Effects of Transglutaminase and Heat Treatment on the Structure and Gelation Properties of Camel Casein Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Camel Casein Protein Samples
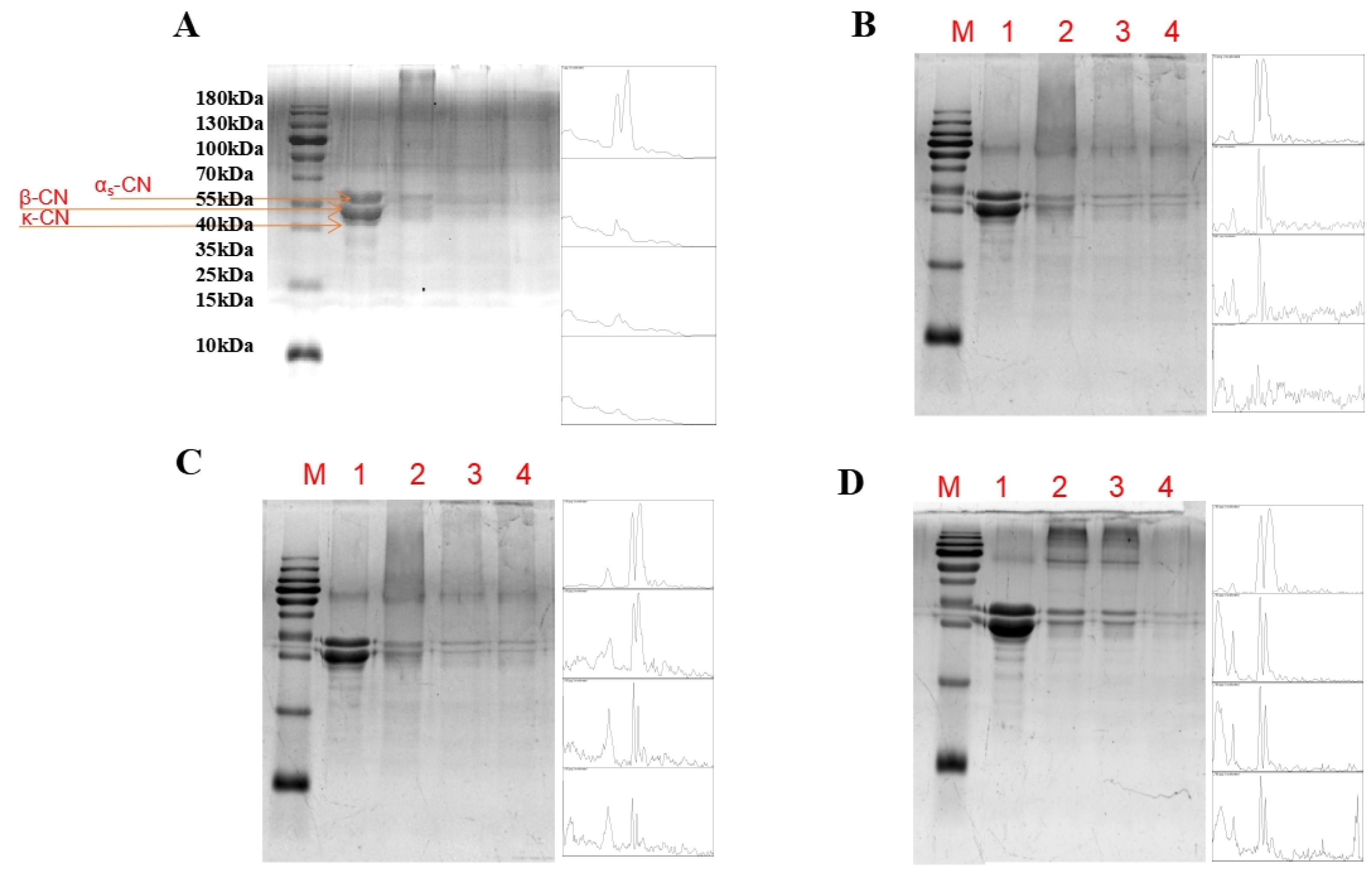
2.3. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS–PAGE)
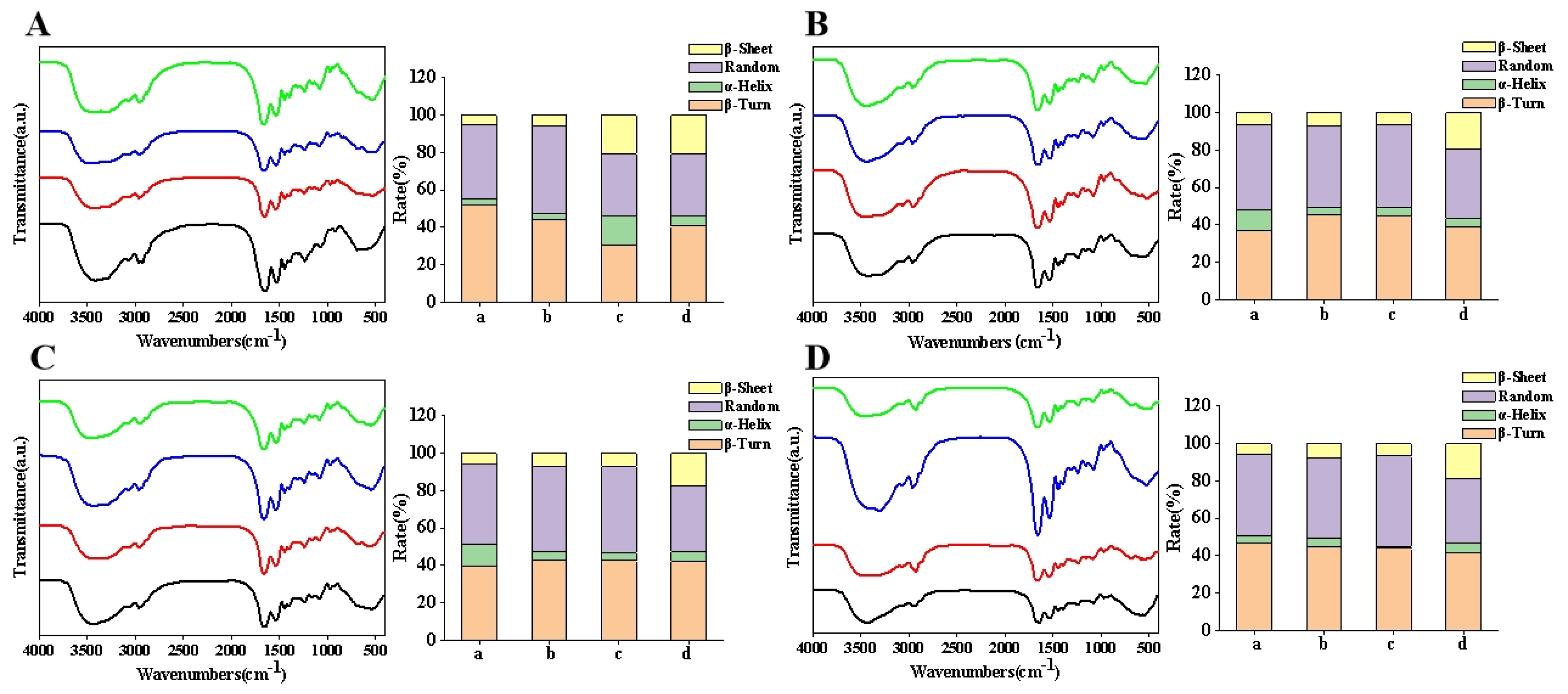
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
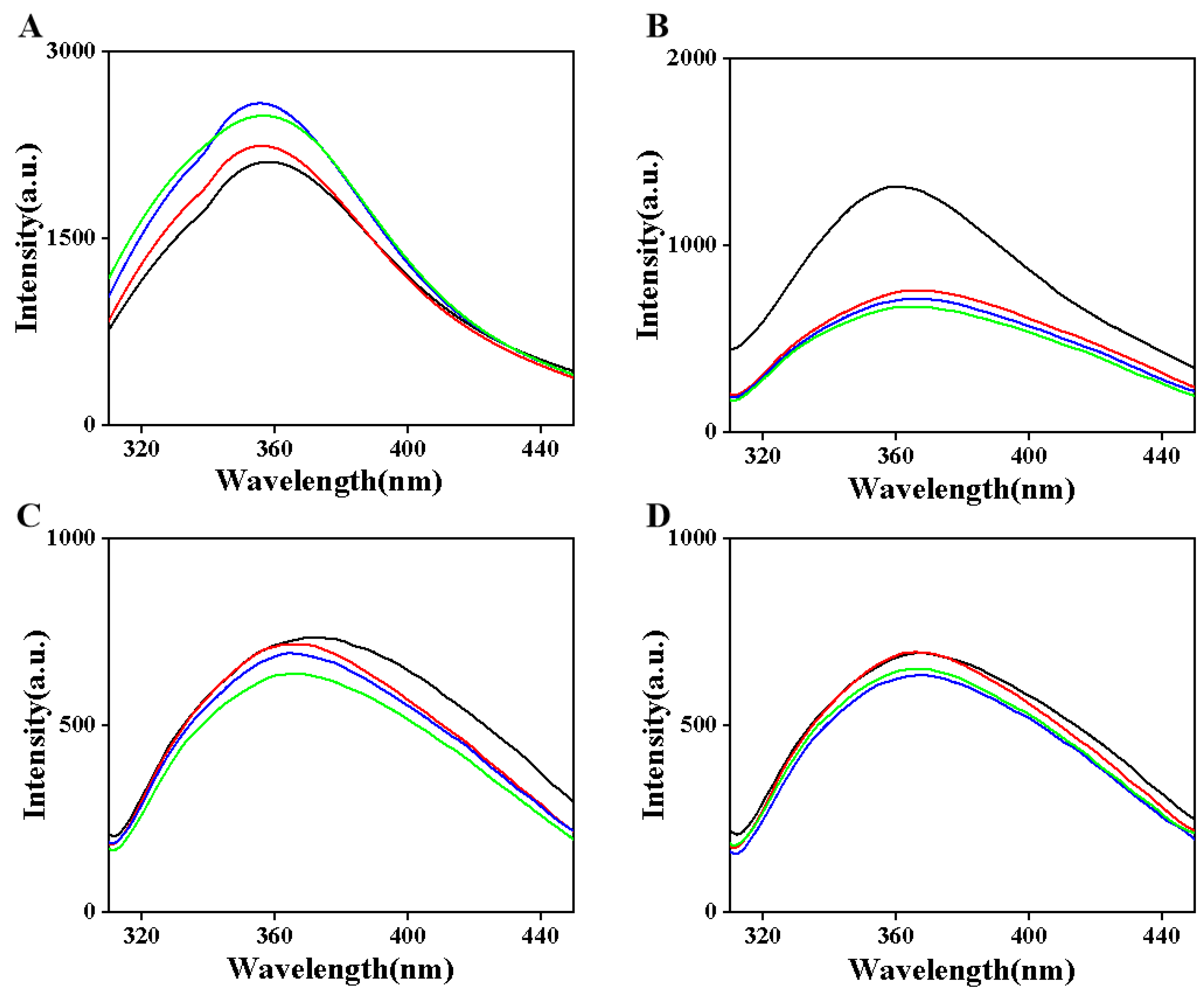
2.5. Intrinsic Fluorescence Spectroscopy
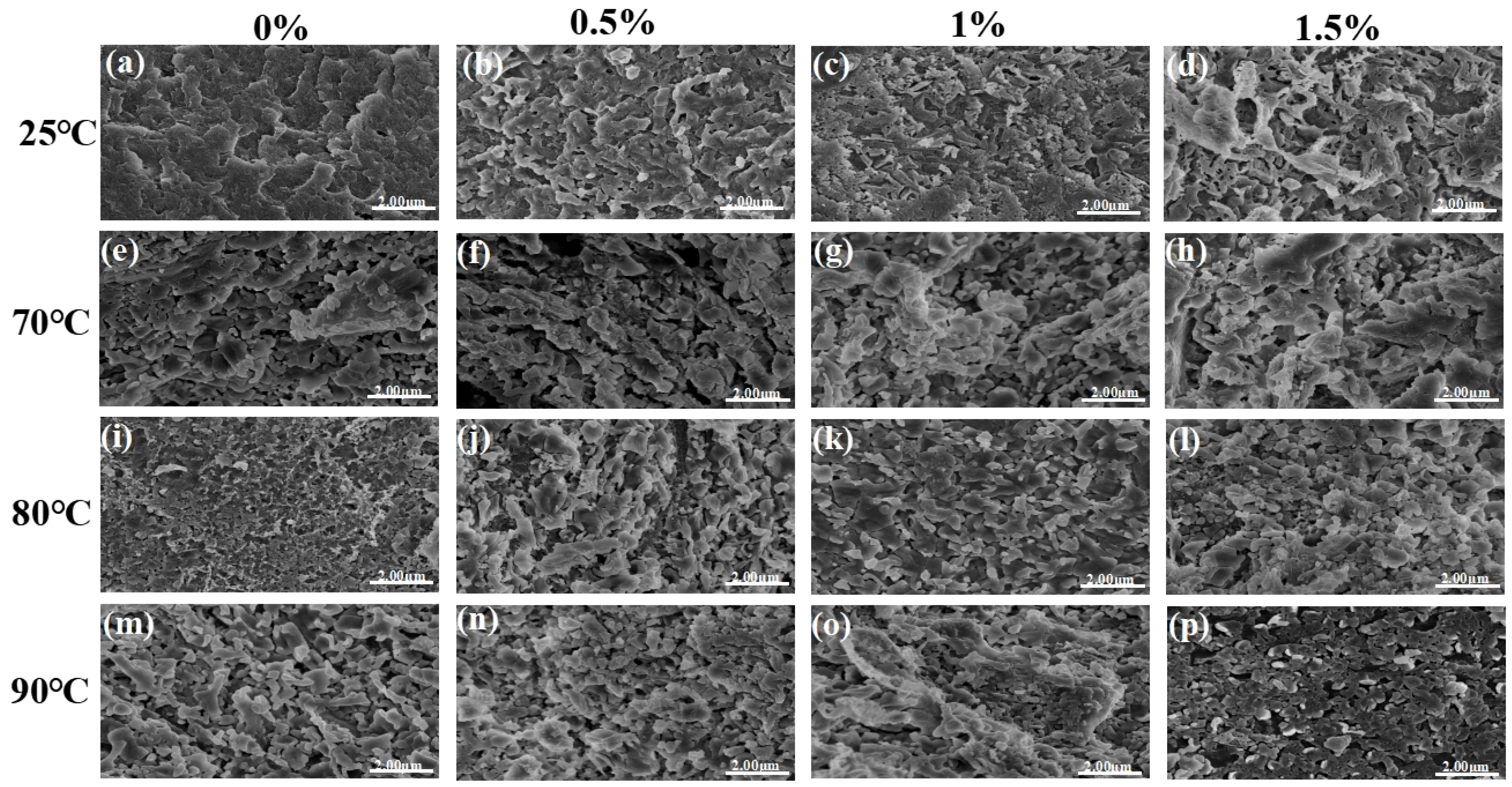
2.6. Observation of Protein Microstructure
2.7. Analysis of Thermodynamic Properties of Proteins
2.8. Surface Hydrophobicity Measurement (Ho)
2.9. Preparation of TGase Enzyme Crosslinked Gel
2.10. Gel Water Retention
2.11. Texture Characteristics
2.12. Dynamic Shear Rheological Properties
2.13. Statistical Analysis
3. Results and Discussion
3.1. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Endogenous Fluorescence Spectrum
3.4. Determination of Protein Microstructure
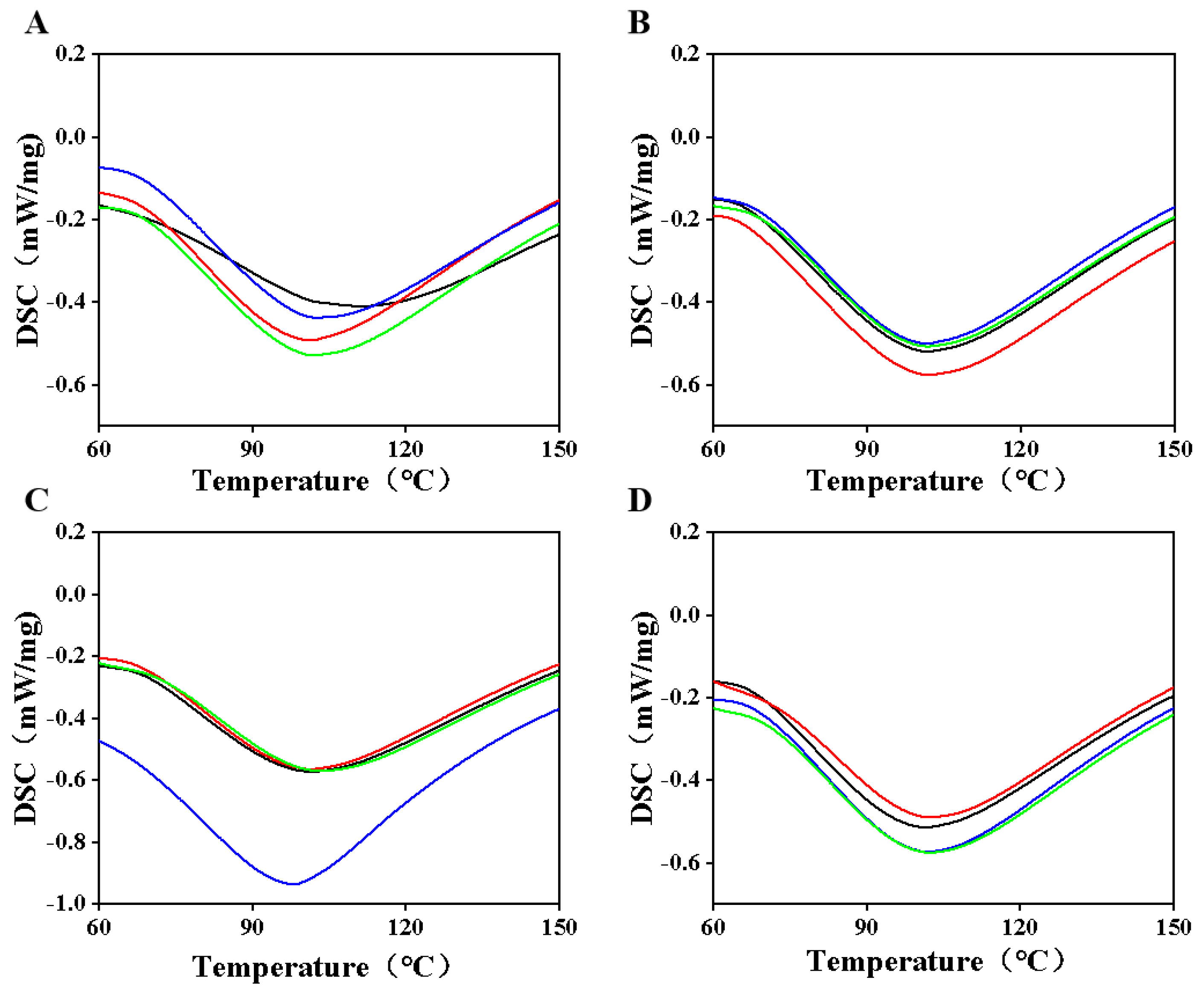
3.5. Analysis of Thermodynamic Properties of Casein Proteins
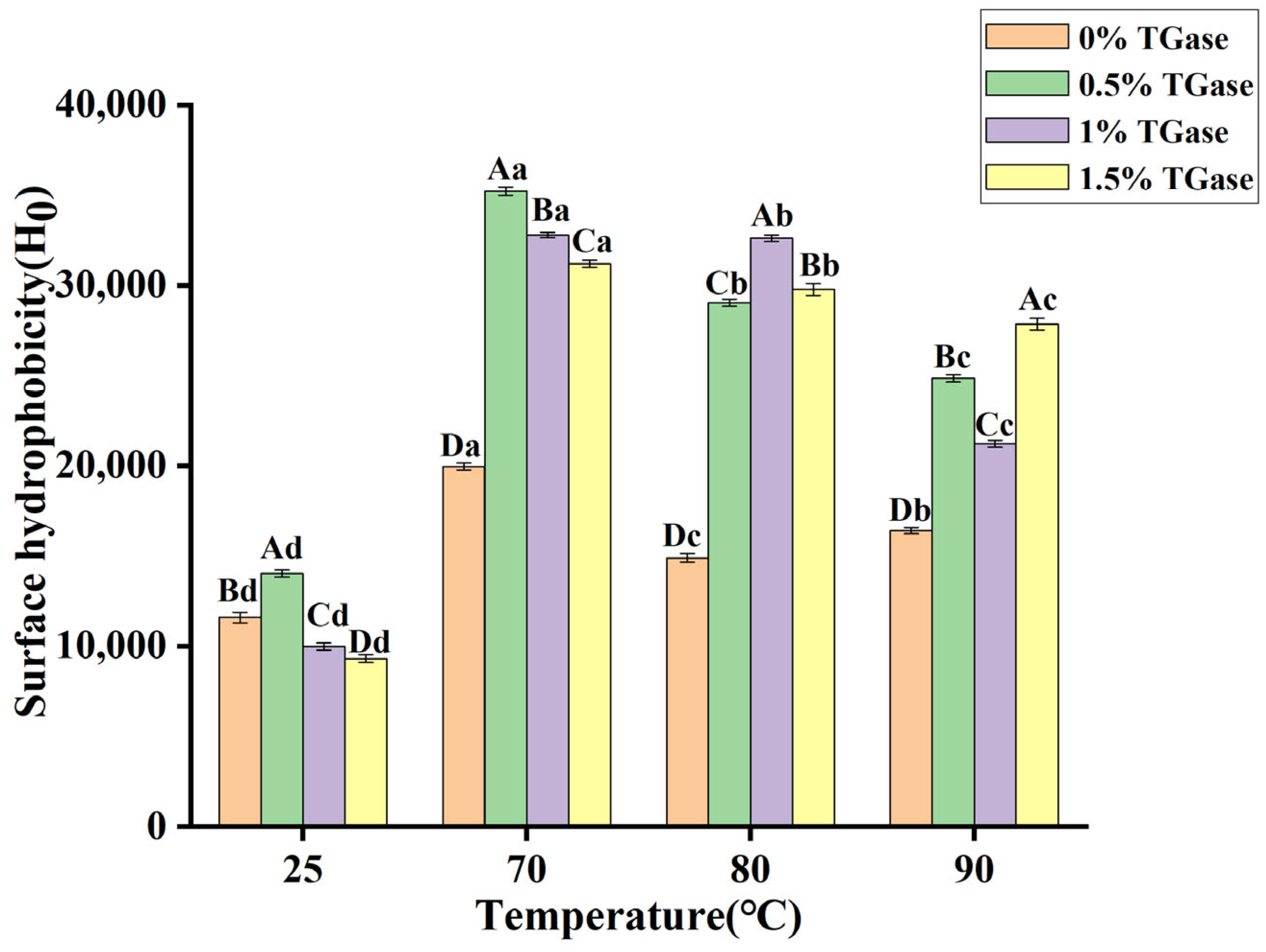
3.6. Surface Hydrophobicity (Ho)
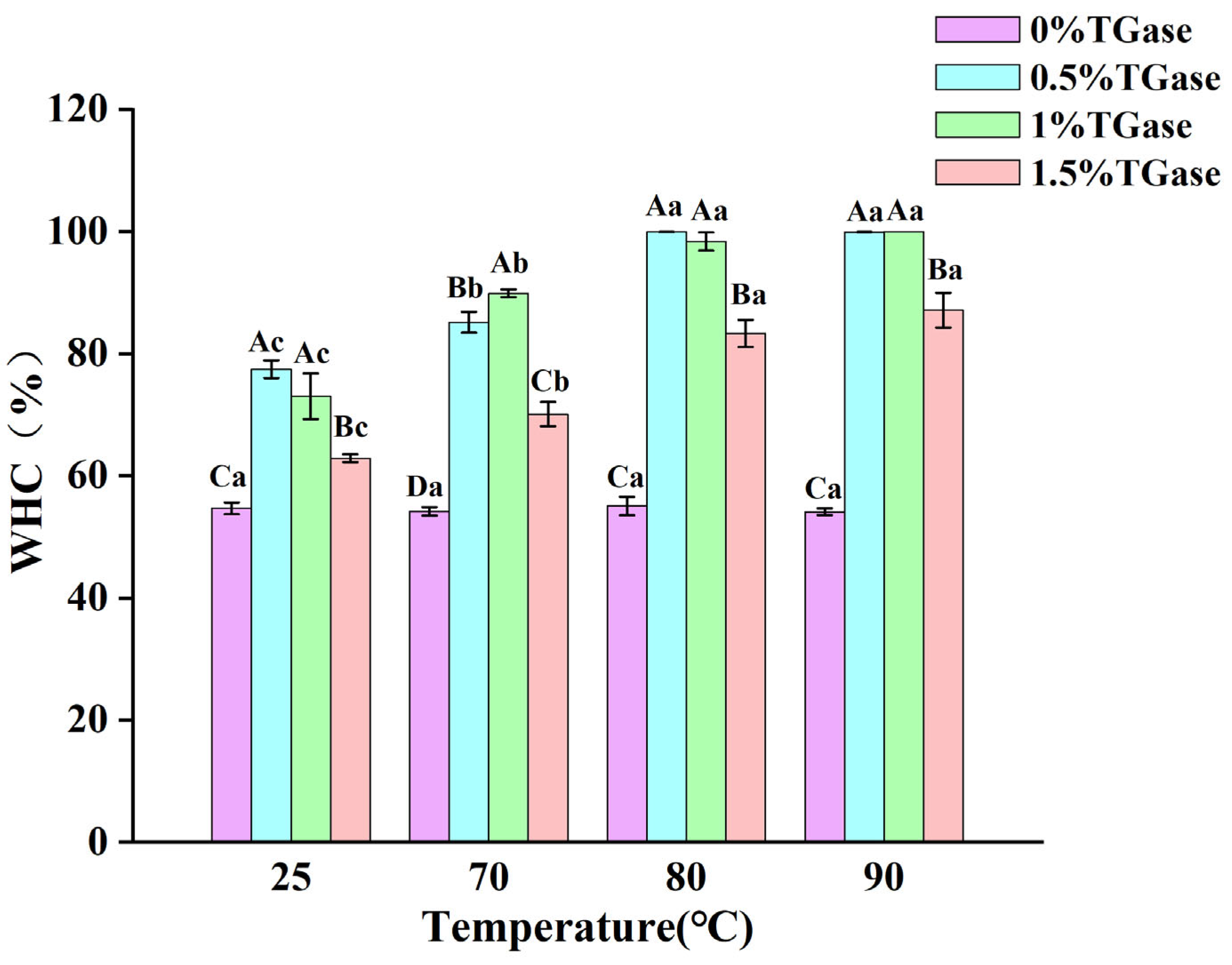
3.7. Gel Water Retention
3.8. Mechanical Properties
3.9. Rheological Properties
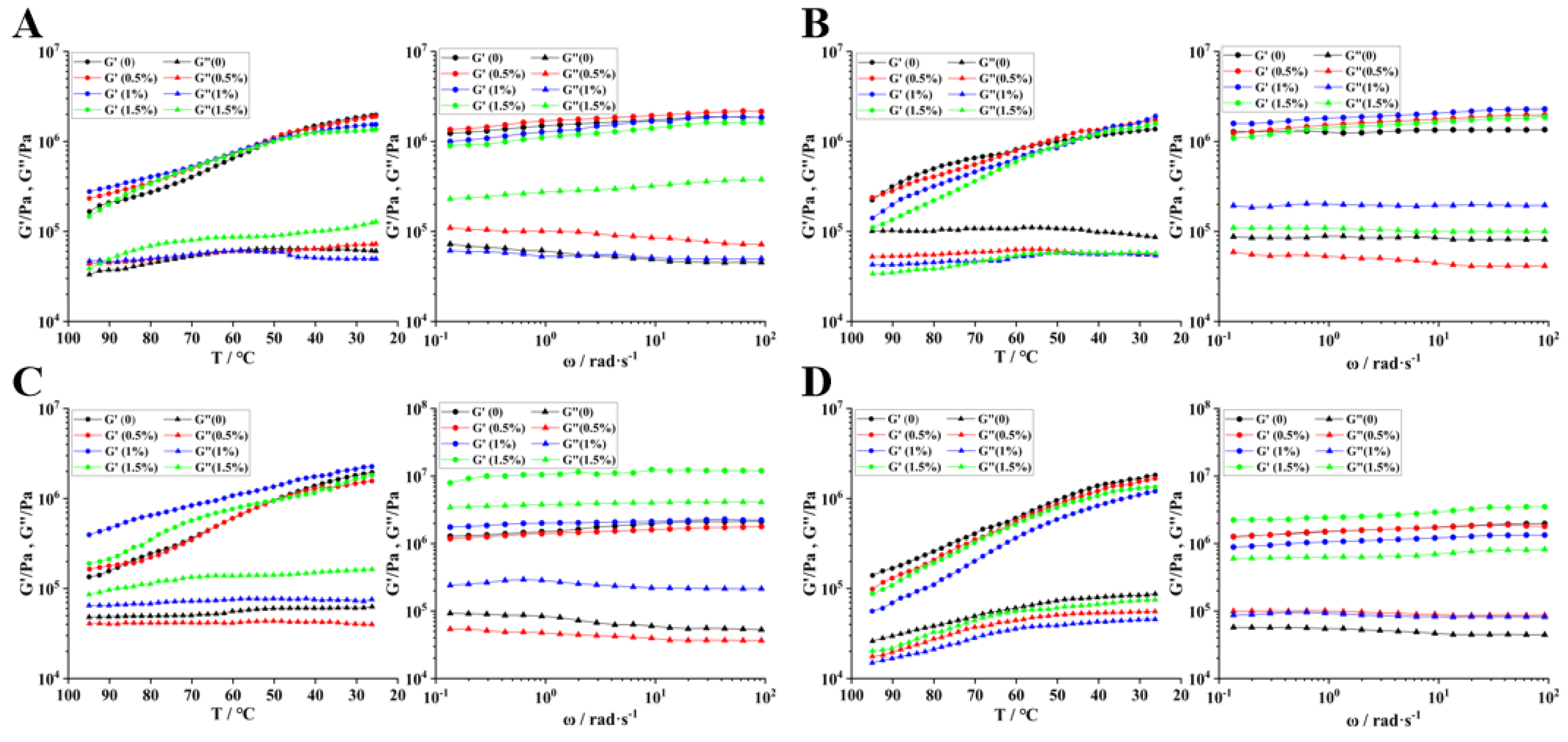
3.9.1. Dynamic Rheological Characteristics
3.9.2. Shear Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, K.; Chen, Q.; Wang, Y. Camel milk modulates the gut microbiota and has anti-inflammatory effects in a mouse model of colitis. J. Dairy Sci. 2022, 105, 3782–3793. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.H.; Isono, H.; Miyata, T. Potential antioxidant bioactive peptides from camel milk proteins. Anim. Nutr. 2018, 4, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Aqib, A.I.; Kulyar, M.F.-E.; Ashfaq, K.; Bhutta, Z.A.; Shoaib, M.; Ahmed, R. Camel milk insuline: Pathophysiological and molecular repository. Trends Food Sci. Technol. 2019, 88, 88497–88504. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Hou, Y.; Zhong, J.; Hettinga, K.; Zhou, P. Phosphoproteomics reveals that camel and goat milk improve glucose homeostasis in HDF/STZ-induced diabetic rats through activation of hepatic AMPK and GSK3-GYS axis. Food Res. Int. 2022, 157, 111254. [Google Scholar] [CrossRef]
- Mudgil, P.; Baby, B.; Ngoh, Y.-Y.; Kamal, H.; Vijayan, R.; Gan, C.-Y.; Maqsood, S. Molecular binding mechanism and identification of novel anti-hypertensive and anti-inflammatory bioactive peptides from camel milk protein hydrolysates. LWT 2019, 112, 108193. [Google Scholar] [CrossRef]
- Kane, J.; Jansen, M.; Hendrix, S.; Bosmans, L.A.; Beckers, L.; van Tiel, C.; Gijbels, M.; Zelcer, N.; de Vries, C.J.; von Hundelshausen, P.; et al. Anti-Galectin-2 Antibody Treatment Reduces Atherosclerotic Plaque Size and Alters Macrophage Polarity. Thromb. Haemost. J. Int. Soc. Thromb. Haemost. 2022, 122, 1047–1057. [Google Scholar] [CrossRef]
- Khan, M.Z.; Xiao, J.; Ma, Y.; Ma, J.; Liu, S.; Khan, A.; Khan, J.M.; Cao, Z. Research Development on Anti-Microbial and Antioxidant Properties of Camel Milk and Its Role as an Anti-Cancer and Anti-Hepatitis Agent. Antioxidants 2021, 10, 788. [Google Scholar] [CrossRef]
- Maqsood, S.; Al-Dowaila, A.; Mudgil, P.; Kamal, H.; Jobe, B.; Hassan, H.M. Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2019, 279, 328–338. [Google Scholar] [CrossRef]
- Zou, Z.; Duley, J.A.; Cowley, D.M.; Reed, S.; Arachchige, B.J.; Koorts, P.; Shaw, P.N.; Bansal, N. Digestibility of proteins in camel milk in comparison to bovine and human milk using an in vitro infant gastrointestinal digestion system. Food Chem. 2022, 374, 131704. [Google Scholar] [CrossRef]
- Zhao, D.; Bai, Y.; Niu, Y. Composition and characteristics of Chinese Bactrian camel milk. Small Rumin. Res. 2015, 127, 58–67. [Google Scholar] [CrossRef]
- Ho, T.M.; Zheng, Z.; Nidhi, B. Camel milk: A review of its nutritional value, heat stability, and potential food products. Food Res. Int. 2022, 153, 110870. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, C.; Yang, X.; Guo, Y. Acid-induced mixed methylcellulose and casein gels: Structures, physical properties and formation mechanism. Food Chem. 2022, 366, 130561. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Structure, gelation mechanism of plant proteins versus dairy proteins and evolving modification strategies. Trends Food Sci. Technol. 2024, 147, 104464. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Holt, C. Casein micelle structure, functions and interactions. In Advanced Dairy Chemistry—1 Proteins: Part A/Part B; Springer: Boston MA, USA, 2003; pp. 233–276. [Google Scholar]
- Mohamed, H.; Nagy, P.; Agbaba, J.; Kamal-Eldin, A. Use of near and mid infra-red spectroscopy for analysis of protein, fat, lactose and total solids in raw cow and camel milk. Food Chem. 2021, 334, 127436. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171, 36–52. [Google Scholar] [CrossRef]
- Katja, V.; Željko, K.; Maja, L. Immobilization of Transglutaminases and Transglutaminase as Immobilization Agent; Elsevier: Amsterdam, The Netherlands, 2024; pp. 293–332. [Google Scholar] [CrossRef]
- Mehdi, A.; Hadi, S.R.; Marek, K. Recent advances in microbial transglutaminase biosynthesis and its application in the food industry. Trends Food Sci. Technol. 2021, 110, 458–469. [Google Scholar] [CrossRef]
- Gao, Y.; Zhuang, D.; Ye, J.; Guo, Y.; Zhu, J. Modulation of sodium distribution and digestion properties in low salt myofibrillar protein gel incorporating γ-polyglutamic acid and transglutaminase: Conformation and microstructure. Food Hydrocoll. 2024, 153, 110007. [Google Scholar] [CrossRef]
- Santhi, D.; Kalaikannan, A.; Malairaj, P.; Prabhu, S.A. Application of microbial transglutaminase in meat foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2071–2076. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Chronakis, I.S. Crosslinking of milk proteins by microbial transglutaminase: Utilization in functional yogurt products. Food Chem. 2018, 245, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, Y.; Huang, Z.; Zhang, W.; Chen, J.; Mu, W. Cold-active enzymes in the dairy industry: Insight into cold adaption mechanisms and their applications. Trends Food Sci. Technol. 2022, 125, 126–135. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Xu, M.F.; Xiang, M.X.; Cheng, X.F. Research on the structural differences of the casein from milk by pasteurization and ultrahigh temperature sterilization. J. South China Agric. Univ. 2013, 34, 192–196. [Google Scholar]
- Yazdi, S.R.; Corredig, M. Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study. Food Chem. 2012, 132, 1143–1149. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, L.-Y.; Li, W.-T.; Chang, K.-L.; Tseng, H.-W.; Chen, B.-Y.; Chen, C.-J.; Hsieh, J.-F. Ultrasound-assisted transglutaminase catalysis of the cross-linking and microstructure of αs-casein, β-casein and κ-casein. Processes 2021, 9, 1630. [Google Scholar] [CrossRef]
- Bulca, S.; Umut, F.; Koc, A. The influence of microbial transglutaminase on camel milk yogurt. LWT 2022, 160, 113339. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Guan, Z.; Jia, Z.; Zhang, Z.; Yang, C.; Wang, J. Transglutaminase-crosslinked cold-set pea protein isolate gels modified by pH shifting: Properties, structure and formation mechanisms. Food Hydrocoll. 2024, 154, 110158. [Google Scholar] [CrossRef]
- Xue, H.Y.; Li, J.Y.; Zhao, X.H.; He, B.Y.; Yi, M.X.; Song, H.X. Analysis of thermal aggregation in goat milk based on protein structure and interaction. Food Ferment. Ind. 2023, 49, 175–181. [Google Scholar]
- Tang, C.; Yang, X.-Q.; Chen, Z.; Wu, H.; Peng, Z.-Y. Physicochemical and structural characteristics of sodium caseinate biopolymers induced by microbial transglutaminase. J. Food Biochem. 2005, 29, 402–421. [Google Scholar] [CrossRef]
- Shirley, B.A. (Ed.) Protein Stability and Folding: Theory and Practice; Humana Press: New York, NY, USA, 1995. [Google Scholar]
- Li, H.; Zhao, T.; Li, H.; Yu, J. Effect of heat treatment on the property, structure, and aggregation of skim milk proteins. Front. Nutr. 2021, 8, 714869. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shao, H.; Yan, Y.; Wu, Y.; Meng, X.; Yang, A.; Wu, Z.; Gao, J.; Li, X.; Chen, H. Changes in structural and functional properties of whey protein cross-linked by polyphenol oxidase. Food Res. Int. 2023, 164, 112377. [Google Scholar] [PubMed]
- Zhao, Y.; Wang, P.; Xu, Y.; Zeng, X.; Xu, X. A study on the mechanisms of nanoparticle-stabilized high internal phase emulsions constructed by cross-linking egg white protein isolate with different transglutaminase concentrations. Foods 2022, 11, 1765. [Google Scholar] [CrossRef]
- Kuraishi, C.; Yamazaki, K.; Susa, Y. Transglutaminase: Its utilization in the food industry. Food Rev. Int. 2001, 17, 221–246. [Google Scholar] [CrossRef]
- Jaros, D.; Partschefeld, C.; Henle, T.; Rohm, H. Transglutaminase in dairy products: Chemistry, physics, applications. J. Texture Stud. 2006, 37, 113–155. [Google Scholar] [CrossRef]
- Bassogog, C.B.B.; Nyobe, C.E.; Ngui, S.P.; Minka, S.R.; Mune, M.A.M. Effect of heat treatment on the structure, functional properties and composition of Moringa oleifera seed proteins. Food Chem. 2022, 384, 132546. [Google Scholar] [CrossRef]
- Wahl, L.A.; Beyer, L.; Hinrichs, J. Understanding the effect of aggregation temperature on molecular interactions in zein gels to tailor the matrix for plant-based cheese alternatives. Food Hydrocoll. 2025, 164, 111169. [Google Scholar] [CrossRef]
- Anema, G.S. Heat-induced changes in caseins and casein micelles, including interactions with denatured whey proteins. Int. Dairy J. 2021, 122, 105136. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Hu, N.; Zhao, Y.; Wu, Y.; Liu, J.; Zhao, Y. Effect of Heat Treatment Combined with TG Enzyme Cross-Linking on the Zein–Pea Protein Complex: Physicochemical and Gel Properties. Gels 2024, 10, 301. [Google Scholar] [CrossRef]
- Huda, M.; Mutamed, A.; Afaf, K. Effect of heat treatments on camel milk proteins—A review. Int. Dairy J. 2022, 133, 105404. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Sancho, A.I.; Nielsen, S.D.; Larsen, L.B.; Gao, Y.; Bøgh, K.L.; Hansen, E.B. Enzymatic hydrolysis and extensive heat treatment induce distinct modifications of cow’s and camel milk proteins of relevance for production of infant formula. LWT 2024, 191, 115591. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Han, J.; Yuan, D.; Lu, Z.; Zhang, L. Influence of transglutaminase-induced modification of milk protein concentrate (MPC) on yoghurt texture. Int. Dairy J. 2018, 78, 65–72. [Google Scholar] [CrossRef]
- Ji, Y.; Silva, M.; Lin, R.; Adhikari, B.; Chandrapala, J. Gelation and Thermal Stability of Camel Milk Protein and Soy protein Blends: A Review. Food Rev. Int. 2024, 40, 3816–3846. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Y.; Zhong, Y.; Wang, D.; Deng, Y. Casein-hempseed protein complex via cross-link catalyzed by transglutaminase for improving structural, rheological, emulsifying and gelation properties. Food Chem. 2022, 383, 132366. [Google Scholar] [CrossRef]
- Chen, Q.; Guan, J.; Wang, Z.; Wang, Y.; Wang, X.; Chen, Z. Improving the Gelation Properties of Pea Protein Isolates Using Psyllium Husk Powder: Insight into the Underlying Mechanism. Foods 2024, 13, 3413. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar]
- Truong, V.-D.; Clare, D.A.; Catignani, G.L.; Swaisgood, H.E. Cross-linking and rheological changes of whey proteins treated with microbial transglutaminase. J. Agric. Food Chem. 2004, 52, 1170–1176. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Salami, M.; Mohammadian, M. Effect of microbial transglutaminase on the mechanical properties and microstructure of acid-induced gels and emulsion gels produced from thermal denatured egg white proteins. Int. J. Biol. Macromol. 2020, 153, 523–532. [Google Scholar] [CrossRef]

| Temperature (°C) | Enzyme Addition (%) | Hardness (gf) | Elasticity (gf) | Chewability (gf) | Adhesion (gf) | Cohesion | Resilience |
|---|---|---|---|---|---|---|---|
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 7.63 ± 0.67 Ab | 0.40 ± 0.52 ABa | 2.31 ± 3.10 Ab | 5.19 ± 0.72 Bb | 0.68 ± 0.12 Bb | 0.59 ± 0.14 Ba | |
| 1 | 7.96 ± 0.61 Ac | 0.22 ± 0.06 Bc | 1.51 ± 0.44 Ab | 6.95 ± 0.31 Ac | 0.87 ± 0.03 Aa | 0.72 ± 0.08 ABa | |
| 1.5 | 4.71 ± 0.23 Bc | 0.93 ± 0.07 Aa | 3.09 ± 0.41 Ab | 3.32 ± 0.24 Cb | 0.71 ± 0.07 ABa | 0.84 ± 0.02 Aa | |
| 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 18.53 ± 2.77 Aa | 0.69 ± 0.04 Aa | 11.92 ± 1.87 Aa | 17.25 ± 1.69 Aa | 0.94 ± 0.05 Aa | 0.74 ± 0.05 Aa | |
| 1 | 12.67 ± 2.30 Bb | 0.57 ± 0.06 Bb | 6.51 ± 1.05 Bb | 11.46 ± 0.79 Bb | 0.92 ± 0.12 Aa | 0.82 ± 0.06 Aa | |
| 1.5 | 10.86 ± 0.51 Bb | 0.49 ± 0.03 Bb | 4.74 ± 0.84 Bab | 9.72 ± 1.23 Ba | 0.89 ± 0.10 Aa | 0.75 ± 0.11 Aa | |
| 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 21.10 ± 0.46 Aa | 0.68 ± 0.02 Aa | 11.87 ± 1.04 Aa | 17.50 ± 0.95 Aa | 0.83 ± 0.03 Aab | 0.76 ± 0.06 Aa | |
| 1 | 18.69 ± 0.67 Ba | 0.71 ± 0.01 Aa | 11.89 ± 0.65 Aa | 14.75 ± 2.75 ABa | 0.90 ± 0.04 Aab | 0.75 ± 0.06 Aa | |
| 1.5 | 12.56 ± 0.38 Ca | 0.53 ± 0.07 Bb | 5.91 ± 1.64 Ba | 11.07 ± 1.52 Ba | 0.88 ± 0.15 Aa | 0.71 ± 0.09 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Q.; Liu, E.; Huo, C.; Zhang, F.; He, R.; Yang, J.; Zhao, Z. Effects of Transglutaminase and Heat Treatment on the Structure and Gelation Properties of Camel Casein Protein. Foods 2025, 14, 1644. https://doi.org/10.3390/foods14091644
Niu Q, Liu E, Huo C, Zhang F, He R, Yang J, Zhao Z. Effects of Transglutaminase and Heat Treatment on the Structure and Gelation Properties of Camel Casein Protein. Foods. 2025; 14(9):1644. https://doi.org/10.3390/foods14091644
Chicago/Turabian StyleNiu, Qing, Enhui Liu, Chenkun Huo, Fei Zhang, Ruiqi He, Jie Yang, and Zhongkai Zhao. 2025. "Effects of Transglutaminase and Heat Treatment on the Structure and Gelation Properties of Camel Casein Protein" Foods 14, no. 9: 1644. https://doi.org/10.3390/foods14091644
APA StyleNiu, Q., Liu, E., Huo, C., Zhang, F., He, R., Yang, J., & Zhao, Z. (2025). Effects of Transglutaminase and Heat Treatment on the Structure and Gelation Properties of Camel Casein Protein. Foods, 14(9), 1644. https://doi.org/10.3390/foods14091644





