Digestate-Based Liquid Growth Medium for Production of Microbial Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Medium Development from Digestate Fractions
2.3. Microorganisms
2.4. Shaken Culture Experiments
2.4.1. Strains Screening for Biomass Producing Capability on ELP Medium
2.4.2. Growth and Chitosan Production Kinetics on ELP Medium
2.5. Analytical Methods
2.5.1. ELP Medium Characterization
2.5.2. Chitosan Extraction
2.5.3. Determination of Minimum Inhibitory Concentration of Fungal Chitosan
2.5.4. Fourier Transform Infrared (FT-IR) Spectroscopy, Elemental Analysis and Thermogravimetric Analysis (TGA) of Chitosan Samples
2.5.5. Intrinsic Viscosity and Average Molecular Mass Determination
3. Results and Discussion
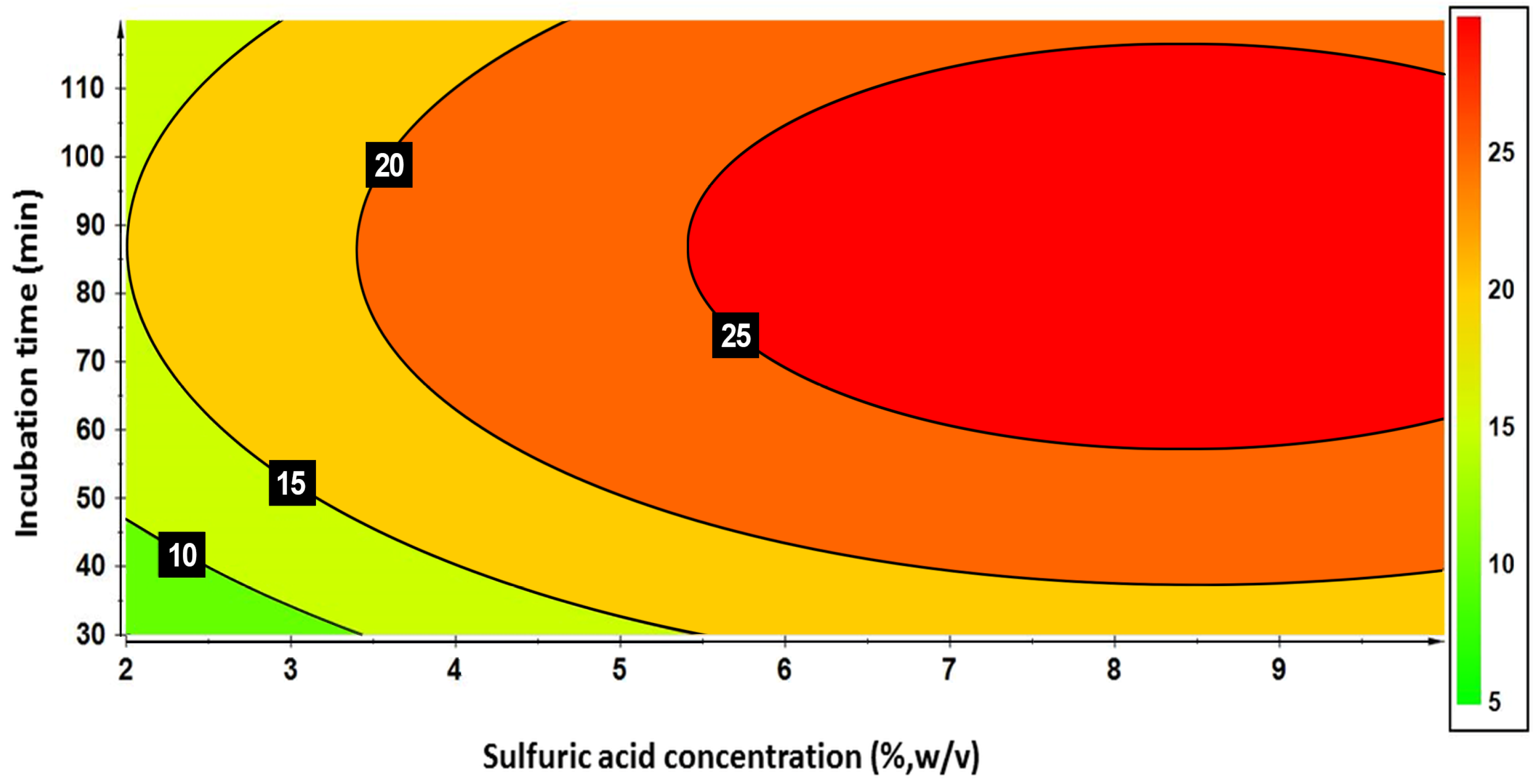
3.1. Production of Growth Medium from Digestate Fractions
3.2. Screening of Strains for Biomass- and Chitosan-Producing Ability on ELP Medium
| Strain | Growth Medium | CVP a (mg L−1) | rc b (mg L−1 d−1) | YC/X c (mg g−1 Biomass) | Reference |
|---|---|---|---|---|---|
| A. blakesleeana NRRL 2696 | ELP d | 444 | 111 | 59 | This study |
| A. blakesleeana NRRL 1340 | GPY e (2.0–1.0–0.1 g L−1) with added 5.0 g L−1 (NH4)2SO4 | 282 | 141 | 170 | [22] |
| A. butleri NCIM977 | GPY e (20.0–10.0–1.0 g L−1) | 570 | 190 | 55 | [32] |
| A. coerulea ATCC14076 | GPY e (10.0–5.0–10.0 g L−1) | 1860 | 624 | 300 | [33] |
| A. glauca | GPY e (20.0–10.0–1.0 g L−1) | 650 | 324 | 74 | [7] |
| A. coerulea CTCC93105 | Glucose-based medium (20 g L−1) with 5% added soybean pomace | 4110 | 684 | 267 | [34] |
| R. oryzae NRRL 1510 | ELP d | 324 | 81 | 40 | This study |
| R. oryzae USBD 0602 | GPY e (20.0–10.0–1.0 g L−1) with added (NH4)2SO4 | 280 | 55 | 49 | [29] |
| R. oryzae USBD 0263 | GPY e (20.0–10.0–1.0 g L−1) with added (NH4)2SO4 | 220 | 71.4 | 44 | [29] |
| R. oryzae USBD 0602 | GPY e (5.0–1.0–0.1 g L−1) | 56 | 19 | 49 | [35] |
| R. oryzae MTCC262 | DW f with added gibberellic acid (0.1 mg L−1) | 1130 | 377 | 136 | [36] |
| R. oryzae PAS17 | Molasses-based medium (7%) with added MgSO4 | 1500 | 187 | 140 | [37] |
| R. oryzae ME-F12 | Corn straw acid hydrolysate | 580 | n. r. | 112 | [38] |
| R. oryzae MTCC262 | DW f with added (NH4)2HPO4 (8 g L−1) and yeast extract | 620 | n. r. | 100 | [39] |
| R. oryzae NRRL395 | Ground corn grains-based liquid medium (10%) | 406 | 135 | n. r. | [41] |
| R. oryzae NRRL395 | Ground rice-based liquid medium (10%) | 700 | 233 | n. r | [41] |
| R. oryzae SWERI | Sugar cane molasses | 940 | 118 | 77 | [42] |
| R. oryzae SWERI | Sugar beet molasses | 713 | 89 | 65 | [42] |
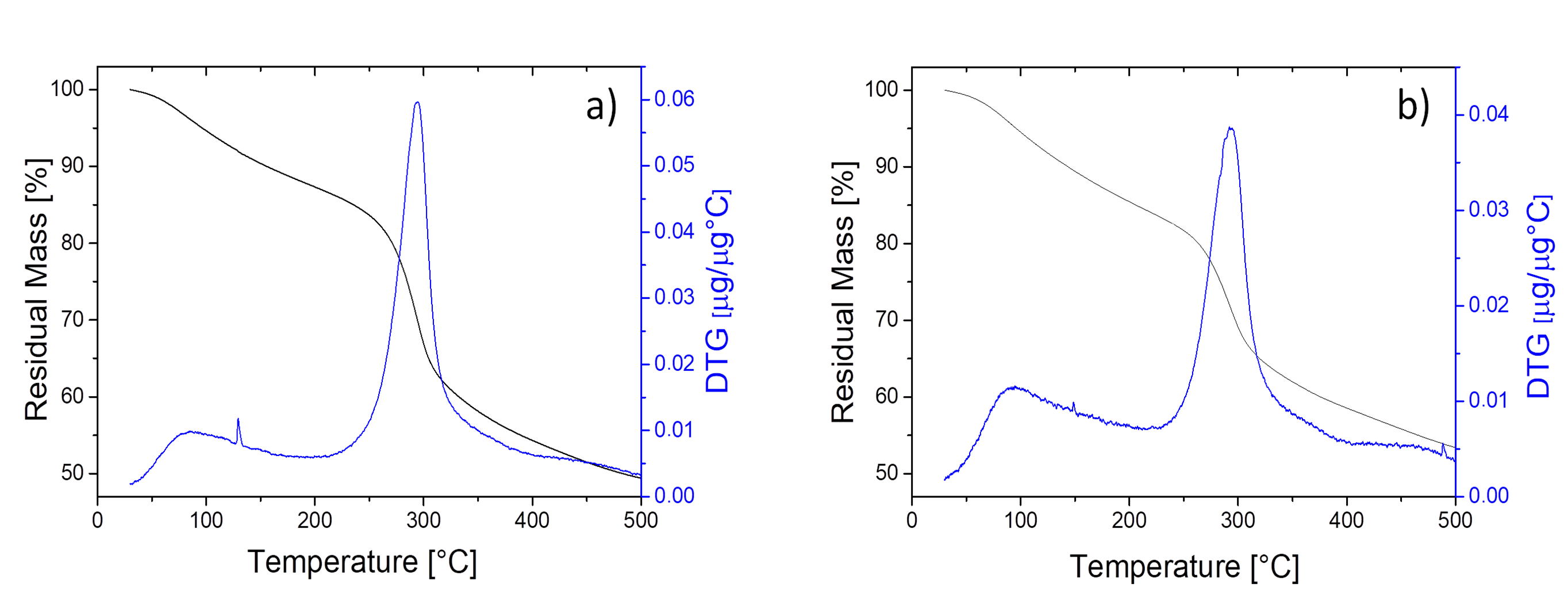
3.3. Characterization of Fungal Chitosans
3.4. Antibacterial Activity of Fungal Chitosans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ab-Cht | Absidia blakesleeana chitosan |
| AIM | Alkali-insoluble material |
| CVP | Chitosan volumetric production |
| DD | Deacetylation degree |
| DLF | Digestate liquid fraction |
| DSF | Digestate solid fraction |
| DTG | Derivative thermogravimetry |
| ELP | Enriched liquid phase |
| FT-IR | Fourier transform infrared |
| GPY | Glucose-peptone-yeast extract |
| HH | Hemicellulose hydrolysate |
| LMWCh | Low-molecular-weight chitosan |
| MIC | Minimum inhibitory concentration |
| Mv | Viscosimetric average molecular weight |
| rc | Average daily productivity |
| Ro-Cht | Rhizopus oryzae chitosan |
| RY | Recovery yield |
| TGA | Thermogravimetric analysis |
| TS | Total sugars |
| Yc/x | Chitosan yield referred to biomass dry weight |
| Yx/s | Biomass yield referred to the substrate consumed |
References
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 43–59. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan production by fungi: Current state of knowledge, future opportunities and constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef]
- Chien, R.; Yen, M.; Mau, J. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Biores. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Hu, K.J.; Hu, J.L.; Ho, K.P.; Yeung, K.W. Fungal screening for chitosan producers and copper adsorption capabilities of fungal chitosan and chitosanaceous materials. Carbohydr. Polym. 2004, 58, 45–52. [Google Scholar] [CrossRef]
- Streit, F.; Koch, F.; Laranjeira, M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Brazil. J. Microbiol. 2009, 40, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.; Lins, C.I.M.; Dos Santos, E.R.; Silva, M.C.F.; Campos-Takaki, G.M. Microbial enhance of chitosan production by Rhizopus arrhizus using agroindustrial substrates. Molecules 2012, 17, 4904–4914. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.R.; Stamford, T.C.; Stamford-Arnaud, T.M.; de Oliveira Franco, L.; do Nascimento, A.E.; Cavalcante, H.M.; Macedo, R.O.; de Campos-Takaki, G.M. Effect of corn steep liquor (CSL) and cassava wastewater (CW) on chitin and chitosan production by Cunninghamella elegans and their physicochemical characteristics and cytotoxicity. Molecules 2014, 19, 2771–2792. [Google Scholar] [CrossRef]
- Namboodiri, M.M.T.; Pakshirajan, K. Sustainable and green approach of chitosan production from Penicillium citrinum biomass using industrial wastewater as a cheap substrate. J. Environ. Manag. 2019, 240, 431–440. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthigadevi, G.; Bharathiraja, B.; Kumar, R.P.; Abo, L.D.; Prabhu, S.V.; Balachandar, R.; Jayakumar, M. Current and prognostic overview on the strategic exploitation of anaerobic digestion and digestate: A review. Environ. Res. 2023, 216, 114526. [Google Scholar] [CrossRef]
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef]
- Carota, E.; Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Lignocellulolytic potential of the recently described species Aspergillus olivimuriae on different solid wastes. Appl. Sci. 2021, 11, 5349. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Deschatelets, L.; Yu, E.K. A simple pentose assay for biomass conversion studies. Appl. Microbiol. Biotechnol. 1986, 24, 379–385. [Google Scholar] [CrossRef]
- Jüttner, F. Interference with ammonium determination by the indophenol-type reaction of salicylate and dichloroisocyanurate. Fresen. J. Anal. Chem. 1999, 363, 128–129. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sun, S.W.; Lin, Y.C.; Weng, Y.M.; Chen, M.J. Efficiency improvements on ninhydrin method for amino acid quantification. J. Food Comp. Anal. 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Ainsworth, E.; Gillespie, K. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Rane, K.D.; Hoover, D.G. Production of chitosan by fungi. Food Biotechnol. 1993, 7, 11–33. [Google Scholar] [CrossRef]
- Standard’s CLSI Document M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Dos Santos, Z.M.; Caroni, A.L.P.F.; Pereira, M.R.; Da Silva, D.R.; Fonseca, J.L.C. Determination of deacetylation degree of chitosan: A comparison between conductometric titration and CHN elemental analysis. Carbohydr. Res. 2009, 344, 2591–2595. [Google Scholar] [CrossRef]
- Amitaye, A.N.; Elemike, E.E.; Akpeji, H.B.; Amitaye, E.; Hossain, I.; Mbonu, J.I.; Aziza, A.E. Chitosan: A sustainable biobased material for diverse applications. J. Environ. Chem. Eng. 2024, 12, 113208. [Google Scholar] [CrossRef]
- Berger, L.R.R.; Stamford, T.C.M.; de Oliveira, K.Á.R.; Pessoa, A.D.M.P.; de Lima, M.A.B.; Pintado, M.M.E.; Câmara, M.P.S.; de Oliveira Franco, L.; Magnani, M.; de Souza, E.L. Chitosan produced from Mucorales fungi using agroindustrial by-products and its efficacy to inhibit Colletotrichum species. Int. J. Biol. Macromol. 2018, 108, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.L.; Bartnicki-Garcia, S. Chitosan synthesis by the tandem action of chitin synthetase and chitin deacetylase from Mucor rouxii. Biochemistry 1984, 23, 1065–1073. [Google Scholar] [CrossRef]
- Tan, S.C.; Tan, T.K.; Wong, S.M.; Khor, E. The chitosan yield of zygomycetes at their optimum harvesting time. Carbohydr. Polym. 1996, 30, 239–242. [Google Scholar] [CrossRef]
- El-Mekawy, A.; El-Baz, A.F.; Soliman, A.E.; Hudson, S. Statistical modeling and optimization of chitosan production from Absidia coerulea using response surface methodology. Curr. Biotechnol. 2013, 2, 125–133. [Google Scholar] [CrossRef]
- Campos-Takaki, G.M.; Beakes, G.W.; Dietrich, S.M.C. Electron microscopic X-ray microprobe and cytochemical study of isolated cell walls of mucoralean fungi. Trans. Brit. Mycol. Soc. 1983, 80, 536–541. [Google Scholar] [CrossRef]
- Vaingankar, P.N.; Juvekar, R.A. Fermentative production of mycelial chitosan from zygomycetes: Soil optimization and chemical-physical characterization. Adv. Biosci. Biotechnol. 2014, 5, 940. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Ilari, P.; Tarsi, R.; Dubini, B.; Xia, W. Chitosan from Absidia coerulea. Carbohydr. Polym. 1994, 25, 45–50. [Google Scholar] [CrossRef]
- Jiang, L.; Pan, S.; Kim, J.M. Influence of nitrogen source on chitosan production by Absidia coerulea CTCC AF 93105. Carbohydr. Polym. 2011, 86, 359–361. [Google Scholar] [CrossRef]
- Nadarajah, K.; Kader, J.; Mazmira, M.; Paul, D.C. Production of chitosan by fungi. Pak. J. Biol. Sci. 2001, 4, 263–265. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Enhancement of growth and chitosan production by Rhizopus oryzae in whey medium by plant growth hormones. Int. J. Biol. Macromol. 2008, 42, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tasar, O.C.; Erdal, S.; Taskin, M. Production of chitosan by psychrotolerant Rhizopus oryzae under non-sterile open fermentation conditions. Int. J. Biol. Macromol. 2016, 89, 428–433. [Google Scholar] [CrossRef]
- Tai, C.; Li, S.; Xu, Q.; Ying, H.; Huang, H.; Ouyang, P. Production of chitosan from maize straw hemicellulose hydrolysate: Impact of degradation products on Rhizopus oryzae growth and chitosan fermentation. Lett. Appl. Microbiol. 2010, 51, 278–284. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guha, A.K.; Chatterjee, B.P. Evaluation of the quantity and quality of chitosan products from Rhizopus oryzae using processing waste from food, whey and molasses. J. Environ. Manag. 2019, 251, 109565. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Lai, C.; Fan, Y.; Ouyang, J.; Yong, Q. Fungal chitosan production using xylose rich of corn stover prehydrolysate by Rhizopus oryzae. Biotechnol. Biotechnol. Equip. 2017, 31, 1160–1166. [Google Scholar] [CrossRef]
- Hang, Y.D. Chitosan production from Rhizopus oryzae mycelia. Biotechnol. Lett. 1990, 12, 911–912. [Google Scholar] [CrossRef]
- Al-Sharnouby, S.; Abd-Elfatah, S.I. Fermentative production, characterization and antimicrobial activity of chitosan from some zygomycetes fungi. Egypt. J. Chem. 2022, 65, 1579–1589. [Google Scholar] [CrossRef]
- Kittur, F.S.; Kumar, A.B.V.; Tharanathan, R.N. Low molecular weight chitosans preparation by depolymerization with Aspergillus niger pectinase, and characterization. Carbohydr. Res. 2003, 338, 1283–1290. [Google Scholar] [CrossRef]
- Nimesh, S.; Thibault, M.M.; Lavertu, M.; Buschmann, M.D. Enhanced gene delivery mediated by low molecular weight chitosan/DNA complexes: Effect of pH and serum. Mol. Biotechnol. 2010, 46, 182–196. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of low molecular weight chitosan and chitooligosaccharides (COS): A review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Niederhofer, A.; Müller, B.W. A method for direct preparation of chitosan with low molecular weight from fungi. Eur. J. Pharm. Biopharm. 2004, 57, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, Y.; Qiu, Y.; Wang, X.; Hu, Y.; Yang, J.; Cai, J.; Kennedy, J.F. A new green technology for direct production of low molecular weight chitosan. Carbohydr. Polym. 2008, 74, 127–132. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Johns, J. Thermal behavior of chitosan/natural rubber latex blends TG and DSC analysis. J. Therm. Anal. Calorim. 2008, 92, 801–806. [Google Scholar] [CrossRef]
- Fai, A.E.C.; Stamford, T.C.; Stamford-Arnaud, T.M.; Santa-Cruz, P.D.A.; da Silva, M.C.F.; Campos-Takaki, G.M.; Stamford, T.L. Physico-chemical characteristics and functional properties of chitin and chitosan produced by Mucor circinelloides using yam bean as substrate. Molecules 2011, 16, 7143–7154. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state-of-the-art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Mellegård, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int. J. Biol. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.; Opwis, K.; Knittel, D.; Schollmeyer, E.; Nickisch-Hartfiel, A. Inhibition of microbial pathogens by fungal chitosan. Int. J. Biol. Macromol. 2010, 47, 10–14. [Google Scholar] [CrossRef]
- Boamah, P.O.; Onumah, J.; Agolisi, M.H.; Idan, F. Application of low molecular weight chitosan in animal nutrition, husbandry, and health: A review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100329. [Google Scholar] [CrossRef]



| Treatment a | T b (°C) | SLR c | Total Sugars | Reducing Sugars | ||
|---|---|---|---|---|---|---|
| VC (g L−1) d | RY (%) | VC (g L−1) d | RY (%) | |||
| Aqueous extraction | 60 | 1:20 | 1.2 ± 0.1 | 1.4 ± 0.4 | 0.9 ± 0.1 | 115.2 ± 10.9 |
| Aqueous extraction | 90 | 1:20 | 1.3 ± 0.1 | 1.9 ± 0.1 | 1.2± 0.1 | 192.0 ± 16.5 |
| Aqueous extraction | 120 | 1:20 | 2.4 ± 0.1 | 5.3 ± 0.2 | 1.5 ± 0.1 | 236.2 ± 19.7 |
| Aqueous extraction | 60 | 1:10 | 1.5 ± 0.3 | 1.3 ± 0.0 | 1.2 ± 0.1 | 95.3 ± 4.9 |
| Aqueous extraction | 90 | 1:10 | 2.1 ± 0.3 | 2.1 ± 0.3 | 1.5 ± 0.1 | 127.2 ± 5.4 |
| Aqueous extraction | 120 | 1:10 | 3.7 ± 0.1 | 4.5 ± 0.1 | 2.0 ± 0.1 | 179.7 ± 5.5 |
| Mild alkaline hydrolysis 6% NaOH (w/v) | 90 | 1:10 | 4.4 ± 0.2 | 5.6 ± 0.2 | 1.4 ± 0.0 | 108.7 ± 1.1 |
| Mild alkaline hydrolysis 6% NaOH (w/v) | 120 | 1:10 | 6.0 ± 0.3 | 8.2 ± 0.4 | 2.6 ± 0.1 | 242.0 ± 12.5 |
| Mild acid hydrolysis of 6% H2SO4 (w/v) | 90 | 1:10 | 7.4 ± 0.4 | 10.3 ± 0.6 | 4.4 ± 0.1 | 433.5 ± 14.9 |
| Mild acid hydrolysis of 6% H2SO4 (w/v) | 120 | 1:10 | 24.1 ± 1.3 | 36.1 ± 2.1 | 22.5 ± 1.4 | 2412.0 ± 153 |
| Run Number | H2SO4 Concentration (X1) (%, w/v) | Incubation Time (X2) (min) | Concentrations of Total Sugars (g L−1) | |
|---|---|---|---|---|
| Observed | Predicted | |||
| 1 | 2 [−1] | 30 [−1] | 4.38 | 4.87 |
| 2 | 2 [−1] | 30 [−1] | 4.49 | 4.87 |
| 3 | 6 [0] | 30 [−1] | 16.80 | 15.89 |
| 4 | 6 [0] | 30 [−1] | 12.01 | 15.89 |
| 5 | 10 [1] | 30 [−1] | 19.30 | 16.87 |
| 6 | 10 [1] | 30 [−1] | 17.5 | 16.87 |
| 7 | 2 [−1] | 60 [−0.333] | 12.38 | 12.74 |
| 8 | 2 [−1] | 60 [−0.333] | 14.22 | 12.74 |
| 9 | 6 [0] | 60 [−0.333] | 25.36 | 23.76 |
| 10 | 6 [0] | 60 [−0.333] | 25.15 | 23.76 |
| 11 | 10 [1] | 60 [−0.333] | 24.40 | 24.74 |
| 12 | 10 [1] | 60 [−0.333] | 24.24 | 24.74 |
| 13 | 2 [−1] | 120 [1] | 10.89 | 11.48 |
| 14 | 2 [−1] | 120 [1] | 11.84 | 11.48 |
| 15 | 6 [0] | 120 [1] | 25.98 | 22.49 |
| 16 | 6 [0] | 120 [1] | 21.83 | 22.49 |
| 17 | 10 [1] | 120 [1] | 20.61 | 23.48 |
| 18 | 10 [1] | 120 [1] | 24.14 | 23.48 |
| 20 | 6 [0] | 75 [0] | 23.02 | 25.56 |
| 21 | 6 [0] | 75 [0] | 24.89 | 25.56 |
| 22 | 6 [0] | 75 [0] | 25.89 | 25.56 |
| Model Terms a and Parameters | Original Model | Refined Model |
|---|---|---|
| Constant (βo) | 25.6 ± 0.8 *** | 25.6 ± 0.8 *** |
| First order coefficient of X1 (β1) | 5.9 ± 0.6 *** | 6.0 ± 0.5 *** |
| First order coefficient of X2 (β2) | 3.3 ± 0.5 *** | 3.3 ± 0.5 *** |
| Second order coefficient of X1 (β1·β1) | −5.0 ± 0.9 *** | −5.0 ± 0.9 *** |
| Second order coefficient of X2 (β2·β2) | 6.4 ± 0.9 *** | 6.4 ± 0.9 *** |
| Interaction coefficient X1×2 (β1·β2) | −0.63 ± 0.7 n.s. | n.i. |
| FMOD | 50.6 ± 1.4 *** | 63.4 ± 1.9 *** |
| R2 | 0.944 | 0.941 |
| R2adj | 0.925 (DF = 16) | 0.926 (DF = 16) |
| Q2 | 0.890 | 0.895 |
| FME | 1.63 (p = 0.230) | 1.54 (p = 0.256) |
| Fungal Strains | Biomass (g L−1) | TS Consumption (%) | YX/S (g g−1) |
|---|---|---|---|
| Absidia blakesleeana NRRL 1304 | 0.55 ± 0.06 E | 15.12 ± 0.22 FG | 0.15 ± 0.01 FG |
| Absidia blakesleeana NRRL 2696 | 7.58 ± 0.08 AB | 75.23 ± 2.12 BC | 0.42 ± 0.02 AB |
| Absidia coerulea NRRL 1312 | 2.59 ± 0.06 D | 29.42 ± 3.28 E | 0.37 ± 0.05 BC |
| Absidia coerulea NRRL 1315 | 0.76 ± 0.04 E | 14.23 ± 0.11 FG | 0.22 ± 0.01 EF |
| Absidia coerulea NRRL A-9483 | 0.59 ± 0.02 E | 10.11 ± 0.16 G | 0.24 ± 0.01 DE |
| Absidia corymbifera NRRL 2798 | 0.61 ± 0.04 E | 11.20 ± 2.23 FG | 0.22 ± 0.06 EF |
| Absidia glauca NRRL 1324 | 0.74 ± 0.03 E | 10.03 ± 1.12 G | 0.31 ± 0.05 CD |
| Absidia glauca NRRL 2799 | 1.02 ± 0.08 E | 92.45 ± 3.08 A | 0.05 ± 0.01 H |
| Absidia glauca NRRL 3010 | 0.61 ± 0.04 E | 20.22 ± 1.08 F | 0.13 ± 0.02 G |
| Absidia repens NRRL 1337 | 0.61 ± 0.02 E | 8.05 ± 0.09 G | 0.31 ± 0.01 CD |
| Aspergillus tubingensis NRRL 4700 | 6.63 ± 0.15 B | 91.23 ± 1.34 A | 0.30 ± 0.01 CD |
| Benjaminiella poitrasii NRRL 2845 | 4.46 ± 0.13 C | 72.47 ± 0.52 C | 0.26 ± 0.01 DE |
| Cunninghamella elegans NRRL1392 | 0.70 ± 0.16 E | 14.3 ± 1.10 FG | 0.20 ± 0.04 EFG |
| Mucor moelleri As. Col E | 4.29 ± 0.41 C | 77.23 ± 2.67 BC | 0.26 ± 0.03 DE |
| Mucor rouxii NRRL 1894 | 3.95 ± 0.16 C | 81.34 ± 3.02 B | 0.20 ± 0.02 EFG |
| Rhizopus oryzae NRRL 1510 | 8.12 ± 0.21 A | 77.11 ± 2.01 BC | 0.44 ± 0.02 AB |
| Rhizopus oryzae NRRL 2625 | 6.89 ± 0.67 AB | 55.54 ± 2.12 D | 0.52 ± 0.07 A |
| Fungal Strains | AIM (mg g−1 Biomass) | Chitosan (% on AIM) | YC/X (mg g−1 Biomass) | CVP (mg L−1) | rc (mg L−1 d−1) |
|---|---|---|---|---|---|
| A. blakesleeana NRRL 2696 | 245.9 ± 12.2 C | 21.0 ± 1.0 A | 59.0 ± 3.0 A | 443.5 ± 17.7 A | 110.9 ± 4.4 A |
| A. coerulea NRRL 1312 | 610.0 ± 21.0 A | 7.0 ± 0.3 C | 44.0 ± 5.0 AB | 49.1 ± 4.9 E | 12.3 ± 1.2 E |
| A. tubingensis NRRL 4700 | 354.5 ± 3.0 B | 4.2 ± 0.3 C | 13.0 ± 1.1 D | 86.0 ± 6.9 DE | 21.5 ± 1.7 DE |
| B. poitrasii NRRL 2845 | 128.0 ± 9.1 E | 16.2 ± 1.0 AB | 20.1 ± 2.0 CD | 91.0 ± 12.0 DE | 22.8 ± 3.0 DE |
| M. moelleri As. Col E | 155.1 ± 42.0 DE | 20.4 ± 1.1 A | 31.3 ± 6.2 BC | 134.3 ± 14.4 CD | 33.6 ± 3.6 CD |
| M. rouxii NRRL 1894 | 205.0 ± 19.1 DE | 13.9 ± 1.1 B | 29.1 ± 1.4 BCD | 139.0 ± 7.4 CD | 34.7 ± 1.1 CD |
| R. oryzae NRRL 2625 | 147.4 ± 26.2 DE | 18.1 ± 3.4 AB | 25.2 ± 1.1 CD | 176.2 ± 22.3 C | 44.1 ± 5.6 C |
| R. oryzae NRRL 1510 | 219.0 ± 6.9 CD | 18.4 ± 1.3 AB | 40.0 ± 8.1 AB | 324.1 ± 34.2 B | 81.0 ± 8.5 B |
| Samples | C (%) | H (%) | N (%) | S (%) | DD (%) |
|---|---|---|---|---|---|
| A blakesleeana NRRL 2696 | 32.91 | 6.61 | 6.12 | 1.25 | 86.45 |
| R oryzae NRRL 1510 | 36.94 | 6.65 | 6.84 | 0.08 | 84.18 |
| Low-molecular-weight chitosan (Fluka) | 39.69 | 7.18 | 7.49 | 0.19 | 90.69 |
| Chitosan Source | Regression Method | R2 | Intrinsic Viscosity (mL g−1) | Mv (Da) |
|---|---|---|---|---|
| A. blakesleeana NRRL 2696 | Huggins | 0.985 | 61.0 ± 2.3 | 19,996 |
| Kraemer | 0.952 | 61.1 ± 2.1 | 20,030 | |
| R. oryzae NRRL 1510. | Huggins | 0.998 | 23.6 ± 1.2 | 5385 |
| Kraemer | 0.997 | 24.1 ± 1.8 | 5525 |
| Chitosan Source | Pseudomonas syringae DSM 21482 (mg L−1) | Escherichia coli ATCC 9637 (mg L−1) | Bacillus subtilis DSM 10 (mg L−1) |
|---|---|---|---|
| A. blakesleeana NRRL 2696 | 160 | 300 | 360 |
| R. oryzae NRRL 1510 | 160 | 420 | 480 |
| Low-molecular-weight chitosan (Fluka) | 120 | 180 | 220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crognale, S.; Russo, C.; Carota, E.; Armentano, I.; Di Gregorio, F.; D’Annibale, A.; Cimini, A.; Petruccioli, M. Digestate-Based Liquid Growth Medium for Production of Microbial Chitosan. Fermentation 2025, 11, 469. https://doi.org/10.3390/fermentation11080469
Crognale S, Russo C, Carota E, Armentano I, Di Gregorio F, D’Annibale A, Cimini A, Petruccioli M. Digestate-Based Liquid Growth Medium for Production of Microbial Chitosan. Fermentation. 2025; 11(8):469. https://doi.org/10.3390/fermentation11080469
Chicago/Turabian StyleCrognale, Silvia, Cristina Russo, Eleonora Carota, Ilaria Armentano, Federico Di Gregorio, Alessandro D’Annibale, Alessio Cimini, and Maurizio Petruccioli. 2025. "Digestate-Based Liquid Growth Medium for Production of Microbial Chitosan" Fermentation 11, no. 8: 469. https://doi.org/10.3390/fermentation11080469
APA StyleCrognale, S., Russo, C., Carota, E., Armentano, I., Di Gregorio, F., D’Annibale, A., Cimini, A., & Petruccioli, M. (2025). Digestate-Based Liquid Growth Medium for Production of Microbial Chitosan. Fermentation, 11(8), 469. https://doi.org/10.3390/fermentation11080469










