Application of Isochoric Impregnation: Effects on Microbial and Physicochemical Parameters and Shelf Life of Strawberries Stored Under Refrigeration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Postharvest Isochoric Cold Storage
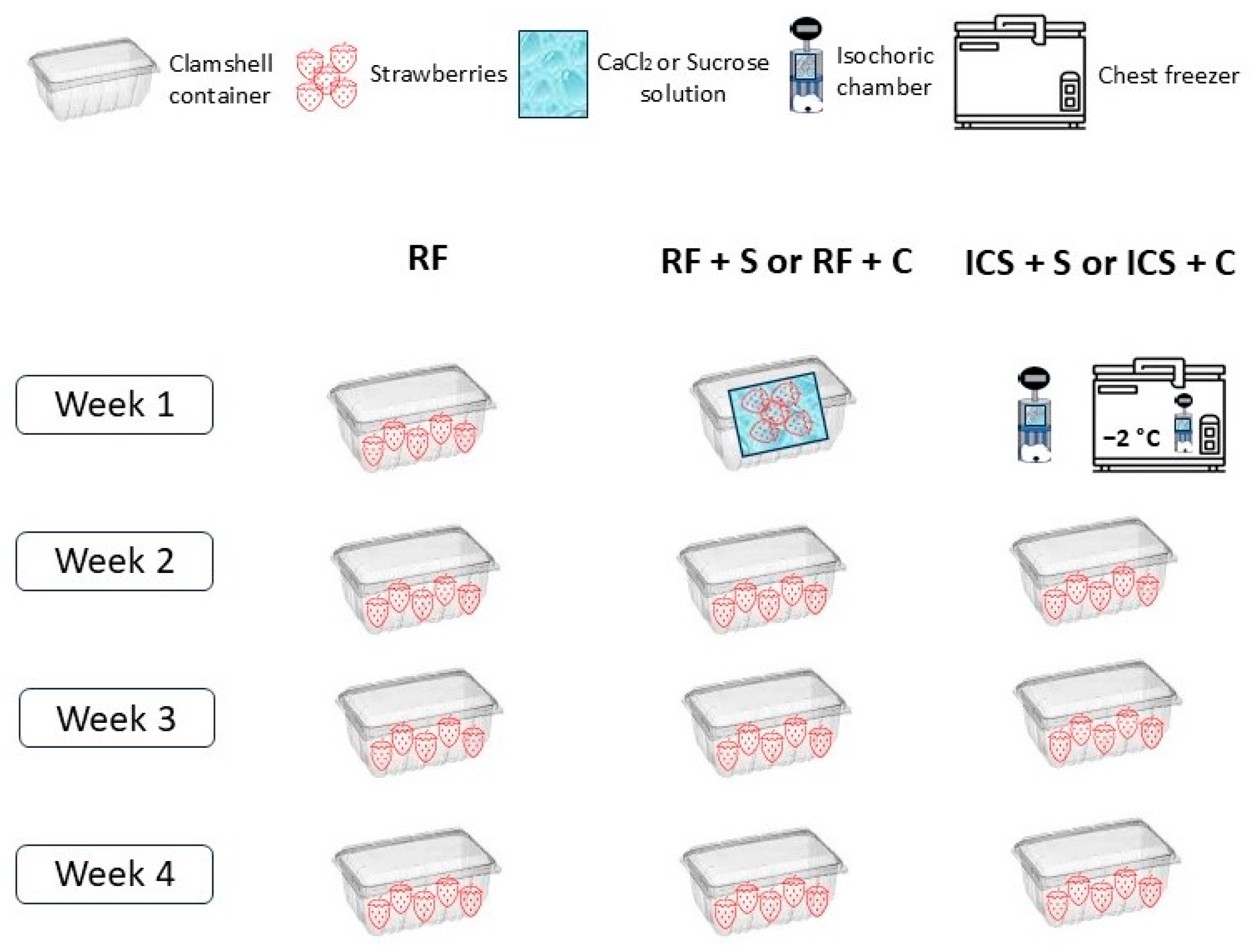
2.3. Experimental Protocol
2.4. Enumeration of Microbial Populations
2.5. Measurement of Physicochemical Parameters
2.5.1. Fungal Decay
2.5.2. Weight Change
2.5.3. Moisture Content, pH, Titratable Acidity (TA), and Total Soluble Solids (TSSs)
2.5.4. Surface Color
2.5.5. Mechanical Properties
2.6. Total Anthocyanins Content
2.7. Ascorbic Acid Determination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Evaluation
3.2. Visible Fungal Growth
3.3. Weight Changes
3.4. Effects of Preservation Method on Moisture Content, pH, Titratable Acidity (TA), and Total Soluble Solids (TSSs)
3.5. Color and Appearance
3.6. Texture
3.7. Total Anthocyanin Content
3.8. Ascorbic Acid Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Muñoz, P.; Almenar, E.; Valle, V.D.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Panou, A.A.; Karabagias, I.K.; Riganakos, K.A. Effect of Gamma-Irradiation on Sensory Characteristics, Physicochemical Parameters, and Shelf Life of Strawberries Stored under Refrigeration. Int. J. Fruit Sci. 2020, 20, 191–206. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT—Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Huma Qureshi, Q.; Waseem, A.; Rafia, A.; Nabila, C.-B.; Abdul, Q.; Asad, A. Post-Harvest Problems of Strawberry and Their Solutions. In Recent Studies on Strawberries; Nesibe Ebru, K., Ed.; IntechOpen: Rijeka, Croatia, 2023; Chapter 13. [Google Scholar]
- Ahmed, L.; Martin-Diana, A.B.; Rico, D.; Barry-Ryan, C. The impact of delactosed whey permeate treatment on shelf-life and antioxidant contents of strawberries. Int. J. Food Sci. Technol. 2012, 47, 1430–1438. [Google Scholar] [CrossRef]
- Ikegaya, A.; Ohba, S.; Nakajima, T.; Toyoizumi, T.; Ito, S.; Arai, E. Practical long-term storage of strawberries in refrigerated containers at ice temperature. Food Sci. Nutr. 2020, 8, 5138–5148. [Google Scholar] [CrossRef]
- Bulut, M.; Bayer, Ö.; Kırtıl, E.; Bayındırlı, A. Effect of freezing rate and storage on the texture and quality parameters of strawberry and green bean frozen in home type freezer. Int. J. Refrig. 2018, 88, 360–369. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, G.; Peng, J.; Salokhe, V. Effects of modified atmosphere package on preservation of strawberries. Int. Agrophysics 2003, 17, 143–148. [Google Scholar]
- Kahramanoğlu, İ. Effects of lemongrass oil application and modified atmosphere packaging on the postharvest life and quality of strawberry fruits. Sci. Hortic. 2019, 256, 108527. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Majid, A.; Shah, A.; Hussain, M. Impact of Low Doses of Gamma Irradiation on Shelf life and Chemical Quality of Strawberry (Fragaria × ananassa) CV. ‘Corona’. J. Anim. Plant Sci. 2014, 24, 1531–1536. [Google Scholar]
- de Jesus Filho, M.; Scolforo, C.Z.; Saraiva, S.H.; Pinheiro, C.J.G.; Silva, P.I.; Della Lucia, S.M. Physicochemical, microbiological and sensory acceptance alterations of strawberries caused by gamma radiation and storage time. Sci. Hortic. 2018, 238, 187–194. [Google Scholar] [CrossRef]
- Vischetti, C.; Feliziani, E.; Landi, L.; De Bernardi, A.; Marini, E.; Romanazzi, G. Effectiveness of Four Synthetic Fungicides in the Control of Post-Harvest Gray Mold of Strawberry and Analyses of Residues on Fruit. Agronomy 2024, 14, 65. [Google Scholar] [CrossRef]
- Moghadas, H.C.; Smith, J.S.; Tahergorabi, R. Recent Advances in the Application of Edible Coatings for Shelf-Life Extension of Strawberries: A Review. Food Bioprocess Technol. 2024, 18, 1079–1103. [Google Scholar] [CrossRef]
- Bridges, D.F.; Bilbao-Sainz, C.; Powell-Palm, M.J.; Williams, T.; Wood, D.; Sinrod, A.J.; Ukpai, G.; McHugh, T.H.; Rubinsky, B.; Wu, V.C. Viability of Listeria monocytogenes and Salmonella Typhimurium after isochoric freezing. J. Food Saf. 2020, 40, e12840. [Google Scholar] [CrossRef]
- Powell-Palm, M.J.; Rubinsky, B. A shift from the isobaric to the isochoric thermodynamic state can reduce energy consumption and augment temperature stability in frozen food storage. J. Food Eng. 2019, 251, 1–10. [Google Scholar] [CrossRef]
- Preciado, J.A. The Fundamentals of Isochoric Freezing and Its Role in the Cryopreservation of Biological Materials. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2007. [Google Scholar]
- Rubinsky, B.; Perez, P.A.; Carlson, M.E. The thermodynamic principles of isochoric cryopreservation. Cryobiology 2005, 50, 121–138. [Google Scholar] [CrossRef]
- Câmpean, Ș.I.; Beșchea, G.A.; Tăbăcaru, M.B.; Scutaru, L.M.; Dragomir, G.; Brezeanu, A.I.; Șerban, A.; Năstase, G. Preservation of black grapes by isochoric freezing. Heliyon 2023, 9, e17740. [Google Scholar] [CrossRef]
- McHugh, T.H.; Bilbao-Sainz, C.; Powell-Palm, M.J.; Rubinsky, B. Isochoric Impregnation of Solid Foods at Subfreezing Temperatures. U.S. Patent 17/692,784, 14 September 2023. [Google Scholar]
- Bilbao-Sainz, C.; Chiou, B.S.; Takeoka, G.; Williams, T.; Wood, D.; Powell-Palm, M.J.; Rubinsky, B.; McHugh, T. Novel isochoric impregnation to develop high-quality and nutritionally fortified plant materials (apples and sweet potatoes). J. Food Sci. 2022, 87, 4796–4807. [Google Scholar] [CrossRef]
- Biegańska-Marecik, R.; Czapski, J. The effect of selected compounds as inhibitors of enzymatic browning and softening of minimally processed apples. Acta Sci. Pol. Technol. Aliment. 2007, 6, 37–49. [Google Scholar]
- Bilbao-Sainz, C.; Sinrod, A.J.; Dao, L.; Takeoka, G.; Williams, T.; Wood, D.; Chiou, B.-S.; Bridges, D.F.; Wu, V.C.; Lyu, C. Preservation of grape tomato by isochoric freezing. Food Res. Int. 2021, 143, 110228. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Sinrod, A.; Powell-Palm, M.J.; Dao, L.; Takeoka, G.; Williams, T.; Wood, D.; Ukpai, G.; Aruda, J.; Bridges, D.F.; et al. Preservation of sweet cherry by isochoric (constant volume) freezing. Innov. Food Sci. Emerg. Technol. 2019, 52, 108–115. [Google Scholar] [CrossRef]
- Năstase, G.; Lyu, C.; Ukpai, G.; Şerban, A.; Rubinsky, B. Isochoric and isobaric freezing of fish muscle. Biochem. Biophys. Res. Commun. 2017, 485, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Molina, F.; Gómez, P.L.; Castro, M.A.; Alzamora, S.M. Storage quality of strawberry fruit treated by pulsed light: Fungal decay, water loss and mechanical properties. Innov. Food Sci. Emerg. Technol. 2016, 34, 267–274. [Google Scholar] [CrossRef]
- O’Grady, L.; Sigge, G.; Caleb, O.J.; Opara, U.L. Effects of storage temperature and duration on chemical properties, proximate composition and selected bioactive components of pomegranate (Punica granatum L.) arils. LWT—Food Sci. Technol. 2014, 57, 508–515. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 2011, 22, 801–804. [Google Scholar] [CrossRef]
- Acedo, J.; Varron, D.; Emnace, I.; Lauzon, R.; Aced, A., Jr. Antimicrobial effects of ascorbic acid and calcium lactate in freshcut jackfruit (Artocarpus heterophyllus Lam.). In Proceedings of the Southeast Asia Symposium on Quality Management in Postharvest Systems and Asia Pacific Symposium on Postharvest Quality 989, Bangkok, Thailand, 1 May 2013; pp. 199–208. [Google Scholar]
- Membré, J.-M.; Kubaczka, M.; Chéné, C. Combined Effects of pH and Sugar on Growth Rate of Zygosaccharomyces rouxii, a Bakery Product Spoilage Yeast. Appl. Environ. Microbiol. 1999, 65, 4921–4925. [Google Scholar] [CrossRef]
- Amal, S.A.; El-Mogy, M.; Aboul-Anean, H.; Alsanius, B. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Alsaiari, A.; Al-Qurashi, A.; Elsayed, M.; Abo-Elyousr, K. Postharvest application of Prohexadione-Ca and calcium chloride for improving storability and controlling mold disease of strawberry fruits. J. Phytopathol. Dis. Manag. 2024, 11, 1–11. [Google Scholar] [CrossRef]
- Vicente, A.R.; Martínez, G.A.; Civello, P.M.; Chaves, A.R. Quality of heat-treated strawberry fruit during refrigerated storage. Postharvest Biol. Technol. 2002, 25, 59–71. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Millé, A.; Chiou, B.-S.; Takeoka, G.; Rubinsky, B.; McHugh, T. Calcium impregnation during isochoric cold storage to improve postharvest preservation of fresh blueberries. Postharvest Biol. Technol. 2024, 211, 112841. [Google Scholar] [CrossRef]
- Maida, A.L.; Bilbao-Sainz, C.; Karman, A.; Takeoka, G.; Powell-Palm, M.J.; Rubinsky, B. Effects of Isochoric Freezing on the Quality Characteristics of Raw Bovine Milk. Foods 2023, 12, 4150. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Sainz, C.; Chiou, B.-S.; Takeoka, G.; Williams, T.; Wood, D.; Powell-Palm, M.J.; Rubinsky, B.; Wu, V.C.; McHugh, T. Isochoric freezing and isochoric supercooling as innovative postharvest technologies for pomegranate preservation. Postharvest Biol. Technol. 2022, 194, 112072. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Olsen, C.; Chiou, B.S.; Rubinsky, B.; Wu, V.C.; McHugh, T. Benefits of isochoric freezing for carrot juice preservation. J. Food Sci. 2024, 89, 1324–1336. [Google Scholar] [CrossRef]
- Atci, S.; McGraw, V.S.; Takeoka, G.; Vu, V.C.H.; McHugh, T.; Rubinsky, B.; Bilbao-Sainz, C. Assessing the impact of isochoric freezing as a preservation method on the quality attributes of orange juice. J. Food Sci. 2024, 89, 3167–3182. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Yang, H.; Lai, S.; Cheng, X.; Xin, Y.; Yang, B.; Hou, H.; Yao, Y.; Zhang, S.; et al. Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment. Food Chem. 2011, 126, 450–459. [Google Scholar] [CrossRef]
- Lara, I.; Garcıa, P.; Vendrell, M. Modifications in cell wall composition after cold storage of calcium-treated strawberry (Fragaria × ananassa Duch.) fruit. Postharvest Biol. Technol. 2004, 34, 331–339. [Google Scholar] [CrossRef]
- Jabbar, A.; East, A.R.; Jenkins, C. Quality Variability of Strawberries in the Southern North Island of New Zealand. In International Symposium Postharvest Pacifica 2009-Pathways to Quality: V International Symposium on Managing Quality in Chains + Australasian Postharvest Horticultural Conference; International Society for Horticultural Science: Leuven, Belgium, 2010; pp. 447–453. Available online: https://www.actahort.org/books/880/880_53.htm (accessed on 3 February 2025).
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Hashmi, M.S.; East, A.R.; Palmer, J.S.; Heyes, J.A. Pre-storage hypobaric treatments delay fungal decay of strawberries. Postharvest Biol. Technol. 2013, 77, 75–79. [Google Scholar] [CrossRef]
- Aday, M.S.; Temizkan, R.; Büyükcan, M.B.; Caner, C. An innovative technique for extending shelf life of strawberry: Ultrasound. LWT—Food Sci. Technol. 2013, 52, 93–101. [Google Scholar] [CrossRef]
- Bellary, A.N.; Rastogi, N.K. Ways and Means for the Infusion of Bioactive Constituents in Solid Foods. Crit. Rev. Food Sci. Nutr. 2016, 56, 1126–1145. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Buchovec, I. Impact of chlorophyllin-chitosan coating and visible light on the microbial contamination, shelf life, nutritional and visual quality of strawberries. Innov. Food Sci. Emerg. Technol. 2019, 52, 463–472. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Wagh, M.D.; Alam, M.S.; Aslam, R. Vacuum Impregnation of Ascorbic Acid and Calcium Lactate Improves Quality Attributes and Functionality of White Button Mushrooms. J. Food Process. Preserv. 2023, 2023, 6728630. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazad Bari, L.; Malekzadeh, S.; Amiri, S.; Mostashari, P.; Ahmadi Gheshlagh, P. Effect of Aloe vera gel-based active coating incorporated with catechin nanoemulsion and calcium chloride on postharvest quality of fresh strawberry fruit. J. Food Process. Preserv. 2022, 46, e15960. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Physical and chemical quality characteristics of strawberries after storage are reduced by a short delay to cooling. Postharvest Biol. Technol. 1995, 6, 17–28. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.B.; Sargent, S.A. Possible Influences of Water Loss and Polyphenol Oxidase Activity on Anthocyanin Content and Discoloration in Fresh Ripe Strawberry (cv. Oso Grande) During Storage at 1 °C. J. Food Sci. 2005, 70, S79–S84. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Zhang, J.; Zhang, Y.; Wang, Y.; Chen, Q.; Luo, Y.; Zhang, Y.; Li, M.; Wang, X.; et al. Identification of Anthocyanins-Related Glutathione S-Transferase (GST) Genes in the Genome of Cultivated Strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2020, 21, 8708. [Google Scholar] [CrossRef]
- Chandra, D.; Lee, J.-S.; Hong, Y.P.; Park, M.-H.; Choi, A.J.; Kim, J.G. Short-term application of CO2 gas: Effects on physicochemical, microbial, and sensory qualities of “Charlotte” strawberry during storage. J. Food Saf. 2019, 39, e12597. [Google Scholar] [CrossRef]
- Gliemmo, M.F.; Latorre, M.E.; Gerschenson, L.N.; Campos, C.A. Color stability of pumpkin (Cucurbita moschata, Duchesne ex Poiret) puree during storage at room temperature: Effect of pH, potassium sorbate, ascorbic acid and packaging material. LWT—Food Sci. Technol. 2009, 42, 196–201. [Google Scholar] [CrossRef]
- Tappi, S.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Rizzi, F.; Rocculi, P. Study on the quality and stability of minimally processed apples impregnated with green tea polyphenols during storage. Innov. Food Sci. Emerg. Technol. 2017, 39, 148–155. [Google Scholar] [CrossRef]
- McGraw, V.S.; Atci, S.; Powell-Palm, M.J.; Rubinsky, B.; Bilbao-Sainz, C. Isochoric Impregnation of Calcium to Extend Postharvest Shelf Life of Blueberries. ACS Food Sci. Technol. 2024, 4, 3007–3015. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Huang, X.; Zhang, L.; Zhao, P.; Ma, H.; Li, X.; Ban, Z.; Liu, X. Label-free quantitative proteomics to investigate strawberry fruit proteome changes under controlled atmosphere and low temperature storage. J. Proteom. 2015, 120, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G.; Sabovics, M.; Kruma, Z.; Kampuse, S.; Straumite, E. Effect of chitosan and chitooligosaccharide on vitamin C and polyphenols contents in cherries and strawberries during refrigerated storage. Eur. Food Res. Technol. 2011, 233, 351–358. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Inanoglu, S.; Barbosa-Cánovas, G.V.; Tang, Z.; Liu, F.; Sablani, S.S.; Zhu, M.-J.; Tang, J. Qualities of High Pressure and Microwave-Assisted Thermally Pasteurized Ready-to-Eat Green Beans During Refrigerated Storage at 2 and 7 °C. Food Bioprocess Technol. 2022, 15, 105–119. [Google Scholar] [CrossRef]
- Ragaert, P.; Devlieghere, F.; Debevere, J. Role of microbiological and physiological spoilage mechanisms during storage of minimally processed vegetables. Postharvest Biol. Technol. 2007, 44, 185–194. [Google Scholar] [CrossRef]
- Nguyen, V.T.B.; Nguyen, D.H.H.; Nguyen, H.V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria × ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Chakraborty, S.; Baier, D.; Knorr, D.; Mishra, H.N. High pressure inactivation of polygalacturonase, pectinmethylesterase and polyphenoloxidase in strawberry puree mixed with sugar. Food Bioprod. Process. 2015, 95, 281–291. [Google Scholar] [CrossRef]
- Chong, J.X.; Lai, S.; Yang, H. Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control 2015, 53, 195–205. [Google Scholar] [CrossRef]
- Liu, Q.; Cedric Tan, C.S.; Yang, H.; Wang, S. Treatment with low-concentration acidic electrolysed water combined with mild heat to sanitise fresh organic broccoli (Brassica oleracea). LWT—Food Sci. Technol. 2017, 79, 594–600. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Q.; Ng, L.Y.; Wang, S. Effects of Vacuum Impregnation with Calcium Lactate and Pectin Methylesterase on Quality Attributes and Chelate-Soluble Pectin Morphology of Fresh-Cut Papayas. Food Bioprocess Technol. 2017, 10, 901–913. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Sogvar, O.B. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. LWT—Food Sci. Technol. 2016, 66, 165–171. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Xu, Y.; Li, L.; Aghdam, M.S.; Luo, Z. Effect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem. 2019, 289, 112–120. [Google Scholar] [CrossRef]
- Turmanidze, T.; Gulua, L.; Jgenti, M.; Wicker, L. Effect of Calcium Chloride Treatments on Quality Characteristics of Blackberry, Raspberry and Strawberry Fruits After Cold Storage. Turk. J. Agric.—Food Sci. Technol. 2016, 4, 1127–1133. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M. Anthocyanin Color Behavior and Stability during Storage: Effect of Intermolecular Copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V. Transpiration and water stress, Postharvest physiology and pathology of vegetables. In Postharvest Physiology and Pathology of Vegetables; CRC Press: Boca Raton, FL, USA, 2002; pp. 143–197. [Google Scholar]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of Nanochitosan-Based Coating With and Without Copper Loaded on Physicochemical and Bioactive Components of Fresh Strawberry Fruit (Fragaria × ananassa Duchesne) During Storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of Drying Methods with the Application of Vacuum Microwaves on the Bioactive Compounds, Color, and Antioxidant Activity of Strawberry Fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Kowalska, J.; Kowalska, H.; Marzec, A.; Brzeziński, T.; Samborska, K.; Lenart, A. Dried strawberries as a high nutritional value fruit snack. Food Sci. Biotechnol. 2018, 27, 799–807. [Google Scholar] [CrossRef]
- Guiamba, I.; Ahrné, L.; Khan, M.A.M.; Svanberg, U. Retention of β-carotene and vitamin C in dried mango osmotically pretreated with osmotic solutions containing calcium or ascorbic acid. Food Bioprod. Process. 2016, 98, 320–326. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Ruoyi, K.; Zhifang, Y.; Zhaoxin, L. Effect of coating and intermittent warming on enzymes, soluble pectin substances and ascorbic acid of Prunus persica (Cv. Zhonghuashoutao) during refrigerated storage. Food Res. Int. 2005, 38, 331–336. [Google Scholar] [CrossRef]
- Oviedo, V.R.S.; Cabral, M.; Garay, C.R.E.; Arredondo, G.A.d.J.C. Postharvest quality of strawberry (Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier) genotypes according to vernalization. Acta Agronómica 2018, 67, 208–214. [Google Scholar] [CrossRef]
- Eroğul, D.; Gundogdu, M.; Sen, F.; Tas, A. Impact of postharvest calcium chloride treatments on decay rate and physicochemical quality properties in strawberry fruit. BMC Plant Biol. 2024, 24, 1088. [Google Scholar] [CrossRef]





| Microbial Population | Fresh | Shelf Life (Weeks) | RF | RF + S | RF + C | ICS + S | ICS + C |
|---|---|---|---|---|---|---|---|
| Total plate count | 1 | 4.2 ± 0.3 efg | 5.4 ± 0.7 cde | 4.2 ± 0.7 efg | <1 h * | <1 h | |
| (TPC) | 3.9 ± 0.3 g | 2 | 4.0 ± 1.1 fg | 6.6 ± 0.4 abc | 6.6 ± 1.4 abc | <1 h | <1 h |
| (log CFU/g) | 3 | 5.2 ± 0.2 def | 7.6 ± 0.3 a | 7.3 ± 0.5 ab | <1 h | <1 h | |
| 4 | 4.8 ± 1.2 ef | 7.3 ± 0.3 ab | 6.3 ± 0.5 bcd | <1 h | <1 h | ||
| Yeast and mold | 1 | 6.1 ± 0.6 bcd | 5.6 ± 0.4 cd | 4.9 ± 0.3 de | <1 g | <1 g | |
| (Y&M) | 3.9 ± 0.3 e | 2 | 4.7 ± 0.5 de | 6.8 ± 0.3 abc | 6.7 ± 1.4 abc | <1 g | <1 g |
| (log CFU/g) | 3 | 5.6 ± 0.2 cd | 7.8 ± 0.3 a | 7.2 ± 0.5 ab | <1 g | <1 g | |
| 4 | 4.8 ± 0.3 de | 7.2 ± 0.3 ab | 7.0 ± 0.5 ab | 1.8 ± 0.9 f | 2.1 ± 2.0 f |
| Parameters | Fresh | Shelf Life (Weeks) | RF | RF + S | RF + C | ICS + S | ICS + C |
|---|---|---|---|---|---|---|---|
| 1 | 85.8 ± 2.6 abcd | 91.5 ± 0.7 a | 89.7 ± 1.1 a | 90.2 ± 2.7 a | 88.6 ± 0.8 ab | ||
| Moisture (%) | 89.7 ± 0.7 a | 2 | 85.0 ± 1.1 abcde | 86.1 ± 0.9 abc | 85.0 ± 0.3 abcde | 86.1 ± 0.6 abc | 85.8 ± 0.7 abcd |
| 3 | 67.6 ± 4.6 f | 78.0 ± 1.6 e | 79.8 ± 2.7 cde | 79.3 ± 6.3 cde | 82.1 ± 0.4 bcde | ||
| 4 | 58.7 ± 6.4 g | 61.6 ± 9.2 fg | 63.1 ± 5.5 fg | 79.0 ± 2.1 de | 68.1 ± 1.5 f | ||
| 1 | 3.2 ± 0.0 cdef | 3.5 ± 0.0 ab | 3.2 ± 0.0 def | 3.3 ± 0.0 cde | 3.0 ± 0.1 gh | ||
| pH | 3.4 ± 0.0 abc | 2 | 3.3 ± 0.0 bcd | 3.5 ± 0.1 a | 3.1 ± 0.1 ef | 3.4 ± 0.1 abcd | 2.9 ± 0.0 ghi |
| 3 | 3.3 ± 0.1 bcd | 3.4 ± 0.0 abc | 2.8 ± 0.1 ij | 3.0 ± 0.1 fg | 2.8 ± 0.0 hi | ||
| 4 | 3.2 ± 0.1 def | - | - | 3.2 ± 0.2 cdef | 2.6 ± 0.3 j | ||
| TA (%) | 1.8 ± 0.2 ghi | 1 | 2.1 ± 0.4 efg | 1.3 ± 0.1 i | 1.4 ± 0.1 hi | 1.5 ± 0.1 hi | 1.5 ± 0.4 hi |
| 2 | 3.1 ± 0.2 cd | 2.0 ± 0.1 fgh | 2.2 ± 0.2 efg | 2.1 ± 0.1 fg | 2.2 ± 0.1 efg | ||
| 3 | 4.2 ± 0.4 a | 3.6 ± 0.3 bc | 4.0 ± 0.7 ab | 2.6 ± 0.1 ef | 2.7 ± 0.3 de | ||
| 4 | 4.5 ± 0.0 a | - | - | 3.5 ± 0.2 bc | 3.3 ± 0.8 c | ||
| TSS (%) | 8.8 ± 0.3 gh | 1 | 9.6 ± 0.6 gh | 7.5 ± 0.1 h | 8.6 ± 0.3 gh | 8.3 ± 0.3 h | 8.9 ± 0.1 gh |
| 2 | 12.9 ± 2.7 ef | 11.1 ± 0.6 fg | 13.6 ± 0.3 def | 12.5 ± 1.1 ef | 11.0 ± 1.6 fg | ||
| 3 | 17.1 ± 1.1 c | 15.9 ± 2.0 cd | 15.6 ± 0.9 cd | 14.7 ± 2.7 cd | 14.5 ± 1.0 de | ||
| 4 | 23.5 ± 2.0 b | - | - | 21.0 ± 1.9 b | 26.9 ± 0.4 a |
| Color Attribute | Fresh | Shelf Life (Weeks) | RF | RF + S | RF + C | ICS + S | ICS + C |
|---|---|---|---|---|---|---|---|
| 1 | 28.6 ± 3.4 b | 25.4 ± 2.2 b | 27.0 ± 2.1 b | 26.0 ± 1.3 b | 26.3 ± 1.3 b | ||
| L* | 28.5 ± 2.0 b | 2 | 28.3 ± 2.4 b | 28.6 ± 2.4 b | 31.0 ± 2.2 b | 27.0 ± 1.9 b | 25.8 ± 2.9 b |
| 3 | 29.4 ± 7.3 b | 32.8 ± 8.3 b | 25.5 ± 6.4 b | 25.7 ± 1.1 b | 27.0 ± 2.1 b | ||
| 4 | 32.0 ± 9.0 b | 55.5 ± 13.7 a | 61.8 ± 13.9 a | 27.5 ± 1.3 b | 26.7 ± 2.7 b | ||
| 1 | 14.7 ± 3.0 abc | 10.4 ± 2.9 cde | 14.6 ± 3.4 abc | 11.0 ± 1.0 cde | 11.5 ± 0.3 cde | ||
| a* | 15.2 ± 3.1 abc | 2 | 14.0 ± 3.1 abc | 14.7 ± 3.2 abc | 19.4 ± 2.8 ab | 11.5 ± 1.9 cde | 15.5 ± 3.2 abc |
| 3 | 11.9 ± 6.2 cd | 13.3 ± 6.8 abc | 19.5 ± 2.9 a | 11.8 ± 1.7 cde | 14.6 ± 3.4 abc | ||
| 4 | 5.9 ± 5.4 def | 1.3 ± 0.9 f | 5.0 ± 4.7 ef | 12.6 ± 2.6 bcd | 19.2 ± 5.3 ab | ||
| b* | 7.8 ± 2.1 abc | 1 | 6.8 ± 2.8 abcd | 3.8 ± 1.8 d | 5.8 ± 1.5 abcd | 3.7 ± 0.9 d | 5.0 ± 1.0 bcd |
| 2 | 10.4 ± 3.6 a | 6.8 ± 1.2 abcd | 8.8 ± 1.3 abc | 4.9 ± 1.1 bcd | 6.1 ± 1.8 abcd | ||
| 3 | 10.5 ± 2.9 a | 7.7 ± 2.6 abcd | 9.8 ± 3.5 ab | 4.7 ± 1.2 cd | 5.8 ± 1.5 abcd | ||
| 4 | 8.8 ± 1.9 abc | 8.9 ± 1.6 abc | 10.0 ± 3.3 a | 6.0 ± 2.0 abcd | 9.3 ± 4.2 abc | ||
| Chroma (C*) | 17.2 ± 3.2 abc | 1 | 16.3 ± 3.6 abcd | 11.2 ± 2.9 cd | 15.7 ± 3.6 abcd | 11.6 ± 1.0 cd | 12.6 ± 0.5 cd |
| 2 | 17.7 ± 3.9 abc | 16.3 ± 3.3 abcd | 21.3 ± 2.9 ab | 12.5 ± 2.0 cd | 16.7 ± 3.6 abc | ||
| 3 | 16.4 ± 5.2 abc | 15.6 ± 6.9 abcd | 21.9 ± 3.9 a | 12.7 ± 1.9 cd | 15.7 ± 3.6 abcd | ||
| 4 | 11.2 ± 4.2 cd | 9.1 ± 1.5 d | 11.8 ± 4.1 cd | 14.0 ± 3.2 bcd | 21.3 ± 6.7 ab | ||
| Hue (h°) | 27.2 ± 6.8 def | 1 | 24.4 ± 6.8 def | 20.3 ± 9.5 ef | 21.6 ± 2.3 ef | 18.6 ± 4.1 f | 23.3 ± 4.4 def |
| 2 | 36.1 ± 9.1 cd | 24.4 ± 4.8 def | 24.7 ± 2.8 def | 23.2 ± 4.3 def | 21.2 ± 2.5 ef | ||
| 3 | 40.9 ± 12.5 c | 33.2 ± 12.6 cde | 26.0 ± 4.6 def | 21.4 ± 3.5 ef | 21.6 ± 2.3 ef | ||
| 4 | 55.8 ± 16.6 b | 79.8 ± 6.8 a | 60.5 ± 17.9 b | 25.3 ± 3.0 def | 25.2 ± 2.7 def |
| Hardness | Fresh | Shelf Life (Weeks) | RF | RF + S | RF + C | ICS + S | ICS + C |
|---|---|---|---|---|---|---|---|
| 1 | 237 ± 101 bcd | 123 ± 35 d | 185 ± 48 cd | 163 ± 30 d | 243 ± 81 bcd | ||
| 194 ± 95 bcd | 2 | 142 ± 76 d | 150 ± 44 d | 177 ± 47 d | 160 ± 59 d | 218 ± 99 bcd | |
| 3 | 295 ± 126 bcd | 202 ± 133 bcd | 209 ± 111 bcd | 267 ± 42 bcd | 185 ± 48 cd | ||
| 4 | 703 ± 589 ab | 273 ± 142 bcd | 416 ± 179 abc | 232 ± 41 bcd | 567 ± 194 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atci, S.; Bilbao-Sainz, C.; McGraw, V.S.; Li, J.; Takeoka, G.; McHugh, T.; Rubinsky, B. Application of Isochoric Impregnation: Effects on Microbial and Physicochemical Parameters and Shelf Life of Strawberries Stored Under Refrigeration. Foods 2025, 14, 540. https://doi.org/10.3390/foods14030540
Atci S, Bilbao-Sainz C, McGraw VS, Li J, Takeoka G, McHugh T, Rubinsky B. Application of Isochoric Impregnation: Effects on Microbial and Physicochemical Parameters and Shelf Life of Strawberries Stored Under Refrigeration. Foods. 2025; 14(3):540. https://doi.org/10.3390/foods14030540
Chicago/Turabian StyleAtci, Sumeyye, Cristina Bilbao-Sainz, Valerie S. McGraw, Jiayuan Li, Gary Takeoka, Tara McHugh, and Boris Rubinsky. 2025. "Application of Isochoric Impregnation: Effects on Microbial and Physicochemical Parameters and Shelf Life of Strawberries Stored Under Refrigeration" Foods 14, no. 3: 540. https://doi.org/10.3390/foods14030540
APA StyleAtci, S., Bilbao-Sainz, C., McGraw, V. S., Li, J., Takeoka, G., McHugh, T., & Rubinsky, B. (2025). Application of Isochoric Impregnation: Effects on Microbial and Physicochemical Parameters and Shelf Life of Strawberries Stored Under Refrigeration. Foods, 14(3), 540. https://doi.org/10.3390/foods14030540







