Clinical Potential of Curcuma longa Linn. as Nutraceutical/Dietary Supplement for Metabolic Syndrome: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol Registration
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
2.3.1. Exclusion Criteria
2.3.2. Prognostic Criteria for Inclusion
2.4. Data Retrieval and Synthesis
2.5. Quality Assessment Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Selection
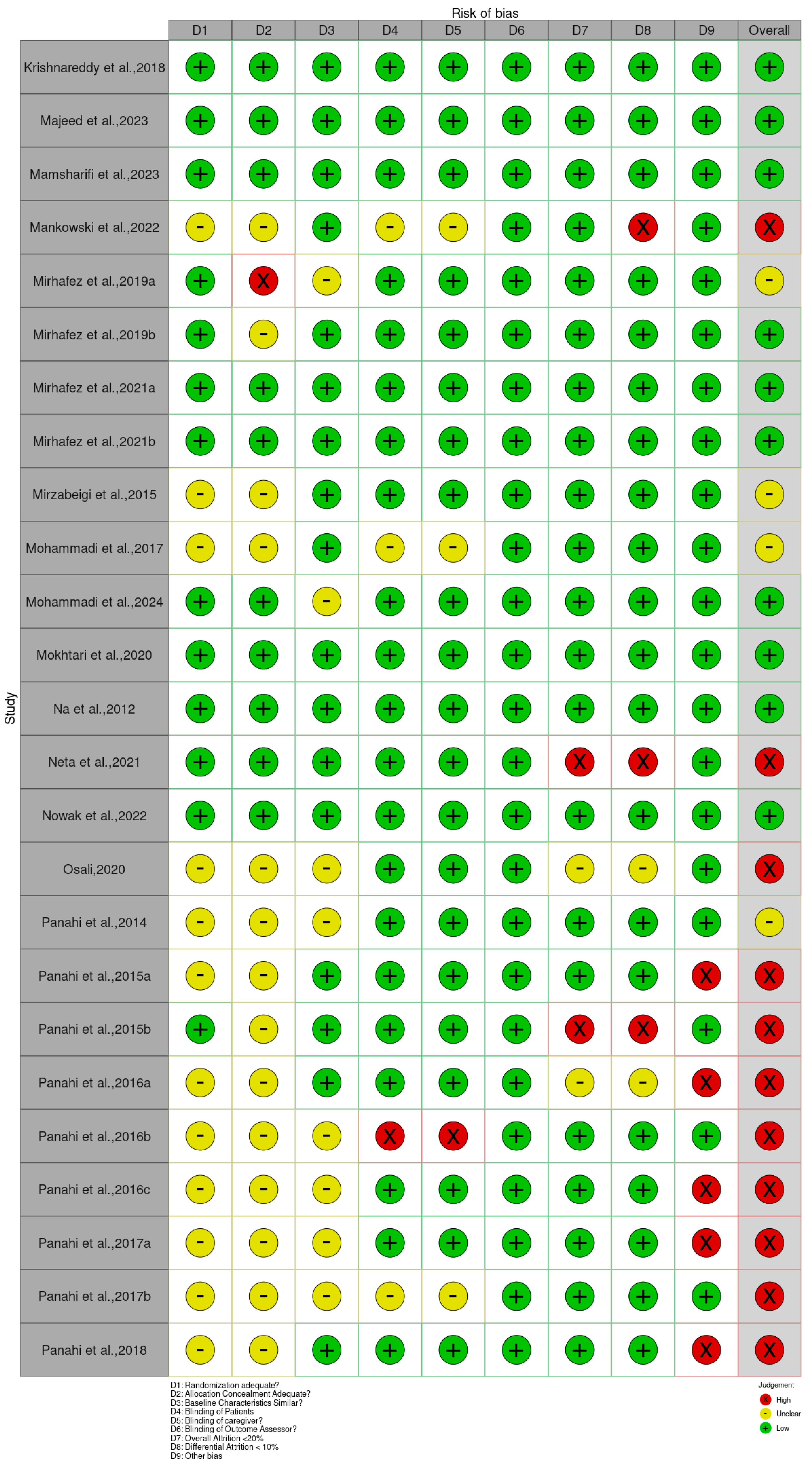
3.2. Assessment of the Risk of Bias of the Included Studies
3.3. Study Characteristics
3.3.1. Publication Year
3.3.2. Demographic Distribution
3.4. Population Characteristics
3.4.1. Gender Distribution
3.4.2. Age of Participants
3.4.3. Sample Size
3.5. Intervention Characteristics
3.5.1. Sample Size Distribution
3.5.2. Type of Formulations
3.5.3. Treatment Dosage
3.5.4. Treatment Duration
3.6. Clinical Efficacy Outcomes
3.6.1. Metabolic Parameters
Anthropometric and Body Composition Indices
Glycemic Control and Insulin Resistance Markers
Lipid Profile Components
Blood Pressure Indices
3.6.2. Inflammatory Parameters
Acute-Phase Reactants and Pro-Inflammatory Cytokines
Adipokines and Other Inflammatory Mediators
3.6.3. Oxidative Parameters
Markers of Oxidative Damage
Endogenous Antioxidant Defenses
3.7. Meta-Analysis of RCTs Regarding the Clinical Potential of Curcuma longa Linn. as a Nutraceutical for Metabolic Syndrome
3.7.1. Anthropometry: BMI, Waist Circumference (WC), Waist-to-Hip Ratio (WHR)
3.7.2. Blood Pressure: SBP and DBP
3.7.3. Glycemic Control: Fasting Blood Sugar (FBS), Blood Glucose, HbA1c
3.7.4. Insulin Resistance and Beta-Cell Indices: HOMA-IR, HOMA-B, QUICKI, Serum Insulin
3.7.5. Lipids: TC, LDL-C, HDL-C, TG
3.7.6. Inflammation: CRP and TNF-Alpha
3.7.7. Oxidative Stress: TAC, GSH, MDA
3.7.8. Pooled Effects and Quality of Evidence for Curcumin Supplementation
4. Discussion
5. Limitations
6. Recommendation for Future Trials
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BP | Blood pressure |
| CAT | Catalase |
| CCNP | Curcumin Nanoparticle |
| CRP | C-reactive protein |
| Glu | Glucose |
| GSH | Glutathione |
| GST | Glutathione S-Transferase |
| Hb1Ac | Hemoglobin A1C |
| HDL | High-density lipoprotein |
| HFD | High fat diet |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin-6 |
| INF-γ | Interferon-gamma |
| INS | Insulin |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| MMP-9 | Matrix metalloproteinase-9 |
| MPO | Myeloperoxidase |
| NAFLD | Non-alcoholic fatty liver disease |
| NCUR | Nanocurcumin |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOx | Total nitrites and nitrates |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TC | Total cholesterol |
| TG | Triglyceride |
References
- Azhdari, M.; Karandish, M.; Mansoori, A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1289–1301. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, C.; Wang, H.; Ren, Y.; Li, J.; Li, M.; Du, X.; Li, W.; Zhang, J. Effects of dietary polyphenol curcumin supplementation on metabolic, inflammatory, and oxidative stress indices in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1216708. [Google Scholar] [CrossRef]
- Kehinde, S.A.; Qaisrani, Z.N.; Pattanayaiying, R.; Lin, W.P.; Lay, B.B.; Phyo, K.Y.; San, M.M.; Awaeloh, N.; Aunsorn, S.; Kitkangplu, R.; et al. Preclinical evidence of Curcuma longa Linn. as a Nutraceuticals in the management of metabolic syndrome: A systematic review and meta-analysis of rodent studies. Biomedicines 2025, 13, 1911. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Preza-Rodríguez, L.; Camacho-Luis, A.; Simental-Mendía, L.E.; Martínez-Aguilar, G.; Gamboa-Gomez, C.I.; Guerrero-Romero, F. Turmeric plus black pepper for the improvement and remission of metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. J. Herb. Med. 2024, 44, 100845. [Google Scholar] [CrossRef]
- Kehinde, S.A.; Lin, W.P.; Lay, B.B.; Phyo, K.Y.; San, M.M.; Pattanayaiying, R.; Chusri, S. Curcumin and dementia: A systematic review of its effects on oxidative stress and cognitive outcomes in animal models. Int. J. Mol. Sci. 2025, 26, 7026. [Google Scholar] [CrossRef] [PubMed]
- Lou, H. Exploring the role of the core mechanism of curcumin in the regulation of fat metabolism. Biomed. Data Sci. 2025, 5, 30–34. [Google Scholar] [CrossRef]
- Cui, J.; Li, H.; Zhang, T.; Lin, F.; Chen, M.; Zhang, G.; Feng, Z. Research progress on the mechanism of curcumin anti-oxidative stress based on signaling pathway. Front. Pharmacol. 2025, 16, 1548073. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The modulatory effects of curcumin on the gut microbiota: A potential strategy for disease treatment and health promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Feng, J. Role of curcumin in altering gut microbiota for anti-obesity and anti-hyperlipidemic effects. Front. Microbiol. 2025, 16, 1625098. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Vakili, S.; Lankarani, K.B.; Akbari, M.; Mirhosseini, N.; Ghayour-Mobarhan, M.; Ferns, G.; Karamali, F.; Karamali, M.; Taghizadeh, M.; et al. The effects of curcumin on glycemic control and lipid profiles among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des. 2018, 24, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. MetaAnalysisOnline.com: Web-based tool for the rapid meta-analysis of clinical and epidemiological studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Sterne, J.A. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Abed, A.B.; Abdulridha, M.K. Clinical assessment of oral curcumin adjuvant in post-ischemic stroke patients. Clin. Schizophr. Relat. Psychoses 2021, 16, 15S. [Google Scholar] [CrossRef]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef]
- Afshar, G.V.; Rasmi, Y.; Yaghmaei, P.; Khadem-Ansari, M.; Makhdomii, K.; Rasooli, J. The effects of nano-curcumin supplementation on serum level of HS-CRP, adhesion molecules, and lipid profiles in hemodialysis patients: A randomized controlled clinical trial. PubMed 2020, 14, 52–61. Available online: https://pubmed.ncbi.nlm.nih.gov/32156842 (accessed on 16 December 2025).
- Alizadeh, F.; Javadi, M.; Karami, A.A.; Gholaminejad, F.; Kavianpour, M.; Haghighian, H.K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother. Res. 2017, 32, 514–521. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.; Santos, R.S.; De Brito, J.S.; Kemp, J.A.; Reis, D.; De Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- Alvarenga, L.; Cardozo, L.F.M.F.; Da Cruz, B.O.; Paiva, B.R.; Fouque, D.; Mafra, D. Curcumin supplementation improves oxidative stress and inflammation biomarkers in patients undergoing hemodialysis: A secondary analysis of a randomized controlled trial. Int. Urol. Nephrol. 2022, 54, 2645–2652. [Google Scholar] [CrossRef]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Asan, S.A.; Baş, M.; Eren, B.; Karaca, E. The effects of curcumin supplementation added to diet on anthropometric and biochemical status in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. Prog. Nutr. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Asghari, K.M.; Saleh, P.; Salekzamani, Y.; Dolatkhah, N.; Aghamohammadzadeh, N.; Hashemian, M. The effect of curcumin and high-content eicosapentaenoic acid supplementations in type 2 diabetes mellitus patients: A double-blinded randomized clinical trial. Nutr. Diabetes 2024, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Sahebkar, A.; Soleimani, D.; Mahdavi, A.; Rafiee, S.; Majeed, M.; Khorvash, F.; Iraj, B.; Elyasi, M.; Rouhani, M.H.; et al. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: A randomized double-blind, placebo-controlled trial. Trials 2022, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- Atakan, A.; Kabaran, S.; Eren, F.H. The effects of dietary intervention with turmeric on lipid profile and anthropometric indices in overweight/obese women with hyperlipidemia: A randomized controlled clinical trial. Prog. Nutr. 2022, 24, 4. [Google Scholar] [CrossRef]
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial variability across individuals in the vascular and nutrigenomic response to an acute intake of curcumin: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 62, 1700418. [Google Scholar] [CrossRef]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef]
- Boshagh, K.; Khorvash, F.; Sahebkar, A.; Majeed, M.; Bahreini, N.; Askari, G.; Bagherniya, M. The effects of curcumin-piperine supplementation on inflammatory, oxidative stress and metabolic indices in patients with ischemic stroke in the rehabilitation phase: A randomized controlled trial. Nutr. J. 2023, 22, 69. [Google Scholar] [CrossRef]
- Campbell, M.S.; Ouyang, A.; Im, K.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht MGh, B.F. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2013, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2019, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: A clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Namiranian, K.; Amerian, R.; Mansouri, S.; Arshadi, S.; Azarbayjani, M.A. The effect of curcumin supplementation and aerobic training on anthropometric indices, serum lipid profiles, C-reactive protein and insulin resistance in overweight women: A randomized, double-blind, placebo-controlled trial. J. Obes. Metab. Syndr. 2020, 29, 47–57. [Google Scholar] [CrossRef]
- Dolati, S.; Farzad, L.; Far, A.H. The effect of aerobic training and supplementation of curcumin on the glycemic markers and level of myeloperoxidase enzyme of overweight women. J. Rom. Diabet. Nutr. Metab. Dis. 2020, 27, 281–288. Available online: http://www.rjdnmd.org/index.php/RJDNMD/article/download/755/546 (accessed on 16 December 2025).
- Ferguson, J.J.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals: A randomized controlled trial. Metabolism 2017, 82, 22–35. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; Wada, H.; et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. J. Diabetes Res. 2019, 2019, 8208237. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Kato, T.; Funada, J.; Ajiro, Y.; Komiyama, M.; Akao, M.; Yasoda, A.; Yamakage, H.; et al. Effects of high-absorption curcumin for the prevention of hypertensive heart disease: A double-blind, placebo-controlled, randomized clinical study. Eur. Heart J. Open 2022, 2, oeac057. [Google Scholar] [CrossRef]
- Garg, A.X.; Devereaux, P.; Hill, A.; Sood, M.; Aggarwal, B.; Dubois, L.; Hiremath, S.; Guzman, R.; Iyer, V.; James, M.; et al. Oral curcumin in elective abdominal aortic aneurysm repair: A multicentre randomized controlled trial. CMAJ 2018, 190, E1273–E1280. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Sepehri, B.; Asghari-Jafarabadi, M.; Ghavami, S. Turmeric and chicory seed have beneficial effects on obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). Int. J. Vitam. Nutr. Res. 2019, 89, 293–302. [Google Scholar] [CrossRef]
- Ghazimoradi, M.; Saberi-Karimian, M.; Mohammadi, F.; Sahebkar, A.; Tavallaie, S.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Moohebati, M.; Esmaeili, H.; et al. The effects of curcumin and curcumin–phospholipid complex on the serum pro-oxidant–antioxidant balance in subjects with metabolic syndrome. Phytother. Res. 2017, 31, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: A comparative, randomized, double-blind, placebo-controlled study. BMC Complement. Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Forman, J.; Chabanova, E.; Svenningsen, J.S.; Holst, J.J.; Gillum, M.P.; Vilsbøll, T.; Knop, F.K. The effect of curcumin on hepatic fat content in individuals with obesity. Diabetes Obes. Metab. 2022, 24, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Hansen, K.B.; Forman, J.L.; Størling, J.; Chabanova, E.; Holst, J.; Vilsbøll, T.; Knop, F.K. No effect of the turmeric root phenol curcumin on prednisolone-induced glucometabolic perturbations in men with overweight or obesity. Endocr. Connect. 2023, 12, e220334. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine 2020, 80, 153395. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Ismail, N.A.; Ragab, S.; El-Baky, A.N.E.A.; Hamed, M.; Ibrahim, A.A. Effect of oral curcumin administration on insulin resistance, serum resistin and fetuin-A in obese children: Randomized placebo-controlled study. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 887–896. [Google Scholar]
- Ismail, N.A.; Abd El Dayem, S.M.; Salama, E.; Ragab, S.; Abd El Baky, A.N.; Ezzat, W.M. Impact of curcumin intake on gluco-insulin homeostasis, leptin and adiponectin in obese subjects. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1891–1897. [Google Scholar]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Jarhahzadeh, M.; Alavinejad, P.; Farsi, F.; Husain, D.; Rezazadeh, A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: A randomized double-blind clinical trial. Diabetol. Metab. Syndr. 2021, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Javandoost, A.; Afshari, A.; Saberi-Karimian, M.; Sahebkar, A.; Safarian, H.; Moammeri, M.; Dizaji, B.F.; Tavalaei, S.; Ferns, G.A.; Pasdar, A.; et al. The effects of curcumin and a modified curcumin formulation on serum cholesteryl ester transfer protein concentrations in patients with metabolic syndrome: A randomized, placebo-controlled clinical trial. Avicenna J. Phytomedicine 2018, 8, 330–337. [Google Scholar]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M. Nano-curcumin improves glucose indices, lipids, inflammation, and nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; De Oca-Solano, H.M.; Madero, M.; Pedraza-Chaverri, J. The effect of dietary supplementation with curcumin on redox status and NRF2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: A phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 2021, 35, 4377–4387. [Google Scholar] [CrossRef]
- Karandish, M.; Mozaffari-Khosravi, H.; Mohammadi, S.M.; Cheraghian, B.; Azhdari, M. Curcumin and zinc co-supplementation along with a loss-weight diet can improve lipid profiles in subjects with prediabetes: A multi-arm, parallel-group, randomized, double-blind placebo-controlled phase 2 clinical trial. Diabetol. Metab. Syndr. 2022, 14, 22. [Google Scholar] [CrossRef]
- Kelardeh, B.M.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kisiolek, J.N.; Kheredia, N.; Flores, V.; Ramani, A.; Lisano, J.; Johnston, N.; Stewart, L.K. Short term, oral supplementation with optimized curcumin does not impair performance improvements associated with high intensity interval training. J. Diet. Suppl. 2021, 19, 733–746. [Google Scholar] [CrossRef]
- Kocher, A.; Bohnert, L.; Schiborr, C.; Frank, J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016, 60, 1555–1563. [Google Scholar] [CrossRef]
- Krishnareddy, N.T.; Thomas, J.V.; Nair, S.S.; Mulakal, J.N.; Maliakel, B.P.; Krishnakumar, I.M. A novel curcumin-galactomannoside complex delivery system improves hepatic function markers in chronic alcoholics: A double-blinded, randomized, placebo-controlled study. Biomed. Res. Int. 2018, 2018, 9159281. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Devarajan, T.V.; Saklecha, S.; Reddy, S.V.K.; Mundkur, L. A minor metabolite from Curcuma longa effective against metabolic syndrome: Results from a randomized, double-blind, placebo-controlled clinical study. Food Funct. 2023, 14, 4722–4733. [Google Scholar] [CrossRef]
- Mamsharifi, P.; Farokhi, B.; Hajipoor-Taziani, R.; Alemi, F.; Hazegh, P.; Masoumzadeh, S.; Jafari, L.; Ghaderi, A.; Dehkohneh, S.G. Nano-curcumin effects on nicotine dependence, depression, anxiety and metabolic parameters in smokers: A randomized double-blind clinical study. Heliyon 2023, 9, e21249. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Sibille, K.; Leeuwenburgh, C.; Lin, Y.; Hsu, F.; Qiu, P.; Sandesara, B.; Anton, S. Effects of Curcumin C3 Complex® on physical function in moderately functioning older adults with low-grade inflammation—A pilot trial. J. Frailty Aging 2022, 12, 143–149. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Gholami, A.; Hooshmand, E.; Tavallaie, S.; Gh, B.F.N.M. The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Drug Metab. Pers. Ther. 2019, 34, 20180040. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Gh, B.F.N.M.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2021, 91, 278–286. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The effect of curcumin phytosome on the treatment of patients with non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled trial. Adv. Exp. Med. Biol. 2021, 1308, 25–35. [Google Scholar] [CrossRef]
- Mirzabeigi, P.; Mohammadpour, A.H.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The effect of curcumin on some traditional and non-traditional cardiovascular risk factors: A pilot randomized, double-blind, placebo-controlled trial. Iran. J. Pharm. Res. 2015, 14, 479–486. Available online: https://pubmed.ncbi.nlm.nih.gov/25901155 (accessed on 16 December 2025).
- Mohammadi, A.; Sadeghnia, H.R.; Saberi-Karimian, M.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Effects of curcumin on serum vitamin E concentrations in individuals with metabolic syndrome. Phytother. Res. 2017, 31, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Mohsenpour, M.A.; Sohrabi, Z.; Niakousari, M.; Jeddi, M.; Hassanzadeh, J.; Ferns, G.A.; Eftekhari, M.H. The effects of powdered drinks enriched with curcumin and probiotics on lipid profile and atherogenic indices in patients with metabolic syndrome: A randomized, double-blinded, placebo-controlled clinical trial. Food Sci. Nutr. 2023, 12, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2020, 35, 2099–2107. [Google Scholar] [CrossRef]
- Na, L.; Li, Y.; Pan, H.; Zhou, X.; Sun, D.; Meng, M.; Li, X.; Sun, C. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Neta, J.F.D.F.; Veras, V.S.; Sousa, D.F.D.; Cunha, M.D.C.D.S.O.; Queiroz, M.V.O.; Neto, J.C.G.L.; Damasceno, M.M.C.; Araújo, M.F.M.D.; Freitas, R.W.J.F.D. Effectiveness of the piperine-supplemented Curcuma longa L. in metabolic control of patients with type 2 diabetes: A randomised double-blind placebo-controlled clinical trial. Int. J. Food Sci. Nutr. 2021, 72, 968–977. [Google Scholar] [CrossRef]
- Nowak, K.L.; Farmer-Bailey, H.; Wang, W.; You, Z.; Steele, C.; Cadnapaphornchai, M.A.; Klawitter, J.; Patel, N.; George, D.; Jovanovich, A.; et al. Curcumin therapy to treat vascular dysfunction in children and young adults with ADPKD. Clin. J. Am. Soc. Nephrol. 2021, 17, 240–250. [Google Scholar] [CrossRef]
- Osali, A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Hosseini, M.S.; Abbasinazari, M.; Sahebkar, A. Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement. Ther. Med. 2014, 22, 851–857. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: Results of a randomized controlled trial. J. Diet. Suppl. 2015, 13, 209–220. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid–piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2016, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: A randomized controlled trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: A randomized controlled trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.; Majeed, M.; Sahebkar, A. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Pashine, L.; Singh, J.V.; Vaish, A.K.; Ojha, S.K.; Mahdi, A.A. Effect of turmeric (Curcuma longa) on overweight hyperlipidemic subjects: Double blind study. Indian J. Community Health 2012, 24, 113–117. [Google Scholar]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Porasgari, Z.; Sakri, H.; Arshadi, S. The effect of eight weeks of Pilates with curcumin supplementation on liver enzymes and lipid profile in overweight and obese women. Obes. Med. 2022, 36, 100448. [Google Scholar] [CrossRef]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. Available online: https://pubmed.ncbi.nlm.nih.gov/27761427 (accessed on 16 December 2025).
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.C.D.S.G.; Alves, A.G.P.; Guillo, L.A.; Sousa Neto, M.A.D.; Trindade, N.R.; Silva, M.S. Curcumin supplementation reduces blood glucose and serum lipids of Brazilian women with high waist circumference: A randomized clinical trial. Arch. Endocrinol. Metab. 2022, 66, 800–807. [Google Scholar] [CrossRef]
- Rezaei, M.; Soltani, M.; Alipoor, E.; Rezayat, S.M.; Vasheghani-Farahani, A.; Yaseri, M.; Firouzi, A.; Hosseinzadeh-Attar, M.J. Effect of nano-curcumin supplementation on angina status and cardiovascular risk factors in overweight or obese patients with coronary slow flow phenomenon: A randomized double-blind placebo-controlled clinical trial. BMC Nutr. 2024, 10, 73. [Google Scholar] [CrossRef]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized placebo-controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M.; Salahshooh, M.M.; Dizaji, B.F.; Safarian, H.; Javandoost, A.; Ferns, G.A.; Sahebkar, A.; Ahmadinejad, M. Evaluation of the effects of curcumin in patients with metabolic syndrome. Comp. Clin. Pathol. 2018, 27, 555–563. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Karimian, M.; Ghazizadeh, H.; Mohammadzadeh, E.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Does curcumin have an effect on sleep duration in metabolic syndrome patients? Avicenna J. Phytomed. 2021, 11, 190–198. [Google Scholar] [PubMed]
- Sadeghzadeh, Z.; Ostadrahimi, A.; Ranjbar, M.; Farshbaf-Khalili, A. The efficacy of Nigella sativa L. and curcumin nanomicelle alone or together on lipid profile, glycemic control indices, and serum 17-β estradiol in postmenopausal women. J. Caring Sci. 2023, 12, 163–173. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Taghdir, M.; Mirahmadi, J.; Sepandi, M.; Parastouei, K. Effects of curcumin and/or coenzyme Q10 supplementation on metabolic control in subjects with metabolic syndrome: A randomized clinical trial. Nutr. J. 2022, 21, 62. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Sedighiyan, M.; Abdolahi, M.; Jafari, E.; Vahabi, Z.; Athar, S.S.; Hadavi, S.; Zamanabadi, M.N.; Yekaninejad, M.; Djalali, M. The effects of nano-curcumin supplementation on adipokines levels in obese and overweight patients with migraine: A double-blind clinical trial study. BMC Res. Notes 2022, 15, 189. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef]
- Shirmohammadi, L.; Ghayour-Mobarhan, M.; Saberi-Karimian, M.; Iranshahi, M.; Tavallaie, S.; Emamian, M.; Sahebkar, A. Effect of Curcumin on Serum Cathepsin D in Patients with Metabolic Syndrome. Cardiovasc. Haematol. Disord. Drug Targets 2019, 20, 116–121. [Google Scholar] [CrossRef]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The Effects of Curcumin Supplementation on Glycemic Status, Lipid Profile and hs-CRP Levels in Overweight/Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef]
- Soltani, M.; Hosseinzadeh-Attar, M.J.; Rezaei, M.; Alipoor, E.; Vasheghani-Farahani, A.; Yaseri, M.; Rezayat, S.M. Effect of Nano-Curcumin Supplementation on Cardiometabolic Risk Factors, Physical and Psychological Quality of Life, and Depression in Patients with Coronary Slow Flow Phenomenon: A Randomized Double-Blind Clinical Trial. Trials 2024, 25, 515. [Google Scholar] [CrossRef]
- Srinivasan, A.; Selvarajan, S.; Kamalanathan, S.; Kadhiravan, T.; Lakshmi, N.C.P.; Adithan, S. Effect of Curcuma longa on Vascular Function in Native Tamilians with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Parallel Arm, Placebo-Controlled Trial. Phytother. Res. 2019, 33, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Tamaddoni, A.; Nasseri, E.; Mohammadi, E.; Qujeq, D.; Zayeri, F.; Zand, H.; Mir, S.M.; Gholami, M. A Double-Blind Randomized Controlled Trial of Curcumin for Improvement in Glycemic Status, Lipid Profile and Systemic Inflammation in β-Thalassemia Major. J. Herb. Med. 2019, 21, 100324. [Google Scholar] [CrossRef]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduces Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef]
- Uchio, R.; Muroyama, K.; Okuda-Hanafusa, C.; Kawasaki, K.; Yamamoto, Y.; Murosaki, S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1822. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2414. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Y.; Yang, H.; Lee, Y.; Chou, J.I.; Ueng, K. Lipid-Lowering Effects of Curcumin in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Zohrabi, T.; Nadjarzadeh, A.; Jambarsang, S.; Sheikhha, M.H.; Aflatoonian, A.; Mozaffari-Khosravi, H. Effect of Dietary Approaches to Stop Hypertension and Curcumin Co-Administration on Glycemic Parameters in Polycystic Ovary Syndrome: An RCT. Int. J. Reprod. Biomed. 2024, 22, 689–700. [Google Scholar] [CrossRef]
- Sartore, G.; Ragazzi, E.; Caprino, R.; Lapolla, A. Long-Term HbA1c Variability and Macro-/Micro-Vascular Complications in Type 2 Diabetes Mellitus: A Meta-Analysis Update. Acta Diabetol. 2023, 60, 721–738. [Google Scholar] [CrossRef]
- Tandon, N.; Ali, M.K.; Venkat Narayan, K.M. Pharmacologic Prevention of Microvascular and Macrovascular Complications in Diabetes Mellitus: Implications of the Results of Recent Clinical Trials in Type 2 Diabetes. Am. J. Cardiovasc. Drugs 2012, 12, 7–22. [Google Scholar] [PubMed]
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New Mechanisms and the Anti-Inflammatory Role of Curcumin in Obesity and Obesity-Related Metabolic Diseases. Eur. J. Nutr. 2011, 50, 151–161. [Google Scholar]
- Bradford, P.G. Curcumin and Obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Xu, R. Anti-Inflammatory Effects of Curcumin in Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [PubMed]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-Mediated Regulation of Intestinal Barrier Function: The Mechanism Underlying Its Beneficial Effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef]
- Mahran, R.I.; Hagras, M.M.; Sun, D.; Brenner, D.E. Bringing Curcumin to the Clinic in Cancer Prevention: A Review of Strategies to Enhance Bioavailability and Efficacy. AAPS J. 2017, 19, 54–81. [Google Scholar]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Aggarwal, B.B. Is Curcumin Bioavailability a Problem in Humans: Lessons from Clinical Trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]












| Component | Description |
|---|---|
| Population (P) | Healthy people at risk of developing metabolic syndrome, pre-diabetic patients, diabetic patients, CVD patients, and patients with one of the conditions of metabolic syndrome. |
| Intervention (I) | Supplementation with Curcuma longa Linn. (turmeric) or curcumin extracts, including enhanced bioavailability formulations (e.g., nanocurcumin, curcumin-piperine). Administered as capsules, tablets, powders, or nutraceutical formulations. |
| Comparator (C) | Placebo, no treatment, or standard care/control diet. RCTs with active comparators (e.g., another supplement) will be included only if curcumin-specific outcomes can be extracted. |
| Outcomes (O) | Primary outcomes: Core components of MetS such as fasting blood glucose, HbA1c, triglycerides, total cholesterol, HDL-C, LDL-C, systolic and diastolic blood pressure, and waist circumference. Secondary outcomes: Inflammatory markers (CRP, TNF-α, IL-6), oxidative stress markers (MDA, TAC), insulin resistance indices (HOMA-IR), liver enzymes, and body mass index (BMI). |
| Author | Year | Country | Population | Gender | Age | Sample Size | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||
| Mean | SD | Mean | SD | Intervention | Control | |||||
| Abed et al. [18] | 2021 | Iraq | Post-ischemic stroke patients | M, F | 51.37 | 9.35 | 50.95 | 10.03 | 24 | 18 |
| Adibian et al. [19] | 2019 | Iran | Type 2 Diabetes patients | M, F | 58 | NM | 60 | 7 | 21 | 23 |
| Afshar et al. [20] | 2020 | Iran | Hemodialysis patients | M, F | 55.33 | 12.95 | 59.05 | 7.68 | 27 | 27 |
| Alizadeh et al. [21] | 2017 | Iran | Infertile men | Male | 30.54 | 4.03 | 30 | 3.96 | 28 | 28 |
| Alvarenga et al. [22] | 2020 | Brazil | Hemodialysis Patients | M, F | 54 | 15 | 53 | 12 | 14 | 14 |
| Alvarenga et al. [23] | 2022 | Brazil | Patients undergoing hemodialysis | M, F | 54 | 15 | 53 | 12 | 14 | 14 |
| Asadi et al. [24] | 2019 | Iran | Type-2 diabetic patients | M, F | 53.3 | 6.5 | 54.6 | 6.2 | 40 | 40 |
| Asan et al. [25] | 2020 | Turkey | Women with polycystic ovary syndrome | F | 27.6 | 3.6 | 28.3 | 5.9 | 15 | 15 |
| Asghari et al. [26] | 2024 | USA | Type 2 Diabetes Mellitus patients (Eicosapentaenoic acid EPA group) Curcumin group Curcumin + EPA group | M, F | 56.88 54.56 56.68 | 8.36 8.3 10.25 | 57.48 | 11.27 | 25 | 25 |
| Askari et al. [27] | 2022 | Iran | COVID-19 outpatient | M, F | 43.74 | 12.9 | 51.52 | 13.8 | 23 | 23 |
| Atakan et al. [28] | 2022 | Turkey | Overweight and obese women (hyperlipidemia) | F | 25–65 | 35 | 35 | |||
| Barber-Chamoux et al. [29] | 2017 | France | Healthy Smokers | M, F | 56 | 4.1 | 56 | 4.1 | 9 | 9 |
| Bateni et al. [30] | 2021 | Iran | Patients with metabolic syndrome | M. F | 50 | 9 | 54 | 7 | 22 | 21 |
| Boshagh et al. [31] | 2023 | Iran | Patients with ischemic stroke | M, F | 59.48 | 5.15 | 60.12 | 3.12 | 27 | 29 |
| Campbell et al. [32] | 2019 | USA | Young obese men | M | 18–35 (range) | 11 | 11 | |||
| Chashmniam et al. [33] | 2019 | Iran | Non-Alcoholic Fatty Liver Disease Patient | M, F | 46.56 | 11.25 | 37.75 | 14.4 | 25 | 20 |
| Chuengsamarn et al. [34] | 2012 | Thailand | Subjects with the criteria of prediabetes | M, F | 56.95 | 12 | 57.93 | 12.71 | 119 | 116 |
| Chuengsamarn et al. [35] | 2014 | Thailand | Type 2 Diabetes patients | M, F | 59.16 | 11.03 | 59.58 | 10.71 | 107 | 106 |
| Cicero et al. [36] | 2019 | Italy | Overweight subjects | M, F | 54 | 3 | 53 | 5 | 40 | 40 |
| Darmian et al. [37] | 2022 | Iran | Middle-aged women with hyperlipidemia and type 2 diabetes (Turmeric capsule group) Aerobic training group Aerobic training + Turmeric capsule group | F | 44.33 42.13 43.02 | 1.23 2.39 3.04 | 44.22 | 3.07 | 11 10 11 | 10 |
| Dolati et al. [38] | 2020 | Iran | Overweight women (Curcumin group) (Curcumin + Training group) (Placebo + training group) | F | 38.9 35.80 38.20 | 5.4 3.22 5.67 | 40.8 | 3.55 | 10 10 10 | 10 |
| Farzad et al. [39] | 2020 | Iran | Overweight women (Curcumin group) Curcumin + Training group Training group | F | 38.9 35.8 38.2 | 5.4 3.22 5.67 | 40.6 | 3.71 | 10 10 | 10 |
| Fergusona et al. [40] | 2018 | Australia | Patients with hypercholesterolemia (Curcumin group) (Phytosterol+ Curcumin group) (Phytosterol group) | M, F | 51 50.35 51.35 | 2.34 3.36 3.62 | 50.11 | 2.96 | 18 17 17 | 18 |
| Funamoto et al. [41] | 2019 | Japan | Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus | M, F | 70 | 6 | 69 | 7 | 15 | 18 |
| Funamoto et al. [42] | 2016 | Japan | Patients with mild COPD | M, F | 69.6 | 6.6 | 69.9 | 6.3 | 22 | 17 |
| Funamoto et al. [43] | 2022 | Japan | Patients exhibiting initial signs of hypertensive heart disease | M, F | 67 (median) | 66 (median) | 73 | 69 | ||
| Garg et al. [44] | 2018 | Canada | Patients with elective repair of an abdominal aortic aneurysm | M, F | 76 (median) | 304 | 302 | |||
| Ghaffari et al. [45] | 2019 | Iran | Patients with non-alcoholic fatty liver disease (Turmeric only group) (Chicory seed only group) (Turmeric + Chicory seed group) | M, F | 42.5 41 41.5 | 6.93 8.61 7.68 | 40.3 | 9.26 | 21 21 21 | 21 |
| Ghazimoradi et al. [46] | 2017 | Iran | Subjects with metabolic syndrome (Phosholipidated Curcumin group) (Curcumin group) | M/F | 40.05 37.52 | 10.48 9.87 | 38.59 | 10.28 | 37 36 | 36 |
| Hariri et al. [47] | 2020 | Iran | Non-Alcoholic Fatty Liver Disease Patient | M, F | 40.95 | 12.24 | 40.06 | 13.69 | 23 | 22 |
| Haroyan et al. [48] | 2018 | Armenia | Osteoarthritis patients (Curamed group) (Curcumin group) | M, F | 54.65 57.91 | 8.84 9.02 | 56.04 | 8.55 | 57 66 | 54 |
| Hellmann et al. [49] | 2022 | Denmark | Obese, non-diabetic individuals | M, F | 44.8 | 15.8 | 47.7 | 12.1 | 18 | 19 |
| Hellmann et al. [50] | 2023 | Denmark | Prednisolone-induced glucometabolic perturbations in men with overweight or obesity (Prednisolone + Curcumin placebo group) Prednisolone + Curcumin group | M | 44 47 | 13.5 17.8 | 41.6 | 9.8 | 8 8 | 8 |
| Heshmati et al. [51] | 2020 | Iran | Patients with Polycystic Ovary Syndrome | F | 30.97 | 5.2 | 30.75 | 7.97 | 34 | 33 |
| Heshmati et al. [52] | 2020 | Iran | Polycystic ovarian syndrome (PCOS) patients | F | 31 (Median) | 29 (Median) | 34 | 33 | ||
| Hodge et al. [53] | 2019 | Iran | Type 2 Diabetes patients | M, F | 58 | 8 | 60 | 7 | 21 | 23 |
| Ismail et al. [54] | 2014 | Egypt | Obese Children | M, F | 15.5714 | 5.7974 | 16.5357 | 8.69674 | 14 | 11 |
| Ismail et al. [55] | 2016 | Egypt | Obese subjects (Pediatrics) (Adults) | M, F | 14.707 37.552 | 4.52 9.934 | 14.707 37.552 | 4.52 9.552 | 15 15 | 14 14 |
| Jamilian et al. [56] | 2020 | Iran | Women with polycystic ovary syndrome | F | 28.6 | 4.7 | 27.2 | 3.4 | 24 | 26 |
| Jarhahzaden et al. [57] | 2021 | Iran | Nonalcoholic fatty liver disease | M, F | 44.12 | 8.35 | 38.56 | 10.43 | 32 | 32 |
| Javandoosi et al. [58] | 2018 | Iran | Patients with metabolic syndrome (Curcumin group) (Complex Curcumin Group) | M, F | 18–65 (range) | 36 37 | 36 | |||
| Jazayeri-Tehrani et al. [59] | 2019 | Iran | Overweight/obese patients with non-alcoholic fatty liver disease | M, F | 41.8 | 5.6 | 42.5 | 6.2 | 42 | 42 |
| Jimenez-Osorio et al. [60] | 2016 | Mexico | Patients with non-diabetic or diabetic proteinuria, chronic kidney disease (Diabetes Group) (Non-Diabetes Group) | M, F | 55 36.8 | 1.6 2.7 | 56.2 44.3 | 1.5 3.4 | 28 24 | 23 26 |
| Karandish et al. [61] | 2021 | Iran | Overweight or obese prediabetes subjects (Curcumin group) Zinc group Zinc + Curcumin group | M, F | 36.95 38.19 34.48 | 7.23 4.87 6.45 | 34.19 | 7.03 | 21 21 20 | 20 |
| Karandish et al. [62] | 2022 | Iran | overweight or obese patients with prediabetes (Curcumin group) Zinc group Curcumin + Zinc group | M, F | 36.95 38.19 34.48 | 7.23 4.87 6.45 | 34.19 | 7.03 | 21 21 20 | 20 |
| Kelardeh et al. [63] | 2020 | Iran | Older women with non-alcoholic fatty liver disease (Resistance training group) Curcumin group Resistance training + Curcumin group | F | 65.91 66.72 64.09 | 3.31 3.03 3.03 | 64.36 | 2.97 | 12 11 11 | 11 |
| Khajehdehi et al. [64] | 2011 | Iran | Type 2 Diabetes patients | M, F | 52.9 | 9.2 | 52.6 | 9.7 | 20 | 20 |
| Khajehdehi et al. [65] | 2012 | Iran | Patients with relapsing or refractory biopsy-proven lupus nephritis | M, F | 32.2 | 11.4 | 35 | 10.4 | 12 | 12 |
| Kisiolek et al. [66] | 2021 | USA | Healthy and physically active subjects (Curcumin Fast Cycling Time Trial Group) (Curcumin Slow Cycling Time Trial Group) | M, F | 25 25.6 | 3.6 5.1 | 23.1 | 3.7 | 12 12 | 12 |
| Kocher et al. [67] | 2016 | Germany | Hyperlipidemia individuals (Men) (Women) | M, F | 50 52 | 20 16 | 500 52 | 20 16 | N/M | N/M |
| Krishnareddy et al. [68] | 2018 | India | Healthy subjects with chronic alcohol intake | M | 45 | 9.1 | 45 | 9.1 | 23 | 22 |
| Majeed et al. [69] | 2023 | India | Obese adults | M, F | 45.25 | 7.11 | 41.25 | 7.11 | 47 | 47 |
| Mamsharifi et al. [70] | 2023 | Iran | Smokers | M, F | 33.05 | 10.05 | 32.14 | 9.55 | 35 | 35 |
| Mankowski et al. [71] | 2022 | USA | Moderately functioning older adults with low-grade inflammation | M, F | 79.4 | 10.1 | 76.2 | 5.6 | 9 | 8 |
| Mirhafez et al. [72] | 2019 | Iran | Non-Alcoholic Fatty Liver Disease Patient | M, F | 44.8 | 11.14 | 40.7 | 11.83 | 32 | 29 |
| Mirhafez et al. [73] | 2019 | Iran | Non-Alcoholic Fatty Liver Disease Patient | M, F | 41.2 | 14.1 | 40.7 | 11 | 22 | 22 |
| Mirhafez et al. [74] | 2019 | Iran | Patients with non-alcoholic fatty liver diseases | M, F | 38.82 | 2.95 | 43.29 | 2.21 | 24 | 23 |
| Mirhafez et al. [75] | 2021 | Iran | Non-Alcoholic Fatty Liver Disease patient | M, F | 45 | 11.1 | 43.1 | 11.6 | 35 | 37 |
| Mirzabeigi et al. [76] | 2015 | Iran | Volunteers >18 years old with a diagnosis of CAD | M, F | 61.5 | 8.7 | 64.3 | 8.42 | 17 | 16 |
| Mohammadi et al. [77] | 2017 | Iran | Individuals with metabolic syndrome (Curcumin phospholipid complex group) (Curcumin group) | M/F | 40.05 37.52 | 10.48 9.87 | 38.59 | 10.28 | 37 36 | 36 |
| Mohammadi et al. [78] | 2024 | Iran | Patients with metabolic syndrome (Curcumin group) Probiotic group Curcumin + Probiotic group | M, F | 49.39 49.5 47.39 | 1.31 1.13 1.67 | 48.96 | 1.28 | 28 30 28 | 28 |
| Mokhtari et al. [79] | 2020 | Iran | Patients with diabetic foot ulcer | M, F | 57.4 | 11.7 | 55.8 | 9.4 | 25 | 25 |
| Na et al. [80] | 2012 | China | Overweight or Obese with T2D Patients | M, F | 55.42 | 6.4 | 54.72 | 8.34 | 50 | 50 |
| Neta et al. [81] | 2021 | Brazil | Type 2 Diabetes patients | M, F | 63.1 | 11.1 | 61.9 | 11 | 33 | 28 |
| Nowak et al. [82] | 2022 | USA | Autosomal dominant polycystic kidney disease patients | M, F | 18 | 6 | 19 | 5 | 28 | 29 |
| Osali et al. [83] | 2020 | Iran | Elderly female with metabolic syndrome (Exercise group) Nano-curcumin group) Exercise + Nano-curcumin group) | F | 62.3 | 1.23 | 62.3 | 1.23 | 11 11 11 | 11 |
| Panahi et al. [84] | 2018 | Iran | Patients with type 2 Diabetes Mellitus | M, F | 43 | 8 | 41 | 7 | 50 | 50 |
| Panahi et al. [85] | 2014 | Iran | Patients with metabolic syndrome | M, F | 44.8 | 8.67 | 43.46 | 9.7 | 50 | 50 |
| Panahi et al. [86] | 2015 | Iran | Metabolic syndrome patients | M, F | 44.8 | 8.67 | 43.46 | 9.7 | 50 | 50 |
| Panahi et al. [87] | 2015 | Iran | Osteoarthritis patients | M, F | 57.32 | 8.78 | 57.57 | 9.05 | 19 | 21 |
| Panahi et al. [88] | 2016 | Iran | Type 2 Diabetes Patients | M, F | 43 | 8 | 41 | 7 | N/M | N/M |
| Panahi et al. [89] | 2016 | Iran | Non-Alcoholic Fatty Liver Disease Patients | M, F | 44.98 | 12.59 | 47.21 | 10.29 | 44 | 43 |
| Panahi et al. [90] | 2016 | Iran | Subjects with metabolic syndrome | M, F | 44.8 | 8.67 | 43.46 | 9.7 | 50 | 50 |
| Panahi et al. [91] | 2017 | Iran | Type 2 Diabetes Patients | M, F | 43 | 8 | 41 | 7 | 50 | 50 |
| Panahi et al. [92] | 2017 | Iran | Non-Alcoholic Fatty Liver Disease Patients | M, F | 44.98 | 12.59 | 47.21 | 10.29 | 44 | 43 |
| Pashine et al. [93] | 2012 | India | Overweight hyperlipidemia subjects | M, F | 21–60 | 53 | 52 | |||
| Pierro et al. [94] | 2015 | Italy | Overweight people with metabolic syndrome | M, F | 39.1 | 16.8 | 41.85 | 15.91 | 22 | 22 |
| Porasgari et al. [95] | 2022 | Iran | Overweight and obese women (Curcumin group) Pilates group Curcumin + Pilates group | F | 36.37 36.62 37.37 | 21.97 19.47 24.99 | 37.75 | 22.73 | 14 14 15 | 13 |
| Rahimi et al. [96] | 2016 | Iran | Diabetic subjects | M, F | 56.34 | 11.17 | 60.95 | 10.77 | 35 | 35 |
| Rahmani et al. [97] | 2016 | Iran | Patients with symptoms of metabolic syndrome | M, F | 46.37 | 11.57 | 48.95 | 9.78 | 37 | 40 |
| Reis et al. [98] | 2022 | Brazil | Brazilian women | F | 47 | 10.52 | 50 | 12.58 | 15 | 20 |
| Rezaei et al. [99] | 2024 | Iran | Overweight or obese patients with the coronary slow flow phenomenon | M, F | 54.3 | 9.1 | 54.6 | 8.4 | 21 | 21 |
| Saadati et al. [100] | 2019 | Iran | Non-Alcoholic Fatty Liver Disease Patient | M, F | 11.5 | 46.19 | 10.9 | 45.13 | 25 | 23 |
| Saadati et al. [101] | 2019 | Iran | Patients with non-alcoholic fatty liver disease | M, F | 46.19 | 11.5 | 45.13 | 10.9 | 27 | 23 |
| Saberi-Karimian et al. [102] | 2018 | Iran | Patients with metabolic syndrome (Curcumin phospholipid group) (Curcumin group) | N/M | 40.05 37.52 | 10.48 9.47 | 38.59 | 10.28 | 37 36 | 36 |
| Saberi-Karimian et al. [103] | 2019 | Iran | Metabolic syndrome patients (Curcumin phospholipid group) (Curcumin group) | M, F | 40.05 37.52 | 10.48 9.47 | 38.59 | 10.28 | 37 36 | 36 |
| Saberi-Karimian et al. [104] | 2020 | Iran | Non-Alcoholic Fatty Liver Disease Patients | M, F | 18–70 | 23 | 26 | |||
| Sadeghzadeh et al. [105] | 2023 | Iran | Postmenopausal women (Curcumin Group) Nigella sativa Group Curcumin + Nigella sativa Group | F | 58 57.2 57.4 | 3.4 4.3 3.8 | 58.4 | 3.4 | 30 28 28 | 29 |
| Sangouni et al. [106] | 2022 | Iran | Subjects with metabolic syndrome (Curcumin CP group) Coenzyme + Placebo QP group Curcumin + Coenzyme CQ group | M, F | 38.8 39 37.7 | 4.9 4.5 5 | 39.5 | 5 | 22 22 22 | 22 |
| Saraf-Bank et al. [107] | 2019 | Iran | Overweight adolescent girls | F | 16.03 | 1.56 | 15.98 | 1.72 | 30 | 30 |
| Saraf-Bank et al. [108] | 2019 | Iran | Healthy overweight and obese adolescent girls | F | 16.03 | 1.56 | 15.98 | 1.72 | 30 | 30 |
| Sedighiyan et al. [109] | 2022 | Iran | Obese and overweight patients with migraine | M, F | 39.27 | 10.07 | 41 | 11.35 | 22 | 22 |
| Shafabakhsh et al. [110] | 2020 | Iran | T2D Mellitus and coronary heart disease patients | M, F | 64.9 | 7.8 | 66.5 | 7.7 | 25 | 24 |
| Shirmohammadi et al. [111] | 2019 | Iran | Patients with metabolic syndrome | M, F | 40.05 | 10.48 | 38.59 | 10.28 | 37 | 36 |
| Sohaei et al. [112] | 2019 | Iran | Overweight and obese women with PCOS | F | 29.4 | 5.33 | 29.58 | 5 | 27 | 24 |
| Soltani et al. [113] | 2024 | Iran | Overweight or obese patients with the coronary slow flow phenomenon | M, F | 54 | 9 | 55 | 8 | 21 | 21 |
| Srinivasan et al. [114] | 2019 | India | Type 2 Diabetes patients | M, F | 51.32 | 8.61 | 49.94 | 8.72 | 60 | 54 |
| Tamaddoni et al. [115] | 2019 | Iran | Beta thalassemia major patients | M, F | 25.97 | 6.92 | 27.61 | 6.23 | 31 | 30 |
| Thota et al. [116] | 2020 | Australia | Adults with high risk of T2 Diabetes and Alzheimer’s disease | M, F | 54.5 | 2.9 | 50.4 | 2.6 | 14 | 15 |
| Uchio et al. [117] | 2019 | Japan | Subjects with overweight or prehypertension/mild hypertension | M, F | 58.8 | 5.3 | 58.5 | 5.5 | 43 | 44 |
| Yaikwawong et al. [118] | 2024 | Thailand | Type 2 Diabetes Patients | M, F | 60.27 | 8.82 | 62.26 | 8.65 | 113 | 114 |
| Yaikwawong et al. [119] | 2024 | Thailand | Obese patients with Type 2 Diabetes | M, F | 60.27 | 8.82 | 62.26 | 8.65 | 113 | 114 |
| Yang et al. [120] | 2014 | Taiwan | Metabolic syndrome patients | M, F | 59.03 | 10.1 | 59.61 | 14.09 | 30 | 29 |
| Zohrabi et al. [121] | 2023 | Iran | Polycystic ovary syndrome patients (Curcumin group) Curcumin + DASH diet group DASH diet group | F | 18–45 | 25 24 24 | 24 | |||
| Outcome Domain | Parameter | Pooled SMD (95% CI) | p-Value | I2 (%) | Remarks |
|---|---|---|---|---|---|
| Anthropometry | BMI | −0.27 (−0.57 to 0.02) | >0.05 | 79 | No significant effect; heterogeneous results |
| Waist Circumference (WC) | −0.33 (−0.81 to 0.15) | >0.05 | 75 | Non-significant; trend toward reduction | |
| Waist–Hip Ratio (WHR) | −0.11 (−0.37 to 0.15) | >0.05 | 60+ | No effect | |
| Blood Pressure | Systolic BP (SBP) | −0.65 (−1.21 to −0.08) | <0.05 | 65 | Significant SBP reduction (mainly in MetS) |
| Diastolic BP (DBP) | −0.40 (−0.79 to −0.01) | <0.05 | 63 | Mild DBP reduction; modest heterogeneity | |
| Glycemic Control | Fasting Blood Sugar (FBS) | −0.25 (−0.48 to −0.03) | <0.05 | 55 | Small but significant reduction |
| Blood Glucose | −0.53 (−0.82 to −0.23) | <0.05 | 58 | Moderate improvement | |
| HbA1c | −0.33 (−0.58 to −0.09) | <0.05 | 66 | Significant improvement in long-term control | |
| Insulin Function | HOMA-IR | −0.01 (−0.78 to 0.76) | >0.05 | 95 | No consistent effect; highly heterogeneous |
| HOMA-B | 0.09 (−0.19 to 0.36) | >0.05 | 0 | No effect on β-cell function | |
| QUICKI | 0.41 (−0.39 to 1.21) | >0.05 | 80+ | Non-significant; high variability | |
| Serum Insulin | −0.33 (−0.77 to 0.11) | >0.05 | 75 | No consistent reduction | |
| Lipid Profile | Total Cholesterol (TC) | −0.22 (−0.45 to 0.01) | >0.05 | 60+ | No consistent change |
| LDL-C | −0.36 (−0.64 to −0.07) | <0.05 | 56 | Modest reduction (not uniform) | |
| HDL-C | 0.40 (0.03 to 0.77) | <0.05 | 72 | Significant increase | |
| Triglycerides (TG) | −0.27 (−0.49 to −0.05) | <0.05 | 68 | Mild reduction, especially in NAFLD | |
| Inflammation | CRP | −0.39 (−0.64 to −0.14) | <0.05 | 40 | Consistent anti-inflammatory effect |
| TNF-α | −1.07 (−2.05 to −0.09) | <0.05 | 91 | Large but heterogeneous reduction | |
| Oxidative Stress | TAC | 0.68 (−0.30 to 1.67) | >0.05 | 85 | Inconsistent antioxidant improvement |
| GSH | 0.91 (−0.88 to 2.69) | >0.05 | 92 | Non-significant; sparse data | |
| MDA | −0.22 (−0.54 to 0.09) | >0.05 | 45 | No consistent effect |
| Outcome Category | Specific Outcomes | No. of Trials (Approx.) | Effect Direction | Certainty (GRADE) | Reasons for Downgrading |
|---|---|---|---|---|---|
| Glycemic control | Fasting blood sugar (FBS) | 18 | ↓ Significant reduction | Moderate | Moderate heterogeneity, some unclear RoB |
| HbA1c | 10 | ↓ Significant reduction | Moderate | Some inconsistency and small-trial effects | |
| Insulin resistance indices | HOMA-IR, HOMA-B, fasting insulin, QUICKI | 15 | ↔/↓ Inconsistent or null | Low–Very Low | Very high heterogeneity, imprecision, small samples |
| Lipid profile | Triglycerides (TG) | 16 | ↓ Significant reduction | Moderate | Moderate heterogeneity (dose/formulation) |
| Total cholesterol (TC) | 15 | ↓ Modest reduction | Low–Moderate | Heterogeneity and imprecision | |
| LDL-C | 14 | ↓ Inconsistent reduction | Low | Small-study bias, variable results | |
| HDL-C | 14 | ↑ Significant increase | Moderate | Moderate heterogeneity | |
| Anthropometric measures | BMI | 17 | ↓ Slight reduction | Low | Short intervention durations, small samples |
| Waist circumference | 14 | ↓ Moderate reduction | Moderate | Some heterogeneity, limited long-term data | |
| Waist-to-hip ratio | 7 | ↓ Minimal reduction | Low | Imprecision and limited sample size | |
| Inflammatory markers | C-reactive protein (CRP) | 18 | ↓ Significant reduction | Moderate | High heterogeneity, possible publication bias |
| TNF-α | 10 | ↓ Moderate reduction | Low | Fewer studies, wide CIs | |
| Oxidative stress markers | Total antioxidant capacity (TAC) | 7 | ↑ Moderate increase | Low | Inconsistent assays, small samples |
| Glutathione (GSH) | 4 | ↑ Inconsistent | Very Low | Scarce data, high heterogeneity | |
| Malondialdehyde (MDA) | 5 | ↓ Inconsistent | Low | Imprecision, limited data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kehinde, S.A.; Qaisrani, Z.N.; Pattanayaiying, R.; Lay, B.B.; Phyo, K.Y.; Lin, W.P.; San, M.M.; Awaeloh, N.; Aunsorn, S.; Kitkangplu, R.; et al. Clinical Potential of Curcuma longa Linn. as Nutraceutical/Dietary Supplement for Metabolic Syndrome: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2026, 15, 60. https://doi.org/10.3390/foods15010060
Kehinde SA, Qaisrani ZN, Pattanayaiying R, Lay BB, Phyo KY, Lin WP, San MM, Awaeloh N, Aunsorn S, Kitkangplu R, et al. Clinical Potential of Curcuma longa Linn. as Nutraceutical/Dietary Supplement for Metabolic Syndrome: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods. 2026; 15(1):60. https://doi.org/10.3390/foods15010060
Chicago/Turabian StyleKehinde, Samuel Abiodun, Zahid Naeem Qaisrani, Rinrada Pattanayaiying, Bo Bo Lay, Khin Yadanar Phyo, Wai Phyo Lin, Myat Mon San, Nurulhusna Awaeloh, Sasithon Aunsorn, Ran Kitkangplu, and et al. 2026. "Clinical Potential of Curcuma longa Linn. as Nutraceutical/Dietary Supplement for Metabolic Syndrome: Systematic Review and Meta-Analysis of Randomized Controlled Trials" Foods 15, no. 1: 60. https://doi.org/10.3390/foods15010060
APA StyleKehinde, S. A., Qaisrani, Z. N., Pattanayaiying, R., Lay, B. B., Phyo, K. Y., Lin, W. P., San, M. M., Awaeloh, N., Aunsorn, S., Kitkangplu, R., & Chusri, S. (2026). Clinical Potential of Curcuma longa Linn. as Nutraceutical/Dietary Supplement for Metabolic Syndrome: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods, 15(1), 60. https://doi.org/10.3390/foods15010060







