lactiplantibacillus plantarum NRRL B-4496, Lactobacillus acidophilus NRRL B-4495, Lacticaseibacillus casei NRRL B1922, Levilactobacillus brevis ATCC 367, Lacticaseibacillus casei 21/1, Lactobacillus amylovorus ATCC 33621, Fructilactobacillus sanfranciscensis ATCC 27651, and Lacticaseibacillus rhamnosus NRRL B-442. | - | Antimicrobials in vitro against Escherichia coli, Staphylococcus aureus, Shigella sonnei, Pseudomonas fluorescens, Salmonella typhimurium, or Listeria monocytogenes | [66] |
| Lactobacillus spp. | - | Produces exopolysaccharides that have interesting film-forming properties and may be used to produce edible packaging. | [67] |
| Enterococcus mundtii STw38 | Tehuelche scallop (Aequipecten tehuelchus), Patagonian Argentinean clam (Ameghinomya antique), Patagonian blue mussel (Mytilus edulis platensis), sea cucumber (Hemiodema spectabilis), geoduck (Panopea generosa) and razor clam (Solen tehuelchus) of the Argentine coast | Reducing the development of native flora of fish paste. | [68] |
| P. pentosaceus FP3, Ligilactobacillus salivarius FP35, Ligilactobacillus salivarius FP25, and E. faecium FP51 | Collected from 17 healthy infant feces samples in the hospital of Chiang Mai. | The use of these probiotics may be suitable as an alternative bioprophylactic and biotherapeutic strategy for colon cancer. | [69] |
| 7 Lacticaseibacillus casei, 27 Lacticaseibacillus paracasei subsp. paracasei, 15 Lactiplantibacillus plantarum subsp. plantarum, 7 Lactobacillus delbrueckii subsp. lactis, 1 E. faecium, and 1 Enterococcus lactis. | Traditional Italian cheeses. | These strains with proven in vitro properties are good candidates for novel probiotic-containing formulations and could be used to functionalize foods such as dairy fermented products. | [70] |
| S. thermophilus, Lactococcus lactis SL242, Lactobacillus delbrueckii subsp. Lactis SL28 and Lactobacillus delbrueckii subsp. lactis IO-1 | - | Development method presented is natural and low-cost and allows for the production of clean-label and lactose-free dairy products without using commercial enzymes from recombinant microorganisms. | [71] |
| Streptococcus salivarius subsp. thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, and Lactobacillus acidophilus, Acetobacter aceti, Bifidobacterium bifidum, B. adolescentics, B. longum, B. animalis, Lactobacillus acidophilus, Lactococcus lactis subsp. cremoris, Propionibacterium freudenreichii, Enterococcus faecium and Streptococcus salivarius subsp. thermophilus | Iprovit Bacterial Milk-Yogurt Starter™, Symbilact Vivo Starter™ and Provit Streptosan Milk Starter™ | The development of edible coatings containing LAB to wheat bread diminished the number of mesophilic aerobic and facultative aerobic bacteria in the bread crust and protected it from contamination of mycelium fungi of genera Aspergillus and Penicillium. | [72] |
| Lactiplantibacillus plantarum: TL-1, TL-2, TP-2, TP-5, I-UL4, I 11, RG 11, RG 14, RS 5. 6 Pediococcus pentosaceus: B12m9, TB-1, TL-3, TP-3, TP-4, TP-8. 2 Pediococcus acidilactici: TB-2, TP-6. | Tempeh-fermented soybean cake, apai ubi-fermented cassava, ikan rebus-steam fish, budu-fermented fish sauce, tempeh-fermented soybean cake and empeh-fermented soybean cake | LAB isolates possess versatile extracellular proteolytic system and have vast capability of producing various amino acids including as methionine, lysine, threonine and tryptophan. | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Méndez, M.G.; Morales Martínez, T.K.; Ascacio Valdés, J.A.; Chávez González, M.L.; Flores Gallegos, A.C.; Sepúlveda, L. Application of Lactic Acid Bacteria in Fermentation Processes to Obtain Tannases Using Agro-Industrial Wastes. Fermentation 2021, 7, 48. https://doi.org/10.3390/fermentation7020048
García Méndez MG, Morales Martínez TK, Ascacio Valdés JA, Chávez González ML, Flores Gallegos AC, Sepúlveda L. Application of Lactic Acid Bacteria in Fermentation Processes to Obtain Tannases Using Agro-Industrial Wastes. Fermentation. 2021; 7(2):48. https://doi.org/10.3390/fermentation7020048
Chicago/Turabian StyleGarcía Méndez, Martha Gabriela, Thelma Karina Morales Martínez, Juan Alberto Ascacio Valdés, Mónica Lizeth Chávez González, Adriana Carolina Flores Gallegos, and Leonardo Sepúlveda. 2021. "Application of Lactic Acid Bacteria in Fermentation Processes to Obtain Tannases Using Agro-Industrial Wastes" Fermentation 7, no. 2: 48. https://doi.org/10.3390/fermentation7020048
APA StyleGarcía Méndez, M. G., Morales Martínez, T. K., Ascacio Valdés, J. A., Chávez González, M. L., Flores Gallegos, A. C., & Sepúlveda, L. (2021). Application of Lactic Acid Bacteria in Fermentation Processes to Obtain Tannases Using Agro-Industrial Wastes. Fermentation, 7(2), 48. https://doi.org/10.3390/fermentation7020048







