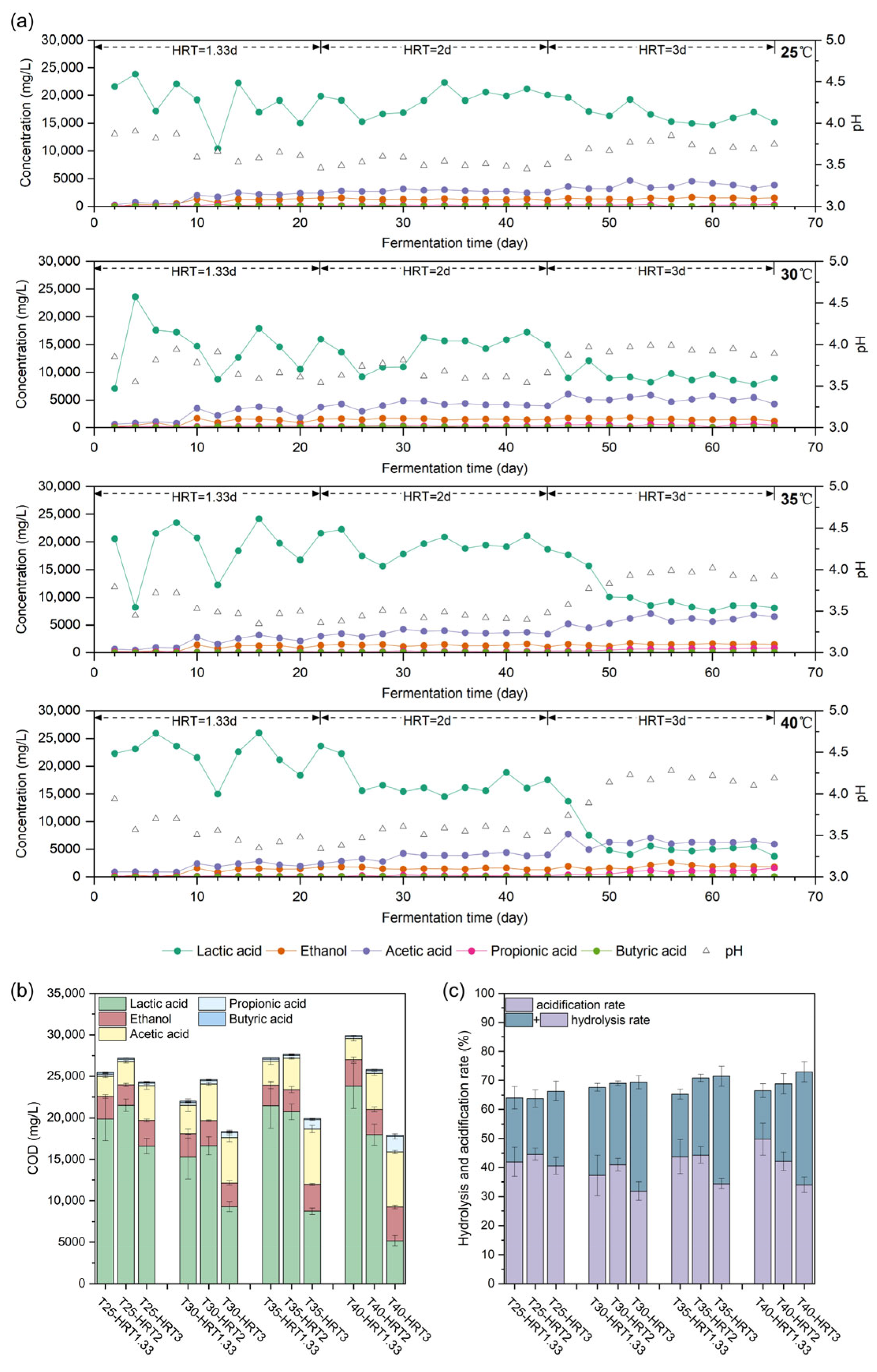
3.1. Fermentation Products Under Different Temperatures and HRTs
Four lab-scale reactors were operated for 66 days using actual FW slurry as the substrate, under controlled combinations of different temperatures and HRTs. Each reactor underwent three sequential fermentation phases with distinct HRT settings. Samples were collected every two days from all reactors to quantify the concentrations of organic acids and alcohols. The temporal profiles of these primary fermentation products throughout the experiment are shown in
Figure 1a, while COD-based product distributions are presented in
Figure 1b.
At an HRT of 1.33 days, all systems exhibited pronounced instability, and the reactor performance at different temperatures was relatively similar. After a short startup period of approximately two days, LA became the dominant product; however, LA concentrations fluctuated substantially in all reactors, with deviations exceeding ±3000 mg/L. This instability was mainly attributed to: (i) considerable variations in FW slurry composition caused by fluctuations in the incoming raw materials and operational conditions at the treatment facility, combined with the extremely short HRT, which made the system behave more like a fed-batch process than a truly semi-continuous one, thereby reducing buffering capacity and increasing sensitivity to feed variations; and (ii) the high dilution rate, which likely caused washout of a large portion of the microbial community during each feeding cycle, necessitating repeated re-establishment of community equilibrium. The stochastic nature of this re-equilibration process contributed to the observed day-to-day variation in fermentation performance, reflected in fluctuating product concentrations and pH values. After 8–10 days, acetic acid and ethanol began to appear alongside LA, presumably due to the adaptation and proliferation of microorganisms responsible for their production. Their yields remained relatively low, with average concentrations ranging from 2269 to 3204 mg/L for acetic acid and from 1190 to 1529 mg/L for ethanol, substantially lower than those of LA (14,342–22,363 mg/L). In all reactors, the pH remained below 3.65. Both the product spectrum and pH values were consistent with typical lactic acid-type fermentation [
14].
Prolonging the HRT exerted a stabilizing effect on the fermentation system. When the HRT was increased to 2 days, all reactors gradually reached a stable operating state after a short adjustment period. Although lactic acid-type fermentation remained the dominant pathway with acetic acid and ethanol as by-products, the average coefficient of variation (standard deviation/mean) of these major products decreased markedly from 9.2–20.0% at an HRT of 1.33 days to 4.2–7.5% at an HRT of 2 days. During this phase, the pH in all reactors remained within a narrow range (3.40–3.60), indicating a stable acidic environment conducive to lactic acid-type fermentation. When the HRT was extended to 3 days, all reactors exhibited a gradual increase in pH, with values rising to approximately 3.65, 3.85, 3.92, and 4.16 at 25, 30, 35, and 40 °C, respectively. This pH rise was accompanied by a decline in LA concentration and an increase in acetic acid, with higher temperatures amplifying these shifts relative to the HRT of 2 days. Additionally, propionic acid began to accumulate in reactors operated at 30 °C or higher.
To further illustrate the effects of temperature and HRT on the fermentation product spectrum, COD-based product concentrations and their relative distributions at each phase were compared (
Figure 1b). When the HRT was 2 days or less, all temperatures yielded similar product profiles: LA was the dominant metabolite with average concentration higher than 14,000 mg/L, followed by acetic acid and ethanol, while propionic and butyric acids remained low (100–300 mg/L and 0–100 mg/L, respectively). At an HRT of 3 days, temperature exerted a pronounced influence: higher temperatures led to a sharper decline in LA and greater increases in acetic and propionic acid concentrations. Spearman’s correlation analysis confirmed a negative association between average LA concentration and HRT (ρ = −0.68,
p < 0.05), and positive associations with acetic acid (ρ = 0.83,
p < 0.01) and propionic acid (ρ = 0.74,
p < 0.01). The magnitude of these shifts increased with temperature; for instance, at 40 °C, LA dropped from 16,842 ± 1211 mg/L (HRT = 2 days) to 4832 ± 600 mg/L (HRT = 3 days), whereas at 25 °C, the decrease was from 20,174 ± 700 mg/L to 15,556 ± 850 mg/L.
3.2. Hydrolysis Rate and Acidification Rate
During acidogenic fermentation, complex solid substrates are first depolymerized by extracellular enzymes into soluble organic compounds, which are subsequently converted into fermentation products by acidogenic microorganisms such as LAB and other fermentative taxa. Therefore, the hydrolysis rate and acidification rate are critical indicators, reflecting the degree of substrate solubilization and its subsequent transformation into target metabolites. These two parameters, expressed on a COD basis, were quantified under different conditions, and the results are presented in
Figure 1c.
The average hydrolysis rate showed a significant positive correlation with both fermentation temperature (ρ = 0.821,
p < 0.01) and HRT (ρ = 0.852,
p < 0.01). Consistent with previous findings, within the ambient-to-mesophilic temperature range relevant to this study (25–40 °C), elevated temperatures enhanced the solubilization of solid substrates [
30,
31]. This enhancement can be attributed to the enrichment of hydrolytic microorganisms and to the acceleration of enzymatic reaction kinetics at higher temperatures. Likewise, longer HRTs allowed substrates to remain in the reactor for extended periods, facilitating more complete interactions with hydrolases and thus improving hydrolysis efficiency. Consequently, when the operating conditions shifted from 25 °C and an HRT of 1.33 days to 40 °C and 3 days, the hydrolysis rate increased markedly from 64.0 ± 3.9% to 72.9 ± 3.4%. Notably, the hydrolysis rate exceeded 60% under all tested conditions, surpassing values reported in comparable FW fermentation studies [
32]. This relatively high efficiency is likely attributable, at least in part, to the pretreatment step applied to the feedstock, which removed a portion of the particulate solids, thereby lowering the initial TCOD and increasing the apparent hydrolysis rate compared with systems fed with untreated FW.
The acidification rate fluctuated markedly at an HRT of 1.33 days, reflecting the instability of the fermentation system under these operating conditions. Increasing the HRT to 2 days stabilized the fermentation process, resulting in more consistent acidification performance. However, further extending the HRT to 3 days did not enhance acidification; on the contrary, a statistically significant decrease (t-test, p < 0.05) was observed across all temperatures compared with HRT = 2 days. This decline may be attributed to the diversion of part of the soluble substrate toward secondary metabolic pathways or to the further conversion of lactic and acetic acids into other, as yet undetected, metabolites under prolonged retention conditions.
3.3. Microbial Community
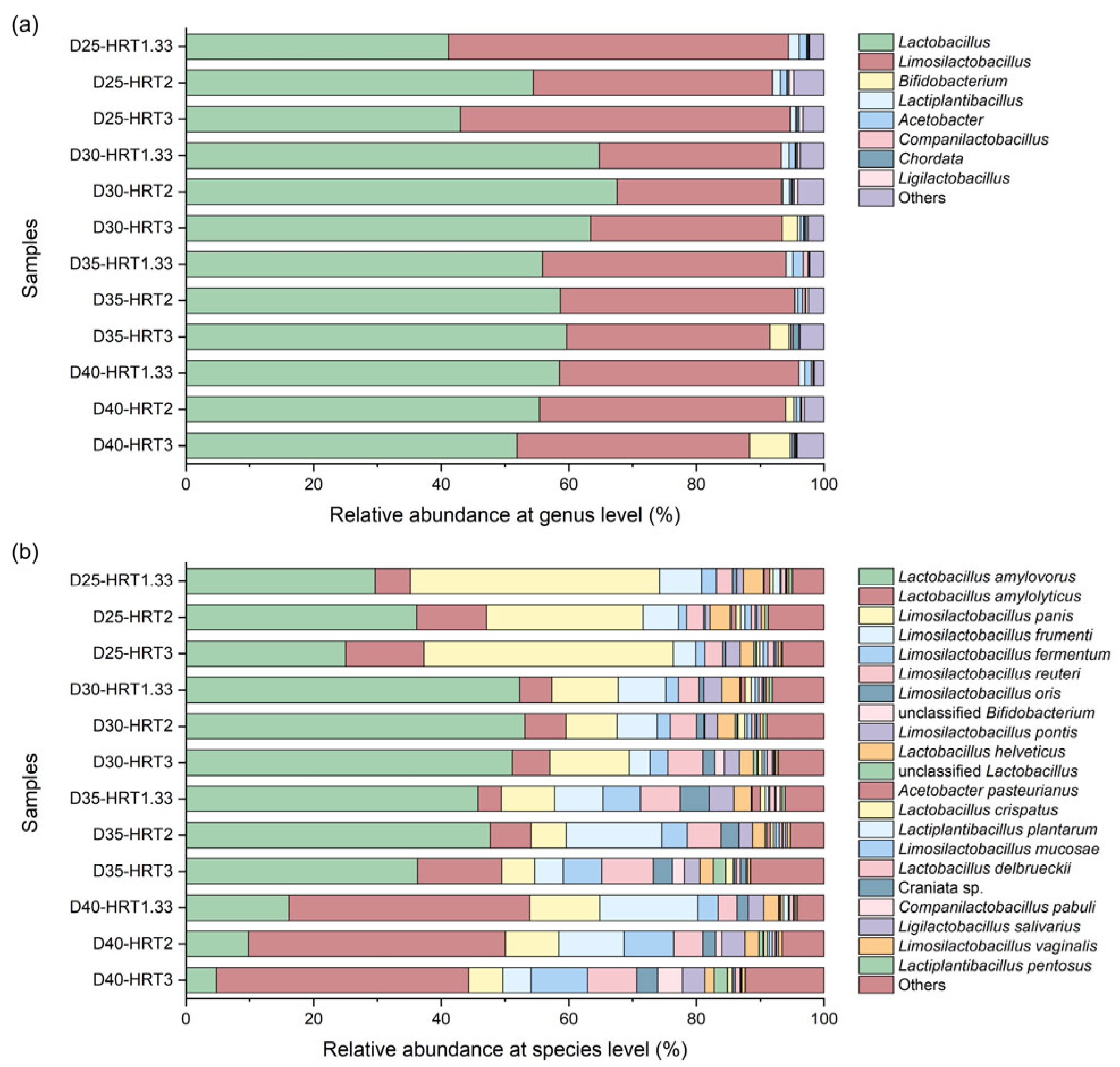
Metagenomic sequencing was conducted on samples collected during the steady-state phase of each fermentation condition. Taxonomic classification revealed that over 99% of assigned reads belonged to the bacterial domain, with the family Lactobacillaceae dominating and accounting for approximately 90% of total bacterial reads.
Figure 2 presents the microbial composition at both genus and species levels.
At the genus level,
Lactobacillus and
Limosilactobacillus were predominant across all samples, together comprising more than 88% of the bacterial community. Both are typical LAB that ferment carbohydrates anaerobically into LA [
33], forming the microbial foundation for lactic acid-type fermentation under all tested conditions. Notably, higher temperatures and longer HRTs promoted the enrichment of
Bifidobacterium (Spearman’s ρ > 0.764,
p < 0.05), whose relative abundance reached 2.42%, 2.96%, and 6.36% in samples D30-HRT3, D35-HRT3, and D40-HRT3, respectively. This enrichment may also have been influenced by the relatively higher pH observed during these fermentation phases, as Bifidobacterium species have been reported to proliferate optimally at a pH of around 4.0 [
14].
At the species level, 2664 microbial species were identified, with 21 species exhibiting relative abundances above 0.5% in at least one sample (
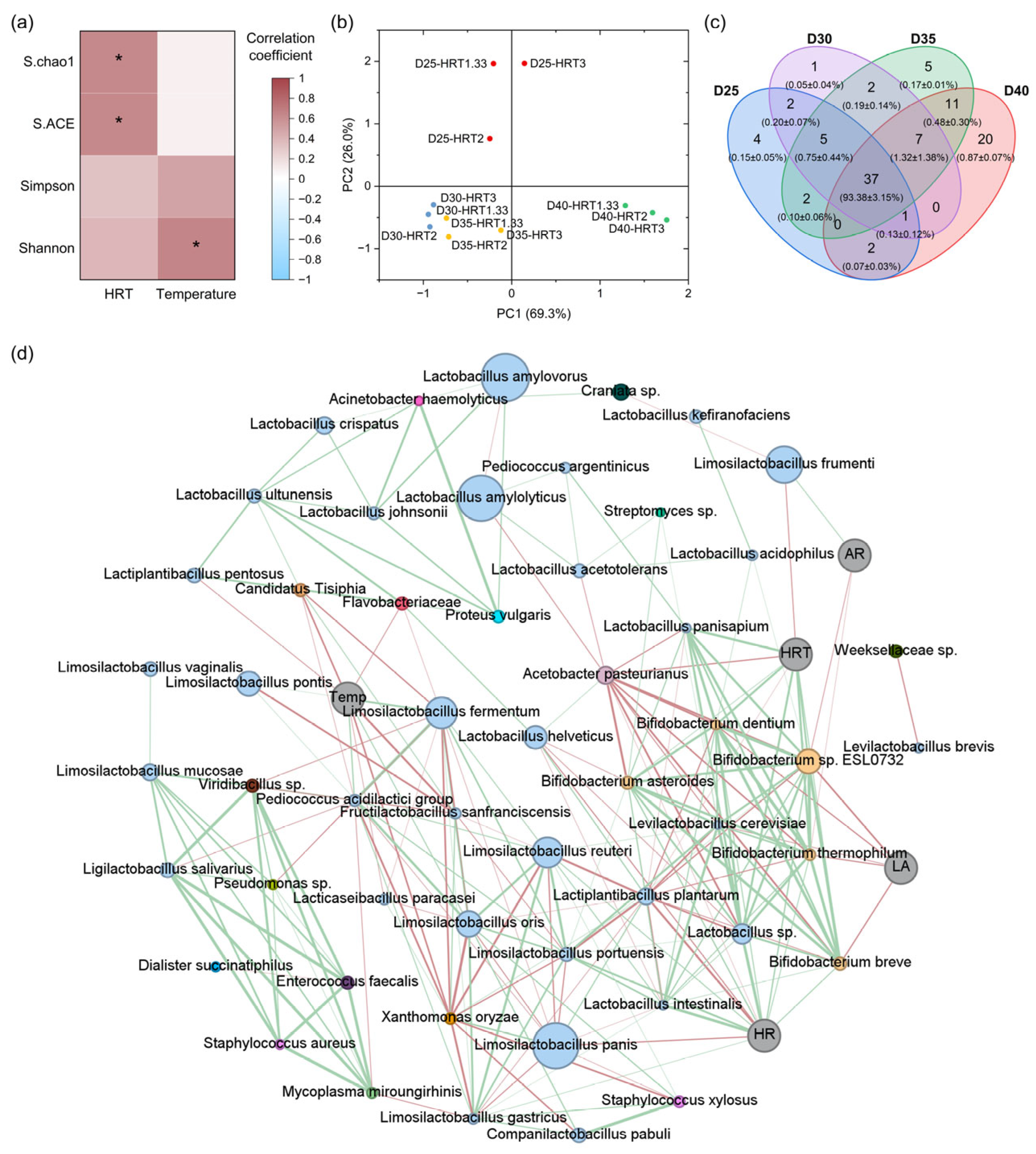
Figure 2b). Alpha diversity analysis indicated that HRT was significantly associated with richness indices (Chao1, ACE), whereas temperature correlated significantly with the Shannon diversity index, which is strongly influenced by community evenness (
Figure 3a). These findings suggest that extended HRTs favor the proliferation of slow-growing taxa, increasing richness, whereas higher temperatures in the range of 25–40 °C enhance evenness by supporting a broader range of fermentative microorganisms. Principal component analysis further demonstrated that temperature exerted a stronger influence on community structure than HRT, grouping samples into three clusters—(i) 25 °C, (ii) 30 °C and 35 °C, and (iii) 40 °C (
Figure 3b)—and the communities at 30 °C and 35 °C are more similar to each other than to those at 25 °C or 40 °C. Furthermore, the shared and unique taxa among temperature conditions were further examined using Venn diagram analysis (
Figure 3c). The results showed that 79 species were shared across all temperature conditions, representing 98.79 ± 0.78% of the total community, thereby constituting a core microbiome. While the number of unique species increased with temperature (from 1 at 25 °C to 35 at 40 °C), their relative abundances remained low (<0.5%), reinforcing the conclusion that temperature primarily shapes evenness rather than richness.
Beyond diversity indices, clear temperature-driven species turnover was observed. At 25 °C,
Lactobacillus amylovorus and
Lactobacillus panis co-dominated, with comparable relative abundances (24.49–39.08%). At 30–35 °C,
L. amylovorus became predominant as
L. panis declined, whereas at 40 °C,
Lactobacillus amylolyticus replaced
L. amylovorus as the dominant species. Both
L. amylolyticus and
L. amylovorus are commonly associated with lactic acid-type fermentation of FW, with the former exhibiting superior thermotolerance, making it a frequent dominant species under high-temperature conditions [
14]. In addition to temperature effects, HRT exerted further selective pressure:
Limosilactobacillus frumenti consistently showed lower abundance at an HRT of 3 days than at shorter HRTs across all temperatures (
t-test,
p < 0.01).
To elucidate the relationships among the relative abundances of microbial species, operational conditions (temperature, HRT), and process performance (hydrolysis rate, acidification rate, LA concentration), a microbe–environment–function association network was constructed using Spearman’s correlations for species with relative abundance ≥ 0.1% (
Figure 3d). In total, 53 species exhibited significant associations with one another or with environmental/functional variables. Although
L. amylovorus and
L. amylolyticus were highly abundant—and even dominant in fermentations above 30 °C—their degree values were only 4 (excluding nodes representing operational conditions and process performance), indicating limited network connectivity. This suggests that despite their numerical dominance, these taxa exert relatively weak influence on/by other community members. Such taxa can be regarded as numerically dominant producers that occupy ecological niches primarily through massive proliferation, yet contribute little to overall network cohesion, potentially making them more susceptible to environmental fluctuations. In contrast,
L. panis, another high-abundance species, exhibited a degree of 10, suggesting a potentially more central role in maintaining network structure and functional stability.
Lactiplantibacillus plantarum displayed the highest degree (18) and was thus identified as a hub species. Despite its relatively low abundance (0.20–0.96% in all samples), this taxon serves as a bridging node connecting multiple species or functional groups, thereby playing a key role in sustaining overall community stability.
Regarding operational parameters, temperature was positively correlated with Limosilactobacillus fermentum, Limosilactobacillus pontis, and Limosilactobacillus oris, but negatively correlated with Fructilactobacillus sanfranciscensis and Lactiplantibacillus pentosus. HRT was negatively correlated with Acetobacter pasteurianus and L. frumenti, yet positively correlated with several Bifidobacterium species. These patterns potentially reflect differences in optimal growth temperature and intrinsic growth rate for these species. Functionally, the hydrolysis rate was positively correlated with multiple Bifidobacterium species as well as with Limosilactobacillus portuensis and Limosilactobacillus reuteri. In contrast, the acidification rate exhibited limited associations, showing significant negative correlations only with Bifidobacterium sp. ESL0732 and Bifidobacterium thermophilum. There is no evidence that these species produce alternative metabolites capable of directly reducing the acidification rate, suggesting that potential product diversion mechanisms warrant further investigation. LA production correlated negatively with Bifidobacterium species, likely reflecting their intrinsically lower LA yields compared with other LAB.
3.4. Lactic Acid Production Pathway
Carbohydrates, lipids, and proteins constitute the major organic components of FW. During fermentation, hydrolytic and acidogenic microorganisms primarily metabolize carbohydrates to organic acids and alcohols [
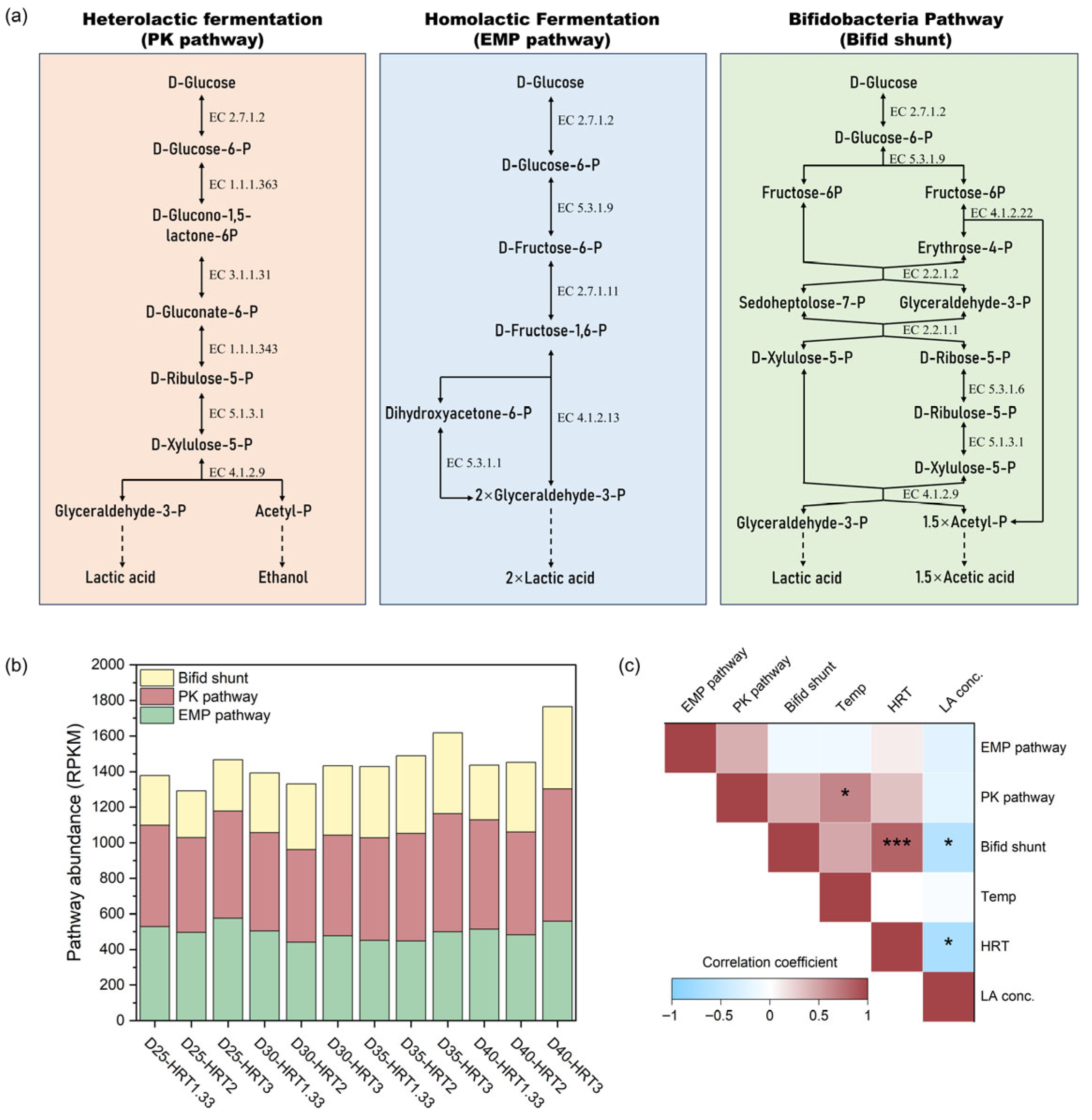
34]. Taking glucose—the most typical carbohydrate—as an example, microorganisms convert it to LA via three main pathways: homolactic fermentation, heterolactic fermentation, and the Bifidobacteria pathway.
Figure 4a illustrates these three metabolic routes. In homolactic fermentation, homofermentative LAB metabolize hexose through the glycolytic (Embden–Meyerhof–Parnas, EMP) pathway to generate pyruvate, which is subsequently reduced to LA by lactate dehydrogenase (EC 1.1.1.27). Stoichiometrically, 1 mol of glucose yields 2 mol of LA [
35]. In heterolactic fermentation, heterofermentative LAB metabolize glucose via the phosphoketolase (PK) pathway (EC 4.1.2.9) to produce equimolar amounts of LA, ethanol, and CO
2 (1 mol each per mol of glucose) [
35]. The Bifidobacterium pathway involves the “bifid shunt” (fructose-6-phosphate phosphoketolase pathway), in which
Bifidobacterium spp. utilize fructose-6-phosphate phosphoketolase (F6PPK, EC 4.1.2.22) as a key enzyme. Here, 1 mol of hexose is converted into 1 mol of LA and 1.5 mol of acetate, a yield profile distinct from the other two pathways [
36].
Using metagenomic data, we estimated the potential distribution of glucose into the three LA-producing pathways under different fermentation conditions. Pathway abundance was calculated as the geometric mean of the relative abundances (RPKM) of key enzymes (annotated in
Figure 4a) involved in the conversion process from glucose to glyceraldehyde-3-phosphate, the precursor of LA (
Figure 4b). It is important to note that because this estimate is derived from metagenomic data, it represents the functional potential rather than the actual metabolic flux of each pathway.
In most samples, the combined abundances of the three pathways ranged from 1291 to 1489 RPKM, with higher values in D35-HRT3 (1618 RPKM) and D40-HRT3 (1765 RPKM). Across all samples, the PK pathway was most abundant (39.0–42.9% of total), followed by the EMP pathway (30.1–39.2%) and the bifid shunt (19.7–29.3%).
Figure 4c illustrates correlations between pathway abundances and fermentation parameters. Temperature was positively correlated with the PK pathway (ρ = 0.64,
p = 0.02), suggesting that elevated temperature enhances the potential for heterolactic fermentation, consistent with the higher relative abundances of heterofermentative species such as
Limosilactobacillus fermentum and
Limosilactobacillus pontis (see
Section 3.3) [
37]. Given that heterolactic fermentation also produces ethanol, this association aligns with the observed temperature-dependent increase in ethanol concentration (ρ = 0.71,
p = 0.01). HRT correlated positively with the abundance of the bifid shunt, likely due to the enrichment of
Bifidobacterium spp. under longer HRT conditions (
Figure 3d). At 40 °C with an HRT of 3 days, the bifid shunt reached its highest abundance (462 RPKM), coinciding with the highest acetic acid concentration observed in the experiment. Moreover, the abundance of the bifid shunt was negatively correlated with LA concentration (ρ = −0.60,
p = 0.04). Notably, LA concentration in the effluent showed no significant correlation with the total abundance of LA-producing pathways. For instance, the highest total abundance observed at 40 °C with an HRT of 3 days corresponded to the lowest LA concentration. This suggests that substrate competition or further LA conversion may limit accumulation—a hypothesis that warrants further investigation.
3.5. Carbohydrate Depolymerization and Utilization
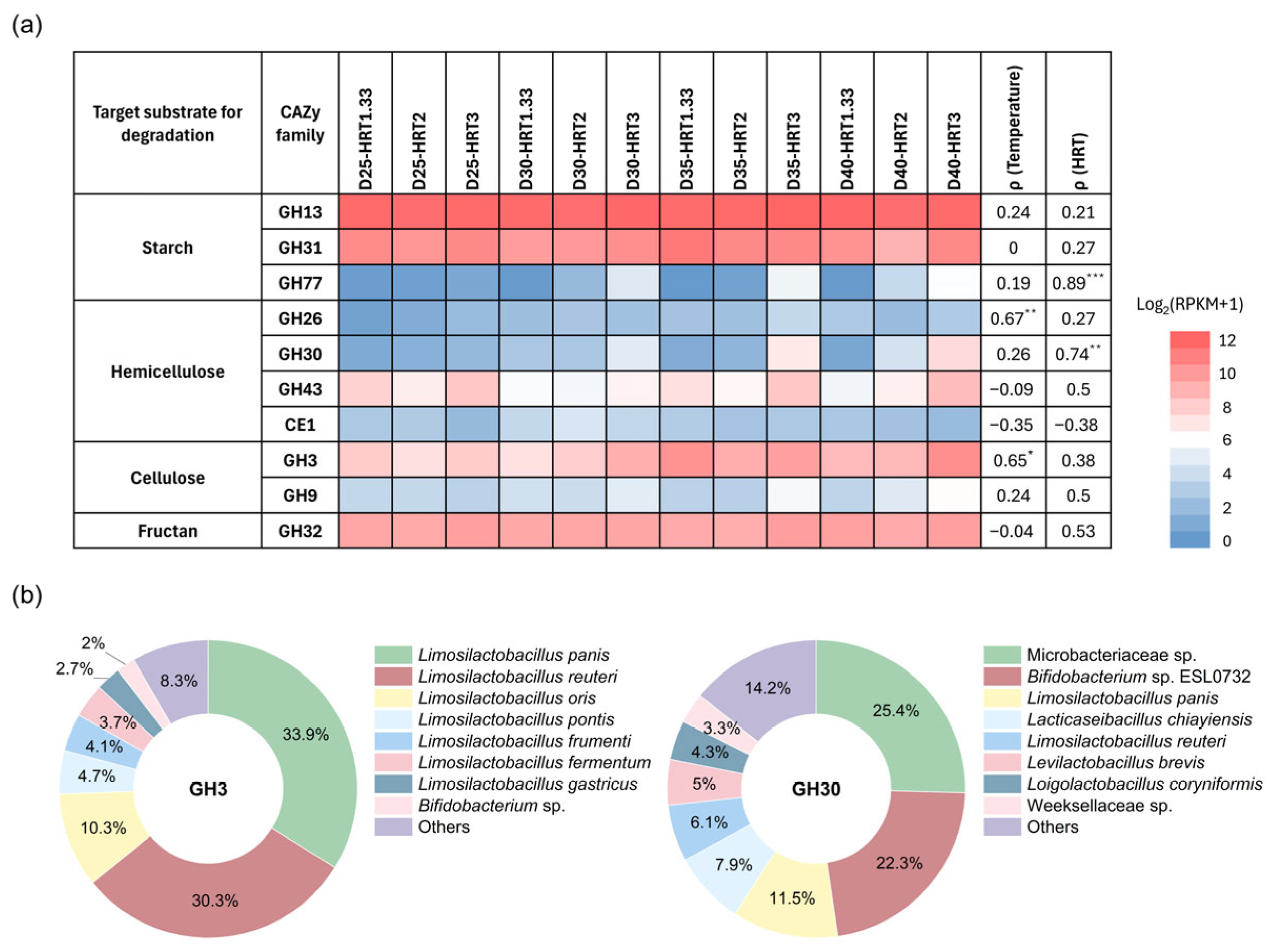
Fermentation systems generally rely primarily on carbohydrate hydrolysis to supply substrates for microbial metabolism. However, the rates at which microorganisms hydrolyze different carbohydrates—such as starch, cellulose, and hemicellulose—vary considerably. To evaluate the microbial potential for degrading various carbohydrates under different fermentation conditions, the metagenomic data were annotated against the CAZy database, which catalogs key enzyme families involved in carbohydrate metabolism.
Typical CAZymes involved in the hydrolysis of various carbohydrates were summarized, and their abundances in each sample are presented in a heatmap (
Figure 5a). CAZymes associated with starch hydrolysis (GH13, GH31) exhibited markedly higher abundances in all samples than those linked to cellulose and hemicellulose hydrolysis. This pattern indicates that starch is the primary hydrolyzed and utilized substrate during acidogenic fermentation, whereas cellulose and hemicellulose undergo limited degradation due to their recalcitrant structural properties, consistent with previous studies [
33]. Notably, GH32, an enzyme family with transfructosylation activity, also showed relatively high abundance, suggesting a substantial potential for fructan hydrolysis. Fructan, an oligo- or polysaccharide composed of fructose units, serves as an important energy reserve and contributes to stress tolerance in certain plants. Bulbous roots of
Allium and
Asteraceae plants are particularly rich in fructan; for example, garlic contains up to 45 g per 100 g dry weight [
38]. Although fructan has often been overlooked in previous studies of FW fermentation, the present results suggest that it may constitute an important substrate for lactic acid-type fermentation under a broad range of temperature and HRT conditions.
Correlation analysis revealed that the abundances of GH26 and GH3 (cellulose-associated members) were significantly positively correlated with temperature, while GH77 (starch-associated) and GH30 (hemicellulose-associated) were significantly positively correlated with HRT. These trends suggest that higher temperatures may enhance the potential for cellulose and hemicellulose hydrolysis, whereas longer HRT may increase the potential for starch and hemicellulose degradation. Such shifts could partly explain the observed increases in overall hydrolysis rates under elevated temperature or extended HRT (see
Section 3.2).
To further elucidate the microbial basis for the differential abundances of GH3 and GH30, we performed taxonomic assignment of their genes and mapped reads back to these targets to quantify the contributions of individual microbial taxa (
Figure 5b). For GH3, although
L. panis and
L. reuteri were the two largest contributors, their relative abundances were not significantly correlated with temperature. In contrast,
L. oris,
L. pontis, and
L. fermentum—together contributing 19.1% to GH3—were positively correlated with temperature (
Figure 3d), suggesting that their enrichment at higher temperatures may contribute to enhanced cellulose hydrolysis. For GH30, the main contributors were
Microbacteriaceae sp.,
Bifidobacterium sp. ESL0732, and
L. panis. Among these,
Bifidobacterium sp. ESL0732 (contributing 22.3%) showed a significant positive correlation with HRT (
Figure 3d), suggesting that its enrichment under longer HRT conditions may enhance the hydrolysis capability of the system for hemicellulose.
3.6. Practical Implications for Industrial Applications
In practical FW treatment, achieving processing objectives at the lowest possible cost is a priority. Shorter HRTs reduce reactor volume requirements, thereby lowering capital investment and footprint, while lower operating temperatures minimize heating demand and associated operating costs. Findings from this study demonstrate that LAB have the ability to proliferate rapidly and convert substrates to LA at high rates. Even at an HRT as short as 1.33 days—corresponding to daily replacement of 75% of the reactor’s effective volume—lactic acid-type fermentation was achieved. However, excessively short HRTs resulted in system instability, as reflected by substantial fluctuations in LA concentrations in the effluent. Increasing the HRT to 2 days markedly enhanced process stability and modestly improved the hydrolysis rate, making it the optimal choice for industrial application. Under this HRT, stable and efficient LA fermentation was achieved across all tested temperatures. Nonetheless, the high animal-derived lipid content of FW posed operational challenges at lower temperatures. In our experiments, coagulated lipids were observed to adhere to reactor walls at 25 °C, potentially clogging pipelines and impeding mass transfer. Therefore, slightly higher temperatures offer greater operational reliability. Taking into account operating costs, capital investment, and fermentation performance, an HRT of 2 days combined with a temperature of 30–35 °C is recommended for industrial-scale application.
This study also yielded findings that diverge from previous reports. Earlier work using canteen FW or synthetic FW typically indicated that longer HRTs promote more complete fermentation and higher LA accumulation without pH control [
16,
39]. In contrast, our results showed that increasing the HRT to 3 days led to a reduction in both LA yield and acidification rate. This may be attributed to a greater role of the bifid shunt pathway in LA production under extended HRTs, or to potential re-conversion of LA into other metabolites. These results highlight the complexity of open mixed-culture fermentation systems and underscore the necessity of substrate-specific process optimization.
Despite the considerable potential of LA production from FW, this technological route remains some distance from commercial viability. One of the primary barriers to industrial application lies in the downstream separation and recovery of LA. Due to the highly heterogeneous nature of FW—which is rich in lipids, proteins, and other interfering substances—traditional separation methods such as calcium salt precipitation often result in low product purity and poor recovery efficiency, rendering them unsuitable for this substrate [
40]. Therefore, the development of novel LA separation and purification strategies tailored to the unique characteristics of FW is expected to be a key focus of future research.