Abstract
In this study, the non-destructive determination of pH, total soluble solids (TSS), total acidity (TA), reducing sugars (RS), seed total phenolic content (TPCD), and skin total phenolic content (TPCN) in Ugni Blanc grapes was performed using visible/near-infrared (Vis/NIR) spectroscopy coupled with chemometric quantitative analysis. Diffuse reflectance spectra in the 400–1507 nm range were measured using a handheld Vis–NIR spectrometer, after which the dataset was partitioned using the SPXY algorithm, accounting for joint X-Y distances. Six spectral preprocessing methods and three modeling algorithms, Partial Least Squares (PLS), Support Vector Machine Regression (SVR), and Convolutional Neural Network (CNN), were used to construct quantitative models based on full-wavelength and feature-wavelength data. Feature-based models outperformed full-spectrum models for TA, RS, and TPCN, whereas full-spectrum models performed better for pH, TSS, and TPCD. The optimal models achieved values of 0.940, 0.957, 0.913, 0.889, 0.917, and 0.871 and RPD values of 4.074, 4.798, 3.397, 2.998, 2.904, and 2.786, correspondingly. The findings highlight the applicability of Vis/NIR spectroscopy for the accurate and non-destructive prediction of key physicochemical indicators in Ugni Blanc grapes.
1. Introduction
Ugni Blanc is a strategically vital white grape variety in the Cognac region, whose ripeness directly influences the acidity balance, flavor precursor content, and overall quality stability of the distilled spirit [1]. Strict control of ripeness at harvest is crucial for brandy quality: under-ripe grapes produce an astringent profile due to acid–sugar imbalance, while overripe grapes lose aromatic precursors and develop off-flavors, compromising freshness and typical style [2,3,4]. Accurate and rapid monitoring of Ugni Blanc ripeness is vital for optimal harvest. Key indicators include total soluble solids content (TSS), total acidity (TA), and total phenolics content (TPC), which collectively influence potential alcohol, flavor development, freshness, and oxidative stability [5,6,7]. Currently, routine analytical methods for these components generally rely on destructive sampling, which is complex, time-consuming, and costly, and requires trained personnel. These limitations restrict their efficient application in harvest decision-making [8,9].
In recent years, advances in chemometrics and spectroscopic instrumentation have provided new approaches for evaluating the inner quality of fruits without physical damage. Studies have shown that visible–near-infrared (Vis–NIR) spectra in the 350–1800 nm range can effectively predict the TSS of pears [10,11,12]. Fernández-Novales et al. successfully analyzed dynamic changes in amino acids during grape ripening using Vis-NIR spectroscopy [13]. Xiao et al. achieved a classification accuracy of over 77% for grapes based on TSS and TPC using spectra in the 400–1100 nm range [14]. In addition, Yu et al. developed a near-infrared probe that enabled non-destructive prediction of grape TSS [15]. The near-infrared models developed by Basile et al. also demonstrated strong predictive capability for grape quality parameters [16]. Collectively, research confirms that Vis–NIR spectroscopy, applied as a fast and non-invasive technique, has great potential for assessing fruit ripeness and key quality attributes [17,18,19,20].
Despite the broad adoption of Vis–NIR spectroscopy as a non-destructive tool for evaluating fruit ripeness and quality attributes, its combination with deep learning approaches remains insufficiently explored, particularly for the simultaneous prediction of multiple grape quality parameters, including pH, TSS, TA, reducing sugars (RS), and total phenolic compounds present in grape skins and seeds (TPCN and TPCD) [21]. Spectral modeling has often relied on classical machine learning methods, for instance, partial least squares regression (PLS) and support vector regression (SVR), to suppress noise interference and improve prediction accuracy. In recent years, deep learning approaches, particularly convolutional neural networks (CNNs), have advanced rapidly in the field of artificial intelligence, demonstrating excellent feature learning and pattern recognition capabilities and significant potential for extracting and modeling complex spectral information [22]. However, the utilization of neural network techniques for spectral analysis remains relatively limited. Therefore, integrating Vis–NIR spectroscopy with neural network algorithms to systematically enable fast, non-invasive estimation of multiple grape attribute parameters holds significant theoretical value and practical potential [23,24].
This study aimed to evaluate the feasibility of using visible–near infrared (Vis–NIR) spectroscopy combined with multivariate modeling and deep learning methods for rapid, non-destructive assessment of quality parameters in Ugni Blanc grapes during ripening. The specific objectives included: (1) collecting Vis–NIR spectra and reference quality data of grapes from véraison to the ripening stage, and developing PLS, SVR, and CNN models; (2) investigating spectral preprocessing methods and characteristic wavelength selection using the successive projections algorithm (SPA) to reduce data dimensionality while maintaining prediction accuracy; and (3) comparing the predictive performance of different models to determine the optimal model for each quality parameter.
2. Materials and Methods
2.1. Grape Samples
From July to the end of August 2024, Ugni Blanc grapes were collected by experienced grape growers in Laizhou, Shandong, China (37°12′3″ N, 120°1′23.9″ E). To account for adequate variability, a stratified sampling technique was employed [25]. To establish fixed sampling points, three rows within the vineyard were randomly selected. For each cluster, grapes were systematically sampled from the top, middle, and bottom sections, and wine grape clusters were partitioned into corresponding strata [26]. Two to three berries were collected from each designated position, resulting in a total of 6–9 berries per grape bunch. Based on grape development characteristics and cultivation experience, samples were collected every 3–5 days, with 500 berries harvested between 8:00 and 10:00 a.m. at each sampling event. Subsequently, the grapes were hand-divided into 8–15 groups, grouping berries with analogous color and firmness [27].
2.2. Spectroscopic Measurements
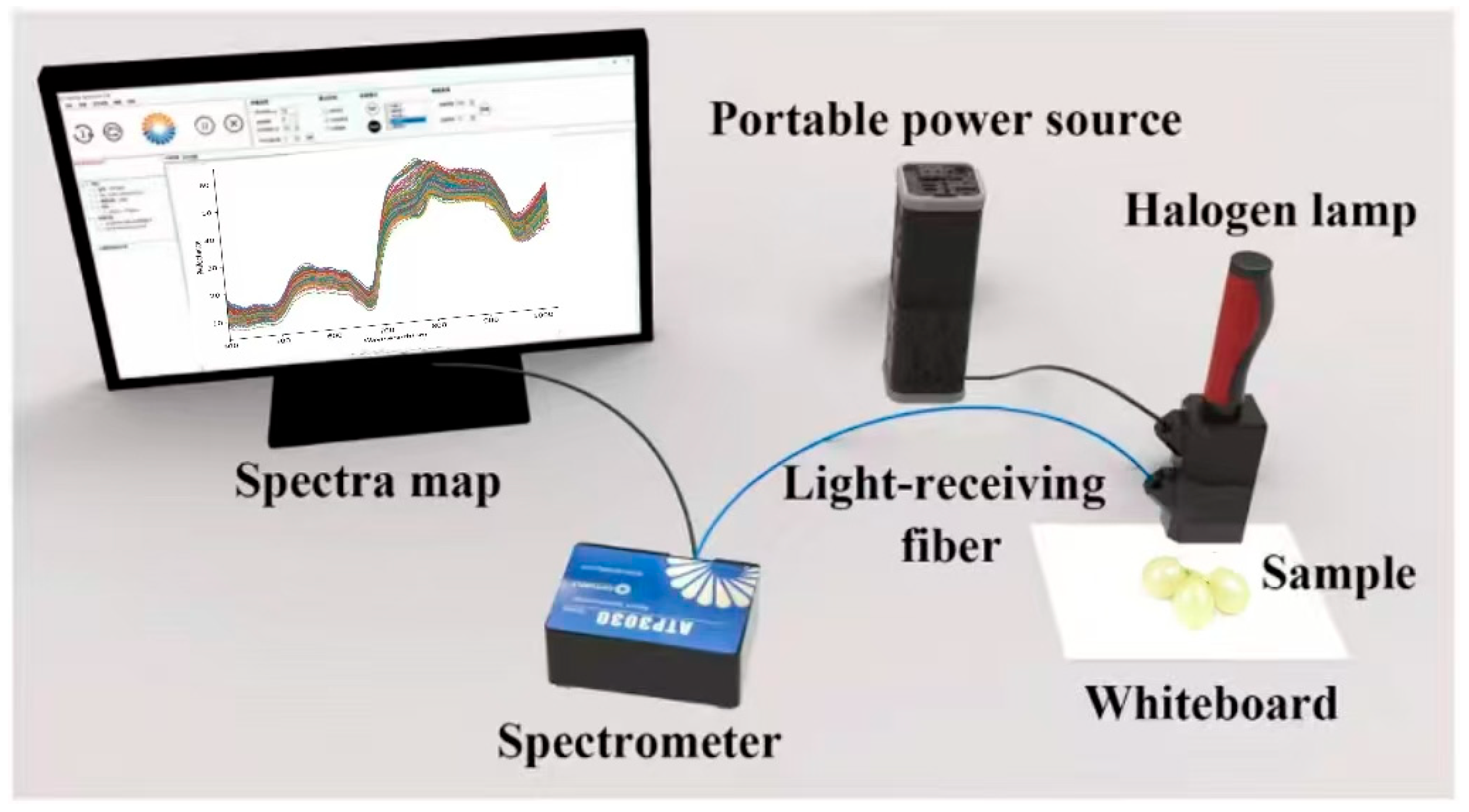
The system utilized a high-resolution miniature spectrometer ATP 3030 (Optosky Photonics Inc., Xiamen, China), equipped with an internal circuit capable of synchronously triggering and controlling the xenon lamp. The spectrometer operates over a wavelength range of 200–1100 nm with a resolution of 0.05–2 nm. All spectral measurements were conducted at an ambient room temperature of approximately 22 ± 2 °C, and the spectrometer was powered on and allowed to warm up for at least 30 min prior to data acquisition to ensure measurement stability and spectral consistency. Other elements of the setup consisted of a 12 V halogen lamp light source (HL 2000), a dark chamber, optical fibers, and a computer equipped with Optosky Spectra V3.1.25 software. A standard whiteboard (Guangzhou Jingyi Optoelectronics Technology Co., Ltd., Xiamen, China) was utilized as the white reference, and calibration was carried out before each measurement according to the defined standard. Each sample underwent ten repeated measurements from its sun-facing and shaded sides [28], and the Euclidean distance was calculated. The spectrum showing the least deviation from other measurements was designated as the representative spectrum for each sample. Grapes were subsequently placed on a standard whiteboard for spectral acquisition. Figure 1 shows a schematic diagram of the spectral acquisition.
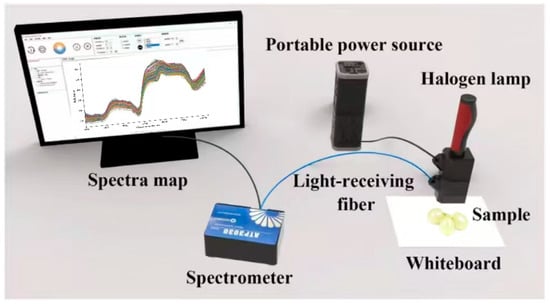
Figure 1.
Reflectance spectral acquisition system.
2.3. Chemical Analysis
2.3.1. Sample Preparation
After completion of spectral measurements, the samples were kept at −40 °C to allow for subsequent reference analyses. The grape skins, pulp, and seeds were then manually separated. The pulp was processed for juice extraction from grapes for the measurable determination of TSS and TA. For phenolic compound analysis, the skins and seeds were first ground and freeze-dried. Phenolics were repeatedly extracted three times with a hydrochloric methanol solution (0.1% v/v HCl: 60% v/v methanol), after which the supernatants were merged and stored at −40 °C for further determination [29].
2.3.2. TSS and the Reducing Sugar Content
The TSS of grape juice from each sample was measured using a digital handheld refractometer (PAL-1; Atago Co. Ltd., Tokyo, Japan). RS content was determined using the Fehling reagent hot titration method. Before measurement, the grape juice was prepared by dilution to reach a sugar content within the range of 2–4 g/L [30].
2.3.3. pH and the Total Acid Content
Measurement of the pH in grape samples was conducted using a pH meter (Shanghai, China, Leici, PHS-3E). To determine the TA of each sample, 2 mL of grape juice was analyzed by titration using a standard NaOH solution, with a color change indicator marking the endpoint, and the volume of NaOH consumed was recorded. A blank test was conducted simultaneously for reference [30].
2.3.4. Total Phenolic Content
The total phenolic content in grape skins (TPCN) and seeds (TPCD) was quantified using a slightly modified Folin–Ciocalteu procedure [30,31]. Briefly, 0.1 mL of grape extract was mixed with 0.5 mL of Folin–Ciocalteu reagent and allowed to react for 5 min at room temperature. Subsequently, 1.5 mL of 20% (w/v) sodium carbonate solution was added, after which the mixture was diluted with distilled water to a final volume of 10 mL. The results were expressed as milligrams of gallic acid equivalents per gram (mg GAE/g).
2.4. NIR Spectra Acquisition
2.4.1. Spectral Preprocessing
During the process of spectral acquisition, different types of noise introduced by environmental conditions may compromise both model accuracy and its predictive reliability [32]. Therefore, spectral preprocessing is essential before model development. In this study, six preprocessing methods were applied: Detrending (DT), First Derivative (FD), Multiplicative Scatter Correction (MSC), Savitzky–Golay Smoothing (SG), Standard Normal Variate (SNV), and Standardization or Standard Scaling (SS).
2.4.2. Dataset Partitioning
To ensure that the modeling dataset adequately represented both spectral characteristics and chemical properties of the samples, the SPXY (Sample set Partitioning based on joint X-Y distances) algorithm was implemented to allocate the dataset into training and test groups [33]. The SPXY method considers not only the differences in the spectral space (X) but also the distribution of the response variables (Y), effectively avoiding uneven sample distribution caused by traditional random partitioning and thereby enhancing model robustness and generalization. In this study, a total of 192 Ugni Blanc samples were partitioned into training and test sets at a ratio of 7:3.
2.4.3. Feature Wavelength Selection
Vis–NIR spectral data usually consist of hundreds to thousands of wavelengths, with a large number exhibiting limited correlation with the predicted reference values [34]. Therefore, feature selection of wavelengths is essential for improving the predictive ability of calibration models. In this study, selection of wavelengths was conducted using the Successive Projections Algorithm (SPA).
The SPA method is a sequential projection-based approach for variable selection. It aims to determine the wavelengths that most effectively capture spectral information while minimizing multicollinearity. The algorithm iteratively selects a subset of variables that can maximally capture the spectral information, which are then used for model development [22,35].
2.4.4. Modeling Methods
Three different predictive models were employed in this study: Partial Least Squares Regression (PLS), Support Vector Regression (SVR), and one-dimensional Convolutional Neural Networks (CNNs). The spectral dataset was utilized as input, whereas physicochemical measurements were designated as the target variables.
PLS develops a mathematical relationship connecting spectral variables to the target chemical indicators. By extracting latent variables that maximize the shared covariance between the independent matrix X and the response variable y, PLS compresses high-dimensional spectral data into a limited set of latent variables, controlling model complexity while retaining the main information relevant to the response [36,37]. To determine the optimal latent variable dimensionality, this study employed the integration of a combined fivefold cross-validation with grid-based hyperparameter optimization, in which n_components ranged from 1 to the smaller of the number of training samples or spectral variables, allowing automatic selection of the most suitable n_components. After fitting the model using the training dataset, it was applied to the test set for prediction, enabling quantitative modeling from spectral data to chemical indicators while effectively addressing the issue of high dimensionality and multicollinearity of the spectral variables.
SVR was used to establish quantitative predictive models between spectral data and target chemical indicators [38]. By employing a kernel function, SVR projects input features into an elevated-dimensional feature representation, where a linear regression-based model is constructed to capture the nonlinear association linking spectral data and response outputs. During model training, key hyperparameters, namely the regularization coefficient C, kernel type, and the kernel parameter ϒ, were automatically optimized using randomized search (RandomizedSearchCV) combined with five-fold cross-validation to achieve the best fit. The search was performed over a predefined parameter grid: C = [0.1, 1, 10, 100], ϒ = [‘scale’, ‘auto’, 0.01, 0.1, 1], and kernel types = [‘linear’, ‘rbf’, ‘sigmoid’], with 10 random iterations. Once trained, the model was applied to the test set for prediction, enabling quantitative modeling from spectra to chemical indicators while effectively handling high-dimensional and nonlinear spectral data.
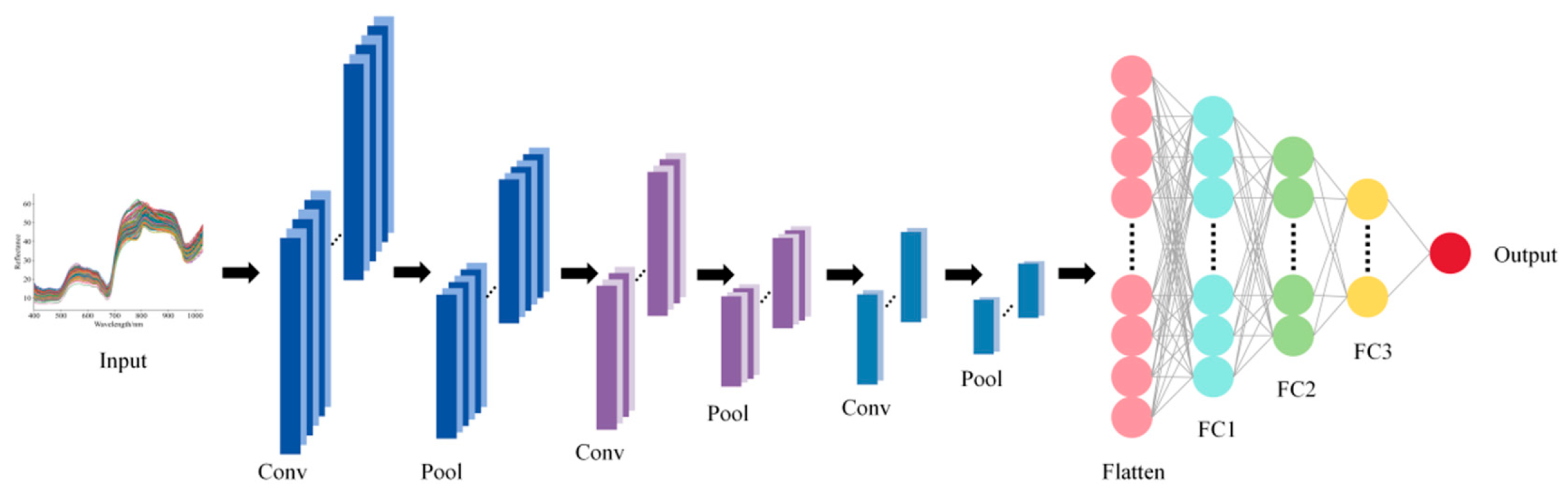
A CNN was used to construct regression models for spectral data. The network consists of three convolutional layers, which extract local features from the spectral sequence through convolutional filtering, batch normalization layers, ReLU-based nonlinear activation, and pooling mechanisms. The first convolutional layer has 16 output channels, kernel size 5, and stride 2, followed by max pooling (window 2, stride 1); the second convolutional layer has 32 output channels, kernel size 3, and stride 2, followed by max pooling (window 2, stride 1); the third convolutional layer has 64 output channels, kernel size 1, and stride 1, followed by adaptive max pooling with output length 1. Detailed parameters are listed in Table S1. Figure 2 illustrates the CNN architecture described above. The extracted features are then aggregated into a fixed-length feature vector via adaptive pooling [39]. The convolutional features are subsequently fed into a multilayer fully connected network to map and output continuous response variables: the first fully connected layer has 64 neurons with dropout 0.2, the second has 32 neurons with dropout 0.2, and the output layer has 1 neuron. During training, mean squared error (MSE) served for loss calculation, and network parameters were updated using the Adam optimizer. This network can effectively capture both local and global patterns in spectral data, enabling nonlinear quantitative prediction of high-dimensional spectra.
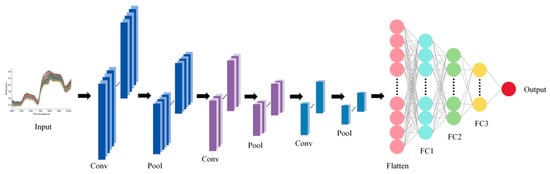
Figure 2.
Architecture of CNN model.
2.4.5. Evaluation of Model Performance
The performance of the model was comprehensively assessed with several metrics, including the coefficient of determination (R2), root mean square error (RMSE), and residual predictive deviation (RPD) [40]. Among them, R2 is used to assess how well the predicted values correspond to the measured values [8], calculated as follows:
where is the i-th measured value, is the i-th predicted value, is the mean of the measured values, and n is the number of samples. An R2 value closer to 1 indicates stronger predictive ability. RMSE reflects the average magnitude of prediction errors and is calculated as follows:
A smaller RMSE value indicates lower prediction error. The RPD serves to evaluate the consistency of the model’s predictions and is defined as the quotient of the standard deviation of the response variable to the residuals of the predicted values, calculated as follows:
In general, RPD values above 2 are indicative of robust predictive performance, while those between 1.4 and 2.0 are acceptable for initial predictions. By comprehensively analyzing these metrics, the performance differences and applicability of the three modeling approaches, PLS, SVR, and CNN, in spectral prediction tasks can be fully compared.
2.5. Software and Data Analysis Tools
This study employs different software tools for data processing and visualization, leveraging their respective strengths: data organization and basic statistical analysis utilize Excel software (version 2019; Microsoft Corporation, Washington, DC, USA); complex data analysis, algorithm implementation, and statistical chart generation were performed in the Python environment (Anaconda distribution, Python 3.12), primarily utilizing scientific computing libraries such as Pandas, NumPy, Matplotlib, and Seaborn; and final publication-grade images were refined and generated using Origin 2021 software (OriginLab, Northampton, MA, USA).
3. Results and Discussion
3.1. Spectral Characteristics of Grapes from Veraison to Maturity
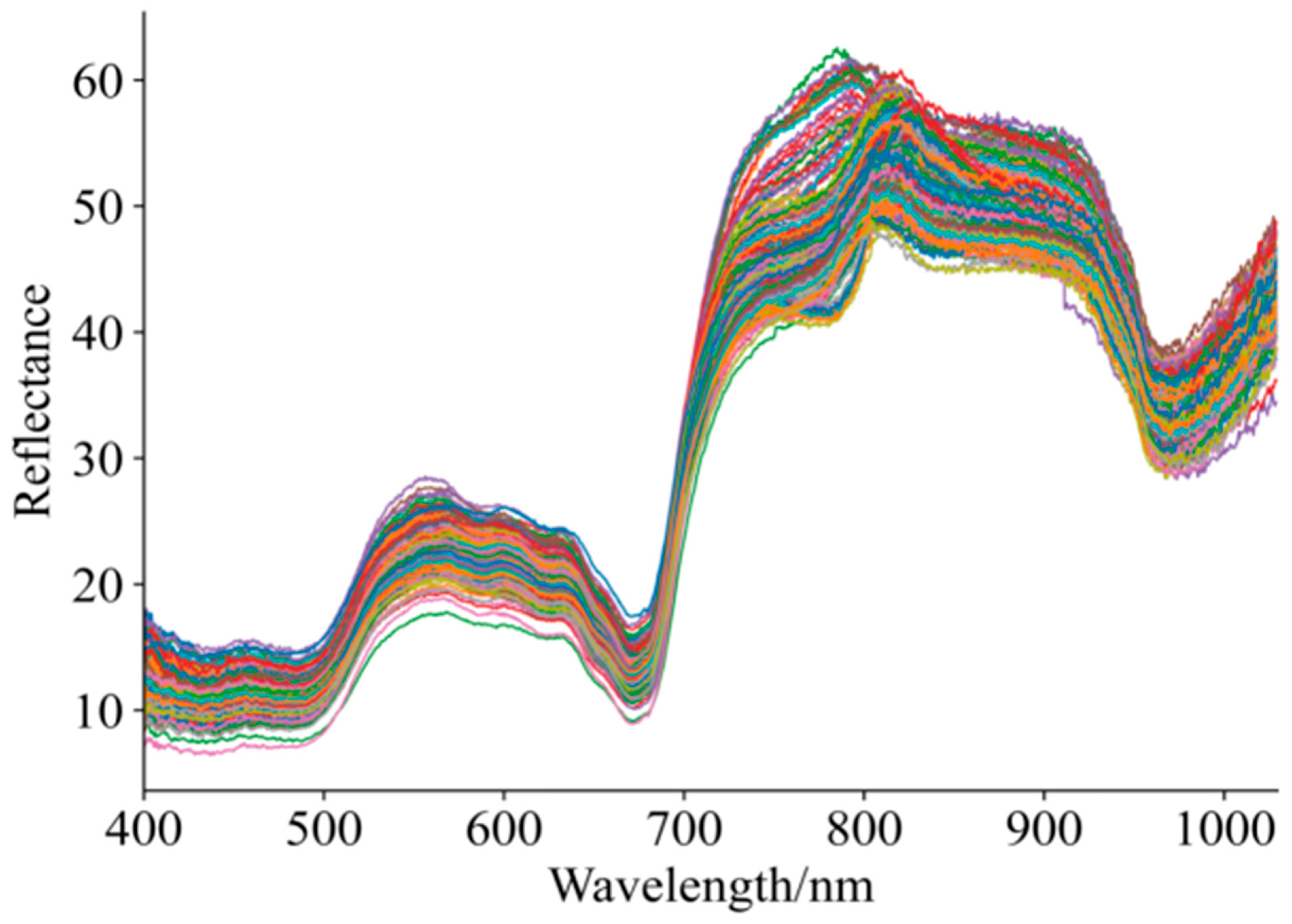
Figure 3 shows the diffuse reflectance profiles for 192 grape samples with varying maturity and quality. As shown in Figure 3, although there is overlap and crossover in the unprocessed Vis–NIR spectral profiles of the grape samples, the overall spectral trends are generally consistent. Within the 400–1000 nm spectral range, noticeable differences exist among the curves, which may be attributed to variations in grape maturity affecting skin color and, consequently, the absorption characteristics of the spectra [5,41,42]. Within the 800–920 nm spectral range, absorption bands were observed, which may be related to the absorption of phenolic compounds, cellulose, and sucrose [41,42]. Overall, the dynamic changes in spectral profiles clearly reflect the physiological processes occurring during fruit maturation, including chlorophyll degradation, synthesis of phenolic compounds, and transformation of structural components.
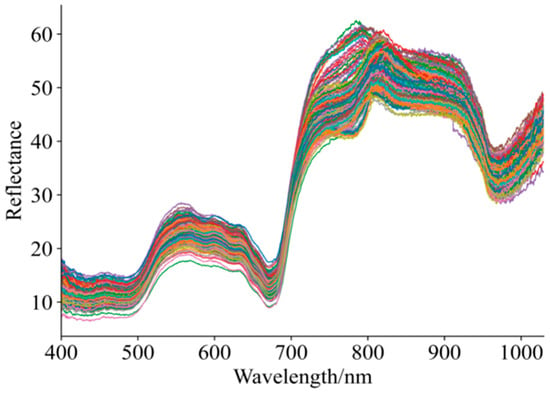
Figure 3.
Spectral curves of grapes in ripening period.
3.2. Construction of Full-Wavelength Prediction Models Under Different Preprocessing Methods
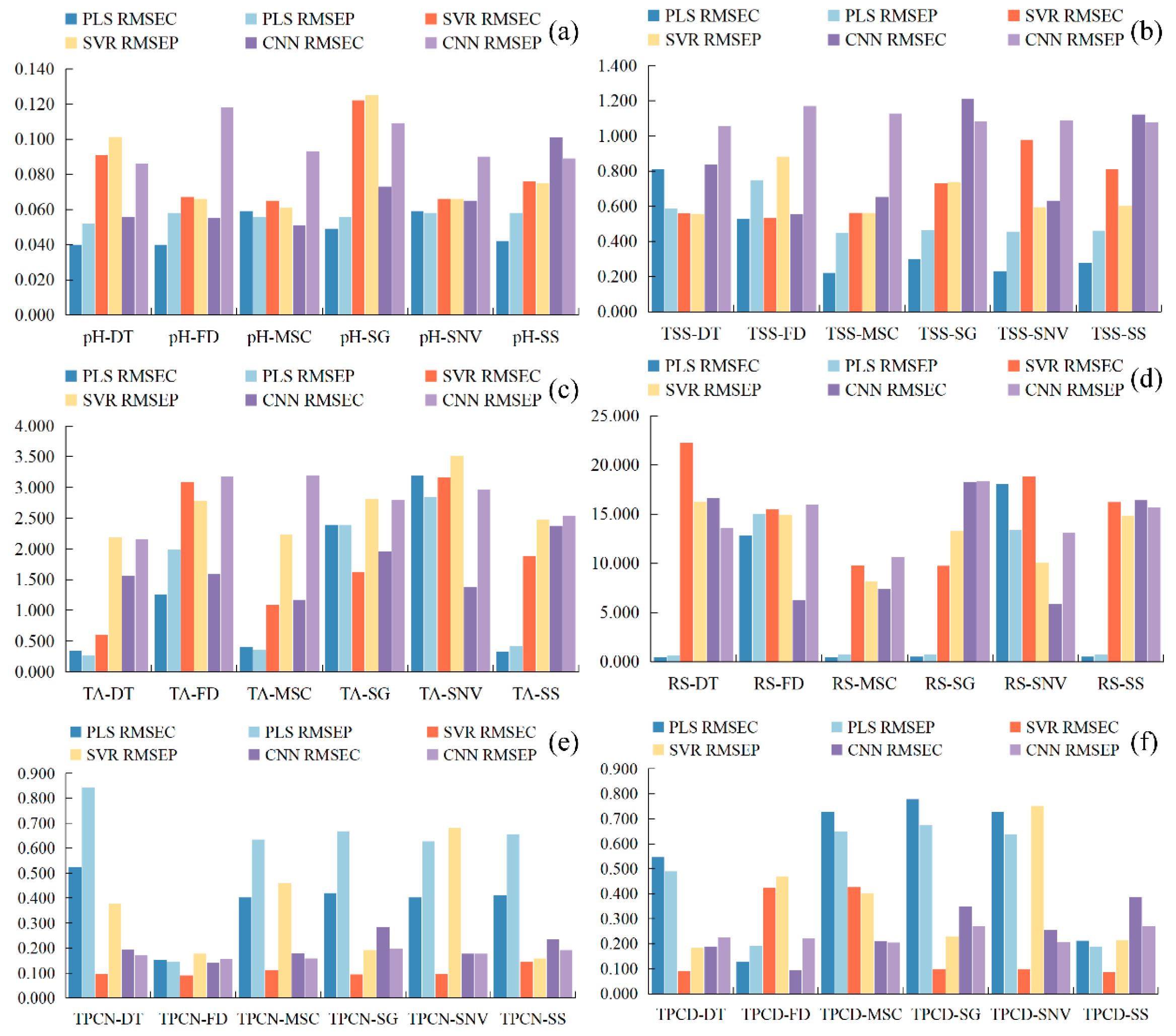
Previous studies have shown that visible–near infrared spectroscopy can be employed to predict grape quality attributes, and traditional machine learning approaches have been widely applied to model gluconic acid and other indices in red grape cultivars [25]. Conventional physicochemical analyses, such as measurements of sugars, acids, and phenolic compounds, typically require extensive laboratory procedures, long processing times, and substantial reagent consumption [43]. Therefore, developing a simple and rapid method for determining the physicochemical properties of Ugni Blanc grapes is of particular importance. In this study, PLS, SVR, and CNN algorithms were used to construct prediction models for pH, TSS, TA, RS, TPCN, and TPCD based on spectral data subjected to various preprocessing techniques. The model performance metrics (R2 and RPD) are presented in Figure 4, and the RMSE values are shown in Figure 5. The details of the sample set division, including the number of samples in calibration and validation sets for each indicator, are provided in Table S2.
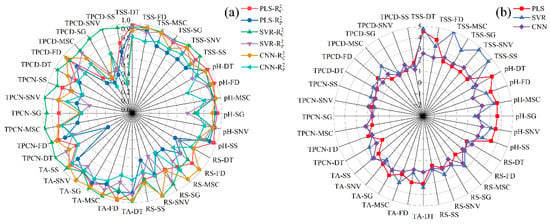
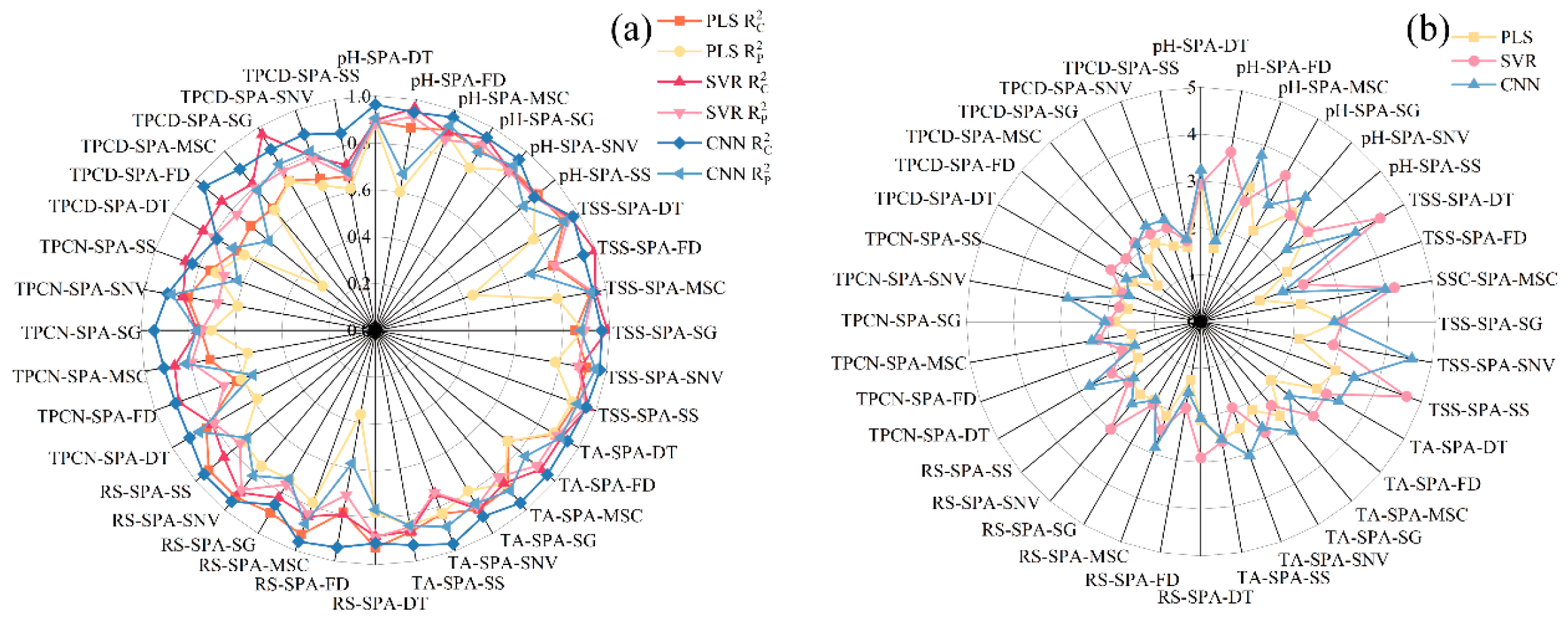
Figure 4.
R2 and RPD values of PLS, SVR and CNN models by using different preprocessing methods. (a) Correlation coefficients of the calibration and prediction sets ; (b) Residual Predictive Deviation RPD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCN = total phenolic content of the skin, TPCD = total phenolic content of the seed, PLS = partial least squares, SVR = support vector machine regression, CNN = convolutional neural network.
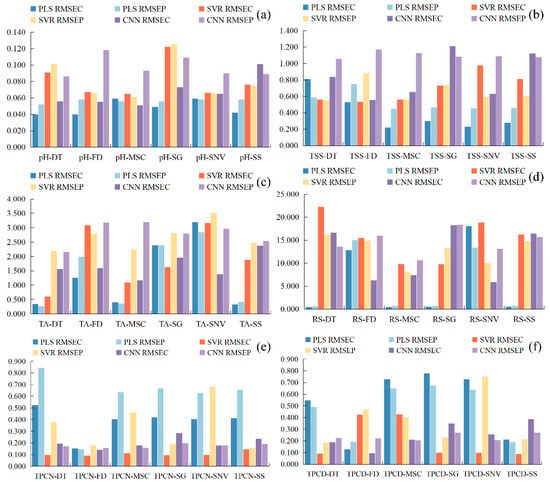
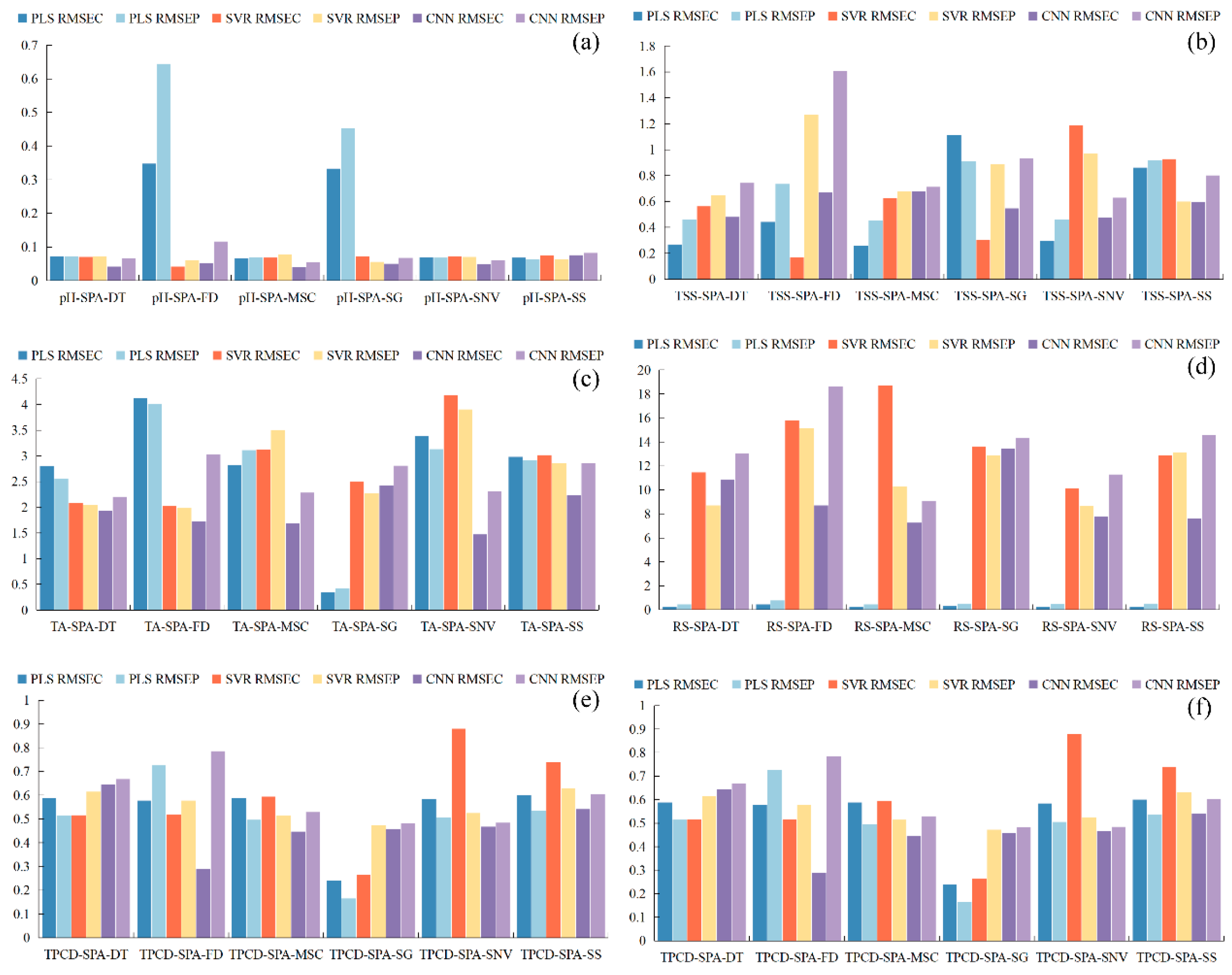
Figure 5.
Root mean square error RMSE of the correction set. (a) pH, (b) TSS, (c) TA, (d) RS, (e) TPCN, (f) TPCD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCN = total phenolic content of the skin, TPCD = total phenolic content of the seed, RMSE = Root Mean Square Error, PLS = partial least squares, SVR = support vector machine regression, PLS = partial least squares, SVR = support vector machine regression, CNN = convolutional neural network.
As shown in Figure 4 and Figure 5a–c, for the models established for pH, TSS, and TA, all three modeling approaches under different preprocessing methods achieved calibration and validation R2 values greater than 0.7. Notably, for pH, the R2 values of both calibration and validation sets exceeded 0.9 across all modeling methods. In addition, the corresponding RPD values were higher than 2, in conjunction with low RMSEC and RMSEP values, indicating strong model reliability and predictive performance [30].
Figure 5d shows that, for RS, the RMSEC and RMSEP values of the PLS models based on DT, MSC, SG, and SNV preprocessing are consistently lower and similar to each other, indicating that these models capture the general patterns effectively while maintaining strong predictive ability for new samples. Among them, the PLS-SNV model exhibits R2 > 0.7 for both calibration and test sets, with an RPD greater than 1.5, demonstrating good goodness-of-fit and reliable predictive capability. This model is therefore suitable for rapid detection and preliminary quantitative analysis of RS. Other RS models may provide rough quantitative predictions but still show risks of underfitting, making them insufficient for high-precision prediction [44].
For the TPCN, models developed using FD-PLS, DT-SVR, and MSC-CNN demonstrated good performance, achieving R2 > 0.75. Among the models, FD-PLS achieved the best performance, yielding R2 values of 0.876 and 0.798 for the calibration and test sets, with RMSEC and RMSEP of 0.153 mg/g and 0.146 mg/g, respectively, and an RPD of 2.223, indicating strong predictive capability. These results indicate that the PLS model combined with FD preprocessing not only provides excellent fitting for the training data but also exhibits robust predictive performance for unknown samples [45]. The calibration and prediction sets showed similar performance, with RMSEP slightly lower than RMSEC [30], indicating that the model did not exhibit overfitting and possesses good generalization ability. An RPD exceeding 2.0 further confirms the model’s outstanding quantitative predictive capability, demonstrating its suitability for accurate determination of total phenolics in peel in practical applications [20].
The performance of PLS, SVR, and CNN models in predicting TPCD content varied significantly under different preprocessing methods. PLS was sensitive to preprocessing, with the first derivative (FD) markedly improving model performance, while MSC, SNV, and SG provided limited enhancement. SVR exhibited strong fitting ability but was prone to overfitting under SNV and SG preprocessing; MSC, however, could improve its generalization. CNN demonstrated stable performance across all preprocessing conditions, achieving the best prediction results under FD preprocessing (RPD = 4.868), highlighting its superior capability in extracting nonlinear features compared to conventional methods. Overall, FD was identified as the most effective preprocessing approach, and the combination of CNN with FD yielded the optimal balance of predictive accuracy and stability.
Significant differences in prediction performance were observed across multiple quality parameters, including seed total phenolics, peel total phenolics, pH, reducing sugars, total acidity, and SSC, when different preprocessing methods were combined with PLS, SVR, and CNN modeling algorithms. Regarding R2, several preprocessing combinations, such as SSC-DT and SSC-SNV, achieved relatively high calibration and prediction coefficients of determination, approaching 0.9. The CNN models showed a clear advantage in capturing complex nonlinear features. Most RPD values were relatively high, approaching 5, which indicates strong predictive reliability. In addition, both RMSEC and RMSEP remained within low ranges, demonstrating small errors in the calibration and prediction sets and reflecting good model stability [46]. Overall, the CNN models displayed superior performance across multiple parameters and proved to be highly suitable for spectroscopic data modeling.
3.3. Feature Wavelength Extraction
Owing to the large number of collected reflectance wavelengths (1507) and the strong interdependencies between them, a feature-wavelength selection approach was implemented to enhance the accuracy and computational efficiency of the detection models. Accordingly, SPA was employed to identify key wavelengths. SPA was applied to the optimally preprocessed spectral data to extract informative wavelengths to predict grape pH, TSS, TA, RS, TPCN, and TPCD.
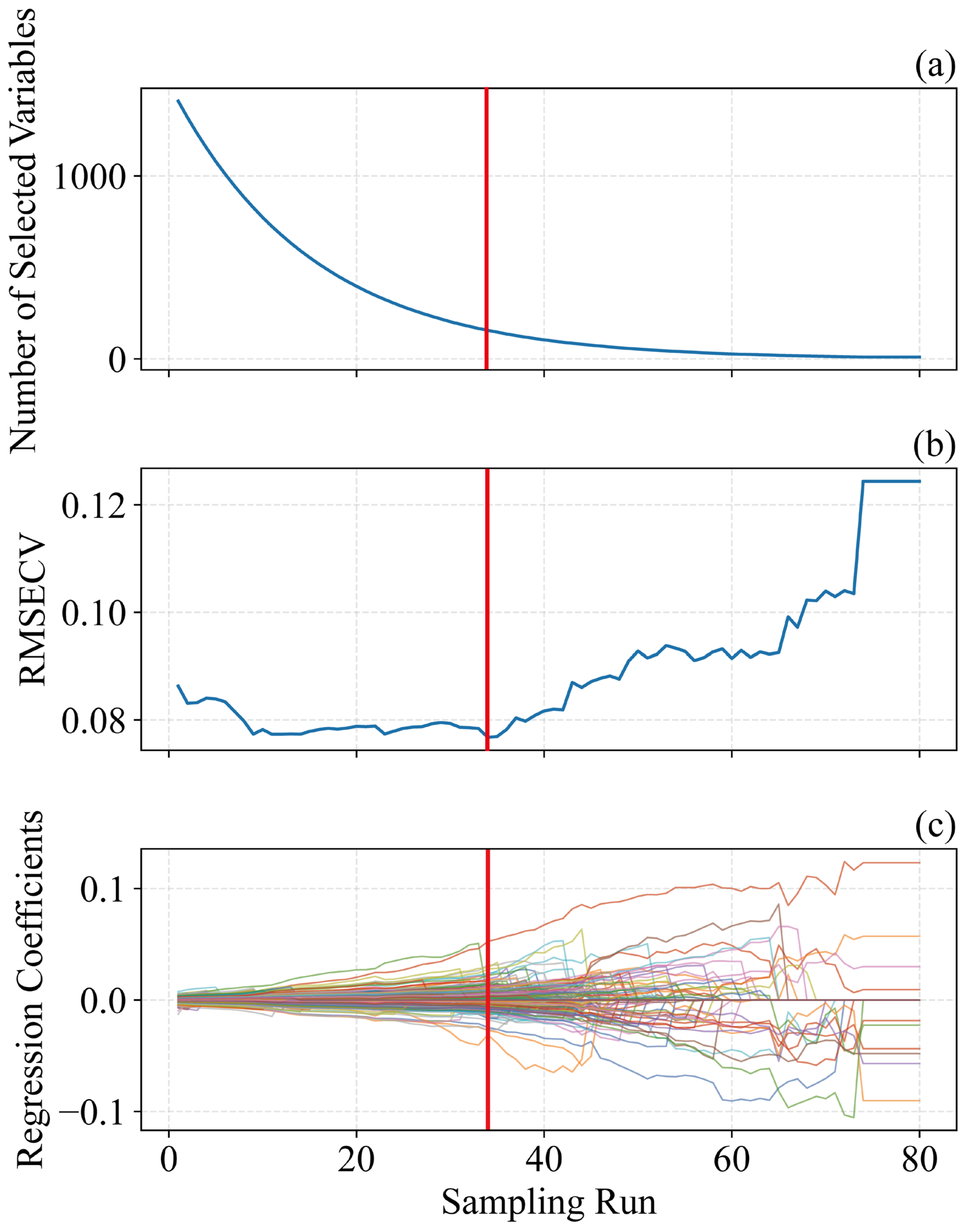
Figure 6 illustrates the SPA-based procedure for selecting informative wavelengths, using pH as a case study. In this study, the number of Monte Carlo samplings was set to 80, and a 10-fold cross-validation approach was used to determine the significance of each variable [47]. As shown in Figure 6b, the RMSECV plot reflects the feature selection process by SPA. RMSECV initially decreases and then stabilizes, reaching a minimum at the red line [30]. Figure 6c shows the regression coefficients across iterations, indicating that wavelengths corresponding to the optimal iteration contribute most to the model [20]. With increasing sampling iterations, RMSECV initially declines and then rises: the initial decrease indicates the removal of irrelevant information, while the subsequent increase signals the elimination of useful information [22,35]. Therefore, the sampling run associated with the lowest RMSECV (80 runs) was chosen as the optimal outcome. Based on Figure 6b,c, the optimal iteration occurs at 34 runs, where the regression coefficients of the selected variables align with the vertical line in Figure 6c.
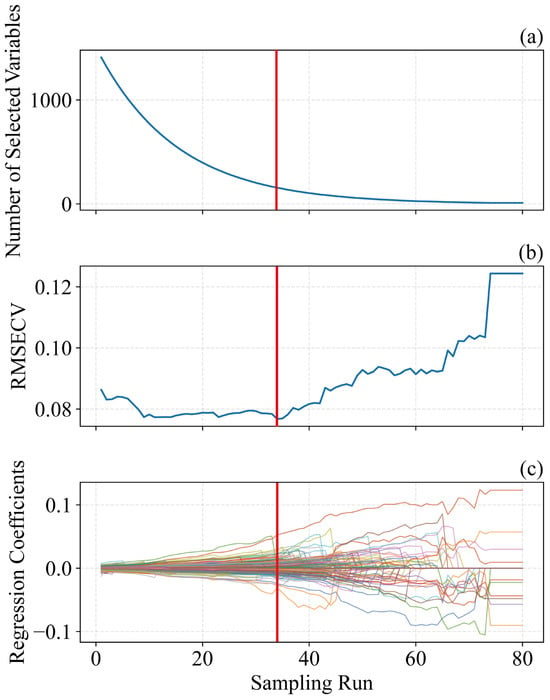
Figure 6.
Characteristic wavelength selection charts of pH content in Ugni Blanc grape extraction based on SPA algorithm. (a) Number of sampled variables, (b) 10-fold RMSECV values, (c) regression coefficient path of each variable with an increase in the number of sampling runs. SPA = successive projections algorithm, RMSECV = root mean squared error of cross-validation.
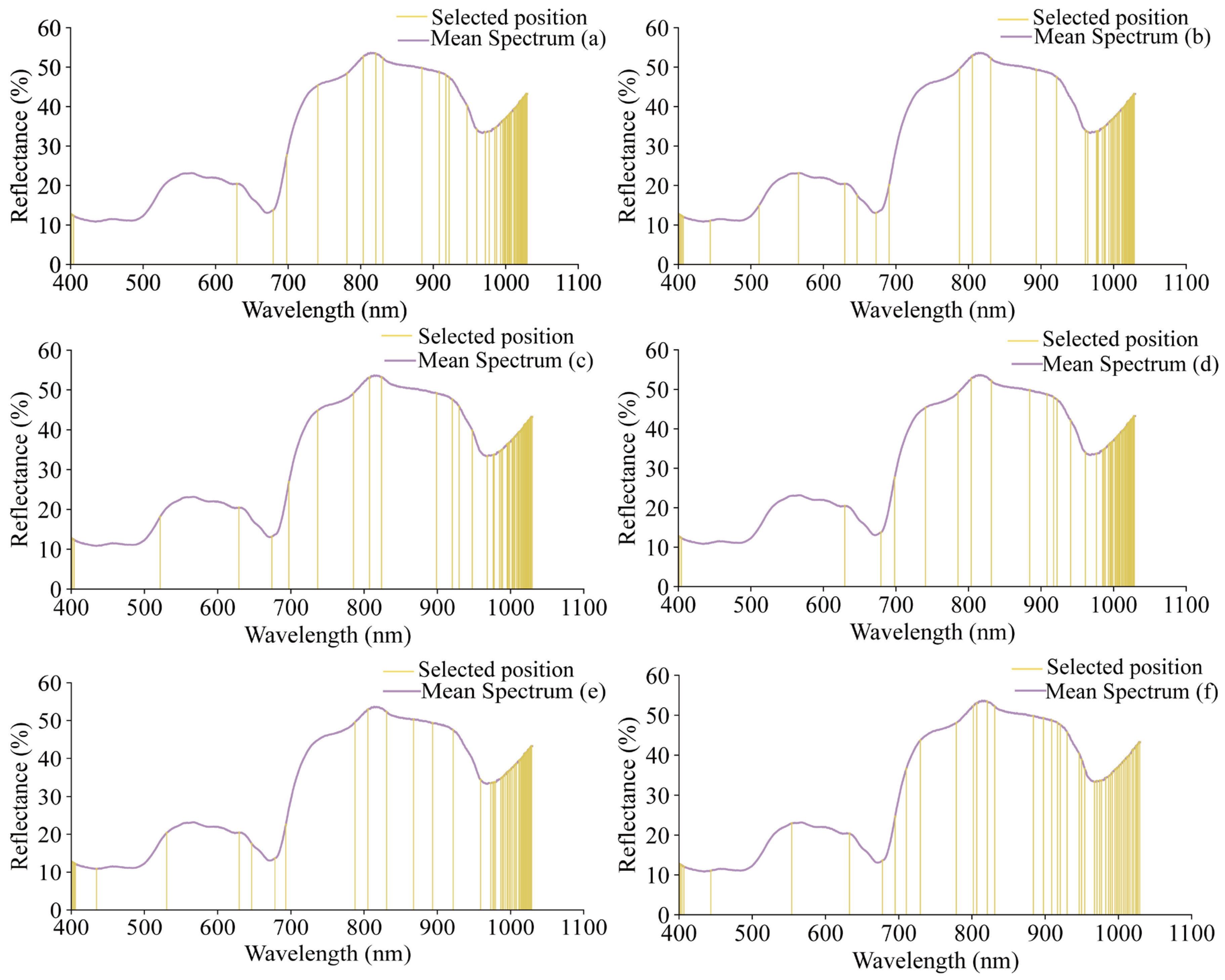
The selected wavelengths are shown in Figure 7. Using SPA, 50 wavelength variables were selected for each of the grape quality indicators (pH, TSS, TA, RS, TPCN, and TPCD), representing 3.3% of the total wavelengths and reducing the data by over 90%. The detailed list of these characteristic wavelengths and their corresponding chemical functional groups is provided in Table S3, offering a comprehensive reference for interpreting the spectral information used in the models.
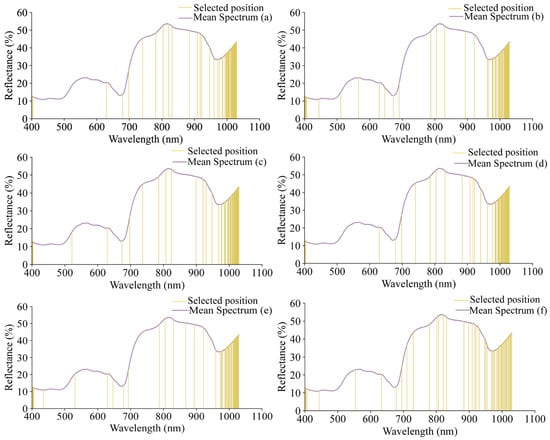
Figure 7.
The positions of the characteristic wavelengths of different indicators selected by SPA. (a) pH, (b) TSS, (c) TA, (d) RS, (e) TPCN, (f) TPCD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCN = total phenolic content of the skin, TPCD = total phenolic content of the seed.
3.4. Construction of Spectral Prediction Models Based on Feature Wavelengths
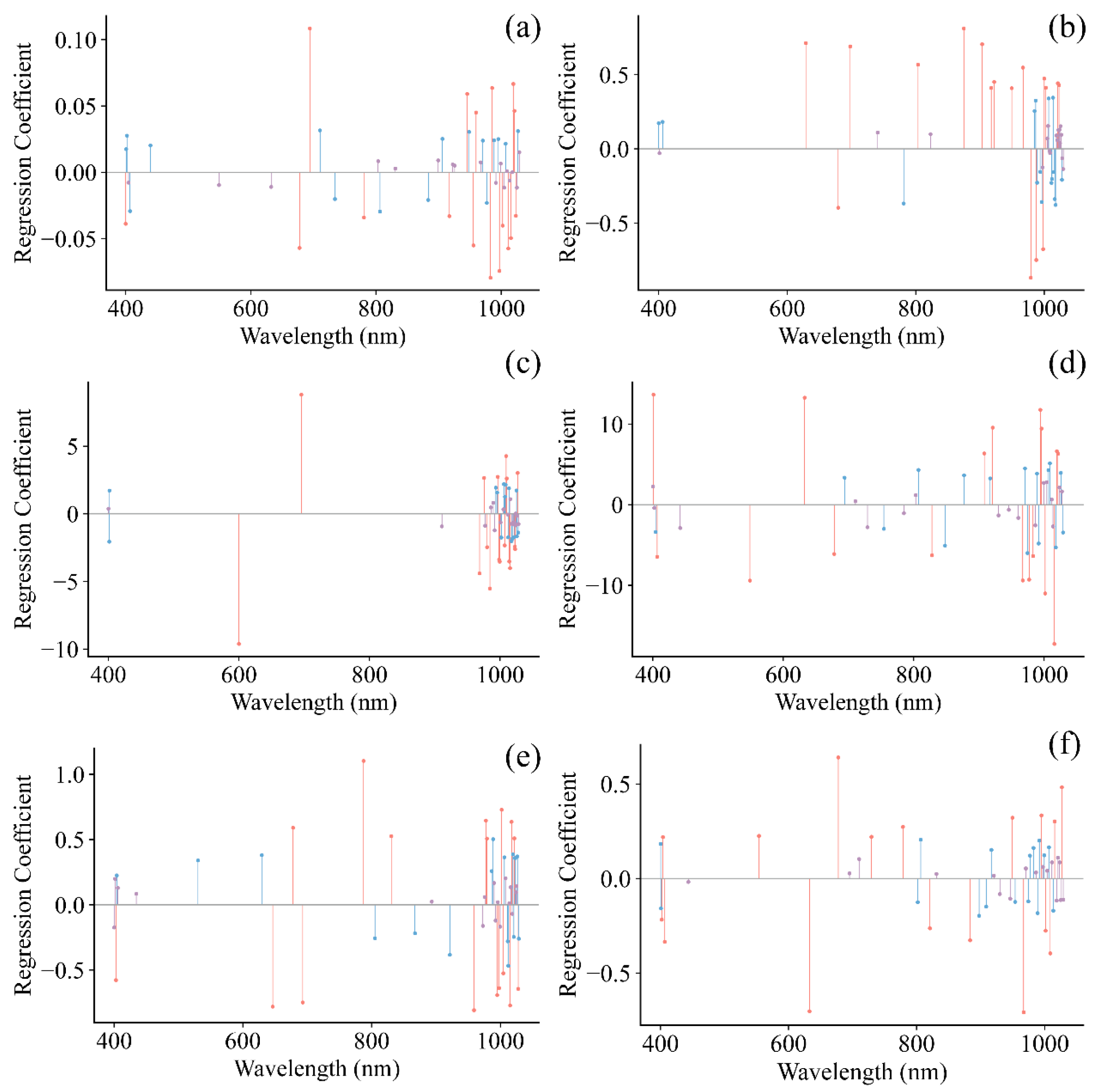
The SPA method was used to identify key spectral regions, minimizing the dimensionality of the input data and facilitating the development of more robust and streamlined models [22,35]. Following the selection of informative wavelengths for grape pH, TSS, TA, RS, TPCN, and TPCD, the spectral data were employed to build models using PLS, SVR, and CNN. Figure 8 shows the regression coefficients of the established PLS models, where the red lines indicate wavelengths with high influence on the quality indicators (top 25%), blue lines represent moderately influential wavelengths (middle 50%), and purple lines denote wavelengths with low influence (bottom 25%).
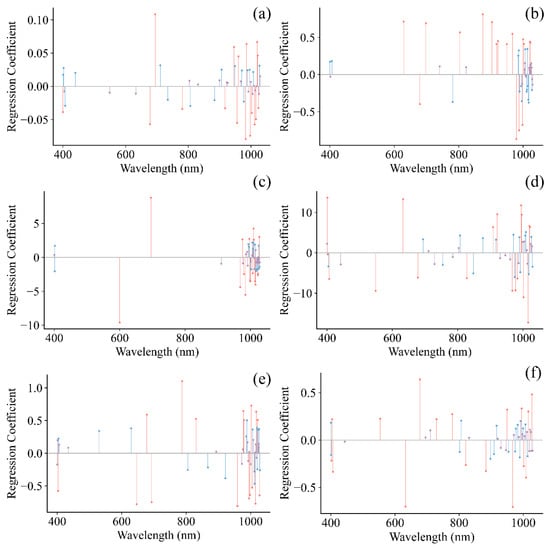
Figure 8.
Regression coefficients of PLS model. (a) pH, (b) TSS, (c) TA, (d) RS, (e) TPCD, (f) TPCN. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCD = total phenolic content of the seed, TPCN = total phenolic content of the skin.
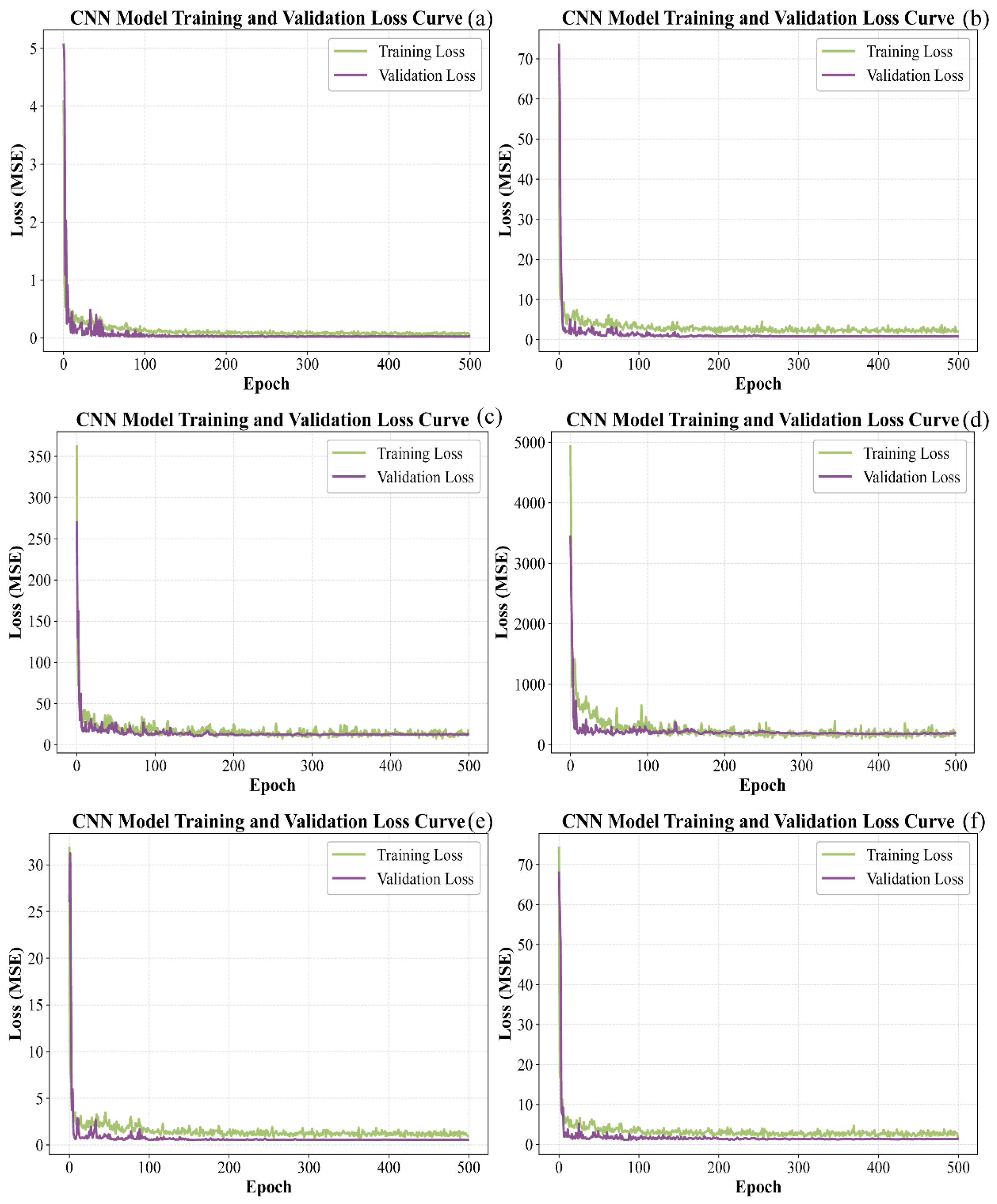
Figure 9 shows the progression of loss for CNN models applied to the calibration and prediction subsets. It is evident that the loss for all six models decreased rapidly at the initial stage, then declined more slowly and exhibited some fluctuations. After 500 iterations, the loss for the training and test datasets gradually stabilized. For pH, the loss values of both sets approached zero. For TSS, TA, TPCN, and TPCD, the prediction set loss values approached zero while the calibration set loss values remained slightly higher. In contrast, the calibration and prediction set loss values for RS remained relatively high.
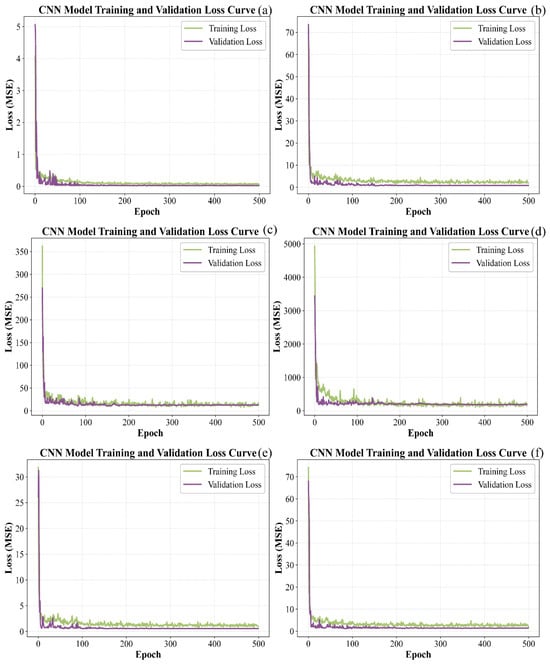
Figure 9.
Loss curves of CNN models by calibration set and prediction set. (a) pH, (b) TSS, (c) TA, (d) RS, (e) TPCN, (f) TPCD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCD = total phenolic content of the seed, TPCN = total phenolic content of the skin, CNN = convolutional neural network.
The performance metrics of the PLS, SVR, and CNN models developed from the selected feature wavelengths for pH, SSC, TA, RS, TPN, and TPD are shown in Figure 10 and Figure 11. The findings indicate that model performance using the selected feature wavelengths for pH, SSC, and TA was generally superior to that for RS, TPN, and TPD. However, for pH, SSC, and TPD, the prediction accuracy of all three models decreased compared with the full-wavelength models after feature wavelength selection. In contrast, for TA, RS, and TPN, the three models exhibited improved prediction performance after selecting key wavelengths relative to the models based on the full spectrum.
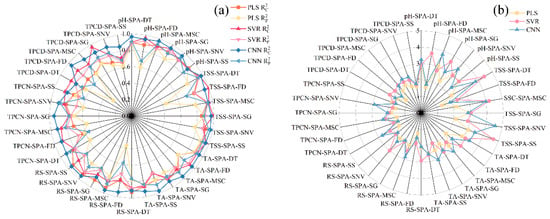
Figure 10.
Comparison of model performance (R2 and RPD) using feature wavelengths. (a) Correlation coefficients of the calibration and prediction sets , (b) Residual Predictive Deviation RPD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCN = total phenolic content of the skin, TPCD = total phenolic content of the seed, PLS = partial least squares, SVR = support vector machine regression, CNN = convolutional neural network.
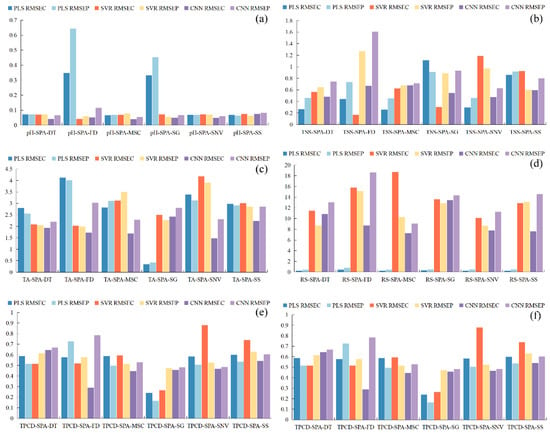
Figure 11.
Performance comparison of PLS, SVR, and CNN models based on feature wavelengths using RMSE. RMSE = Root Mean Square Error, (a) pH, (b) TSS, (c) TA, (d) RS, (e) TPCN, (f) TPCD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCN = total phenolic content of the skin, TPCD = total phenolic content of the seed, PLS = partial least squares, SVR = support vector machine regression, CNN = convolutional neural network.
Analysis of the quantitative spectral prediction models for pH, TSS, and TPCD (Figure 10 and Figure 11a,b,f) showed that the overall prediction accuracy decreased after SPA-based feature wavelength selection. For pH, the PLS model yielded values of 0.877–0.910 and values of 0.600–0.893; the SVR model achieved values of 0.890–0.963 and values of 0.864–0.926; and the CNN model showed values of 0.885–0.968 and values of 0.678–0.930. Although predictive performance slightly decreased relative to the full-spectrum models, the CNN model still maintained the highest fitting accuracy and strong robustness, with most RPD values exceeding 3. For TSS, the overall model accuracy also declined after SPA selection. The CNN model remained the best performer, with = 0.972, = 0.952, and an RPD of 4.565. However, its performance was still marginally inferior to that of the full-wavelength model, suggesting that excessive wavelength compression may compromise the completeness of the spectral information. TPCD exhibited a similar trend. Although the CNN model achieved an of up to 0.955, the prediction performance decreased, with values of only 0.593–0.820. Compared with the full-wavelength model, this decline indicates that feature selection reduces redundant wavelengths but may also remove critical spectral information, ultimately limiting prediction accuracy [22].
For TA, RS, and TPCN (Figure 10 and Figure 11c–e), all three models constructed using feature wavelengths showed improved performance compared with the full-wavelength models. In predicting TA, the CNN model outperformed the other models, with R2 values of 0.970 and 0.913 for the calibration and prediction sets, respectively, an RPD of 3.397, and minimal prediction error. These results demonstrate that deep feature extraction combined with wavelength selection provides a clear advantage for total acidity prediction [22]. For RS, the PLS model performed less effectively on the prediction set (: 0.364–0.776; RPD: 1.254–2.111) compared with the SVR model (: 0.713–0.889; RPD: 1.867–2.998). The CNN model again achieved the best predictive performance (: 0.576–0.878; RPD: 2.860). These findings indicate that feature-wavelength selection can effectively extract key information and remove redundant variables, enabling the construction of more efficient and reliable predictive models for RS than full-wavelength approaches [20]. A similar behavior was noted in the prediction of TPCN. The PLS model (: 0.553–0.724; RPD: 1.495–1.903) showed relatively weak performance, while the SVR model (: 0.682–0.793; RPD: 1.773–2.904) performed better. Once again, the CNN model exhibited superior predictive accuracy and robustness, achieving values ranging from 0.625 to 0.880, RPD values between 2.051 and 2.883, and the lowest RMSEP [30].
The results indicate that the key wavelengths selected by SPA provided effective information for all three modeling approaches [22,35]. The CNN models were able to capture complex nonlinear relationships more fully when processing these high-dimensional features, whereas PLS was constrained by its linear nature, and SVR showed slightly lower prediction accuracy than CNN for certain samples. These findings demonstrate that, when applied to feature-selected high-dimensional spectral data, convolutional neural networks offer clear advantages in nonlinear mapping and modeling robustness [30,35].
3.5. Determination of the Optimal Models
Based on the results shown in Figure 3 and Figure 9, the prediction models developed for the six quality parameters were comprehensively analyzed. For pH, the full-wavelength PLS model built using DT-preprocessed spectra was identified as the optimal model, with , and RPD values of 0.967, 0.940, and 4.074, respectively. For SSC, the SVR model constructed after DT preprocessing exhibited the best performance, achieving = 0.963, = 0.957, and an RPD of 4.798. For TPCN, the SVR model performed best when MSC-preprocessed spectra were used, yielding = 0.907, = 0.871, and an RPD of 2.786. For TA, the best-performing model was the CNN model constructed using DT-preprocessed spectra, followed by SPA-based feature wavelength selection, with = 0.946, = 0.913, and an RPD of 3.397. For RS, the most effective model was identified as the SVR model developed from SNV-preprocessed spectra combined with feature wavelength selection, achieving = 0.903, = 0.889, and an RPD of 2.998. For TPN, the optimal model was again an SVR model, constructed using MSC-preprocessed spectra together with feature wavelength selection, resulting in = 0.917, = 0.907, and an RPD of 2.904.
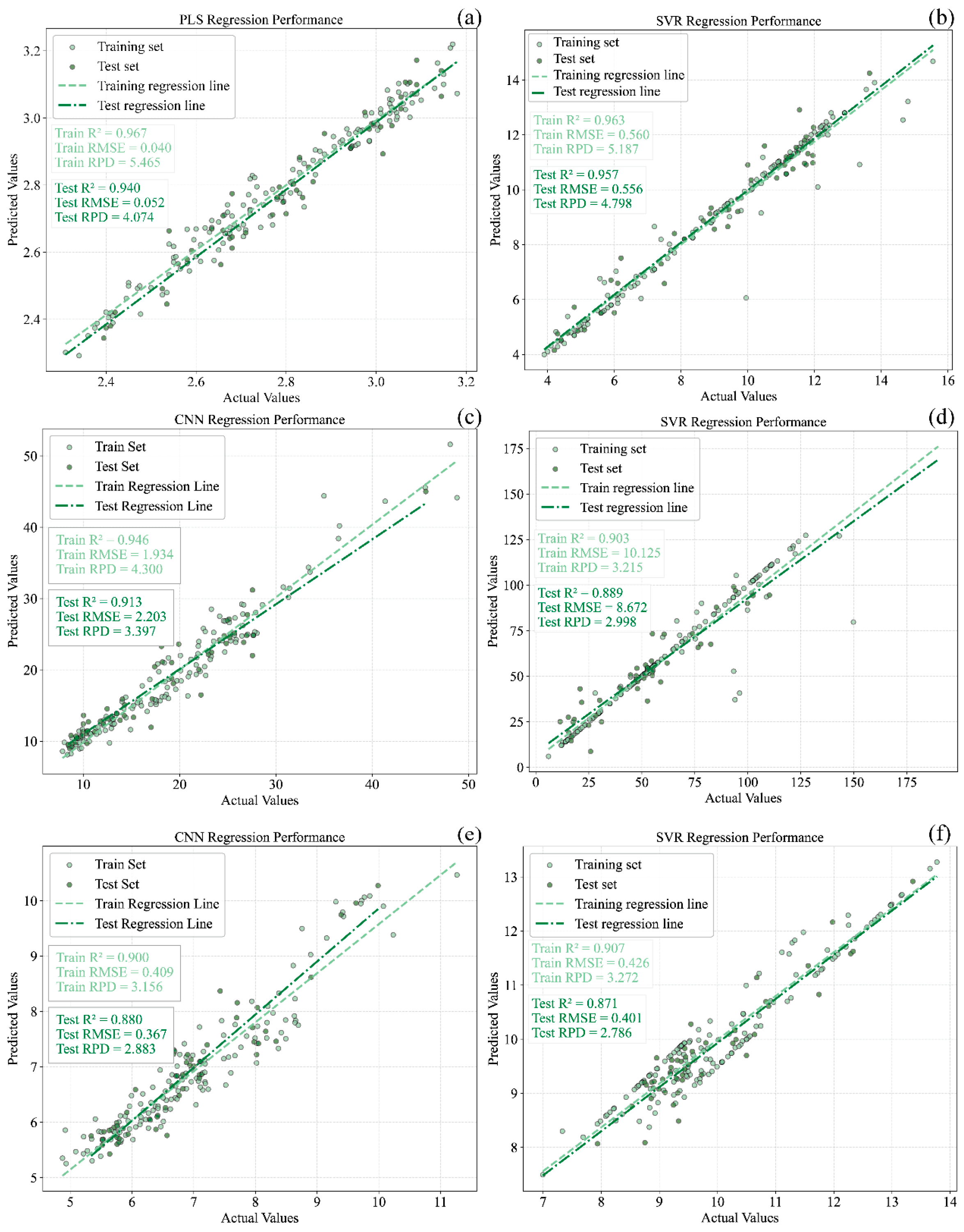
Scatter plots illustrating the correspondence between predicted and reference values for the best calibration and prediction models of pH, TSS, TA, RS, TPCN, and TPCD in grape samples are presented in Figure 12. The CNN-based deep learning approach employed in this study is capable of autonomously learning and extracting features through multiple convolutional layers. Previous spectroscopic studies have demonstrated that CNNs often exhibit superior predictive performance compared with traditional machine learning methods [30,48,49]. After key feature wavelengths were selected, the CNN model exhibited marginally superior performance to PLS and SVR in predicting TA in Ugni Blanc grapes. This improvement may be attributed to the ability of CNNs to capture complex nonlinear relationships and their inherent robustness to noise arising from illumination or sample variability. The feature wavelength selection further reduced data redundancy and noise, providing a more concise input for the CNN model and thereby improving prediction accuracy.
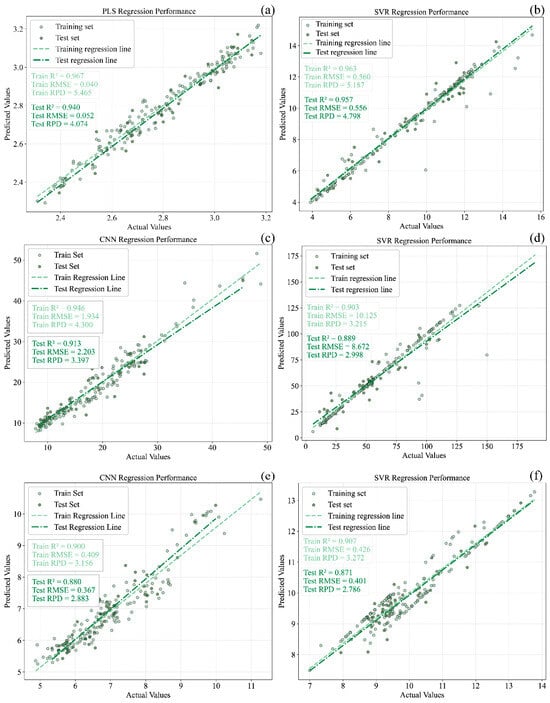
Figure 12.
The best models of pH, TSS, TA, RS and total phenols in grape samples. (a) pH, (b) TSS, (c) TA, (d) RS, (e) TPCN, (f) TPCD. TSS = total soluble solid content, TA = total acid, RS = reducing sugar, TPCN = total phenolic content of the skin, TPCD = total phenolic content of the seed, PLS = partial least squares, SVR = support vector machine regression, CNN = convolutional neural network.
For the optimal prediction models of the six physicochemical parameters, all values exceeded 0.85, and those for pH, TSS, TA, and TPCN were above 0.90, indicating strong predictive capability. However, the CNN model in this study was deliberately kept shallow, as spectral data are one-dimensional, and overly deep networks tend to cause overfitting, whereas deeper neural architectures typically perform better on larger datasets.
4. Conclusions
The raw spectral data were first subjected to six preprocessing methods for this analysis, followed by modeling using PLS, SVR, and CNN. The results showed that for pH, TSS, and TA, the models based on DT-preprocessed spectra performed best, while the prediction accuracy of TPCN and TPCD was significantly improved after MSC preprocessing. For RS, model performance was enhanced following SNV preprocessing. Further, feature wavelengths were extracted from the optimally preprocessed spectra using the SPA algorithm. For each parameter, 50 feature wavelengths were extracted, representing only 3.3% of the full spectral range and reducing the data volume by over 95%. A comprehensive comparison of PLS, SVR, and CNN models constructed with both full-spectrum and selected feature wavelengths revealed that for TA, RS, and TPCN, feature wavelength selection not only substantially reduced computational cost but also improved prediction accuracy. In contrast, model accuracy for pH, TSS, and TPCD showed a slight decline. The optimal models for each parameter were determined as follows: DT-PLS (pH), DT-SVR (TSS), DT-SPA-CNN (TA), SNV-SPA-SVR (RS), MSC-SPA-SVR (TPCN), and MSC-SVR (TPCD). The corresponding prediction set evaluation metrics were: = 0.940, 0.957, 0.913, 0.889, 0.917, and 0.871; RMSEP = 0.052, 0.585; Brix, 2.203 g/L, 8.672 g/L, 2.904 mg/g, and 0.401 mg/g; RPD = 4.074, 4.798, 3.397, 2.998, 2.904, and 2.786, respectively. Despite the promising results, the applicability of these models to other grape varieties remains to be validated. Additionally, the current study only includes samples from a single vineyard and a single season, which may limit the generalizability of the models. Future work should incorporate samples from multiple sources and harvest years to enhance the robustness and reliability of the predictive models.
5. Future
For future research, the collection of grape samples could be further expanded to include samples from different sources and years, enhancing model comprehensiveness. Additionally, fine-tuning and training models using multi-year datasets could improve model stability and generalizability. This would allow for deeper CNN architectures, further increasing model robustness and predictive accuracy and promoting the use of deep learning in this field.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods15030475/s1, Table S1. Layers and parameters of the one-dimensional convolutional neural network architecture. Table S2. Dataset Division Using SPXY Method. Table S3. Characteristic wavelengths of grape quality indicators and their corresponding chemical functional groups.
Author Contributions
Writing—original draft, C.S.; Methodology, C.S., J.C. and W.L.; Investigation, C.S., J.C., Z.W. and K.L.; Software, C.S. and J.C.; Data Curation, C.S., Z.W. and W.L.; Formal analysis, C.S. and W.L., Validation, C.S., W.L., X.S. and Y.F.; Writing—review and editing, C.S., J.C., Z.W., X.S., W.L. and Y.F.; Supervision, X.S. and W.L.; Resources, X.S. and W.L.; Funding acquisition, W.L.; Project administration and Conceptualization, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key R&D Program of Shandong Province, China (2023TZXD062), the special project of scientific and technological innovation of Xinjiang Research Institute of Arid Area Agriculture (XJHQNY-2025-7), the National Natural Science Foundation of China (32201678), and the Key R&D Program of Shanxi Province, China (2025NC-YBXM-219).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rolle, L.; Giacosa, S.; Gerbi, V.; Novello, V. Comparative Study of Texture Properties, Color Characteristics, and Chemical Composition of Ten White Table-Grape Varieties. Am. J. Enol. Vitic. 2011, 62, 49–56. [Google Scholar] [CrossRef]
- Niu, S.Y.; Hao, F.G.; Mo, H.Z.; Jiang, J.F.; Wang, H.; Liu, C.H.; Fan, X.C.; Zhang, Y. Phenol profiles and antioxidant properties of white skinned grapes and their coloured genotypes during growth. Biotechnol. Biotechnol. Equip. 2017, 31, 58–67. [Google Scholar] [CrossRef]
- Ferrero-del-Teso, S.; Arias, I.; Escudero, A.; Ferreira, V.; Fernández-Zurbano, P.; Sáenz-Navajas, M.P. Effect of grape maturity on wine sensory and chemical features: The case of Moristel wines. LWT-Food Sci. Technol. 2020, 118, 108848. [Google Scholar] [CrossRef]
- Butron-Benitez, D.; Valcárcel-Muñoz, M.J.; García-Moreno, M.V.; Rodriguez-Dodero, M.C.; Guillén-Sanchez, D.A. Effect of the alcoholic strength of unaged wine distillates on the final composition of Brandy de Jerez aged in Sherry Casks®. Oeno One 2023, 57, 269–285. [Google Scholar] [CrossRef]
- Benelli, A.; Cevoli, C.; Ragni, L.; Fabbri, A. In-field and non-destructive monitoring of grapes maturity by hyperspectral imaging. Biosyst. Eng. 2021, 207, 59–67. [Google Scholar] [CrossRef]
- Yang, S.H.; Zheng, Y.J.; Liu, X.X.; Zhang, T.G.; Zhang, X.S.; Xu, L.M. Cabernet Gernischt Maturity Determination Based on Near-Ground Multispectral Figures by Using UAVs. Spectrosc. Spectr. Anal. 2021, 41, 3220–3226. [Google Scholar]
- Guerrero-Chanivet, M.; Ortega-Gavilán, F.; Bagur-González, M.G.; Valcarcel-Muñoz, M.J.; García-Moreno, M.V.; Guillén-Sánchez, D.A. Influence of the use of sulfur dioxide, the distillation method, the oak wood type and the aging time on the production of brandies. Curr. Res. Food Sci. 2023, 6, 100486. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, D.; Hu, X.J.; Tong, W.H.; Han, B.L.; Tian, J.P.; Luo, H.B. Application of hyperspectral technology in detection of agricultural products and food: A Review. Food Sci. Nutr. 2020, 8, 5206–5214. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wang, J.; Liu, X.; Gu, Y.H.; Ren, Z.H. The Application of Optical Nondestructive Testing for Fresh Berry Fruits. Food Eng. Rev. 2024, 16, 85–115. [Google Scholar] [CrossRef]
- Li, J.B.; Huang, W.Q.; Zhao, C.J.; Zhang, B.H. A comparative study for the quantitative determination of soluble solids content, pH and firmness of pears by Vis/NIR spectroscopy. J. Food Eng. 2013, 116, 324–332. [Google Scholar] [CrossRef]
- Li, J.B.; Tian, X.; Huang, W.Q.; Zhang, B.H.; Fan, S.X. Application of Long-Wave Near Infrared Hyperspectral Imaging for Measurement of Soluble Solid Content (TSS) in Pear. Food Anal. Methods 2016, 9, 3087–3098. [Google Scholar] [CrossRef]
- Li, J.B.; Zhang, H.L.; Zhan, B.S.; Wang, Z.L.; Jiang, Y.L. Determination of TSS in pears by establishing the multi-cultivar models based on visible-NIR spectroscopy. Infrared Phys. Technol. 2019, 102, 103066. [Google Scholar] [CrossRef]
- Fernández-Novales, J.; Garde-Cerdán, T.; Tardáguila, J.; Gutiérrez-Gamboa, G.; Pérez-Alvarez, E.P.; Diago, M.P. Assessment of amino acids and total soluble solids in intact grape berries using contactless Vis and NIR spectroscopy during ripening. Talanta 2019, 199, 244–253. [Google Scholar] [CrossRef]
- Xiao, H.; Feng, L.; Song, D.J.; Tu, K.; Peng, J.; Pan, L.Q. Grading and Sorting of Grape Berries Using Visible-Near Infrared Spectroscopy on the Basis of Multiple Inner Quality Parameters. Sensors 2019, 19, 2600. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Sun, X.Y.; Huang, W.D. Parameter optimization in soluble solid content prediction of entire bunches of grape based on near infrared spectroscopic technique. J. Food Meas. Charact. 2017, 11, 1676–1680. [Google Scholar] [CrossRef]
- Basile, T.; Marsico, A.D.; Perniola, R. NIR Analysis of Intact Grape Berries: Chemical and Physical Properties Prediction Using Multivariate Analysis. Foods 2021, 10, 113. [Google Scholar] [CrossRef]
- Gehlken, J.; Nikfardjam, M.P.; Zörb, C. Determination of aroma compounds in grape mash under conditions of tasting by on-line near-infrared spectroscopy. Eur. Food Res. Technol. 2022, 248, 2325–2337. [Google Scholar] [CrossRef]
- Duckena, L.; Alksnis, R.; Erdberga, I.; Alsina, I.; Dubova, L.; Duma, M. Non-Destructive Quality Evaluation of 80 Tomato Varieties Using Vis-NIR Spectroscopy. Foods 2023, 12, 1990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.X.; Sun, Q.; Liu, Y.; Yang, Y.T.; Zhuang, Z.L. Prediction of Solid Soluble Content of Green Plum Based on Improved CatBoost. Agriculture 2023, 13, 1122. [Google Scholar] [CrossRef]
- Zhou, X.J.; Liu, W.Z.; Li, K.; Lu, D.Q.; Su, Y.; Ju, Y.L.; Fang, Y.L.; Yang, J.H. Discrimination of Maturity Stages of Cabernet Sauvignon Wine Grapes Using Visible-Near-Infrared Spectroscopy. Foods 2023, 12, 4371. [Google Scholar] [CrossRef]
- Li, L.; Hu, D.Y.; Tang, T.Y.; Tang, Y.L. Non-destructive detection of the quality attributes of fruits by visible-near infrared spectroscopy. J. Food Meas. Charact. 2023, 17, 1526–1534. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Cao, Y.C.; Yang, J.; Xie, L.J. Enhancing model robustness through different optimization methods and 1-D CNN to eliminate the variations in size and detection position for apple TSS determination. Postharvest Biol. Technol. 2023, 205, 112513. [Google Scholar] [CrossRef]
- Aherwadi, N.; Mittal, U.; Singla, J.; Jhanjhi, N.Z.; Yassine, A.; Hossain, M.S. Prediction of Fruit Maturity, Quality, and Its Life Using Deep Learning Algorithms. Electronics 2022, 11, 4100. [Google Scholar] [CrossRef]
- Wei, X.J.; Xie, F.X.; Wang, K.; Song, J.; Bai, Y. A study on Shine-Muscat grape detection at maturity based on deep learning. Sci. Rep. 2023, 13, 4587. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.D.; Mesa, N.F.O.; Freire, M.S.; Ramos, R.P.; Mederos, B.J.T. Development of predictive models for quality and maturation stage attributes of wine grapes using vis-nir reflectance spectroscopy. Postharvest Biol. Technol. 2019, 150, 166–178. [Google Scholar] [CrossRef]
- Armstrong, C.E.J.; Gilmore, A.M.; Boss, P.K.; Pagay, V.; Jeffery, D.W. Machine learning for classifying and predicting grape maturity indices using absorbance and fluorescence spectra. Food Chem. 2023, 403, 134321. [Google Scholar] [CrossRef] [PubMed]
- Escribano, S.; Biasi, W.V.; Lerud, R.; Slaughter, D.C.; Mitcham, E.J. Non-destructive prediction of soluble solids and dry matter content using NIR spectroscopy and its relationship with sensory quality in sweet cherries. Postharvest Biol. Technol. 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Tosin, R.; Monteiro-Silva, F.; Martins, R.; Cunha, M. Precision maturation assessment of grape tissues: Hyperspectral bi-directional reconstruction using tomography-like based on multi-block hierarchical principal component analysis. Biosyst. Eng. 2023, 236, 147–159. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah). Antioxidants 2020, 9, 800. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yang, J.H.; Su, Y.; He, K.; Fang, Y.L.; Sun, X.Y.; Ju, Y.L.; Liu, W.Z. Aggregation and assessment of grape quality parameters with visible-near-infrared spectroscopy: Introducing a novel quantitative index. Postharvest Biol. Technol. 2024, 218, 113131. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compos. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Adesokan, M.; Alamu, E.O.; Fawole, S.; Maziya-Dixon, B. Prediction of functional characteristics of gari (cassava flakes) using near-infrared reflectance spectrometry. Front. Chem. 2023, 11, 1156718. [Google Scholar] [CrossRef]
- Gao, T.; Hu, L.N.; Jia, Z.Z.; Xia, T.N.; Fang, C.; Li, H.Z.; Hu, L.H.; Lu, Y.H.; Li, H. SPXYE: An improved method for partitioning training and validation sets. Clust. Comput.-J. Netw. Softw. Tools Appl. 2019, 22, S3069–S3078. [Google Scholar] [CrossRef]
- de Brito, A.A.; Campos, F.; Nascimento, A.D.; Damiani, C.; da Silva, F.A.; Teixeira, G.H.D.; Cunha, L.C., Jr. Non-destructive determination of color, titratable acidity, and dry matter in intact tomatoes using a portable Vis-NIR spectrometer. J. Food Compos. Anal. 2022, 107, 104288. [Google Scholar] [CrossRef]
- Li, S.Y.; Song, Q.M.; Liu, Y.J.; Zeng, T.H.; Liu, S.Y.; Jie, D.F.; Wei, X. Hyperspectral imaging-based detection of soluble solids content of loquat from a small sample. Postharvest Biol. Technol. 2023, 204, 112454. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Partial Least Squares Regression (PLSR) Applied to NIR and HSI Spectral Data Modeling to Predict Chemical Properties of Fish Muscle. Food Eng. Rev. 2017, 9, 36–49. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, Q.Q. Online Quality-Relevant Monitoring with Dynamic Weighted Partial Least Squares. Ind. Eng. Chem. Res. 2020, 59, 21124–21132. [Google Scholar] [CrossRef]
- Jain, P.; Rahman, I.; Kulkarni, B.D. Development of a soft sensor for a batch distillation column using support vector regression techniques. Chem. Eng. Res. Des. 2007, 85, 283–287. [Google Scholar] [CrossRef]
- Malek, S.; Melgani, F.; Bazi, Y. One-dimensional convolutional neural networks for spectroscopic signal regression. J. Chemom. 2018, 32, 2977. [Google Scholar] [CrossRef]
- Hamad, H. Validation of metamodels in simulation: A new metric. Eng. Comput. 2011, 27, 309–317. [Google Scholar] [CrossRef]
- Lleó, L.; Barreiro, P.; Ruiz-Altisent, M.; Herrero, A. Multispectral images of peach related to firmness and maturity at harvest. J. Food Eng. 2009, 93, 229–235. [Google Scholar] [CrossRef]
- Scano, P. Characterization of the medium infrared spectra of polyphenols of red and white wines by integrating FT IR and UV-Vis spectral data. LWT-Food Sci. Technol. 2021, 147, 111604. [Google Scholar] [CrossRef]
- Fernández-Novales, J.; Tardáguila, J.; Gutiérrez, S.; Diago, M.P. On-The-Go VIS plus SW—NIR Spectroscopy as a Reliable Monitoring Tool for Grape Composition within the Vineyard. Molecules 2019, 24, 2795. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Dong, X.L. Improved partial least squares regression for rapid determination of reducing sugar of potato flours by near infrared spectroscopy and variable selection method. J. Food Meas. Charact. 2015, 9, 95–103. [Google Scholar] [CrossRef]
- Mishra, P.; Passos, D. Deep chemometrics: Validation and transfer of a global deep near-infrared fruit model to use it on a new portable instrument. J. Chemom. 2021, 35, 3367. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Guo, Z.M.; Wang, A.C.; Zhou, R.Y.; Cai, J.R. Influence of the peel on online detecting soluble solids content of pomelo using Vis-NIR spectroscopy coupled with chemometric analysis. Food Control 2025, 167, 110777. [Google Scholar] [CrossRef]
- Wei, X.; He, J.C.; Zheng, S.H.; Ye, D.P. Modeling for SSC and firmness detection of persimmon based on NIR hyperspectral imaging by sample partitioning and variables selection. Infrared Phys. Technol. 2020, 105, 103099. [Google Scholar] [CrossRef]
- Iqbal, S.; Qureshi, A.N.; Alhussein, M.; Aurangzeb, K.; Anwar, M.S. AD-CAM: Enhancing Interpretability of Convolutional Neural Networks With a Lightweight Framework—From Black Box to Glass Box. IEEE J. Biomed. Health Inform. 2024, 28, 514–525. [Google Scholar] [CrossRef]
- Held, M.; Rabe, A.; Senf, C.; van der Linden, S.; Hostert, P. Analyzing Hyperspectral and Hypertemporal Data by Decoupling Feature Redundancy and Feature Relevance. IEEE Geosci. Remote Sens. Lett. 2015, 12, 983–987. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.