Abstract
Metabolic syndrome (MetS) and its associated conditions, namely, type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), obesity, and polycystic ovary syndrome (PCOS) are characterized by insulin resistance, dyslipidemia, and low-grade inflammation. Curcumin, a polyphenolic compound derived from Curcuma longa Linn., exhibits pleiotropic metabolic and anti-inflammatory properties and has thus been evaluated as a nutraceutical intervention for these conditions, but findings remain inconsistent. This systematic review and meta-analysis evaluated the clinical efficacy of Curcuma longa supplementation on anthropometric, glycemic, lipid, inflammatory, and oxidative stress parameters in adults with MetS or related disorders. A comprehensive search of databases (PubMed, Scopus, AMED, LILACS, and Google Scholar) identified 104 eligible randomized controlled trials (RCTs). The included trials primarily assessed standardized oral turmeric/curcumin supplements and bioavailability-enhanced formulations rather than whole culinary turmeric. Pooled standardized mean differences (SMDs) with 95% confidence intervals (CIs) were computed using random-effects models. Subgroup analyses were conducted by disease category, dose, and formulation. Risk of bias was assessed using the Cochrane RoB 2 tool. Curcumin supplementation significantly reduced fasting blood sugar (SMD = −0.54, 95% CI −0.72 to −0.36) and HbA1c (SMD = −0.41, 95% CI −0.60 to −0.23) in T2DM; decreased triglycerides (SMD = −0.48; 95% CI: −0.70 to −0.25), and LDL cholesterol (SMD = −0.39; 95% CI: −0.59 to −0.18) while elevating HDL cholesterol (SMD = 0.45; 95% CI: 0.25 to 0.65) and total antioxidant capacity (SMD = 0.73; 95% CI: 0.51 to 0.94). Curcuma longa also attenuated systemic inflammation, lowering C-reactive protein (SMD = −0.62; 95% CI: −0.81 to −0.43), TNF-α (SMD = −0.57; 95% CI: −0.80 to −0.34), and IL-6 (SMD = −0.50; 95% CI: −0.70 to −0.29). Heterogeneity was moderate-to-high, reflecting some differences in the formulation, dosage, and duration. Collectively, these findings affirm that Curcuma longa exerts measurable, clinically relevant improvements on glycemic regulation, lipid metabolism, and inflammatory−oxidative balance, supporting its role as a nutraceutical adjunct in metabolic health management, while its bioavailability-enhanced formulations show superior efficacy. Larger, long-term, multicenter RCTs are warranted to confirm durability, optimal dosing, and safety.
1. Introduction
Metabolic syndrome (MetS) induces a constellation of cardiometabolic risk factors such as central obesity, dyslipidemia, poor glycemic control, and hypertension that increase the risk of type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease [1,2,3]. The rate of its spread is increasing across the globe due to increases in urbanization, sedentary lifestyles, and high-energy diets. One in every four adults qualifies as experiencing MetS; this places an extra burden on health systems that are already under pressure from non-communicable diseases [4]. MetS is particularly dangerous, since each of its components support each other. High visceral fats lead to dysfunction in the adipocytes, releasing pro-inflammatory cytokines like TNF-α and IL-6 and reducing beneficial adipokines like adiponectin [5]. This inflammatory condition disrupts insulin signaling, drives oxidative stress, compromises endothelial activity, and disrupts lipid metabolism. These changes, in combination, cause insulin resistance, atherosclerosis, and organ damage. Thus, multi-tasking treatments targeting inflammation, oxidative stress, and metabolic dysregulation are highly promising for the treatment of MetS.
A traditional medicinal ingredient and a culinary spice that has been used for centuries is Turmeric (Curcuma longa Linn.). It contains antioxidant, anti-inflammatory, and metabolism-regulating properties due to its main active compounds, curcumin and curcuminoids [2,6,7]. In preclinical models, curcumin has been shown to cause an inhibition in the activation of NF-κB, downregulate proinflammatory mediators such as COX-2 and iNOS, stimulate AMP-activated protein kinase (AMPK) signaling, inhibit lipid peroxidation, and enhance mitochondrial function [8,9]. Beyond these, a growing body of research suggests curcumin also modulates gut microbiota composition, strengthens intestinal barrier integrity, and invariably influences host–microbiome metabolic crosstalk [10,11,12].
Over the past decade, a considerable number of randomized controlled trials (RCTs) have been carried out on individuals with MetS or related metabolic diseases. Some trials have described an increase in fasting glucose, HbA1c, triglycerides, total cholesterol, and inflammatory markers [1,2,13]. Others have reported negligible or inconsistent effects. These discrepancies may arise from the differences in study design, dosage, curcumin formulation, intervention duration, or baseline metabolic status. Consequently, the overall clinical relevance of C. longa in metabolic regulation remains uncertain.
Metabolic syndrome presents a major clinical and public health challenge because its management often requires long-term, multi-drug therapy targeting individual components of the disease, leading to polypharmacy, variable adherence, and cumulative adverse effects. This has driven increasing interest in safe, cost-effective adjunctive approaches capable of simultaneously modulating key pathogenic mechanisms, including chronic low-grade inflammation, oxidative stress, insulin resistance, and dyslipidemia. Nutraceuticals derived from bioactive food constituents have therefore gained attention as integrative strategies for metabolic risk reduction, provided their clinical efficacy is supported by robust randomized evidence.
Curcuma longa Linn., particularly its principal bioactive compound, curcumin, has emerged as a leading nutraceutical candidate due to its pleiotropic anti-inflammatory, antioxidant, and metabolic regulatory effects. Advances in formulation technologies have improved its bioavailability and clinical applicability; however, findings from randomized controlled trials investigating metabolic syndrome and related conditions remain heterogeneous and at times, inconsistent. Accordingly, the present systematic review and meta-analysis quantitatively synthesizes randomized evidence to clarify the clinical relevance of Curcuma longa supplementation across metabolic, inflammatory, and oxidative stress outcomes, thereby providing evidence to inform its rational integration into metabolic health management.
Given this heterogeneity, a rigorous quantitative synthesis of relevant data is needed to clarify the therapeutic potential of Curcuma longa as a dietary supplement in the aforementioned metabolic disorders. Although turmeric has a long history of culinary and traditional medicinal use and can be considered a nutraceutical, most contemporary randomized clinical trials evaluate standardized turmeric/curcumin supplements or bioavailability-enhanced curcumin formulations rather than whole-food turmeric consumed as part of a habitual diet. Accordingly, this review focuses specifically on supplemental and formulated curcumin interventions and not on the dietary use of whole turmeric. Therefore, this systematic review and meta-analysis aims to evaluate the pooled effects of C. longa supplementation on glycemic, lipid, anthropometric, inflammatory, and oxidative stress markers in adults with MetS or related conditions; explore subgroup differences by disease type, dosage, and formulation; and assess the quality and consistency of the available clinical evidence. Establishing a consolidated evidence base for curcumin’s efficacy may inform its rational integration into dietary and preventive strategies for improved metabolic health.
2. Materials and Methods
2.1. Study Design and Protocol Registration
The systematic review and meta-analysis were performed according to the Preferred Items to Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [14]. Figure 1 demonstrates the PRISMA checklist for every reporting domain. The a priori registration of the study protocol is in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD420251184355. The study was aimed at assessing the clinical effectiveness of Curcuma longa Linn. (curcumin) supplementation in metabolic syndrome and associated conditions, such as type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), obesity, and polycystic ovary syndrome (PCOS). The inclusion criteria were randomized controlled trials (RCTs) that investigated Curcuma longa or curcumin preparations in the oral form, either as a mono-therapy or an adjunctive to standard care, and reported an outcome on anthropometric, glycemic, lipid, inflammatory, or oxidative stress measurements. MetaAnalysisOnline.com (https://www.metaanalysisonline.com; accessed 15 July 2025) [15] was used to conduct a meta-analysis of the extracted quantitative data. The disease subtype and outcome categories were used to stratify the pooled analyses in order to identify heterogeneity. The effects were measured as standardized mean differences (SMDs) in the form of 95% confidence intervals (CIs) based on a random-effects model. The tau2 and I2 statistics were used to assess between-study variance. Sensitivity and subgroup analyses were performed to determine the effect of the study quality, intervention formulation (standard, piperine-enhanced, phospholipidated, or nanocurcumin), and the duration of dosage on the overall effects.
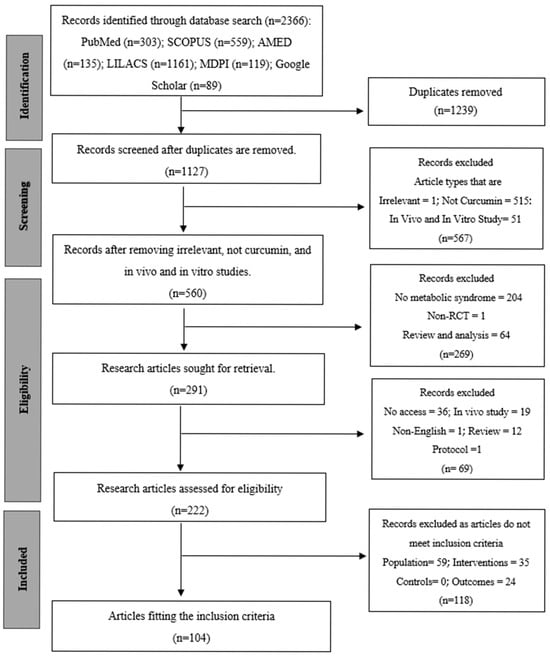
Figure 1.
PRISMA flow diagram of the systematic review process.
2.2. Information Sources and Search Strategy
An extensive literature review was performed to explore the existing randomized controlled trials (RCTs) exploring the impact of Curcuma longa Linn. (curcumin) supplementation on the elements of metabolic syndrome and its associated conditions. Also, databases such as Pubmed (http://www.pubmed.gov (accessed on 23 August 2024)), Scopus (http://www.scopus.com (accessed on 28 August 2024)), AMED (https://www.ebsco.com/products/research-databases (accessed on 28 August 2024)), LILACS (https://lilacs.bvsalud.org (accessed on 30 August 2024)), MDPI (www.mdpi.com (accessed on 28 August 2024)), and Google Scholar (www.scholar.google.com (accessed on 2 September 2024)) without time restrictions (Supplementary Table S1). Searches were conducted from database inception through 9 September 2024. No additional update searches were performed after 9 September 2024, prior to final analysis, to avoid selective inclusion and to maintain methodological consistency. The search strategy was a combination of Medical Subject Headings (MeSH) and the free-text terms of Curcuma longa, curcumin, and metabolic syndrome. Sensitivity and specificity were improved with the use of the Boolean operators (“AND” or “OR”) and truncation symbols. All databases had the search strategies adapted to them, making them sensitive and specific. There were also no limitations on the year of publication. Only English studies that were carried out on human populations were considered. The retrieved articles and relevant reviews were manually screened to discover more eligible studies in their reference lists. To ensure search transparency and reproducibility, the complete database search history and query strings are provided in Supplementary Table S1. The search process and record selection are visually summarized in the PRISMA flow diagram (Figure 1)
2.3. Eligibility Criteria
For the purposes of this review, we defined ‘Nutraceutical/Dietary Supplement’ as an oral, standardized preparation delivered as a capsule, tablet, or liquid formulation containing curcumin or curcumin-enriched Curcuma longa extracts. This definition includes formulations designed to enhance systemic bioavailability (e.g., curcumin–piperine combinations, phytosomal or phospholipid complexes, and nano-formulations). Studies of whole-food turmeric preparations incorporated into meals or habitual diets were not the focus of this review. This distinction is critical because supplement formulations provide concentrated, standardized curcuminoid doses and pharmacokinetic profiles that differ substantially from culinary turmeric. We included studies based on the following predefined eligibility criteria, which were structured according to the PICOS framework (Table 1).

Table 1.
Eligibility criteria for the selected studies.
2.3.1. Exclusion Criteria
Studies that were not randomized (for example, observational studies), in vitro experiments, and animal studies were excluded because they do not provide the randomized clinical evidence required for this review. We also excluded case reports, narrative reviews, editorials, and conference abstracts lacking full text, as these sources do not present extractable trial data. Trials that administered curcumin together with other herbal extracts or multi-ingredient formulations were excluded when curcumin-specific effects could not be disentangled. Finally, we excluded trials that did not apply clear diagnostic criteria for metabolic syndrome or that failed to report sufficient outcome data for extraction and synthesis.
2.3.2. Prognostic Criteria for Inclusion
To ensure consistency, eligible studies must report baseline prognostic indicators of MetS such as waist circumference, BMI, or measures of central obesity; blood pressure (systolic/diastolic); lipid profile (triglycerides, HDL-C, LDL-C, total cholesterol); glycemic control (fasting plasma glucose, HbA1c, insulin resistance indices); and presence of at least three diagnostic risk factors for MetS (per ATP III, IDF, or WHO). These baseline criteria allow for pooling of comparable populations and enable subgroup analyses based on the severity of metabolic dysfunction, geographic region, or curcumin formulation.
2.4. Data Retrieval and Synthesis
Two independent reviewers screened titles and abstracts. Potentially eligible studies were identified, and the full contents were strictly examined according to the predetermined inclusion and exclusion criteria. Where the effect sizes were not extractable or calculable based on the published data, the relevant authors were contacted through email to obtain further statistical data. Disputes were resolved by consensus or a third reviewer. Only manuscripts describing original clinical studies were considered. All articles found during the search were imported into Microsoft Excel 2019, and duplicates were eliminated. The first screening included a title and abstract review, which led to the exclusion of irrelevant articles. The rest of the records underwent complete-text critical appraisal. Articles that had no complete text or were irrelevant were eliminated.
In order to improve data visualization, results were arranged and represented in figures, with other data summarized in tables. A standardized form of data extraction was created and pilot tested. Three reviewers extracted data from the selected studies. Variables of interest were as follows: study characteristics (author(s), year of publication, country, study design, sample size, randomization method, and blinding), population characteristics (age, gender distribution, duration and severity, baseline MetS symptoms), intervention (name of curcumin formulation, composition, form, route of administration, frequency, duration, dosage), comparator (type of control, e.g., no treatment, standard care/control diet, placebo), and outcomes assessed as core components of MetS, such as fasting blood glucose, HbA1c, triglycerides, total cholesterol, HDL-C, LDL-C, systolic and diastolic blood pressure, waist circumference, inflammatory markers (CRP, TNF-a, IL-6), oxidative stress markers (MDA, TAC), insulin resistance indices (HOMA-IR), liver enzymes, and body mass index (BMI). Any difference between data extraction was sorted out by discussion.
2.5. Quality Assessment Risk of Bias Assessment
The risk of bias in each included study was independently assessed by three (3) reviewers via predefined criteria adapted from the Cochrane Risk of Bias 2 (RoB 2) to assess the studies’ risk of bias [16]. This evaluation examined various factors, including the risk of bias in individual studies, the directness of the evidence, the precision of effect estimates, the heterogeneity among studies, and the potential for publication bias. Bias, in this context, refers to systematic deviations from accurate findings or inferences that can distort study results. This instrument comprises items categorized into randomization, allocation concealment, baseline characteristics, patient blinding, caregiver blinding, blinding of outcome assessor, overall attrition, differential attrition, other bias, and overall bias. Each domain was evaluated using one of three responses: “Low” (indicating low risk of bias), “High” (indicating high risk of bias), or “Unclear” (not clear due to insufficient information). Two reviewers independently evaluated each study and classified the risk of bias as “low,” “unclear,” or “high” in each domain. Upon concluding their assessments, the reviewers engaged in a comparative analysis and discussion of their findings. Discrepancies in scoring were addressed through consultation with a third reviewer to achieve consensus.
2.6. Statistical Analysis
Quantitative synthesis (meta-analysis) was performed using an online tool, MetaAnalysisOnline.com (https://metaanalysisonline.com; accessed 15 July 2025) when sufficient homogeneity in study design, intervention, and outcome measures was present. The intervention and control group sample sizes, standard deviations (SDs), and mean values were immediately uploaded into the application. Before data entry, the standard errors (SEs) provided in the studies were converted to standard deviations (SDs). Continuous outcome analysis was conducted via a random effects model because of the expected methodological and biological heterogeneity of studies. Effect sizes were calculated using the standard mean difference (SMD) with a 95% confidence interval (CI) due to the various measurement scales that were used in the study. The method of moments (Der-Simonian and Laird) was used to determine the variation between studies, and heterogeneity was measured using the Cochran Q test and quantified with the I2 statistic. An I2 value of more than 50% meant that there was a lot of heterogeneity. The assessment of biases in the publications was carried out visually (using funnel plots) and statistically (using the Egger regression test) [15]. A p-value of less than 0.05 was considered a manifestation of an important asymmetry. Publication bias was evaluated both visually through funnel plots and statistically through the Egger regression test [15], which was developed on MetaAnalysisOnline.com. The overall quality of evidence obtained for each outcome was evaluated via the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework [17]. The domains assessed included risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of the evidence was categorized as high, moderate, low, or very low.
3. Results
3.1. Study Selection
The process of study selection is represented in the PRISMA flow diagram (Figure 1). As of 9 September 2024, a total of 2366 records were first retrieved via electronic database searches, comprising contributions from PubMed (n = 303), SCOPUS (n = 559), AMED (n = 135), LILACS (n = 1161), MDPI (n = 119), and Google Scholar (n = 89). Following the elimination of 1239 duplicate records, 1127 distinct records were retained for screening. In the title and abstract screening step, 567 records were rejected because they did not meet the inclusion criteria. The exclusions were mainly attributed to the articles being reviews, nonclinical outcome measures, non-metabolic syndrome, non-curcumin, including animal or in vitro data, or lacking a comparative arm. This resulted in 291 records for additional evaluation. A total of 69 articles were eliminated because they were non-English, irrelevant, or lacked full-text access after a comprehensive assessment, resulting in 222 articles being evaluated for eligibility. After a comprehensive evaluation, 118 additional publications were removed for failing to meet the specified inclusion criteria: 59 did not have the required population, 35 did not satisfy the intervention criteria, and 24 lacked outcome data. Finally, 104 studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] were included in the final systematic review and fulfilled all the set inclusion criteria. These studies were identified by searching for statistical data, either in published publications or by emailing the corresponding author. Articles that had insufficient information to be used in meta-analysis were only included in the qualitative section of the systematic review. The earliest publication was from 2011, and over 90% of studies published were performed in the last decade (2014–2024).
3.2. Assessment of the Risk of Bias of the Included Studies
The Cochrane Risk of Bias 2 (RoB 2) tool for RCTs was applied to all included studies to evaluate their methodological quality. The assessment revealed a range of bias levels across domains (bias arising from the randomization, allocation concealment, baseline characteristics, patient blinding, caregiver blinding, blinding of outcome assessor, overall attrition, differential attrition, other bias, and overall bias) (Figure 2 and Supplementary Table S2). Overall, the risk of bias assessment indicates that a substantial majority of the included randomized controlled trials (RCTs) involving Curcuma longa are considered to have a low risk of bias in domains related to blinding and the completeness of outcome data. Specifically, risks associated with the Blinding of Patients, Blinding of Caregivers, and Blinding of Outcome Assessors were judged to be low across almost all studies, suggesting robust implementation of double-blind methodologies. Furthermore, the domains addressing Attrition Bias, represented by both Overall Attrition <20% and Differential Attrition <10%, also showed minimal concern, confirming that participant dropout and differential loss between groups were generally well-managed or reported effectively. This consistency across blinding and attrition domains strengthens the reliability of the observed treatment effects.
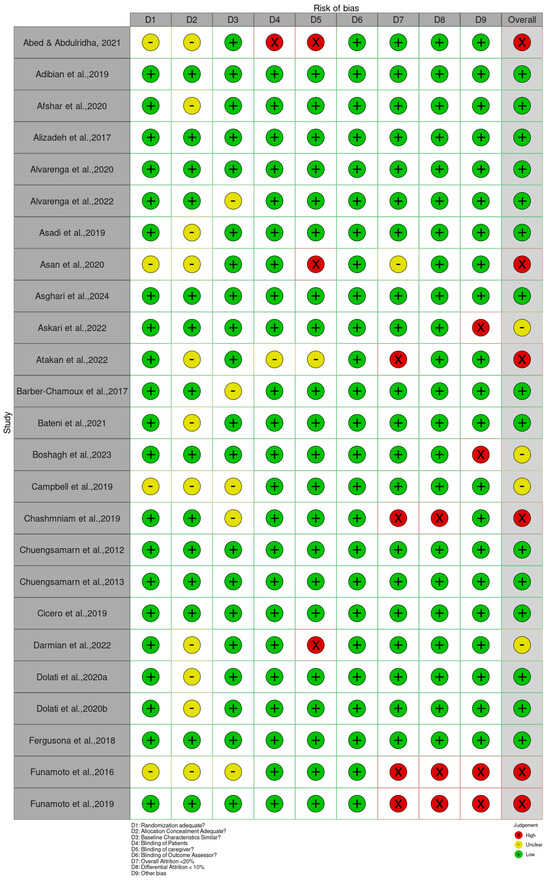
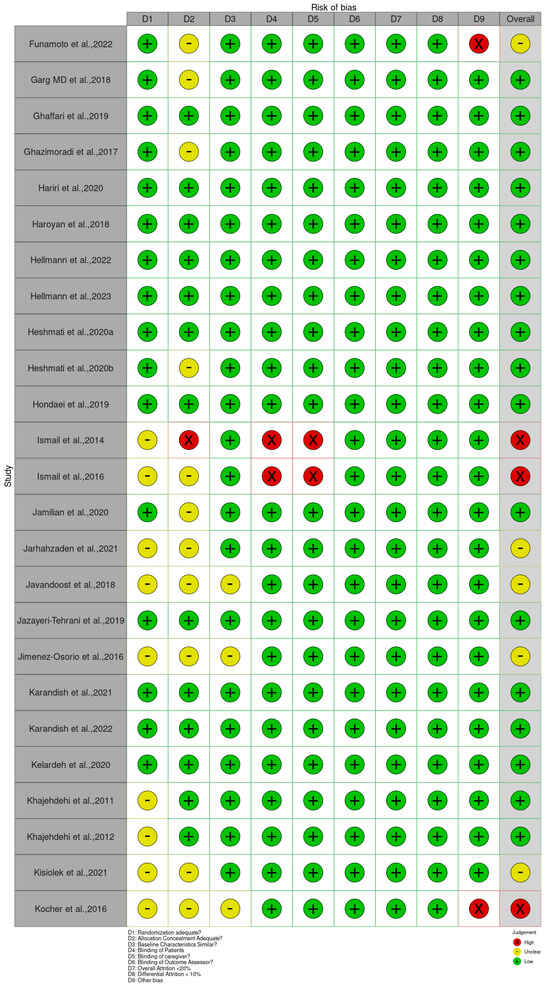
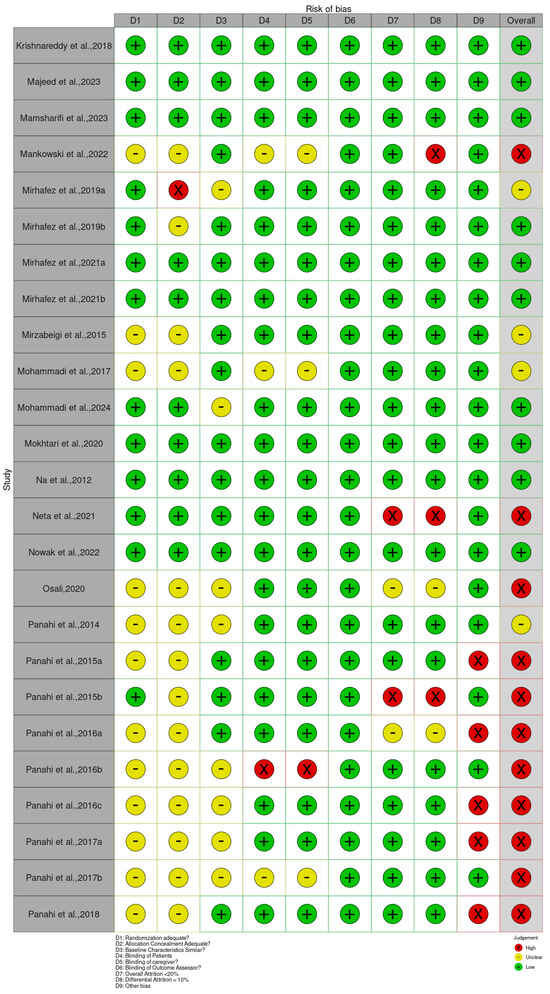
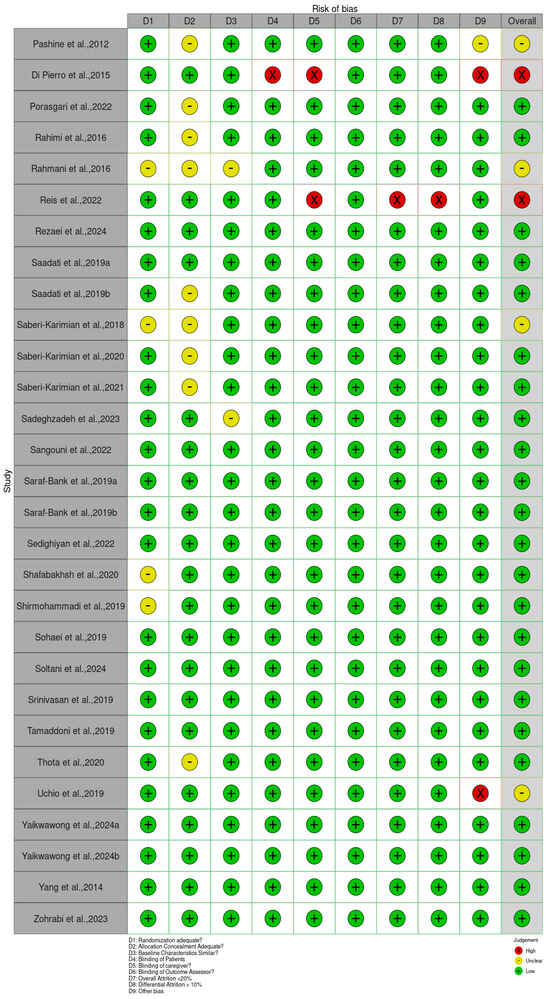
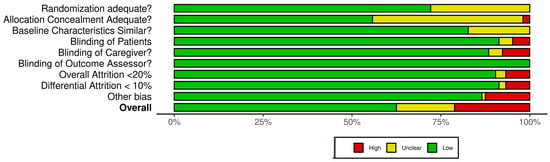
Figure 2.
Quality of reporting and bias evaluation conducted with the risk of bias tool. The upper panel illustrates the quality of reporting and bias risk in the included studies, while the lower panel evaluates biases related to selection, performance, detection, attrition, and other factors.
Bias arising from the randomization process was generally low, as many studies clearly reported adequate random sequence generation and allocation concealment [34,36,47,49,80]. However, a few trials, such as Abed et al. [19], Asan et al. [25], and Ismail et al. [54], showed some concerns or high risk due to insufficient reporting or unclear randomization procedures. Bias due to deviations from intended interventions showed the greatest variability. While studies such as Haroyan et al. [48], Hellmann et al. [50], and Mirhafez et al. [75] adequately implemented blinding and adherence monitoring, others, including Ismail et al. [54,55] and several trials by Panahi et al. [85,87], were rated as having some concerns or high risks, primarily because of open-label designs and potential protocol deviations.
Bias due to missing outcome data was mostly low across studies, with manageable attrition rates reported in trials such as Adibian et al. [19], Chuengsamarn et al. [35], and Cicero et al. [36]. Nevertheless, incomplete or unclearly handled data in studies such as Abed et al. [18], Asan et al. [25], and Mankowski et al. [71] resulted in the determination of there being some concern for this domain. Bias in measurement of the outcome was predominantly low in trials using objective biochemical or anthropometric measures [34,47,49,74]. In contrast, studies that relied on subjective outcomes without assessor blinding, such as Funamoto et al. [41,42], Chashmniam et al. [33], and Neta et al. [81], were rated as high or having some risks for outcome measurement. Bias in the selection of the reported result was low in most trials, indicating consistent reporting of prespecified outcomes [35,47,50,75]. A few studies, particularly those lacking a published protocol or showing incomplete reporting of secondary outcomes, were marked as having some concerns [85,87].
Overall, most trials demonstrated a low or moderate risk of bias, supporting confidence in the evidence base. High-risk studies [25,28,33,41,42,54,55,67,71,81,83,85,86,87,88,89,90,91,92,94,98] tended to show combined weaknesses in randomization, blinding, and outcome assessment. In contrast, a large number of studies [19,20,21,22,23,24,26,29,30,34,35,36,38,39,40,44,45,46,47,48,49,50,51,52,53,56,59,61,62,63,64,65,68,69,70,73,74,75,78,79,80,82,95,96,99,100,101,103,104,105,106,107,108,109,110,111,112,113,114,115,116,118,119,120,121] consistently demonstrated low risk across domains, contributing the most reliable evidence in this review.
3.3. Study Characteristics
A total of 104 randomized controlled trials (RCTs) investigating the clinical effects of Curcuma longa Linn. or its formulations in populations related to metabolic syndrome were included in this review. The included trials were published between 2011 and 2024, highlighting a steady and global rise in curcumin-focused clinical research over the past decade. The general characteristic table is illustrated in Table 2.

Table 2.
General characteristics of included studies.
3.3.1. Publication Year
The temporal distribution of studies revealed a sharp increase in research output after 2015. Only about 12.5% (n = 13) of the studies were published between 2011 and 2015 [34,35,54,64,65,76,80,84,85,86,93,94,120] while 81.7% (n = 85) were published between 2016 and 2023 [18,19,20,21,22,23,24,25,27,28,29,30,31,32,33,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,55,56,57,58,59,60,61,62,63,66,67,68,69,70,71,72,73,74,75,77,79,81,82,83,87,88,89,90,91,92,95,96,97,98,100,101,102,103,104,105,106,107,108,109,110,111,112,114,115,116,117,121]. The remaining 5.76% (n = 6) were published in 2024, demonstrating sustained and accelerating interest in curcumin as a supplement and nutraceutical ingredient for metabolic health. The year intervals used for reporting publication frequency (2011–2015, 2016–2023, and 2024) were chosen to reflect the empirically observed inflection in the temporal distribution of included trials that occurs after 2015 (Figure 3).
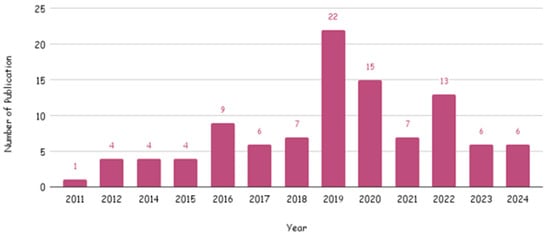
Figure 3.
Number of publications per year of included studies.
3.3.2. Demographic Distribution
The studies were conducted in nineteen (19) countries, with Iran as a leading country, with 63% (n = 65) of the studies, e., refs. [73,74,78,84,97] followed by the USA (n = 5). There were 4 studies each from India, Thailand, Brazil, and Japan, and 2 studies each from Australia, Egypt, Italy, Denmark, and Turkey, while other countries like Iraq, China, Armenia, Germany, Taiwan, Canada, France, and Mexico had only 1 study each (Figure 4). The selected studies were published between 2001 and 2024. The year 2019 had the highest number of publications [21, 20.2%], while the years 2016–2024 each had six or more publications. The regional distribution of publications shows a marked dominance of research output from the Middle East (67.4%), indicating a strong regional interest and investment in Curcuma longa research. North and South America contributed 10.6%, while Asia followed closely with 9.6% of studies. Europe accounted for 6.7%, India for 3.8%, and Australia for 1.9%. This distribution highlights limited global representation, with most of the evidence emerging from the Middle East, suggesting potential geographical bias and also the need for broader international research participation to enhance the generalizability of these findings.
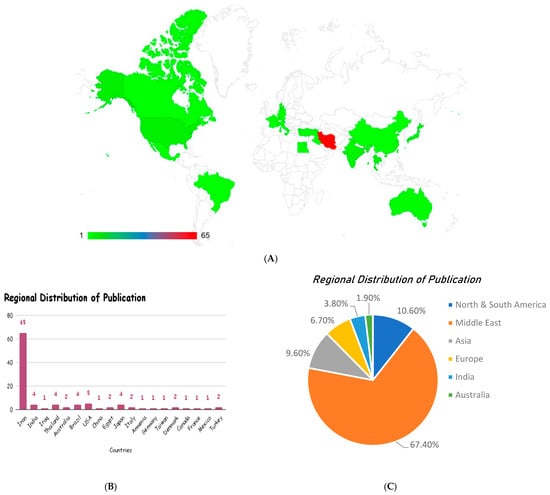
Figure 4.
Geographical distribution (A) and regional distribution of the publication (B,C) of the included studies, reflecting the demographics of the studies assessing the beneficial effects of curcumin/Curcuma longa extracts in research on metabolic syndrome.
3.4. Population Characteristics
The target populations were diverse and were unified by their metabolic relevance (Figure 5). About 70% (n = 73) of the studies targeted patients diagnosed with metabolic syndrome or its main elements, which included type 2 diabetes, dyslipidemia, obesity, or non-alcoholic fatty liver disease, e.g., refs. [75,80,85,96]. Approximately 18% (n = 19) of the studied patients had associated metabolic or inflammatory conditions like chronic kidney disease (60), ischemic stroke (18, 31), osteoarthritis (85), or hypertension (43). The remaining 12 and 0 percent (n = 12) were seemingly healthy but overweight or obese adults, as a measure of curcumin’s preventive efficacy, e.g., refs. [32,36,49]. This pattern shows that the majority of these research studies were performed on a population that is already presenting with metabolic dysfunctions or risk factors.
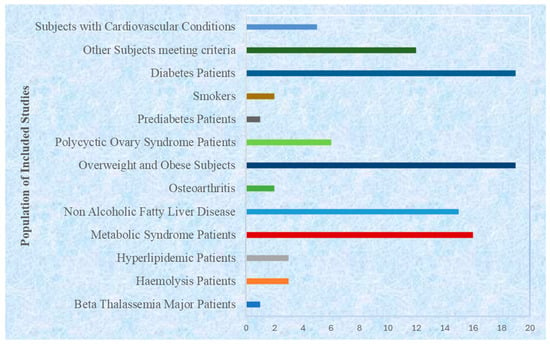
Figure 5.
Populations of included studies with reference to associated diseases.
3.4.1. Gender Distribution
Out of the study populations used in the review, 78.9% of studies had both male and female participants, thus making them more generalizable, e.g., refs. [47,73,74,87]. Another 16.3% of the trials had only female subjects, with most of them addressing a reproductive or hormonal disorder, including polycystic ovary syndrome [25,52,56,121], postmenopausal health [105], or postmenopausal obesity in women [38,95]. A very small minority of trials used only male subjects (3.8%) and generally focused on male infertility or exercise metabolism [21,32]. The gender of the participants was not specified in a small percentage (1%) (Figure 6). This distribution shows that most randomized controlled trials that have measured the clinical impact of Curcuma longa on metabolic syndrome have mixed-gender sample designs.
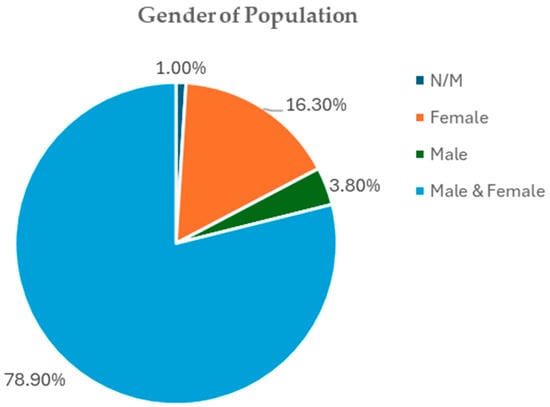
Figure 6.
Gender distribution of included studies.
3.4.2. Age of Participants
The age of the participants ranged between 15 and 79 years, with the majority of studies centered on adults of middle age. Approximately 76% (n = 79) of studies enrolled adults aged between 35 and 60 years, the age at which metabolic syndrome is most impacted [75,80,85]. Adolescents or younger adults were represented in about 10% of the studies (n = 10), such as Ismail et al. [54], Saraf Bank et al. [107], and Campbell et al. [32]. The rest 14% (n = 15) were older adults with ages above 65 years, including the articles by Mankowski et al. [71], Funamoto et al. [42], and Kelardeh et al. [63]. This distribution illustrates a strong clinical emphasis on the adult and aging population, where curcumin’s metabolic and anti-inflammatory actions are most relevant.
3.4.3. Sample Size
The studies that were included had diverse numbers of participants. Small-scale trials (<40 participants) represented 31% (n = 32) of the total, primarily serving as pilot or mechanistic investigations [29,38,50]. Trials of moderate size (40–99 participants) were the most frequent, comprising 52% (n = 54) of all studies [47,72,85]. Large-scale trials (≥100 participants) accounted for 17% (n = 18), including Chuengsamarn et al. [34], n = 235, Chuengsamarn et al. [35], n = 213, and Yaikwawong et al. [118], n = 227. The increasing number of larger RCTs in recent years reflects enhanced methodological rigor and growing investment in clinical validation of Curcuma longa’s therapeutic potential. Together, these studies encompass a wide demographic and clinical spectrum that spans continents, age groups, and metabolic conditions, thereby demonstrating the versatility of Curcuma longa as a nutraceutical candidate. These characteristics demonstrate both the scientific maturity and translational potential of Curcuma longa as a dietary supplement for the prevention and management of metabolic syndrome and its complications, thereby ensuring the internal validity and generalizability of these findings across diverse populations.
3.5. Intervention Characteristics
The analysis of the included RCTs in the study revealed a consistent pattern that spanned across the intervention parameters, specifically concerning sample size, formulation type, and treatment duration.
3.5.1. Sample Size Distribution
Most of the studies that were included in this review had comparatively small sample sizes in the intervention and control groups. As depicted in Figure 7, the intervention group analysis showed that 60.6% of the included studies had a sample size of ≤30 participants, and 37.5% of the included studies had a sample size of >30 participants. Equally, in the placebo/control groups, 63.5% of the studies provided a sample size of ≤30, and 34.6% reported a sample size of >30. The minimal proportion of studies that did not state or calculate the sample size (N/M) was 1.9% in both groups. This trend points to the fact that much of the existing clinical evidence on Curcuma longa is based on smaller studies, and possibly single-center studies.
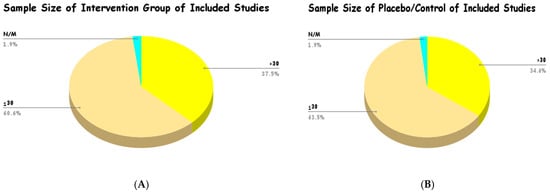
Figure 7.
Sample size of intervention group (A) and placebo/control (B) of included studies.
3.5.2. Type of Formulations
The formulation of Curcuma longa used varied but was largely dominated by conventional oral dosage preparations, as observed in Figure 8 and Supplementary Table S3. The most frequently used modes of delivery were pills, tablets, and capsules, which represented 79.8% of the overall reported interventions. It is worth noting that a large percentage of the studies focused on new formulations aimed at improving bioavailability, with nano-micelle preparations comprising 13.46% of the total. Powder (3.85%), juice (1.92%), and pellets (0.96%) were also less frequently used. To give an example, many studies by Panahi et al. [85,86,87,88,89,90,91] involved capsules with 500 mg of curcuminoids and doses of 5 mg of piperine (Bioperile) in doses of one to two capsules per day. The other widespread approach was phospholipidated curcumin (e.g., Meriva or phytosomal preparations), which authors, including Mirhafez et al. [72,73,74,75] and Hariri et al. [47] used, in some instances providing 50 mg of pure curcumin per 250 mg or 500 mg. The upper limit of these increased forms, including two pills of 1000 mg Meriva that were studied by Hellmann et al. [49], points to the tendency to optimize absorption. These data indicate that most clinical evidence derives from defined supplemental forms of curcuminoids rather than habitual dietary turmeric consumption, and their results should be interpreted accordingly. It is apparent that the shift towards capsules/tablets and the specific delivery systems is an indication that the clinical literature is focused on optimizing the systemic absorption and efficacy of curcuminoids.
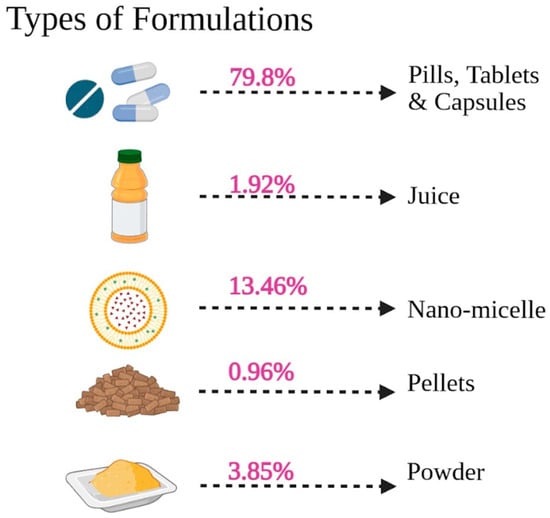
Figure 8.
Types of formulations used in the selected studies.
3.5.3. Treatment Dosage
The review of the treatment protocols demonstrates a high degree of heterogeneity in the daily dosage and schedule of administration of Curcuma longa interventions, but the preference was for a middle-term period (Supplementary Table S3). The total daily dose of curcuminoids (or curcumin equivalent) was extremely variable, with a minimum of 80 mg/day and a maximum of 4000 mg/day. Nano-micelle (nano-curcumin) formulations that were created to ensure high bioavailability were most often used in the lowest therapeutic doses, and were usually used at a dose of 80 mg/day in single or divided doses (studies) [21,79,96]. On the other hand, the maximum dose was 4000 mg of curcumin per day, which was used in the short-term trial performed by Garg et al. [44]. The range of moderate doses was, however, agreed upon by most studies, often in the range of 500 mg/day [54] to 1000 mg/day [77,110] of regular or somewhat modified preparations. Bioavailability enhancers were typical, and most of the trials used 500 mg of curcuminoids in combination with 5 mg of piperine, which Panahi et al. studied in most trials [84,85,86,87,88,89,90,91], or phospholipidated curcumin (e.g., Meriva) at an equivalent dose of 50 mg of pure curcumin per day [72,73,74,75].
The dosing schedules were mostly categorized into two groups: once a day and twice a day. Many of the studies chose the convenience of once-daily administration [32,54,97], especially with ultra-low-dose nano-curcumin formulations. Nevertheless, the schedule of administration in two separate doses per day was also widespread, as it was commonly used to enhance tolerance or stable plasma concentrations. Examples of treatments studied with twice-a-day schedules included 500 mg capsules [58,84], 30 mg Theracurmin capsules [41], or augmented preparations, such as 1000 mg Meriva tablets [49]. Some of the studies that employed a thrice-a-day schedule included those by Yang et al. [120] and Heshmati et al. [51,52], or in one case, a 4-times-a-day schedule [76]. The general range of treatment ranged between 6 weeks and 12 weeks (3 months), which is the average intervention period of this systematic review.
3.5.4. Treatment Duration
The Curcuma longa interventions were not homogeneous in terms of time, making it possible to observe both acute and short-term treatments (not longer than 2 h) as well as long-term interventions (up to 12 months) (Figure 9 and Supplementary Table S3). The data, however, were very much concentrated in short-to-medium-term regimens. The most commonly used treatment time was 12 weeks (mentioned in 27 publications), with an average of 8 weeks (25 publications). There were other similar durations of 3 months (13 publications) and 6 weeks (11 publications). This dispersion gives an indication that the majority of the trials measured the impact of Curcuma longa within two and three months, which is standard practice when it comes to evaluating the therapeutic efficacy of a treatment for a metabolic disorder. Long-term studies (e.g., 6, 9, and 12 months) on the impact of this treatment were significantly less prevalent, which may imply a gap in evidence on the long-term safety and efficacy of Curcuma longa after three months.
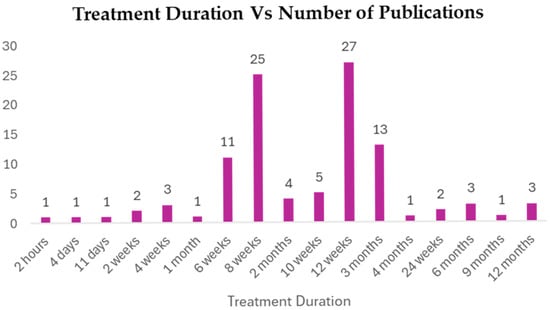
Figure 9.
Treatment duration in reference to number of publications.
3.6. Clinical Efficacy Outcomes
3.6.1. Metabolic Parameters
The clinical efficacy of Curcuma longa was measured across a wide spectrum of metabolic parameters that were systematically tested in the RCTs involved. This review is centered on the major indicators of the five parameters of Metabolic Syndrome (MetS): central obesity, dyslipidemia, hyperglycemia, and hypertension (Supplementary Table S4).
Anthropometric and Body Composition Indices
One of the central points of the research was the impact that Curcuma longa has on anthropometric measures that show the presence of central obesity. Body Mass Index (BMI), Waist Circumference (WC), and the Waist-to-Hip Ratio (W/H Ratio) were the major measures to be considered (Supplementary Table S5). The patient groups in the studies included mostly reported being overweight or mildly obese at baseline, with the means of the BMI values often around 30 kg/m2, such as in the intervention groups included in Saraf-Bank et al. [107] (31.0 ± 2.85 kg/m2), and Asadi et al. [24] (31.0 ± 14.1 kg/m2). In line with this, anthropometric measures of abdominal adiposity, including WC, were also regularly measured, with both baseline values being around 100 cm, as witnessed in the curcumin groups from Sangouni et al. [106] (100.2 ± 8.5 cm) and Asadi et al. [24] (101.1 ± 9.5 cm).
Glycemic Control and Insulin Resistance Markers
Body glucose homeostasis was evaluated with the indices of Fasting Blood Sugar (FBS), Hemoglobin A1c (HbA1c), Fasting Plasma Insulin (FPI) (Supplementary Table S6), and calculation of the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR). Most of the evidence espoused the use of Curcuma longa in enhancing the glucose homeostasis mechanism and insulin sensitivity. There was a tendency for patients in the trials that were included to have signs of impaired glucose metabolism or diabetes at baseline. As an example, in several intervention groups, initial FBS levels were in the normoglycemic range of values, including in Asan et al. [25] (87.30 ± 8.20 mg/dL), which ranged to levels that were significantly higher, as seen in research such as that by Asadi et al. [24] (150.90 ± 58.10 mg/dL). Equally, long-term glycemic control as indicated by the HbA1c values showed significant heterogeneity, ranging between 5.95%, as indicated by Panahi et al. [87], and 8.18%, as indicated by Asadi et al. [24]. There was a high prevalence of markers of insulin resistance, including FPI [92] and HOMA-IR [100], which indicated the predisposition of the MetS population to being insulin-resistant. HbA1c is the most important long-term indicator, and was significantly reduced in several studies [26,34,80,118]. Similarly, the reduction in FBS in the trials by Asghari et al. [26], Chuengsamarn et al. [34], and Zohrabi et al. [121] was also significant; however, an increase in FPS was indicated in the trials by Khajehdehi et al. [64]. Moreover, HOMA-IR was repeatedly and considerably reduced in many studies, among them, Chuengsamarn et al. [34,35], Na et al. [80], Ismail et al. [54], Zohrabi et al. [121], Asghari et al. [26], and Yaikwawong et al. [118], which shows a strong influence on this pathogenic mechanism of MetS.
Lipid Profile Components
The lipid profiles of study participants were strictly measured by measuring Triglycerides (TG), Total Cholesterol (TC), LDL Cholesterol (LDL), and HDL Cholesterol (HDL) (Supplementary Table S7). A state of dyslipidemia, which is one of the essential elements of MetS, was verified with the help of the baseline lipid status of the participants. The intervention groups in most of the studies reported their baseline TG levels as exceeding 150 mg/dL The MetS diagnostic criteria include a range between 109.00 mg/dL [19] and 66.70 mg/dL [106]. The TC levels also were stable throughout the trials, and representative curcumin group baselines were 163 mg/dL [24] to 170.19 mg/dL [100]. Also, the level of HDL was usually low and was often below 40 mg/dL (e.g., [19]: 30 ± 2 mg/dL; [20]: 33.81 ± 8.14 mg/dL), highlighting the common lipid-related risk factors in the population. This benefit extended to the atherogenic lipoproteins, as LDL Cholesterol (LDL) also showed significant reductions in the same studies, with additional support from Rezaei M. (2024) [99]. Crucially, the beneficial HDL Cholesterol (HDL) level was reported to be significantly increased by Na et al. [80], Mansharifi et al. [70], and Pashine et al. [93], confirming a positive modulation of the dyslipidemia characteristic of Metabolic Syndrome.
Blood Pressure Indices
Finally, the effect of Curcuma longa on hypertension was evaluated by recording Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) (Supplementary Table S7). Baseline SBP values were often in the hypertensive or pre-hypertensive range, such as the 142.07 ± 20.73 mmHg reported in the treatment group by Abed et al. [18]. Similarly, baseline DBP values generally indicated elevated pressure, with the curcumin groups in the studies by Abed et al. [18] and Sangouni et al. [106] reporting mean DBP values of 92.27 mmHg and 91.5 mmHg, respectively, prior to intervention. However, some studies did report a significant reduction in Diastolic Blood Pressure (DBP) [113], while results for Systolic Blood Pressure (SBP) were highly variable, with some studies reporting increases and others showing no change [64]. The overall effect of curcumin on blood pressure requires careful interpretation due to the inconsistencies observed, which suggest that the primary clinical benefit of Curcuma longa may be more pronounced in the glycemic and lipid profiles than in hypertension in this patient population.
3.6.2. Inflammatory Parameters
The compelling evidence of the inflammatory parameters analyzed is a strong indication of the anti-inflammatory effects of Curcuma longa supplementation, which is a core of the mechanism of its effect on Metabolic Syndrome (MetS). The majority of the eligible Randomized Controlled Trials (RCTs) had a significant reduction in the most important inflammatory markers, which supports its contribution to the prevention of chronic low-grade inflammation, which is the pathology of MetS (Supplementary Table S8).
Acute-Phase Reactants and Pro-Inflammatory Cytokines
C-Reactive Protein (CRP), a widely utilized acute-phase reactant and strong predictor of cardiovascular risk, was the most consistently and significantly modulated inflammatory marker. Many studies have revealed that CRP reduced tremendously with an intervention with Curcuma longa (Supplementary Table S9). The major authors who have illustrated such a strong impact are Panahi et al. [85], Alizadeh et al. [21], Haroyan et al. [48], Adibian et al. [19], Afshar et al. [20], Asan et al. [25], Javandoosi et al. [58], Majeed et al. [69], and Mamsharifi et al. [70]. Although other studies by Kocher et al. [67] and Garg et al. [44] did not show a change, the overall results showed a positive effect of anti-inflammatories as indicated by a reduction in CRP.
The effects on the Interleukin family were more or less to stifle pro-inflammatory species. Interleukin-6 (IL-6) and insulin resistance major mediators were significantly reduced in numerous publications, such as those by Alvarenga et al. [23], Asghari et al. [26], Boshagh et al. [31], Kisiolek et al. [66], Kocher et al. [67], Mamsharifi et al. [70], and Panahi et al. [88], and Interleukin-8 (IL-8) was also reported to be reduced by Khajehdehi et al. Anti-inflammatory cytokine Interleukin-10 (IL-10), on the other hand, had been demonstrated to rise in certain studies like Mamsharifi et al. [70] and indicated a change towards the anti-inflammatory state(Supplementary Table S9).
Tumor Necrosis Factor-alpha (TNF-a), which is an important upstream pro-inflammatory cytokine, was also significantly and consistently decreased in various trials, which supports the mechanism of curcuminoids as an anti-inflammatory. Alizadeh et al. [21], Alvarenga et al. [23], Asan et al. [25], Jazayeri-Tehrani et al. [59], and Panahi et al. [87,88] reported this effect of Curcuma longa, which inhibits systemic inflammation at a critical regulatory point (Supplementary Table S10).
Adipokines and Other Inflammatory Mediators
The action of Curcuma longa is not limited to conventional metabolic markers but also impacts the regulation of adipokine and non-cytokine inflammatory mediators, with emphasis on its multi-targeted action in the treatment of the chronic, low-grade inflammation of Metabolic Syndrome (MetS). Important chemokine recruitment of macrophages to adipose tissue, leading to insulin resistance, was reported to be markedly decreased after supplementation in the studies by Panahi et al. [87] and Sedighiyan et al. [109] using Monocyte Chemoattractant Protein-1 (MCP-1). Also, there was a positive regulation of adipocyte-derived signaling molecules (adipokines) directly associated with insulin resistance: Visfatin and Resistin were highly reduced by Ismail et al. [54] and Sedighiyan et al. [109]. Lastly, Curcuma longa was shown to inhibit other essential inflammation pathways, suggesting a substantial reduction in NLRP3 [54], a central constituent of the inflammasome, and Fetuin-A (Fe-A) [109], an inflammatory glycoprotein associated with insulin resistance and ectopic fat deposition (Supplementary Table S11). All these findings validate that Curcuma longa has a multi-layered anti-inflammatory and immunomodulatory effect, which is fundamental in the management of MetS therapeutically.
3.6.3. Oxidative Parameters
The measurement of the parameters of oxidative stress gives a very important mechanistic interrelation between the clinical efficacy of Curcuma longa and the pathophysiology of Metabolic Syndrome (MetS). Oxidative stress, which is an imbalance between the generation of reactive oxygen species (ROS) and antioxidant mechanisms in the body, is strongly involved in all aspects of MetS. The results of the reviewed RCTs, as a rule, support the presence of a significant antioxidant effect, which is manifested in a decrease in the indicators of oxidative damage and an enhancement in endogenous antioxidant potential (Supplementary Table S12).
Markers of Oxidative Damage
One of the major indicators of oxidative damage is the amount of lipid peroxidation products, which was also consistently and positively altered by Curcuma longa supplementation. Thiobarbituric acid-reactive substances (TBARS) and Malondialdehyde (MDA) constitute important end products of lipid peroxidation and are well-known biomarkers of oxidative stress. Most of the available studies reported a significant reduction in MDA (Supplementary Table S13), including Alizadeh et al. [21], Darmian et al. [37], Jarhahzaden et al. [57], Osali et al. [83], Panahi et al. [85,86,87,88,89], and Saraf-Bank et al. [107,108]. Some trial studies reported a significant reduction in TBARS (Supplementary Table S14), which were significantly reduced in the trial by Krishnareddy et al. [68]. This repeated decrease points out the capacity of Curcuma longa to shield the cellular membranes against oxidative attack. The balance between prooxidants/antioxidants was also checked. Alvarenga et al. [23] found a significant reduction in the prooxidant antioxidant balance (PAB) of the trial, which is an indication of a desirable change in favor of a non-prooxidative state. On the other hand, a single study by Jimenez-Osorio et al. [60] demonstrated that MDA increased, and this observation might have been due to heterogeneity in formulation or patient population.
Endogenous Antioxidant Defenses
The therapeutic action of Curcuma longa is further involved in strengthening the body’s defense mechanism, which is demonstrated by a change in major antioxidant enzymes and molecules. Important enzymatic antioxidants such as Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) were often found to have been significantly raised after intervention (Supplementary Table S13). The studies by Panahi et al. [85,86,87,88,89], Krishnareddy et al. [68], and Heshmati et al. [51] reported an increase in SOD, while GPx was increased in the studies by Krishnareddy et al. [68] and Heshmati et al. [52]. This suggests that bioactive curcuminoids actively upregulate the endogenous enzymatic scavenger system. Glutathione (GSH), the master non-enzymatic antioxidant, was also reported to be significantly increased by Panahi et al. [85] and Krishnareddy et al. [68]. Concurrently, its oxidized form, Glutathione Disulfide (GSSG), was found to be significantly decreased by Jimenez-Osorio et al. [60], as was Glutathione Reductase (GR). This pattern collectively indicates a shift towards a more reduced, and therefore more protective, cellular environment. Measures of the plasma’s overall non-enzymatic antioxidant capability, TAC and TAS, were consistently and significantly increased across multiple trials, including those by Panahi et al. [88], Alizadeh et al. [21], Saraf-Bank et al. [107], and Mokhtari et al. [79] (Supplementary Table S14). This provides compelling evidence that Curcuma longa enhances the systemic antioxidative buffering capacity. In summary, the highly consistent findings of reduced oxidative damage (MDA/TBARS) and augmented anti-oxidant defenses (SOD, GPx, GSH, and TAC/TAS) strongly establish the potent antioxidative mechanism of Curcuma longa, positioning it as a nutraceutical capable of addressing the core component of cellular stress in MetS.
3.7. Meta-Analysis of RCTs Regarding the Clinical Potential of Curcuma longa Linn. as a Nutraceutical for Metabolic Syndrome
We pooled randomized controlled trial data to evaluate the effect of Curcuma longa (curcumin) supplementation on anthropometric measures, glycemic control, blood pressure, lipid profile, insulin resistance and secretion indices, inflammatory markers, and oxidative stress biomarkers across clinical populations relevant to metabolic syndrome (Type 2 diabetes mellitus [T2DM], metabolic syndrome [MetS], non-alcoholic fatty liver disease [NAFLD], obesity, and polycystic ovary syndrome [PCOS]) (Supplementary Figures S1–S19). Analyses used random-effects models and are reported as standardized mean differences (SMD) with 95% confidence intervals (CI). Heterogeneity is described with tau2 and I2; p-values refer to two-sided tests.
3.7.1. Anthropometry: BMI, Waist Circumference (WC), Waist-to-Hip Ratio (WHR)
Across disease-specific subgroups, curcumin supplementation produced notable improvements in body composition, particularly among patients with type 2 diabetes mellitus (T2DM) and obesity (Supplementary Figures S1–S3). Across clinical subgroups, the pooled effects on BMI were inconsistent. In diabetes cohorts (12 studies), the pooled SMD was −0.27 (95% CI −0.57 to 0.02), with a non-significant trend toward lower BMI with curcumin; substantial heterogeneity was present (I2 ≈ 79%, p < 0.01), suggesting results varied across trials. For metabolic syndrome cohorts (7 studies, 195 vs. 195 subjects), the pooled SMD was 0.09 (95% CI −0.23 to 0.41), i.e., no effect and moderate heterogeneity (I2 ≈ 58%, p = 0.03). NAFLD and overweight/obese sub-groups likewise showed no significant pooled BMI effects. Overall, the evidence does not support a consistent clinically meaningful BMI reduction from curcumin; where an effect was suggested (small negative SMD in some diabetes/obese analyses), heterogeneity limited confidence. The wide 95% prediction interval further suggests that while some future studies might detect meaningful BMI reductions, others could show negligible effects. Trials by Panahi et al. [89,90] demonstrated relatively strong reductions, whereas others (e.g., Asadi et al. [24]; Yakawong et al. [119]) reported minimal change.
Findings for WC were mostly null. A pooled SMD of −0.33 (95% CI −0.81 to 0.15), which was non-significant with high heterogeneity (I2 ~ 75%, p < 0.01), was found in diabetes patients (5 studies; 292 vs. 293 subjects). The same was also observed with obese/overweight populations and metabolic syndrome populations, which did not exhibit any consistent WC benefit. This was thus viewed as a non-significant, inaccurate effect that had low between-study significance, since there was no strong evidence to support the hypothesis that curcumin lowers central adiposity. The pooled SMDs across the NAFLD, overweight/obese, and metabolic syndrome subgroups were small and nonsignificant (e.g., NAFLD SMD 0.11, 95% CI 0.37 to 0.15, and overweight SMD 0.47, 95% CI 0.10 to 1.03, respectively, with moderate heterogeneity in the latter). In sum, there was no significant influence on WHR.
3.7.2. Blood Pressure: SBP and DBP
During the analysis of SBP, for diabetes patients (7 studies; 188 vs. 176), pooled SMD was −0.11 (95% CI −0.45 to 0.23), which is not significant; heterogeneity was moderate (I2 ≈ 61%, p = 0.02). A statistically significant difference was found in patients with metabolic syndrome (4 studies; 105 vs. 104), pooled SMD was −0.65 (95% CI −1.21 to −0.08), p = 0.05, but the heterogeneity was observed to be high (I2 ≈ 65, p = 0.03). There were no significant changes in SBP in NAFLD and the overweight/obese subgroups. Accordingly, curcumin could potentially be used to lower SBP in specific trials on metabolic syndrome, but the heterogeneity and the small number of studies warn against generalizing these findings (Supplementary Figure S4). In diabetes cohorts, pooled DBP SMD was −0.34 (95% CI −1.18 to 0.49), which was non-significant, but had high heterogeneity (I2 ≈ 86%, p < 0.01) in both cases of DBP. In patients with metabolic syndrome (3 studies; 94 vs. 93), pooled SMD was −0.40 (95% CI −0.79 to −0.01), p < 0.05, indicating a slight DBP decrease in that group with low inter-study variability reported. No consistent effect of DBP was demonstrated in other sub-groups. Generally, small, positive BP changes seem to be most reproducible in trials involving metabolic syndrome. (Supplementary Figure S5).
3.7.3. Glycemic Control: Fasting Blood Sugar (FBS), Blood Glucose, HbA1c
As depicted in Supplementary Figures S6–S8, fasting blood sugar (FBS) results differed by population. In diabetes patients (9 studies; 323 vs. 320) pooled SMD was −0.61 (95% CI −1.48 to 0.25), which was not statistically significant and very highly heterogeneous (I2 ≈ 88%, p < 0.01). By contrast, in metabolic syndrome cohorts (6 studies; 197 vs. 198), pooled SMD was −0.25 (95% CI −0.48 to −0.03), p < 0.05, indicating a small but statistically significant reduction in FBS with curcumin and low heterogeneity reported. Obese/overweight and NAFLD subgroups were generally null. The pattern suggests curcumin may modestly improve fasting glycemia in metabolic syndrome populations, while effects in established diabetes are heterogeneous and inconclusive.
In blood glucose (post-prandial/other glucose measures) measured in diabetes patients (4 studies; 241 vs. 237), an SMD of −0.53 (95% CI −0.82 to −0.23), p < 0.05, was published with medium effects (I2 ≈ 58%, p≈0.07). This means that measured blood glucose showed a moderate reduction in trials measuring such endpoints. The possible small-study effects of this outcome were proposed by funnel/Egger’s tests. In diabetes cohort designs (10 studies; 505 vs. 504) that reported HbA1c, SMD was −0.33 (95% CI −0.58 to −0.09), p < 0.05, with moderate heterogeneity (I2 ≈ 66%, p < 0.01). This is a minor yet statistically significant increase in long-term glycemic control. The effect of HbA1c was not significant and extremely heterogeneous in trials of metabolic syndrome. On the whole, HbA1c and some of the glucose measures indicate consistent minor changes in the cohorts with diabetes, but inter-study variability dampens confidence.
3.7.4. Insulin Resistance and Beta-Cell Indices: HOMA-IR, HOMA-B, QUICKI, Serum Insulin
Across all clinical populations, curcumin supplementation showed no significant improvement in insulin resistance indices (Supplementary Figures S9–S12). Results for HOMA-IR were largely null across populations. In diabetes (9 studies; 537 vs. 535), pooled SMD was −0.01 (95% CI −0.78 to 0.76), which was non-significant, with extremely high heterogeneity (I2 ≈ 95%, p < 0.01). The subgroups of overweight/obese, PCOS, and NAFLD patients also did not exhibit a reliable pooled benefit. Overweight/obese, PCOS, and NAFLD subgroups likewise showed no reliable pooled benefit. This is indicative that pooled trials do not demonstrate consistent improvements in HOMA-IR that are attributable to curcumin. As for HOMA-B (beta-cell function), in diabetes cohorts (3 studies; 104 vs. 101), pooled SMD was 0.09 (95% CI −0.19 to 0.36), which was non-significant and without notable heterogeneity. There is thus no evidence of a consistent beneficial effect on beta-cell secretion indices. QUICKI across NAFLD (3 studies) and PCOS (4 studies) cohorts revealed SMDs that were non-significant (e.g., NAFLD-QUICKI SMD 0.41, 95% CI −0.39 to 1.21 with very high heterogeneity). No robust signal favoring curcumin was discovered. Diabetes cohorts for serum insulin (5 studies; 154 vs. 151) revealed a pooled SMD of −0.33 (95% CI −0.77 to 0.11), which was non-significant with high heterogeneity (I2 ≈ 75%, p < 0.01). Overweight/obese and PCOS cohorts were likewise non-significant. Taken together, there is no conclusive benefit to fasting insulin or direct insulin-sensitivity indices.
3.7.5. Lipids: TC, LDL-C, HDL-C, TG
Curcumin supplementation produced notable improvements in serum lipid parameters across several metabolic conditions (Supplementary Figures S13–S16). In measurements of total cholesterol (TC) across diabetes, overweight/obese, PCOS, metabolic syndrome, and NAFLD subgroups, most pooled SMDs were small and non-significant (e.g., diabetes SMD −0.22, 95% CI −0.45 to 0.01). Heterogeneity varied by subgroup (some high). There was no observed consistency in the capacity of curcumin to lower TC according to the analysis. In the case of LDL-C, in diabetes cohorts (10 studies; 387 vs. 388) pooled SMD was −0.19 (95% CI −0.45 to 0.08), which was non-significant with moderate–high heterogeneity (I2 ≈ 69%, p < 0.01). The overweight/obese subgroup showed a statistically significant LDL reduction (9 studies; pooled SMD −0.36, 95% CI −0.64 to −0.07, p < 0.05), though with moderate heterogeneity (I2 ≈ 56%, p = 0.02). Some evidence of small-study/publication bias for LDL in diabetes analyses was flagged by Egger’s test. The outcomes for HDL-C were more favorable. For instance, diabetes cohorts showed a pooled SMD of 0.40 (95% CI 0.03 to 0.77), p < 0.05 (10 studies; 285 vs. 284), though heterogeneity was substantial (I2 ≈ 72%, p < 0.01). Overweight/obese cohorts also showed a smaller positive HDL effect (SMD 0.27, 95% CI 0.05 to 0.49). These suggest curcumin supplementation may modestly increase HDL in some metabolic populations, but between-study inconsistency reduces certainty. There were mixed findings for triglycerides (TG). For example, the diabetes cohort displayed a pooled TG SMD of −0.29 (95% CI −0.64 to 0.07), which was non-significant with high heterogeneity. In the NAFLD and some overweight/obese cohorts, analyses showed modest TG reductions (e.g., NAFLD pooled SMD −0.27, 95% CI −0.49 to −0.05, p < 0.05), suggesting context-specific improvements. Overall lipid effects appear modest: some benefit were found for HDL and certain subgroup improvements for LDL and TG but this was inconsistent across populations.
3.7.6. Inflammation: CRP and TNF-Alpha
Across metabolically compromised populations, curcumin substantially reduced CRP (Supplementary Figure S17). CRP pooled analyses frequently favored curcumin with small-to-moderate effects. For diabetes patients (5 studies; 195 vs. 197) pooled SMD was −0.39 (95% CI −0.64 to −0.14), p < 0.05, which is a statistically significant reduction with little between-study variability reported. In obese/overweight patients (3 studies; 98 vs. 98) pooled SMD was −0.40 (95% CI −0.68 to −0.11), p < 0.05, again with low heterogeneity. These consistent CRP reductions across several populations indicate curcumin exerts an anti-inflammatory effect that is measurable through lower CRP. The funnel/Egger’s tests did not indicate publication bias for these CRP pools. TNF-α pooled effects were larger but heterogeneous. In diabetes cohorts (3 studies; 246 vs. 248) pooled SMD was −1.07 (95% CI −2.05 to −0.09), p < 0.05, suggesting a large point estimate reduction; however, heterogeneity was extreme (I2 ≈ 91%, p < 0.01), and the Egger’s test suggested funnel asymmetry, raising concern for bias and instability in the pooled estimate (Supplementary Figure S18). While some trials report sizable TNF-α reductions, this supports a biologically plausible anti-inflammatory mechanism that is relevant to insulin resistance and tissue injury.
3.7.7. Oxidative Stress: TAC, GSH, MDA
As depicted in Supplementary Figure S19, Total Antioxidant Capacity (TAC) for diabetes cohorts (5 studies; 114 vs. 107) revealed a pooled SMD of 0.68 (95% CI −0.30 to 1.67), which is not statistically significant; heterogeneity was high (I2 ≈ 85%, p < 0.01). This wide, imprecise estimate indicates that some trials saw increased TAC with curcumin, but the effects were inconsistent. For Malondialdehyde (MDA), a pooled diabetes analysis (5 studies) yielded an SMD of −0.22 (95% CI −0.54 to 0.09), which is non-significant with low heterogeneity. This suggests little consistent effect on this lipid-peroxidation marker across trials. In the case of Glutathione (GSH). A pooled (3 studies; 61 vs. 59) SMD of 0.91 (95% CI −0.88 to 2.69) was found, which is non-significant, and extreme heterogeneity (I2 ≈ 92%, p < 0.01) was observed. Sparse data and large between-study differences prevent the formation of reliable conclusions for GSH. Overall, oxidative biomarkers show heterogeneous and imprecise effects; although some trials report antioxidant improvements, the pooled evidence is inconsistent.
Across the pooled randomized trials, curcumin/Curcuma longa supplementation showed consistent small anti-inflammatory effects (notably reductions in CRP) and modest improvements in some glucose metrics (HbA1c, certain glucose measures) and HDL in selected populations. Signals for SBP/DBP reductions are present primarily in metabolic syndrome subgroups. Effects on anthropometry, insulin-resistance indices (HOMA-IR, QUICKI), LDL/TC, and many oxidative markers are inconsistent when pooled; many analyses are limited by substantial between-study heterogeneity (I2 frequently >50–75%).
3.7.8. Pooled Effects and Quality of Evidence for Curcumin Supplementation
The pooled evidence (Table 3 and Table 4) suggests that curcumin offers meaningful but selective benefits across metabolic and inflammatory pathways. Glycemic control and inflammation are the most steadily improved. Glucose in the blood after eating, HbA1c, and fasting glucose were all reduced without much difference between short-term and long-term effects. These findings are consistent with a moderate GRADE certainty, despite a certain degree of inconsistency in the trials. Blood pressure was also somewhat the same; a decrease in systolic and diastolic blood pressure, particularly in the case of individuals with metabolic syndrome, is an indication of a clinically significant cardiovascular impact.

Table 3.
Pooled Effects of Curcuma longa (Curcumin) Supplementation on Clinical and Biochemical Parameters.

Table 4.
Overall quality of evidence (GRADE) for outcomes included in the meta-analysis.
The effects on lipids were more ambivalent. The change in total cholesterol was minimal and not significant, but LDL-C decreased slightly, and HDL-C increased considerably. Triglycerides were reduced, particularly in NAFLD research. There was moderate certainty with regard to TG and HDL-C, but certainty with regard to TC and LDL-C was undermined by heterogeneity and small-study bias. In general, there is a small lipid-modifying effect, which, however, is subject to population and formulation.
The anthropometric findings were a different story. There was no significant change in BMI, waist circumference, or waist–hip ratio. The null findings are probably due to the high heterogeneity and short intervention times of the identified studies. The insulin measures responded similarly; HOMA-IR, insulin, QUICKI, and HOMA-B did not respond in a consistent manner. The extremely large I2 values illustrate the inconsistencies in the study designs and formulations, with low to very low GRADE certainty.
The most evident benefits were demonstrated by inflammatory markers. CRP decreased steadily, and heterogeneity was low; TNF-alpha changed, and the trend remained positive. This aligns with the profile of the anti-inflammatory properties of curcumin, which help to promote its metabolic action. On the contrary, oxidative stress indicators (TAC, GSH, MDA) provided inconclusive, primarily non-significant results. Low certainty was caused by low sample sizes, inconsistencies in assays, and broad confidence intervals.
4. Discussion
This comprehensive systematic review and meta-analysis of 104 randomized controlled trials (RCTs) provides the most extensive synthesis to date of the clinical effects of Curcuma longa supplementation on metabolic syndrome (MetS) and related cardiometabolic conditions, including type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), obesity, and polycystic ovary syndrome (PCOS). Although the metabolic and anti-inflammatory properties of Curcuma longa and its principal bioactive constituent, curcumin, have been extensively described in preclinical and clinical literature, the present systematic review and meta-analysis provide important comparative and quantitative insights that extend beyond prior narrative syntheses. Rather than demonstrating uniform benefits across all components of metabolic syndrome, our findings indicate a pattern of selective efficacy, with the most consistent and clinically relevant effects observed in inflammatory modulation and glycemic control, and more variable or context-dependent effects on anthropometric and insulin-resistance indices.
The results of the glycemic control were subtle. Although the impacts on fasting blood glucose were mixed, especially in already-diabetic patients, HbA1c showed significant, yet statistically insignificant, improvements in cohorts with diabetes, suggesting that curcumin might have a cumulative effect on long-term glycemic control, but not on acute glucose metabolism. This difference has the clinical implication of making HbA1c a relatively robust outcome measure in nutraceutical trials, as the subtle effects of metabolic changes may not be detected in the short run. These improvements, though small, are clinically significant: even minor improvements in HbA1c can have significant results on reductions in microvascular and macrovascular outcomes in the long term [122,123]. The same selectivity pattern was shown in lipid-profile modulation. The changes in HDL cholesterol and triglycerides were more favorable in the case of overweight/obese and non-alcoholic fatty liver disease populations, and the total cholesterol and LDL cholesterol were different across the disease categories. These observations imply that the lipid-modifying effect of curcumin is a secondary effect of its anti-inflammatory and antioxidant effects, but not a sign of a lipid-lowering effect comparable to pharmacological compounds. All these findings indicate that curcumin also acts on lipid transport and hepatic lipid metabolism, which are some of the characteristics of metabolic dysfunction.
Anthropometric changes followed a subtler pattern. Anthropometric outcomes like body mass index, waist circumference, and waist-to-hip ratio did not show statistically reliable pooled benefits. This is not a coincidence in some individual trials, but it is probable that it is due to a combination of biological and methodological issues. There is no evidence that curcumin is a major weight-reduction agent, but its metabolic effects can be realized without significant adiposity changes in response to the short-term interventions (6–12 weeks) that prevail in the literature. As central obesity is more prognostically meaningful than total weight, these findings may reflect curcumin’s influence on adipose inflammation rather than generalized weight loss [124,125]. Additional comparative subgroup analyses indicate that the trials that reported significant changes in anthropometry with a modest degree of change were also more likely to have a higher baseline of inflammation or longer periods of intervention, which points to the significance of population traits and study design in the interpretation of such results. Inflammatory markers demonstrated some of the most consistent responses. C-reactive protein (CRP) decreased markedly across multiple trials, supported by reductions in TNF-α, though with significant heterogeneity and some evidence of publication bias for the latter. Given the central role of low-grade inflammation in the pathophysiology of MetS, these anti-inflammatory actions provide mechanistic coherence to the improvements observed in glycemic and lipid variables. Oxidative stress outcomes were also responsive but heterogeneous. Multiple individual RCTs documented reductions in malondialdehyde (MDA) alongside increases in antioxidant enzymes such as SOD, GPx, and GSH, with several studies demonstrating an enhancement in TAC/TAS levels as evidence of systemic antioxidant reinforcement. However, substantial variability in assays, units, and reporting prevented consistent pooled effects. Thus, while mechanistically plausible, oxidative stress remains the least consistent evidence domain. Taken together, the evidence positions curcumin as a broad-spectrum nutraceutical component capable of targeting multiple interconnected metabolic pathways, reflecting the complex multimorbidity embedded in MetS.
These clinical results align closely with the known molecular mechanisms of curcumin. Preclinical evidence supports curcumin’s ability to modulate inflammatory signaling through the inhibition of NF-κB activation, downregulation of iNOS and COX-2, and attenuation of pro-inflammatory cytokines, including TNF-α and IL-6. Curcumin also activates AMPK, promoting glucose uptake in skeletal muscle, inhibiting hepatic gluconeogenesis, and enhancing fatty acid oxidation, which are mechanistic actions consistent with improvements in fasting glucose, HbA1c, triglycerides, and central adiposity [126,127,128]. The antioxidant effects of curcumin, such as the improvement of endogenous enzymatic defenses and the decrease in lipid peroxidation, provide further evidence on the use of curcumin to induce reductions in oxidative stress as a central factor in insulin resistance and endothelial dysfunction. Moreover, new human data show that curcumin regulates the intestinal microbiota, reinforces the intestinal barrier, and affects the intestinal permeability due to the interactions between the microbiome–immune–metabolic axis [11,129]. These gut-based processes can be the reason why metabolic effects, which are of clinical significance, can be implemented even in cases when plasma curcumin levels are low. On the whole, the combination of mechanistic plausibility and clinical observations contributes to the increased confidence in curcumin’s therapeutic potential.
Among the most impressive pieces of evidence that this review has given is the extent to which the effects of curcumin are formulation dependent. Almost 80% of the included trials involved the use of capsule/tablet products, though bioavailability-enhancing strategies (including piperile, phospholipid complexes, e.g., Meriva) and nano-micellar had a high degree of variation. Approximately 13.5% of the studies included in the scope of the study were nano-formulations, and phospholipidated curcumin and piperine-enhanced extracts also formed significant proportions of the study. Clinical trials with enhanced-bioavailability preparations often found greater and more sustained metabolic activities compared to the traditional extracts [130,131]. Dosage was also quite wide, ranging between 80 mg/day and 4000 mg/day, with nano-formulated curcumin demonstrating results at 80 mg/day and above; conventional preparations have generally had to use a dosage of 500–1500 mg/day before they could show any effects. This supports the claim that the therapeutic potential of curcumin is dependent not on nominal dose but on the pharmacokinetics of curcumin. The main range of treatment was 8–12 weeks, with comparatively low numbers of studies beyond 6 months, restricting the findings on durability and long-term safety. Metabolic remodeling can usually come at a cost, and therefore future RCTs need to prolong the period of intervention in order to understand whether temporary biomarker improvements are converted to long term risk reduction.
This review is distinguished by its breadth (104 RCTs), rigorous stratification by disease subtype and formulation, thorough risk-of-bias appraisals, and the use of the GRADE framework to determine some degree of certainty across outcomes. These features are the key features of this review. The integration of anthropometric, glycemic, lipid, inflammatory, and oxidative stress indicators in a single metabolic model can be seen as providing a comprehensive view of the therapeutic profile of curcumin and a source of clarity in the face of inconsistent results in the past. Importantly, because ~80% of trials used concentrated oral supplements (capsules/tablets/advanced delivery systems) and only a few trials tested turmeric in food-style preparations, our findings primarily reflect the efficacy of defined curcumin products and formulations rather than the effects of culinary turmeric as consumed in habitual diets. Bioavailability enhancers (piperine, phytosomes, nano-formats) were associated with larger and more consistent biomarker changes; this pharmacokinetic dependence reduces the external validity of extrapolating our pooled estimates to regular dietary turmeric intake. Future RCTs should evaluate food-based interventions (standardized powder/food matrices) and head-to-head comparisons with supplement formulations to determine the translational relevance of these findings to diet and public health recommendations.
5. Limitations
Although the findings are promising, the heterogeneity within the studies is still one of the key weaknesses. The moderate–high I2s in various pooled analyses indicate the variations in population, curcumin formulations, disease severity, and quality of studies. Even though the Cochrane RoB 2 evaluations showed that the risks of bias were generally low in the domains of blinding and attrition, issues were still present in the randomization procedures, nonadherence to planned interventions, and missing outcome reporting in some studies. The geographical concentration of trials, especially the fact that most of the trials were based in Iran and other Middle Eastern countries, raises other questions in terms of external validity, because 67% of all the included trials were based in the Middle East, with scanty representation in North America, Europe, and other regions in Asia. Regional nutritional backgrounds, genetic distributions, local diets, and local clinical practices can vary widely, affecting the metabolic reactions to nutritional interventions like curcumin. Sample sizes were also mainly small, with about 60% of studies involving ≤30 subjects per arm, which is a source of imprecision risks and small-study effects. These are the methodological shortcomings that point to the necessity of larger, multisite, and internationally diverse RCTs.
6. Recommendation for Future Trials
The synthesis outlines a number of research priorities that are needed in order to advance the potential of curcumin in translational research: Large, multi-center RCTs using standardized formulations and longer follow-up durations to assess long-term clinical effects and the sustainability of biomarker changes; head-to-head comparisons of traditional, piperine-enhanced, nano-formulated, and phospholipidated curcumin to explain exposure–response relationships; the interactions of curcumin with the gut microbiota, systemic absorption, and tissue distribution to determine the mechanisms by which curcumin has metabolic effects in spite of its low plasma bioavailability; consistency in reporting safety outcomes, especially hepatic and gastrointestinal outcomes, drug–nutrient interactions, and long-term tolerability in vulnerable populations; stratified analyses of metabolic phenotypes, such as sex-specific responses and differences depending upon baseline inflammation or insulin resistance.
7. Conclusions
The findings endorse curcumin as an adjunctive treatment for those with MetS, T2DM, NAFLD, or obesity. The pooled evidence from RCTs indicates that curcumin supplementation yields consistent anti-inflammatory effects and modest yet clinically meaningful improvements in glycemic regulation and selected lipid parameters in individuals with metabolic syndrome and related disorders. These benefits are most pronounced with bioavailability-enhanced curcumin formulations, underscoring the importance of formulation in determining therapeutic efficacy. Importantly, curcumin should be viewed as a nutraceutical adjunct to standard care rather than a stand-alone therapeutic alternative, with potential utility in mitigating metabolic risk and low-grade systemic inflammation. Curcumin has a multi-target profile, so it can be especially useful in preventing early metabolic pathology before the disease is incurable and requires intensive pharmacological treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods15010060/s1.
Author Contributions
S.C.: Funding acquisition, conceptualization, supervision, writing—review and editing. S.A.K.: Writing the original draft, data curation, validation, and formal analysis. Z.N.Q.: Data curation and formal analysis: W.P.L., B.B.L., K.Y.P. and M.M.S.: Article searching, screening, and data curation. N.A., S.A. and R.K.: Data curation, validation, and visualization: R.P.: Co-funding acquisition, conceptualization, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The article processing charge (APC) is supported by Mae Fah Luang University. This research received financial support from the funding agency “Program Management Unit for Competitiveness (PMUC)” (Grant No. C02F670127) and the Postdoctoral Fellowship Fund from Mae Fah Luang University, Thailand (Contract No. 10/2025).
Institutional Review Board Statement
Not acceptable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that might influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body mass index |
| BP | Blood pressure |
| CAT | Catalase |
| CCNP | Curcumin Nanoparticle |
| CRP | C-reactive protein |
| Glu | Glucose |
| GSH | Glutathione |
| GST | Glutathione S-Transferase |
| Hb1Ac | Hemoglobin A1C |
| HDL | High-density lipoprotein |
| HFD | High fat diet |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin-6 |
| INF-γ | Interferon-gamma |
| INS | Insulin |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| MMP-9 | Matrix metalloproteinase-9 |
| MPO | Myeloperoxidase |
| NAFLD | Non-alcoholic fatty liver disease |
| NCUR | Nanocurcumin |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOx | Total nitrites and nitrates |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglyceride |
References
- Azhdari, M.; Karandish, M.; Mansoori, A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1289–1301. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, C.; Wang, H.; Ren, Y.; Li, J.; Li, M.; Du, X.; Li, W.; Zhang, J. Effects of dietary polyphenol curcumin supplementation on metabolic, inflammatory, and oxidative stress indices in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1216708. [Google Scholar] [CrossRef]
- Kehinde, S.A.; Qaisrani, Z.N.; Pattanayaiying, R.; Lin, W.P.; Lay, B.B.; Phyo, K.Y.; San, M.M.; Awaeloh, N.; Aunsorn, S.; Kitkangplu, R.; et al. Preclinical evidence of Curcuma longa Linn. as a Nutraceuticals in the management of metabolic syndrome: A systematic review and meta-analysis of rodent studies. Biomedicines 2025, 13, 1911. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Preza-Rodríguez, L.; Camacho-Luis, A.; Simental-Mendía, L.E.; Martínez-Aguilar, G.; Gamboa-Gomez, C.I.; Guerrero-Romero, F. Turmeric plus black pepper for the improvement and remission of metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. J. Herb. Med. 2024, 44, 100845. [Google Scholar] [CrossRef]
- Kehinde, S.A.; Lin, W.P.; Lay, B.B.; Phyo, K.Y.; San, M.M.; Pattanayaiying, R.; Chusri, S. Curcumin and dementia: A systematic review of its effects on oxidative stress and cognitive outcomes in animal models. Int. J. Mol. Sci. 2025, 26, 7026. [Google Scholar] [CrossRef] [PubMed]
- Lou, H. Exploring the role of the core mechanism of curcumin in the regulation of fat metabolism. Biomed. Data Sci. 2025, 5, 30–34. [Google Scholar] [CrossRef]
- Cui, J.; Li, H.; Zhang, T.; Lin, F.; Chen, M.; Zhang, G.; Feng, Z. Research progress on the mechanism of curcumin anti-oxidative stress based on signaling pathway. Front. Pharmacol. 2025, 16, 1548073. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The modulatory effects of curcumin on the gut microbiota: A potential strategy for disease treatment and health promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Feng, J. Role of curcumin in altering gut microbiota for anti-obesity and anti-hyperlipidemic effects. Front. Microbiol. 2025, 16, 1625098. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Vakili, S.; Lankarani, K.B.; Akbari, M.; Mirhosseini, N.; Ghayour-Mobarhan, M.; Ferns, G.; Karamali, F.; Karamali, M.; Taghizadeh, M.; et al. The effects of curcumin on glycemic control and lipid profiles among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des. 2018, 24, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. MetaAnalysisOnline.com: Web-based tool for the rapid meta-analysis of clinical and epidemiological studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Sterne, J.A. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Abed, A.B.; Abdulridha, M.K. Clinical assessment of oral curcumin adjuvant in post-ischemic stroke patients. Clin. Schizophr. Relat. Psychoses 2021, 16, 15S. [Google Scholar] [CrossRef]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef]
- Afshar, G.V.; Rasmi, Y.; Yaghmaei, P.; Khadem-Ansari, M.; Makhdomii, K.; Rasooli, J. The effects of nano-curcumin supplementation on serum level of HS-CRP, adhesion molecules, and lipid profiles in hemodialysis patients: A randomized controlled clinical trial. PubMed 2020, 14, 52–61. Available online: https://pubmed.ncbi.nlm.nih.gov/32156842 (accessed on 16 December 2025).
- Alizadeh, F.; Javadi, M.; Karami, A.A.; Gholaminejad, F.; Kavianpour, M.; Haghighian, H.K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother. Res. 2017, 32, 514–521. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.; Santos, R.S.; De Brito, J.S.; Kemp, J.A.; Reis, D.; De Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- Alvarenga, L.; Cardozo, L.F.M.F.; Da Cruz, B.O.; Paiva, B.R.; Fouque, D.; Mafra, D. Curcumin supplementation improves oxidative stress and inflammation biomarkers in patients undergoing hemodialysis: A secondary analysis of a randomized controlled trial. Int. Urol. Nephrol. 2022, 54, 2645–2652. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Asan, S.A.; Baş, M.; Eren, B.; Karaca, E. The effects of curcumin supplementation added to diet on anthropometric and biochemical status in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. Prog. Nutr. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Asghari, K.M.; Saleh, P.; Salekzamani, Y.; Dolatkhah, N.; Aghamohammadzadeh, N.; Hashemian, M. The effect of curcumin and high-content eicosapentaenoic acid supplementations in type 2 diabetes mellitus patients: A double-blinded randomized clinical trial. Nutr. Diabetes 2024, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Sahebkar, A.; Soleimani, D.; Mahdavi, A.; Rafiee, S.; Majeed, M.; Khorvash, F.; Iraj, B.; Elyasi, M.; Rouhani, M.H.; et al. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: A randomized double-blind, placebo-controlled trial. Trials 2022, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- Atakan, A.; Kabaran, S.; Eren, F.H. The effects of dietary intervention with turmeric on lipid profile and anthropometric indices in overweight/obese women with hyperlipidemia: A randomized controlled clinical trial. Prog. Nutr. 2022, 24, 4. [Google Scholar] [CrossRef]
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial variability across individuals in the vascular and nutrigenomic response to an acute intake of curcumin: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 62, 1700418. [Google Scholar] [CrossRef]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef]
- Boshagh, K.; Khorvash, F.; Sahebkar, A.; Majeed, M.; Bahreini, N.; Askari, G.; Bagherniya, M. The effects of curcumin-piperine supplementation on inflammatory, oxidative stress and metabolic indices in patients with ischemic stroke in the rehabilitation phase: A randomized controlled trial. Nutr. J. 2023, 22, 69. [Google Scholar] [CrossRef]
- Campbell, M.S.; Ouyang, A.; Im, K.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht MGh, B.F. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2013, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2019, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: A clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Namiranian, K.; Amerian, R.; Mansouri, S.; Arshadi, S.; Azarbayjani, M.A. The effect of curcumin supplementation and aerobic training on anthropometric indices, serum lipid profiles, C-reactive protein and insulin resistance in overweight women: A randomized, double-blind, placebo-controlled trial. J. Obes. Metab. Syndr. 2020, 29, 47–57. [Google Scholar] [CrossRef]
- Dolati, S.; Farzad, L.; Far, A.H. The effect of aerobic training and supplementation of curcumin on the glycemic markers and level of myeloperoxidase enzyme of overweight women. J. Rom. Diabet. Nutr. Metab. Dis. 2020, 27, 281–288. Available online: http://www.rjdnmd.org/index.php/RJDNMD/article/download/755/546 (accessed on 16 December 2025).
- Ferguson, J.J.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals: A randomized controlled trial. Metabolism 2017, 82, 22–35. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; Wada, H.; et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. J. Diabetes Res. 2019, 2019, 8208237. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Kato, T.; Funada, J.; Ajiro, Y.; Komiyama, M.; Akao, M.; Yasoda, A.; Yamakage, H.; et al. Effects of high-absorption curcumin for the prevention of hypertensive heart disease: A double-blind, placebo-controlled, randomized clinical study. Eur. Heart J. Open 2022, 2, oeac057. [Google Scholar] [CrossRef]
- Garg, A.X.; Devereaux, P.; Hill, A.; Sood, M.; Aggarwal, B.; Dubois, L.; Hiremath, S.; Guzman, R.; Iyer, V.; James, M.; et al. Oral curcumin in elective abdominal aortic aneurysm repair: A multicentre randomized controlled trial. CMAJ 2018, 190, E1273–E1280. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Sepehri, B.; Asghari-Jafarabadi, M.; Ghavami, S. Turmeric and chicory seed have beneficial effects on obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). Int. J. Vitam. Nutr. Res. 2019, 89, 293–302. [Google Scholar] [CrossRef]
- Ghazimoradi, M.; Saberi-Karimian, M.; Mohammadi, F.; Sahebkar, A.; Tavallaie, S.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Moohebati, M.; Esmaeili, H.; et al. The effects of curcumin and curcumin–phospholipid complex on the serum pro-oxidant–antioxidant balance in subjects with metabolic syndrome. Phytother. Res. 2017, 31, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: A comparative, randomized, double-blind, placebo-controlled study. BMC Complement. Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Forman, J.; Chabanova, E.; Svenningsen, J.S.; Holst, J.J.; Gillum, M.P.; Vilsbøll, T.; Knop, F.K. The effect of curcumin on hepatic fat content in individuals with obesity. Diabetes Obes. Metab. 2022, 24, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Hansen, K.B.; Forman, J.L.; Størling, J.; Chabanova, E.; Holst, J.; Vilsbøll, T.; Knop, F.K. No effect of the turmeric root phenol curcumin on prednisolone-induced glucometabolic perturbations in men with overweight or obesity. Endocr. Connect. 2023, 12, e220334. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine 2020, 80, 153395. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Ismail, N.A.; Ragab, S.; El-Baky, A.N.E.A.; Hamed, M.; Ibrahim, A.A. Effect of oral curcumin administration on insulin resistance, serum resistin and fetuin-A in obese children: Randomized placebo-controlled study. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 887–896. [Google Scholar]
- Ismail, N.A.; Abd El Dayem, S.M.; Salama, E.; Ragab, S.; Abd El Baky, A.N.; Ezzat, W.M. Impact of curcumin intake on gluco-insulin homeostasis, leptin and adiponectin in obese subjects. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1891–1897. [Google Scholar]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Jarhahzadeh, M.; Alavinejad, P.; Farsi, F.; Husain, D.; Rezazadeh, A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: A randomized double-blind clinical trial. Diabetol. Metab. Syndr. 2021, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Javandoost, A.; Afshari, A.; Saberi-Karimian, M.; Sahebkar, A.; Safarian, H.; Moammeri, M.; Dizaji, B.F.; Tavalaei, S.; Ferns, G.A.; Pasdar, A.; et al. The effects of curcumin and a modified curcumin formulation on serum cholesteryl ester transfer protein concentrations in patients with metabolic syndrome: A randomized, placebo-controlled clinical trial. Avicenna J. Phytomedicine 2018, 8, 330–337. [Google Scholar]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M. Nano-curcumin improves glucose indices, lipids, inflammation, and nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; De Oca-Solano, H.M.; Madero, M.; Pedraza-Chaverri, J. The effect of dietary supplementation with curcumin on redox status and NRF2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 2021, 35, 4377–4387. [Google Scholar] [CrossRef]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. Curcumin and zinc co-supplementation along with a loss-weight diet can improve lipid profiles in subjects with prediabetes: A multi-arm, parallel-group, randomized, double-blind placebo-controlled phase 2 clinical trial. Diabetol. Metab. Syndr. 2022, 14, 22. [Google Scholar] [CrossRef]
- Kelardeh, B.M.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kisiolek, J.N.; Kheredia, N.; Flores, V.; Ramani, A.; Lisano, J.; Johnston, N.; Stewart, L.K. Short term, oral supplementation with optimized curcumin does not impair performance improvements associated with high intensity interval training. J. Diet. Suppl. 2021, 19, 733–746. [Google Scholar] [CrossRef]
- Kocher, A.; Bohnert, L.; Schiborr, C.; Frank, J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016, 60, 1555–1563. [Google Scholar] [CrossRef]
- Krishnareddy, N.T.; Thomas, J.V.; Nair, S.S.; Mulakal, J.N.; Maliakel, B.P.; Krishnakumar, I.M. A novel curcumin-galactomannoside complex delivery system improves hepatic function markers in chronic alcoholics: A double-blinded, randomized, placebo-controlled study. Biomed. Res. Int. 2018, 2018, 9159281. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Devarajan, T.V.; Saklecha, S.; Reddy, S.V.K.; Mundkur, L. A minor metabolite from Curcuma longa effective against metabolic syndrome: Results from a randomized, double-blind, placebo-controlled clinical study. Food Funct. 2023, 14, 4722–4733. [Google Scholar] [CrossRef]
- Mamsharifi, P.; Farokhi, B.; Hajipoor-Taziani, R.; Alemi, F.; Hazegh, P.; Masoumzadeh, S.; Jafari, L.; Ghaderi, A.; Dehkohneh, S.G. Nano-curcumin effects on nicotine dependence, depression, anxiety and metabolic parameters in smokers: A randomized double-blind clinical study. Heliyon 2023, 9, e21249. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Sibille, K.; Leeuwenburgh, C.; Lin, Y.; Hsu, F.; Qiu, P.; Sandesara, B.; Anton, S. Effects of Curcumin C3 Complex® on physical function in moderately functioning older adults with low-grade inflammation—A pilot trial. J. Frailty Aging 2022, 12, 143–149. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Gholami, A.; Hooshmand, E.; Tavallaie, S.; Gh, B.F.N.M. The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Drug Metab. Pers. Ther. 2019, 34, 20180040. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Gh, B.F.N.M.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2021, 91, 278–286. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The effect of curcumin phytosome on the treatment of patients with non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled trial. Adv. Exp. Med. Biol. 2021, 1308, 25–35. [Google Scholar] [CrossRef]
- Mirzabeigi, P.; Mohammadpour, A.H.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The effect of curcumin on some traditional and non-traditional cardiovascular risk factors: A pilot randomized, double-blind, placebo-controlled trial. Iran. J. Pharm. Res. 2015, 14, 479–486. Available online: https://pubmed.ncbi.nlm.nih.gov/25901155 (accessed on 16 December 2025).
- Mohammadi, A.; Sadeghnia, H.R.; Saberi-Karimian, M.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Effects of curcumin on serum vitamin E concentrations in individuals with metabolic syndrome. Phytother. Res. 2017, 31, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Mohsenpour, M.A.; Sohrabi, Z.; Niakousari, M.; Jeddi, M.; Hassanzadeh, J.; Ferns, G.A.; Eftekhari, M.H. The effects of powdered drinks enriched with curcumin and probiotics on lipid profile and atherogenic indices in patients with metabolic syndrome: A randomized, double-blinded, placebo-controlled clinical trial. Food Sci. Nutr. 2023, 12, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2020, 35, 2099–2107. [Google Scholar] [CrossRef]
- Na, L.; Li, Y.; Pan, H.; Zhou, X.; Sun, D.; Meng, M.; Li, X.; Sun, C. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Neta, J.F.D.F.; Veras, V.S.; Sousa, D.F.D.; Cunha, M.D.C.D.S.O.; Queiroz, M.V.O.; Neto, J.C.G.L.; Damasceno, M.M.C.; Araújo, M.F.M.D.; Freitas, R.W.J.F.D. Effectiveness of the piperine-supplemented Curcuma longa L. in metabolic control of patients with type 2 diabetes: A randomised double-blind placebo-controlled clinical trial. Int. J. Food Sci. Nutr. 2021, 72, 968–977. [Google Scholar] [CrossRef]
- Nowak, K.L.; Farmer-Bailey, H.; Wang, W.; You, Z.; Steele, C.; Cadnapaphornchai, M.A.; Klawitter, J.; Patel, N.; George, D.; Jovanovich, A.; et al. Curcumin therapy to treat vascular dysfunction in children and young adults with ADPKD. Clin. J. Am. Soc. Nephrol. 2021, 17, 240–250. [Google Scholar] [CrossRef]
- Osali, A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Hosseini, M.S.; Abbasinazari, M.; Sahebkar, A. Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement. Ther. Med. 2014, 22, 851–857. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: Results of a randomized controlled trial. J. Diet. Suppl. 2015, 13, 209–220. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid–piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2016, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: A randomized controlled trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: A randomized controlled trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.; Majeed, M.; Sahebkar, A. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Pashine, L.; Singh, J.V.; Vaish, A.K.; Ojha, S.K.; Mahdi, A.A. Effect of turmeric (Curcuma longa) on overweight hyperlipidemic subjects: Double blind study. Indian J. Community Health 2012, 24, 113–117. [Google Scholar]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Porasgari, Z.; Sakri, H.; Arshadi, S. The effect of eight weeks of Pilates with curcumin supplementation on liver enzymes and lipid profile in overweight and obese women. Obes. Med. 2022, 36, 100448. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. Available online: https://pubmed.ncbi.nlm.nih.gov/27761427 (accessed on 16 December 2025).
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.C.D.S.G.; Alves, A.G.P.; Guillo, L.A.; Sousa Neto, M.A.D.; Trindade, N.R.; Silva, M.S. Curcumin supplementation reduces blood glucose and serum lipids of Brazilian women with high waist circumference: A randomized clinical trial. Arch. Endocrinol. Metab. 2022, 66, 800–807. [Google Scholar] [CrossRef]
- Rezaei, M.; Soltani, M.; Alipoor, E.; Rezayat, S.M.; Vasheghani-Farahani, A.; Yaseri, M.; Firouzi, A.; Hosseinzadeh-Attar, M.J. Effect of nano-curcumin supplementation on angina status and cardiovascular risk factors in overweight or obese patients with coronary slow flow phenomenon: A randomized double-blind placebo-controlled clinical trial. BMC Nutr. 2024, 10, 73. [Google Scholar] [CrossRef]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized placebo-controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M.; Salahshooh, M.M.; Dizaji, B.F.; Safarian, H.; Javandoost, A.; Ferns, G.A.; Sahebkar, A.; Ahmadinejad, M. Evaluation of the effects of curcumin in patients with metabolic syndrome. Comp. Clin. Pathol. 2018, 27, 555–563. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Karimian, M.; Ghazizadeh, H.; Mohammadzadeh, E.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Does curcumin have an effect on sleep duration in metabolic syndrome patients? Avicenna J. Phytomed. 2021, 11, 190–198. [Google Scholar] [PubMed]
- Sadeghzadeh, Z.; Ostadrahimi, A.; Ranjbar, M.; Farshbaf-Khalili, A. The efficacy of Nigella sativa L. and curcumin nanomicelle alone or together on lipid profile, glycemic control indices, and serum 17-β estradiol in postmenopausal women. J. Caring Sci. 2023, 12, 163–173. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Taghdir, M.; Mirahmadi, J.; Sepandi, M.; Parastouei, K. Effects of curcumin and/or coenzyme Q10 supplementation on metabolic control in subjects with metabolic syndrome: A randomized clinical trial. Nutr. J. 2022, 21, 62. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Sedighiyan, M.; Abdolahi, M.; Jafari, E.; Vahabi, Z.; Athar, S.S.; Hadavi, S.; Zamanabadi, M.N.; Yekaninejad, M.; Djalali, M. The effects of nano-curcumin supplementation on adipokines levels in obese and overweight patients with migraine: A double-blind clinical trial study. BMC Res. Notes 2022, 15, 189. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef]
- Shirmohammadi, L.; Ghayour-Mobarhan, M.; Saberi-Karimian, M.; Iranshahi, M.; Tavallaie, S.; Emamian, M.; Sahebkar, A. Effect of Curcumin on Serum Cathepsin D in Patients with Metabolic Syndrome. Cardiovasc. Haematol. Disord. Drug Targets 2019, 20, 116–121. [Google Scholar] [CrossRef]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The Effects of Curcumin Supplementation on Glycemic Status, Lipid Profile and hs-CRP Levels in Overweight/Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef]
- Soltani, M.; Hosseinzadeh-Attar, M.J.; Rezaei, M.; Alipoor, E.; Vasheghani-Farahani, A.; Yaseri, M.; Rezayat, S.M. Effect of Nano-Curcumin Supplementation on Cardiometabolic Risk Factors, Physical and Psychological Quality of Life, and Depression in Patients with Coronary Slow Flow Phenomenon: A Randomized Double-Blind Clinical Trial. Trials 2024, 25, 515. [Google Scholar] [CrossRef]
- Srinivasan, A.; Selvarajan, S.; Kamalanathan, S.; Kadhiravan, T.; Lakshmi, N.C.P.; Adithan, S. Effect of Curcuma longa on Vascular Function in Native Tamilians with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Parallel Arm, Placebo-Controlled Trial. Phytother. Res. 2019, 33, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Tamaddoni, A.; Nasseri, E.; Mohammadi, E.; Qujeq, D.; Zayeri, F.; Zand, H.; Mir, S.M.; Gholami, M. A Double-Blind Randomized Controlled Trial of Curcumin for Improvement in Glycemic Status, Lipid Profile and Systemic Inflammation in β-Thalassemia Major. J. Herb. Med. 2019, 21, 100324. [Google Scholar] [CrossRef]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduces Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef]
- Uchio, R.; Muroyama, K.; Okuda-Hanafusa, C.; Kawasaki, K.; Yamamoto, Y.; Murosaki, S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1822. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2414. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Y.; Yang, H.; Lee, Y.; Chou, J.I.; Ueng, K. Lipid-Lowering Effects of Curcumin in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Zohrabi, T.; Nadjarzadeh, A.; Jambarsang, S.; Sheikhha, M.H.; Aflatoonian, A.; Mozaffari-Khosravi, H. Effect of Dietary Approaches to Stop Hypertension and Curcumin Co-Administration on Glycemic Parameters in Polycystic Ovary Syndrome: An RCT. Int. J. Reprod. Biomed. 2024, 22, 689–700. [Google Scholar] [CrossRef]
- Sartore, G.; Ragazzi, E.; Caprino, R.; Lapolla, A. Long-Term HbA1c Variability and Macro-/Micro-Vascular Complications in Type 2 Diabetes Mellitus: A Meta-Analysis Update. Acta Diabetol. 2023, 60, 721–738. [Google Scholar] [CrossRef]
- Tandon, N.; Ali, M.K.; Venkat Narayan, K.M. Pharmacologic Prevention of Microvascular and Macrovascular Complications in Diabetes Mellitus: Implications of the Results of Recent Clinical Trials in Type 2 Diabetes. Am. J. Cardiovasc. Drugs 2012, 12, 7–22. [Google Scholar] [PubMed]
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New Mechanisms and the Anti-Inflammatory Role of Curcumin in Obesity and Obesity-Related Metabolic Diseases. Eur. J. Nutr. 2011, 50, 151–161. [Google Scholar]
- Bradford, P.G. Curcumin and Obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Xu, R. Anti-Inflammatory Effects of Curcumin in Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [PubMed]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-Mediated Regulation of Intestinal Barrier Function: The Mechanism Underlying Its Beneficial Effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef]
- Mahran, R.I.; Hagras, M.M.; Sun, D.; Brenner, D.E. Bringing Curcumin to the Clinic in Cancer Prevention: A Review of Strategies to Enhance Bioavailability and Efficacy. AAPS J. 2017, 19, 54–81. [Google Scholar]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Aggarwal, B.B. Is Curcumin Bioavailability a Problem in Humans: Lessons from Clinical Trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.