Abstract
This study evaluated the effects of lasalocid sodium (LASA) and essential oils on the fermentation and nutritional quality of total mixed ration (TMR) silages. A 4 × 2 factorial design tested four additives—a control (distilled water), LASA (375 mg/kg DM), limonene essential oil (LEO), and a blend of cinnamaldehyde and carvacrol (EOB), both at 400 mg/kg DM—during summer and autumn. The TMRs were formulated to meet the nutritional requirements of lactating cows producing 20 kg of milk per day. After 110 days of ensiling, silages were analyzed for fermentation losses, pH, short-chain fatty acids, ammoniacal nitrogen (NH3-N), aerobic stability (AS), and chemical composition. The additives significantly improved dry matter recovery (DMR), especially LASA and EOB in autumn. EOB showed the lowest effluent losses and highest AS, with higher acetic acid and lower NH3-N contents. LEO and EOB increased lactic acid, while LASA reduced ethanol and butyric acid levels in summer. Crude protein increased with LEO in autumn, and LASA and LEO improved total digestible nutrients (TDNs) in summer. EOB-treated silages had higher fiber fractions in autumn, without compromising feed value. Therefore, LASA, LEO, and particularly EOB enhanced silage fermentation and nutrient preservation, with EOB showing the most consistent results across seasons.
1. Introduction
During the ensiling process, maintaining an anaerobic environment and a low pH is essential to inhibit the growth of undesirable microorganisms and ensure proper fermentation. The presence of oxygen, especially after silo opening, can compromise the stability of the material by promoting the proliferation of fungi, yeasts, and spoilage bacteria, resulting in both nutritional and economic losses [].
To mitigate these negative effects, the use of additives during ensiling has been widely studied []. These additives mainly act by stimulating lactic fermentation, inhibiting the activity of undesirable microorganisms, and consequently reducing fermentation losses [,]. Additionally, they can contribute to greater aerobic stability, improved silage intake by animals, and better performance. However, it is important to highlight that a single additive rarely possesses all these desirable characteristics, which justifies the ongoing search for new alternatives [].
Among the compounds with antimicrobial potential, ionophores and essential oils stand out. Lasalocid sodium (LASA) is an ionophore known for its selective action on the ruminal microbiota and is commonly used as a performance enhancer in ruminant diets. However, to date, there are no reports in the literature regarding its use as an additive in the ensiling process, making it relevant to evaluate its effects in this context. On the other hand, essential oils, which are secondary plant metabolites with recognized antimicrobial properties, have been explored as natural alternative additives. Compounds such as limonene essential oil (LEO) and blends containing cinnamaldehyde and carvacrol (EOB) have shown potential to inhibit spoilage microorganisms. Nevertheless, the effects of these compounds on the fermentation process and nutritional value of total mixed ration (TMR) silages are still not well understood, particularly with respect to the ideal dose and the interaction among their active components.
Moreover, ambient temperature can influence the success of silage fermentation capacity. Ref. [] reported that microorganisms may not be able to produce sufficient lactic acid to improve silage quality under low-temperature conditions. Most commercial LAB inoculants generally have little or no effect on silage at low temperatures [,].
Given the evidence that antimicrobial substances such as sodium monensin, studied by [,], can enhance lactic fermentation and aerobic stability in silages, there is growing interest in investigating whether other antimicrobial additives, such as essential oils and other ionophores like LASA, may exhibit similar or complementary effects on the fermentation process. Considering that essential oils have selective action against spoilage microorganisms and that LASA is a widely used ionophore in ruminant nutrition, the hypothesis is that the inclusion of these additives in total mixed ration (TMR) silages may improve the fermentative profile by increasing lactic acid production and reducing fermentation losses, without compromising the chemical–bromatological composition or aerobic stability of the silage. Therefore, this study aimed to evaluate the effect of these additives on the fermentative process and nutritional quality of total mixed ration silages.
2. Materials and Methods
2.1. Study Location, Experimental Design, and Treatments
This study was conducted at the Experimental Farm of the Federal University of Grande Dourados (UFGD) (22°13′52.44′95″ S, 54°59′10.53′72″ W) in the municipality of Dourados, MS, Brazil. The experiment followed a completely randomized design in a 4 × 2 factorial scheme, in which four types of additives were evaluated across two seasons of the year. The tested additives were (1) a control (distilled water); (2) LASA at a dose of 375 mg/kg of dry matter (DM); (3) LEO at a dose of 400 mg/kg DM; (4) EOB at a dose of 400 mg/kg DM. Each treatment was replicated five times.
The evaluated seasons were summer and autumn, with ensiling carried out in February 2024 (summer) and May 2024 (autumn). Each treatment included 5 replicates, totaling 40 experimental silos.
The LASA used was Taurotec® (Zoetis, Parsippany, NJ, USA), which contains 15% lasalocid sodium in its formulation. LEO and EOB were supplied by the commercial products Activo Liquid® and Blend Activo Liquid® (GRASP, Curitiba, Paraná, Brazil), respectively, with the latter composed of 75% cinnamaldehyde and 25% carvacrol.
2.2. TMR Composition and Ensiling Methodology
A total mixed ration (TMR) was formulated to meet the nutritional requirements of lactating dairy cows producing an average of 20 kg of milk per day, with an approximate 450 kg of body weight and 14 kg of daily dry matter intake, according to []. The diet consisted of 33% forage and 67% concentrate. The forage used was BRS Capiaçu grass, harvested at 90 days of growth. The concentrate was formulated using ground corn, soybean meal, dicalcium phosphate, and calcitic limestone. The ingredient proportions and chemical composition of the diet are shown in Table 1.

Table 1.
Ingredient proportions and chemical composition of the TMRs formulated for both experimental trials (summer and autumn).
The additives were first mixed into the concentrate and then into the forage. The final mixture was used to fill the experimental silos. The silos consisted of PVC tubes measuring 10 cm in diameter and 50 cm in height, with a usable volume of 3.8 L. At the bottom of each tube, a layer of approximately 4.5 cm of sand (about 300 g) was placed to allow for liquid drainage. To prevent direct contact between the forage and the sand, a fine cotton mesh was inserted. The silos were manually packed using wooden rods to achieve an average density of 700 kg/m3, which was calculated using the following formula: Density = Mass/Volume. After sealing, the silos were closed with double-faced polyethylene plastic sheeting (black and white) and adhesive tape and stored at room temperature in the laboratory for 110 days.
2.3. Laboratory Analyses, Loss Determination, and Aerobic Stability
During the silo filling process, two TMR samples were collected from each treatment and each season: one sample (300 g) for determining dry matter and ash contents, and another (70 g) for pH and buffering capacity.
The pH (before and after ensiling), buffering capacity (only before ensiling), and the profile of short-chain organic acids in the material were determined using an aqueous extract obtained from the TMR. To prepare the aqueous extract, 25 g of TMR was diluted in 225 mL of distilled water and manually homogenized for approximately 30 min. The pH of the extract was measured using a digital pH meter (mPA210, MS Tecnopon, Piracicaba, Brazil), and the buffering capacity was determined according to the method described by [].
To calculate fermentation losses, all silo components (silo, sand, and fabric), as well as the ensiled TMR mass, were weighed before and after ensiling. Dry matter recovery (g/kg of ensiled DM), gas losses (g/kg of ensiled DM), and effluent losses (g/kg of ensiled forage) were calculated according to the equations proposed by []. Dry matter recovery was calculated using the following formula:
Gas losses were calculated using the following formula:
where GLs = gas losses during storage (% of initial DM mass); SWI = weight of the sealed silo at the beginning (kg); SWF = weight of the sealed silo at the time of opening (kg); and DMI = initial DM mass (kg of DM placed in the silos).
Effluent losses were calculated using the following formula:
where ELs = effluent losses (kg/ton of DM); SWF = final weight of the set (silo + sand + fabric) in kg; SWI = initial weight of the set (silo + sand + fabric) in kg; and DMI = initial DM mass (kg of DM placed in the silos).
After opening, all the material contained in each experimental silo was removed and homogenized for sample collection. A sample of approximately 300 g from each experimental silo was sent to the laboratory for chemical and fermentative profile analysis.
The chemical composition of the TMRs was determined using a Foss 5000 spectrophotometer (Eden Prairie, MN, USA) with calibration (WinISI version 4.6.11, FOSS Analytical A/S, Hillerød, Denmark) provided by the Dairy One Forage Laboratory (Ithaca, NY, USA). The following components were determined: dry matter (DM), ash, crude protein (CP), ammoniacal nitrogen (NH3-N), neutral detergent fiber (NDF), acid detergent fiber (ADF), lignin, ether extract (EE), non-fibrous carbohydrates (NFCs), starch, and total digestible nutrients (TDNs).
The concentrations of organic acids were determined using a gas chromatograph with a mass spectrometry detector (GCMS QP 2010 Plus, Shimadzu, Kyoto, Japan), equipped with a capillary column (Stabilwax, Restek, Bellefonte, PA, USA, 60 m, 0.25 mm ID, 0.25 µm polyethylene cross-bond carbowax glycol). The following organic acids were analyzed: acetic acid (AA), propionic acid (PA), isobutyric acid (Iso-But), butyric acid (BA), isovaleric acid (Iso-Val), valeric acid (VA), and ethanol. Lactic acid concentration was also determined by the colorimetric method proposed by [].
Aerobic stability was determined in all experimental silos after opening. Temperature sensors were placed in the center of the silage, and a double layer of gauze was placed over each experimental silo to prevent drying. The ambient temperature as well as the temperature of each silage was recorded every minute and averaged every 20 min using a data logger (RC-4, Elitech®, San Jose, CA, USA). Aerobic stability was defined as the number of hours required for the silage temperature to rise 2 °C above ambient temperature [].
2.4. Statistical Analysis
The data were analyzed using SISVAR statistical software (version 5.6, Build 86–DES/UFLA). The means were compared using the Scott–Knott test at a 5% significance level. The data related to loss parameters, fermentation profile, and chemical composition of the TMRs were analyzed according to the following model:
where Yijk = dependent variable; μ = overall mean; αi = factor A, corresponding to the effect of additives (control, LASA, LEO, EOB); βj = factor B, corresponding to the effect of the season (summer or autumn); α*βij = interaction between additives and seasons; and εijk = random error associated with the observation.
Yijk = μ + αi + βj + (α*βij) + εijk
3. Results
In Table 2, the DMR was significantly influenced by the additives (p < 0.05), being higher in silages treated with LASA and EOB, especially in the autumn. The lowest values were observed in the control, with a notably poor result in the summer (94.22%). The addition of EOB promoted the highest DMR in autumn (97.24%), followed by LASA (96.30%) and LEO (95.18%).

Table 2.
Fermentation losses, pH, fermentative profile, and aerobic stability of TMR silages with different additives.
Effluent losses were significantly lower in silages with EOB and LASA during summer (27.39 and 33.07 kg/ton DM, respectively), with the control showing the highest loss (58.51 kg/ton DM). In autumn, although the differences between treatments were smaller, LEO had the lowest loss (58.16 kg/ton DM) and the control the highest (68.81 kg/ton DM).
The pH of the silages remained stable among treatments in the summer, but in autumn there was a significant increase in silages treated with LEO (3.96), while the control and EOB maintained lower values. The NH3-N content, an indicator of protein degradation, was significantly reduced with the use of EOB in summer (3.06%), while the other treatments did not differ (p > 0.05) from each other.
NH3-N contents, expressed as a percentage of total nitrogen (TN), were significantly influenced by the type of additive used (p = 0.046), with a tendency for interaction with the seasonal period (p = 0.063). During the summer, the EOB showed the lowest NH3-N value (3.06%), which was statistically lower than the other treatments, whose values ranged from 5.10% to 5.40% of TN.
Lactic acid (LA) concentrations were higher in silages with LEO and EOB, especially in autumn (6.40% DM for EOB). Acetic acid (AA) levels were significantly higher in silages with EOB in autumn (2.17% DM), indicating the greater activity of heterofermentative bacteria. Consequently, the LA/AA ratio showed a significant difference only for the EOB treatment during the summer. Compared with the additives, only the control showed the highest values in both seasons (Figure 1).
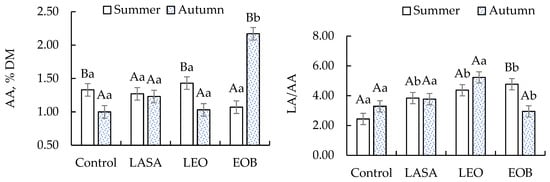
Figure 1.
Concentrations of acetic acid (AA) and lactic-to-acetic acid ratio (LA/AA) in TMR silages with different additives. Different letters indicate significant differences according to the Scott–Knott test at a 5% significance level. Uppercase letters compare the season; lowercase letters compare the additives.
Ethanol contents were significantly reduced with LASA in the summer (0.20% DM) and were also lower in LEO and EOB treatments. In the autumn, the values were generally lower across all treatments, with a notable reduction in LEO (0.03% DM).
The production of butyric acid (BA) and propionic acid (PA) was significantly reduced in silages treated with LASA and LEO during the summer. However, in the autumn, EOB showed higher concentrations of PA (252.83 mg/kg DM) and BA (142.81 mg/kg DM) (Figure 2).
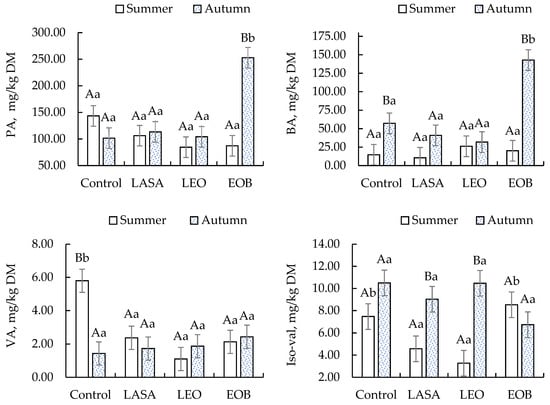
Figure 2.
Concentrations of propionic (PA), butyric (BA), valeric (VA), and isovaleric (Iso-val) acids in TMR silages with different additives. Different letters indicate significant differences according to the Scott–Knott test at a 5% significance level. Uppercase letters compare the season; lowercase letters compare the additives.
Aerobic stability was significantly increased with the use of EOB, reaching the highest stability times in both summer (125.30 h) and autumn (149.30 h). The control silages showed the lowest stability (102.87 h in summer and 121.13 h in autumn), while LASA and LEO had intermediate values.
Table 3 presents the chemical–bromatological composition of TMR silages supplemented with different additives during the summer and autumn. DM content was not affected by the treatments (p > 0.05), although numerically higher values were observed in silages treated with LASA and LEO during the summer (39.70% and 39.27%, respectively). In autumn, the differences between treatments were less pronounced, with averages around 35–37%. Ash content was significantly influenced by the period (p < 0.05), with higher values observed in autumn, especially in the control treatment (8.77% DM). Additives such as LEO resulted in a lower ash content (7.20%).

Table 3.
Chemical–bromatological composition of TMR silages with different additives.
Crude protein (CP) content did not differ between treatments in the summer, but in the autumn, a significant increase was observed with the use of LEO (19.55% DM). The control and the other additives showed similar values, with LASA presenting the lowest.
Although NDF was not significantly different, numerically lower values were recorded for LASA (summer) and LEO (both seasons), suggesting a potential improvement in fiber digestibility. ADF values were significantly higher in EOB-treated silages during autumn (28.73% DM). Lignin content was higher with EOB in autumn (4.30% DM), surpassing the other treatments (p < 0.05). In the other periods, the values remained homogeneous, with no relevant differences.
Ether extract had a significant difference between seasons for the control (3.70%) and LEO (3.47%) treatments, and regarding the effect of additives, only EOB showed the highest value (3.43%) (Figure 3).
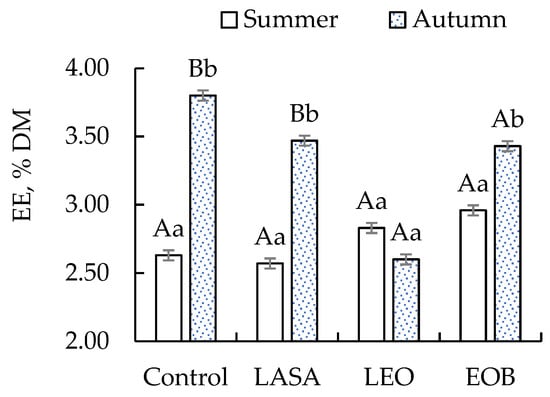
Figure 3.
Concentration of ether extract (EE) in TMR silages with different additives. Different letters indicate significant differences according to the Scott–Knott test at a 5% significance level. Uppercase letters compare the season; lowercase letters compare the additives.
No significant differences were observed in NFC content between treatments. The values ranged from 32% to 42% DM, with a trend towards higher values in LASA during summer.
TDN was significantly influenced by both additives and the period. In summer, LASA resulted in the highest TDN value (73.33% DM), followed by LEO (72.00% DM) and EOB (70.00% DM). The control had the lowest value (69.33% DM). In autumn, no significant differences were observed among treatments, although EOB showed a trend toward reduced energy values (66.67% DM).
4. Discussion
In preserved feeds like silages, DMR is directly associated with GLs and ELs, which depend on the predominant fermentation type. Lactic acid bacteria, producing lactic acid to lower pH, suppress undesirable microbes and reduce energy losses, providing the most efficient fermentation []. According to [], GLs are inevitable but should remain between 2% and 4% of ensiled DM to consider the fermentation efficient. ELs are influenced by the forage DM content and silage packing density []. Additionally, undesirable fermentations can disrupt cell walls, releasing intracellular water and nutrients, which increase effluent losses [], explaining the higher EL values observed in the control treatment. Thus, the use of additives improved the fermentation profile, significantly reduced GLs and ELs, and consequently enhanced DMR. This aligns with [], who reported a significant interaction between silage moisture content and additive type on DMR. They found that in wetter TMR silages (40% moisture), DMR was lower, especially with LEO at 600 mg/kg DM (88% recovery), whereas monensin under the same moisture conditions resulted in 95% DMR. In drier silages, both LEO and monensin yielded a DMR over 97%, with no significant difference between them.
A high DMR is supported in part by pH, which in our study remained within acceptable limits for good-quality silage. Ref. [] state that silages with 30–35% DM should have a pH below 4.2 for efficient fermentation. Ref. [] also noted a significant interaction between additives and moisture: LEO at 600 mg/kg in wetter silages resulted in a higher pH (4.31), while other treatments averaged 4.02.
The antimicrobial effect of LEO is linked to its ability to destabilize microbial cell membranes due to its hydrophobic nature, which alters membrane fluidity and increases permeability, leading to leakage and cell death []. Its action is effective against both Gram-positive and Gram-negative bacteria and is more intense in acidic environments (pH ~4.0), such as those found in well fermented silages. This explains its effectiveness in controlling undesirable microorganisms, especially yeasts resistant to low pH, as observed in the pH and DMR values of the present study [,].
In the current study, LASA, LEO, and EOB enhanced lactic acid production. Ref. [] in a meta-analysis noted that additives can selectively inhibit microbes competing with lactic acid bacteria, promoting lactic acid production. Inhibiting undesirable microbes can also prevent lactic acid’s conversion to butyric acid and ethanol, increasing lactic acid accumulation. Correspondingly, ref. [] evaluated four additives at 400 mg/kg DM in sorghum–Paiaguás grass silages and observed that 62% and 100% thymol treatments reduced Clostridium spp. and Lactobacillus spp. compared with the control.
During fermentation, acetic acid is the second most abundant acid in good-quality silages (1–3% DM) and is essential for aerobic stability due to its antifungal effect. In our study, EOB led to the highest acetic acid concentrations, likely by promoting synthesizing microbes. Ref. [] also reported increased acetic acid with sweet orange oil at 600 mg/kg DM (105.76 g/kg DM vs. 28.03 g/kg DM in control). Ref. [] recorded even greater stability with LEO (297.88 h) and monensin ionophore (242.92 h) in TMR silages. Ref. [] reaffirms the efficacy of EO blends in controlling aerobic microbes, extending silage preservation periods. In this way, TMR, by combining wet and dry ingredients, can minimize the risk of effluent production and undesirable fermentation. The use of ingredients rich in soluble sugars and homofermentative LAB would improve fermentation, while ingredients that promote heterofermentation would enhance the aerobic stability of TMR silages [].
The lactic-to-acetic acid ratio was favored by EOB, supporting heterolactic fermentation. Silages with very high lactic-to-acetic ratios (homofermentation) can sometimes be less aerobically stable than those with lower ratios []. However, the antimicrobial action of EOs and ionophores may have delayed aerobic microbes. Ref. [] observed higher lactic-to-acetic ratios (p < 0.05) with monensin (35–45 mg/kg DM) in lower moisture silages (40% DM); only LEO at 600 mg/kg in lower moisture silages showed a significantly higher ratio (7.8 vs. 2.3).
Cinnamaldehyde exhibits multiple antimicrobial mechanisms, including the permeabilization of the cell membrane, which causes the leakage of ions, proteins, and nucleic acids, compromising cell integrity []. Additionally, it inhibits ATPase enzymes, impairing ATP production and energy metabolism. It also prevents the formation and promotes the destruction of biofilms—structures that protect bacteria in hostile environments. Furthermore, it acts as a quorum sensing inhibitor, hindering cell-to-cell communication and reducing bacterial virulence. Finally, it interferes with cell division by inhibiting the FtsZ protein, which is essential for Z-ring formation, thereby blocking bacterial multiplication [].
Propionic acid is also recognized for its antimicrobial effects []. Propionibacteria are responsible for converting glucose and lactic acid into propionic acid. It is generally undetectable (especially in drier silages) or found at very low concentrations (<0.1%) in high-quality silages []. Season affected its production in this study, though levels remained low and within expected ranges for high-quality silage [].
Ethanol, another fermentation product, was low in this study, consistent with [], who noted that lower temperatures significantly impact fermentation products and epiphytic microbial composition in alfalfa silage. Climatic factors such as precipitation and temperature critically influence epiphytic microbes and silage fermentation. Ref. [] suggest that higher ethanol in silages may relate to forage composition or yeast presence in ensiled TMRs, consistent with higher summer ethanol. Ref. [] showed that lemon grass essential oil (1–3 mL/kg DM) in sugarcane silage significantly reduced ethanol, from 101 g/kg DM in the control to 7.96 g/kg DM with 2 mL/kg DM of lemon grass essential oil.
Butyric, isobutyric, isovaleric, and valeric acids are undesirable protein fermentation products that reduce nutritional value []. The highest butyric acid was found in control and EOB treatments, suggesting Clostridium-favorable conditions. The production of butyric acid is natural and may occur because, at the beginning of the fermentation process, there is still oxygen present that could not be completely removed during compaction. Furthermore, in autumn, the chemical and bromatological composition of the pasture may be altered due to seasonal effects, which further hinders compaction because of the higher presence of fibrous carbohydrates. Another contributing factor may be the lower temperatures, as microbial activity is reduced under such conditions. Therefore, we believe that competition among microorganisms and delayed fermentation may have occurred. However, the values obtained remain within the limits considered acceptable for high-quality silage and do not compromise the interpretation of the data or the conclusions of the study [].
Isovaleric acid varied by treatment and season; the control increased in autumn, suggesting more proteolysis, while EOB showed effective proteolysis inhibition, likely due to rapid acidification.
Plant and microbial proteolytic processes can alter nitrogen compounds in silages. A maximum loss of 10% of total nitrogen to NH3-N is expected []. NH3-N in EOB during summer remained below the safe threshold (<0.1% DM) for high-quality silage []. Ref. [] suggests that essential oils can alter protein structure without significantly compromising silage quality.
Nutritional quality remained consistent for DM, NDF, NFCs, and starch. Ref. [] highlighted that EO addition can alter forage nutritional components. Ref. [] observed that TMR silages often possess a high DM content (>40%), promoting feed preservation. As a rule, well preserved silages have a slightly higher ash content than their fresh crops []. The mineral content was higher in autumn for all treatments. Ref. [] showed that autumn increases N, P, K, Ca, and Mg in plant leaves due to internal nutrient redistribution before winter, enhancing mineral resistance to cold.
During silage fermentation, nitrogen losses may occur; however, they are usually less significant than other soluble fractions and have a smaller impact on total crude protein content. Protein degradation during ensiling occurs in two phases. Primarily, protein hydrolysis is driven by plant and microbial proteases, resulting in peptides and free amino acids. Subsequently, amino acid decarboxylation leads to the formation of biogenic amines and carbon dioxide, while amino acid deamination results in NH3 and organic acids []. The increase in CP during autumn may be related to the antimicrobial action of this compound on microorganisms involved in protein degradation during ensiling. LEO contains monoterpenes with recognized properties of inhibiting microbial protease activity [], which may have reduced the hydrolysis of proteins into peptides and free amino acids, as well as possibly inhibiting deamination [], thus favoring the preservation of the protein fraction in the silage. The milder temperatures in autumn may have enhanced the effects of LEO by limiting the growth of opportunistic proteolytic microorganisms such as Clostridia and enterobacteria. Therefore, the combination of a lower microbial pressure and the inhibitory action of LEO resulted in the reduced degradation of the original protein in the total mixed ration, leading to higher CP levels at the end of the fermentation process. The CP levels in Table 3 are noteworthy; LEO may have inhibited deamination and proteolysis [], preserving CP compared with untreated silages. Ref. [] reported similar findings with 120 mL/kg DM lemon seed LEO in alfalfa silage (12.5% CP vs. 11% in control DM).
ADF represents the least digestible fiber fraction and since BRS Capiaçu grass was harvested at 60 days (summer and autumn), the observed effect may be related to the forage maturity stage []. Furthermore, enzymatic activity may have been stimulated, promoting the breakdown of cell walls and releasing sucrose into the medium, which increased fiber concentrations.
EE was significantly higher in autumn for the control, followed by LASA and EOB, and [] and [] suggest that the bioactive compounds of essential oils, along with the moisture content and chemical composition of the grass, may have compromised fat preservation through oxidation or hydrolysis. The reduction in total fatty acid content during ensiling has been mainly attributed to the breakdown of C18:2n-6 and C18:3n-3 fatty acids, with both plant enzymes and epiphytic microorganisms playing significant roles in lipolysis, as observed in alfalfa silage [].
In summer, LASA and LEO increased the TDNs. Although no differences in NFCs and NDF were observed, it is hypothesized that the combined effects of ADF, lignin, and starch (related to digestibility) contributed to TDN values. The antimicrobial action of additives likely reduced spoilage microbes, resulting in more efficient fermentation and nutrient preservation, thereby increasing the TDNs [], since its mechanism involves the selective transport of mono- and di-valent cations (such as Na+, K+, Ca2+, and Mg2+), promoting osmotic imbalance and cytoplasmic acidification. This forces bacteria to expend energy to restore pH and homeostasis, thereby compromising their growth [].
5. Conclusions
The application of LASA, LEO, and EOB in TMR silages led to notable improvements in fermentative parameters, particularly in dry matter recovery, aerobic stability (AS), and the modulation of volatile fatty acid profiles. EOB demonstrated the highest efficacy by increasing acetic acid levels and reducing the lactic/acetic acid ratio, indicating more stable heterolactic fermentation that is less prone to aerobic spoilage. These effects were more prominent in the autumn season, suggesting that additive performance may vary according to the environmental conditions. Despite the observed shifts in fermentation dynamics, no adverse effects were detected in the chemical composition of the silages, ensuring the stability of key nutritional components.
Additionally, LEO was associated with an increased crude protein content in the autumn, while LASA and LEO contributed to higher total digestible nutrient levels, potentially supporting enhanced productive performance in dairy cows consuming these silages. The superior and consistent performance of EOB across multiple fermentation metrics highlights its promise as a viable strategy to enhance the conservation and nutritional quality of TMR silages. Thus, the use of phytogenic and ionophore additives presents an effective technological approach for improving silage stability and feed value, particularly in high-performance dairy production systems.
Author Contributions
Conceptualization, I.P.d.O.A. and M.A.P.O.J.; methodology, M.R. and R.C.d.A.; validation, A.C.A.O. and T.F.; formal analysis, Y.A.d.S. and M.F.d.O.; investigation, I.P.d.O.A.; resources, M.A.P.O.J.; data curation, T.F.; writing—original draft preparation, I.P.d.O.A.; writing—review and editing, M.A.P.O.J., M.R., and G.R.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES; Brasília, DF, Brazil– No. 0001) and the Development of Education, Science and Technology (FUNDECT; Mato Grosso do Sul, MS, Brazil–TO 007/2023 SIAFIC:32817 and TO 118/2024 SIAFIC 813).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Brazilian Agricultural Research Corporation (EMBRAPA, Mato Grosso do Sul, MS, Brazil) for providing support and assistance.
Conflicts of Interest
Embrapa is a non-profit public institution under the Ministry of Agriculture and Livestock of Brazil. Its work focuses on the development of technologies and knowledge for agriculture and livestock, with an emphasis on sustainability and food security. Dr. Marciana Retore is affiliated with Embrapa, which operates on a non-profit basis. Therefore, the authors state that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Susanto, I.; Rahmadani, M.; Wiryawan, K.G.; Laconi, E.B.; Jayanegara, A. Evaluation of Essential Oils as Additives during Fermentation of Feed Products: A Meta-Analysis. Fermentation 2023, 9, 583. [Google Scholar] [CrossRef]
- Playne, M.J.; Mc Donald, P.T. The Buffering Constituents of Herbage and of Silage. J. Sci. Food Agric. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Jobim, C.C.; Nussio, L.G.; Reis, R.A.; Schimidt, P. Avanços Metodológicos Na Avaliação Da Qualidade Da Forragem Conservada Methodological Advances in Evaluation. Rev. Bras. Zootec. 2007, 36, 101–119. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Wang, Y.; Guo, Z.; Wang, G.; Zhang, Y. The Performance of Plant Essential Oils against Lactic Acid Bacteria and Adverse Microorganisms in Silage Production. Front. Plant Sci. 2023, 14, 1285722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Z.; Li, J.; Chen, L.; Shao, T. Effects of Storage Temperature and Combined Microbial Inoculants on Fermentation End Products and Microbial Populations of Italian Ryegrass (Lolium multiflorum Lam.). Silage. J. Appl. Microbiol. 2018, 125, 1682–1691. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and Microbiota Transplantation to Determine the Role of Microbiota on the Fermentation Type of Oat Silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, M.; Yan, Y.; Sun, P.; Yan, X.; Liu, M.; Na, R.; Jia, Y.; Cha, S.; Guo, G. Characteristics of Isolated Lactic Acid Bacteria and Their Effects on the Silage Quality. Asian Australas. J. Anim. Sci. 2017, 30, 819–827. [Google Scholar] [CrossRef]
- de Andrade, R.C.; Orrico Junior, M.A.P.; da Silva, Y.A.; Retore, M.; Fernandes, T.; Orrico, A.C.A.; Junior, F.M.d.V.; Amaral, I.P.d.O. Impact of Monensin Sodium and Essential Limonene Oil on the Fermentation and Chemical Composition of Total Mixed Ration Silages with Moisture Variations. Agriculture 2024, 14, 1319. [Google Scholar] [CrossRef]
- Lazzari, G.; Poppi, A.C.O.; Machado, J.; Bueno, A.V.I.; Gomes, A.L.M.; Jobim, C.C.; Daniel, J.L.P. Effects of Protein Source and Lipid Supplementation on Conservation and Feed Value of Total Mixed Ration Silages for Finishing Beef Cattle. J. Anim. Sci. 2021, 99, skab032. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Pryce, J.D. A Modification of Barker-Summerson Method for the Determination of Lactic Acid. Analyst 1969, 94, 1151–1152. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Besharati, M.; Palangi, V.; Ghozalpour, V.; Nemati, Z.; Ayaşan, T. Essential Oil and Apple Pomace Affect Fermentation and Aerobic Stability of Alfalfa Silage. S. Afr. J. Anim. Sci. 2021, 51, 371–377. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Lazzari, G.; Jobim, C.C.; Daniel, J.L.P. Ensiling Total Mixed Ration for Ruminants: A Review. Agronomy 2020, 10, 879. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef]
- Amaral, I.P.d.O.; Orrico Junior, M.A.P.; Retore, M.; Fernandes, T.; América, Y.; De Oliveira, M.F.; Orrico, A.C.A. The Fermentative and Nutritional Effects of Limonene and a Cinnamaldehyde–Carvacrol Blend on Total Mixed Ration Silages. Fermentation 2025, 11, 415. [Google Scholar] [CrossRef]
- Susanto, I.; Rahmadani, M.; Wiryawan, K.G.; Jayanegara, A. A Meta-Analysis on the Influence of Essential Oils on Chemical Composition and Fermentative Quality of Silage. IOP Conf. Ser. Earth Environ. Sci. 2023, 1183, 012006. [Google Scholar] [CrossRef]
- de Lana Sousa, B.M.; de Jesus Santos, S.; Backes, A.A.; Silva, C.M.; Fagundes, J.L.; Blank, A.F.; dos Santos Filho, J.R. “Alecrim Pimenta” Nanoformulated Essential oil (Lippia Sidoides) as Additive in Consortium Silages. Cienc. Anim. Bras. 2023, 24, e73623. [Google Scholar] [CrossRef]
- da Silva, V.F.; de Souza, F.J.A.; da Silva, J.R.; Filho, A.S.S.; Miranda, E.S.; de Oliveira, J.C.A.; Mesquita, A.A.; Negrão, F.d.M. Uso de Aditivos Nas. Silagens de Capins Tropicais: Revisão de Literatura. Braz. J. Anim. Environ. Res. 2024, 7, e68716. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Williams, P.; Schmidt, R.J.; Hu, W. A Blend of Essential Plant Oils Used as an Additive to Alter Silage Fermentation or Used as a Feed Additive for Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 4793–4800. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhang, L.; Wei, M.; Wu, B.; Xiao, M.; Zhang, R.; Ju, J.; Dong, C.; Du, L.; Zheng, Y.; et al. Effects of Lactobacillus plantarum (L) and Molasses (M) on Nutrient Composition, Aerobic Stability, and Microflora of Alfalfa Silage in Sandy Grasslands. Front. Microbiol. 2024, 15, 1358085. [Google Scholar] [CrossRef]
- Wang, F.; Nishino, N. Ensiling of Soybean Curd Residue and Wet Brewers Grains with or without Other Feeds as a Total Mixed Ration. J. Dairy Sci. 2008, 91, 2380–2387. [Google Scholar] [CrossRef]
- Cantoia Júnior, R.; Capucho, E.; Garcia, T.M.; Del Valle, T.A.; Campana, M.; Zilio, E.M.C.; Azevedo, E.B.; Morais, J.P.G. Lemongrass Essential Oil in Sugarcane Silage: Fermentative Profile, Losses, Chemical Composition, and Aerobic Stability. Anim. Feed. Sci. Technol. 2020, 260, 114371. [Google Scholar] [CrossRef]
- Orrico, A.C.A.; Lopes, L.S.; Alves, J.P.; Mendes, S.S.; Galeano, E.S.J.; Junior, M.A.P.O.; Fernandes, T.; Retore, M. Yield, Chemical Composition, and Efficiency of Utilization of Applied Nitrogen from BRS Kurumi Pastures. Cienc. Rural 2023, 53, e20210461. [Google Scholar] [CrossRef]
- Kang, N.Q.; Hu, Y.Y.; Zhang, Z.W.; Lü, X.T. Changes of Mineral Nutrition (K, Ca, and Mg) in Soil and Plants Following Historical Nitrogen Inputs in a Temperate Steppe: The Implications for Grass Tetany. Plant Soil 2023, 491, 57–68. [Google Scholar] [CrossRef]
- Foskolos, A.; Cavini, S.; Ferret, A.; Calsamiglia, S. Effects of Essential Oil Compounds Addition on Ryegrass Silage Protein Degradation. Can. J. Anim. Sci. 2016, 96, 100–103. [Google Scholar] [CrossRef]
- de Oliveira, S.A.; da Silva, T.J.H.; Marques, S.A.J.; Bezerra, F.P.; de Pinho, C.K.A.; Marques, C.C.; Chaves, G.A.L.; Gomes, S.A.C.; Pinho, C.J.V.C. Chemical Composition and Fermentation Characteristics of Maize Silage with Citrus Pulp. Rev. Bras. Saude Prod. Anim. 2022, 23, e21352022. [Google Scholar] [CrossRef]
- Hodjatpanah-Montazeri, A.; Danesh Mesgaran, M.; Vakili, A.; Tahmasebi, A.M. Effect of Essential Oils of Various Plants as Microbial Modifier to Alter Corn Silage Fermentation and in Vitro Methane Production. Iran. J. Appl. Anim. Sci. 2016, 6, 269–276. [Google Scholar]
- Ding, W.R.; Long, R.J.; Guo, X.S. Effects of Plant Enzyme Inactivation or Sterilization on Lipolysis and Proteolysis in Alfalfa Silage. J. Dairy Sci. 2013, 96, 2536–2543. [Google Scholar] [CrossRef]
- Zoetis Brazil. Taurotec®–Lasalocida Sódica 15%: Package Insert [Internet]. São Paulo: Zoetis Brazil. 2020. Available online: https://www.zoetis.com.br/especies/bovinos/taurotec/files/bula_bov-alt-40014654-taurotec.pdf (accessed on 28 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).