Abstract
Mechanically separated meat (MSM) is a product of animal origin obtained by applying mechanical force to remove residual meat from bones, a process that results in the loss or modification of muscle fiber structure. Its use is regulated in the European Union. Clearly distinguishing MSM from non-MSM is essential not only to maintain high product standards but also to ensure full compliance with food labeling regulations and to meet consumer expectations for transparency. This study aimed to evaluate a standardized histological protocol for differentiating high-pressure MSM, low-pressure MSM, and non-MSM pork meat using light microscopy, combined with image analysis and statistical evaluation. In total 40 MSM samples (20 high-pressure, 20 low-pressure) and 20 non-MSM samples were analyzed. The histological protocol achieved 100% sensitivity and specificity in distinguishing MSM from non-MSM. Image analysis revealed significant differences between high- and low-pressure MSM in calcium aggregate parameters. The method provides a reliable, cost-effective tool for MSM identification in routine food inspection. However, because the study was conducted exclusively on pork samples, the results should be interpreted within the context of this type of meat. Additional validation across other species and production systems is required to confirm the broader applicability of the method.
1. Introduction
Mechanically separated meat (MSM) is a product of animal origin obtained by removing meat from flesh-bearing bones or poultry carcasses using mechanical means. This process results in the loss or modification of muscle fiber structure and produces a paste-like product. Its use in the European Union must comply with Regulation (EC) No 853/2004 [1], which imposes strict labeling requirements due to differences in composition, microbiological quality, and nutritional value compared to conventionally deboned meat. As specified in Annex I, MSM must be clearly declared in the ingredients list, indicating the species of origin, and products containing MSM are required to provide mandatory information for the final consumer, including a statement that they must be cooked before consumption [1].
Recent market data highlight the relevance of MSM in the European meat supply chain [2]. Although official statistics seldom specify MSM volumes, its use is widespread in processed meat products, particularly in low-cost formulations, due to its cost-effectiveness. Regulatory agencies, including the UK Food Standards Agency, have recently updated guidance on MSM labeling to improve compliance [3].
MSM production parameters, particularly the pressure applied during separation, can significantly influence muscle fiber integrity, bone content, and mineral composition [4]. These factors impact not only product classification but also shelf life and microbiological safety. EU-wide monitoring of Escherichia coli in MSM and meat preparations has been implemented to harmonize hygiene assessment [5]. The EFSA One Health 2023 Zoonoses Report highlighted MSM-related pathogen detections across member states, underscoring public health concerns [6]. High-pressure MSM has been associated with greater mechanical disruption of bone and cartilage, potentially influencing microbial load and physicochemical quality [7]. While MSM helps maximize carcass yield and reduce waste, there are concerns regarding non-compliance with labeling requirements, which may constitute a form of food fraud when MSM is used without proper declaration [8]. Traditional detection methods often rely on calcium quantification; however, this approach can be insufficient due to variability in raw materials and processing [9]. The EFSA BIOHAZ Panel has therefore recommended the development of alternative or complementary methods for MSM identification [10]. For this reason, histology combined with Von Kossa staining was selected, as it offers high specificity for visualizing calcium deposits and outperforms stains such as Masson’s trichrome, Alcian Blue, or Alizarin Red in detecting mineralized particles. Recent technological advances offer promising solutions. Non-thermal treatments such as high-pressure processing (HPP), ultrasound, ozone, organic acids, ultraviolet light, and cold plasma can extend MSM shelf life and reduce bacterial contamination [11]. Specifically, HPP is increasingly used in the meat industry, representing approx. 25–30% of the processing volume, and offers benefits for microbial inactivation, texture, and water-holding capacity [12,13]. Analytical advances include hyperspectral imaging coupled with deep learning models, capable of identifying tissue contaminants on pork products with high accuracy [14], and micro-computed tomography (micro-CT) imaging for detecting residual bone particles [15]. Nevertheless, these technologies often require specialized equipment and expertise, thus excluding their adoption for routine regulatory settings.
In this context, the paper aims to highlight how histological analysis, especially when combined with Von Kossa staining for calcium deposits and quantitative image analysis, represents a cost-effective, widely accessible tool for distinguishing MSM from non-MSM and for assessing processing conditions such as applied pressure. This approach aligns with EFSA recommendations [10] and offers practical advantages for routine food inspection and fraud prevention.
2. Materials and Methods
2.1. Sample Collection
A total of 40 reference MSM pork samples (20 high-pressure, 20 low-pressure) were collected from two authorized Italian production plants. Additionally, 20 fresh ground pork (non-MSM) samples served as negative controls.
2.2. Histological Preparation
Samples were fixed in 10% neutral buffered formalin, routinely processed and paraffin-embedded, sectioned (3 ± 2 μm), and stained with hematoxylin–eosin. Two histological slides were prepared from each sample (80 MSM slides, 40 control slides). Von Kossa staining was also performed on each sample to visualize calcium deposits, with a bovine tuberculous lymph node as positive control.
2.3. Image Analysis
Ten random microscopic fields from each Von Kossa-stained slide were analyzed at 10× magnification using NIS-Elements AR 4.50.00 software (Nikon Europe B.V., Amstelveen, The Netherlands). Parameters considered included the total area occupied by calcium, the number of fields containing aggregates, the total aggregate count, and the mean number of aggregates per field.
2.4. Statistical Analysis
Data were analyzed using Kruskal–Wallis tests to compare high- vs. low-pressure MSM. Sensitivity, specificity, likelihood ratios (LR+ and LR−), and ROC curves were calculated for each parameter. Inter-rater agreement between two blinded pathologists was assessed using Cohen’s kappa. Statistical analysis was performed in Stata/SE 14.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Histological Differentiation of MSM vs. Non-MSM
All 20 non-MSM samples lacked bone and/or cartilage presence within tissues. Calcium deposits (colored in brown) were present in all MSM samples but absent in non-MSM. All 80 MSM samples were correctly identified due to the presence of bone and/or cartilage inside muscle, connective, and/or adipose tissues. (Figure 1) With Von Kossa staining, all calcium deposits appear reddish/brownish in color, while the background is uniformly light pink, much paler than with traditional hematoxylin–eosin staining. Sensitivity and specificity for distinguishing MSM from non-MSM were both 100% (95% CI: 95–100%), with a Cohen’s kappa of 1.00 indicating perfect inter-rater agreement.
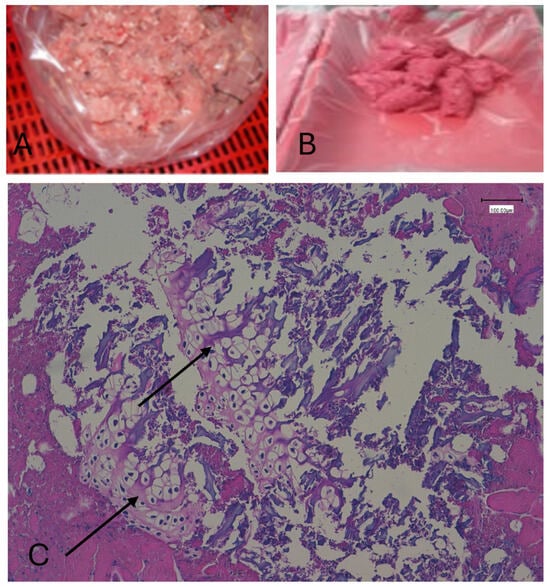
Figure 1.
Low-pressure MSM (A); high-pressure MSM (B); hematoxylin–eosin-stained MSM showing bone/cartilage fragments. The black arrow indicates bone/cartilage (C).
3.2. Image Analysis and Statistical Comparison
Statistical analysis of data obtained by image analysis (see Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8) revealed significant differences in calcium aggregate distribution, especially in high-pressure MSM samples. For instance, Table 2 and Table 3 demonstrate a significantly higher number of fields with aggregates in high-pressure MSM (p < 0.0001, ROC area = 1.000), while Table 5 and Table 7 highlight the diagnostic value of aggregate count and mean aggregates per field (ROC area = 0.9212 and 0.7288, respectively).

Table 1.
Total calcium-occupied area.

Table 2.
Number of fields containing aggregates.

Table 3.
Number of fields containing aggregates. Cutpoint data.

Table 4.
Number of fields containing aggregate: ROC area.

Table 5.
Number of aggregates per slide.

Table 6.
Number of aggregates per slide: ROC area.

Table 7.
Mean aggregates per field.

Table 8.
Mean aggregates per field: ROC area.
4. Discussion
The present study confirms that histological examination, supported by the calcium-specific Von Kossa staining, can distinguish MSM from conventionally deboned pork meat with perfect diagnostic accuracy. These findings are consistent with EFSA recommendations to develop novel microscopy-based approaches when traditional biochemical parameters, such as calcium quantification alone, are insufficient for regulatory purposes [10]. The method demonstrated high reproducibility, with perfect inter-rater agreement, and identified statistically significant differences in calcium aggregate distribution between high- and low-pressure MSM.
From a regulatory perspective, this approach is particularly remarkable in light of updated MSM guidance from the UK Food Standards Agency [3] and recent EU-wide monitoring efforts targeting E. coli in MSM and meat preparations [5]. The EFSA One Health 2023 Zoonoses Report [6] has highlighted the presence of MSM-related pathogens in the food chain, reinforcing the need for reliable, accessible, and cost-effective diagnostic tools to ensure compliance with Regulation (EC) No 853/2004 [1].
The differences observed between high- and low-pressure MSM likely reflect the greater mechanical disruption of bone and cartilage at higher pressures [7], which, while maximizing yield, may also cause increased bone tissue separation and can influence microbiological quality too. This aligns with literature that links the intensity of the process to increased microbial loads and potential quality deterioration in MSM [11]. However, the absence of a significant difference in the total area occupied by calcium between different pressure groups suggests that particle distribution metrics, rather than total mineral content, may offer better discriminatory power.
Compared with emerging technologies such as hyperspectral imaging coupled with deep learning [14], micro-computed tomography [15], and high-pressure processing (HPP) monitoring systems [12], histology offers the advantages of minimal equipment requirements, relatively short turnaround time, and compatibility with existing infrastructure in official control laboratories. While advanced methods provide additional molecular or three-dimensional structural information, their cost and technical complexity limit routine implementation in most inspection settings.
Finally, the method presented here also addresses food fraud prevention: undeclared MSM use in meat preparations not only constitutes a breach of labeling laws but may also mislead consumers regarding product quality [8]. By enabling both the detection of MSM and assessment of processing conditions, the proposed approach supports enforcement actions and consumer health protection.
5. Conclusions
This study validates a standardized histological protocol including Von Kossa staining, for the detection of MSM in pork meat, achieving 100% sensitivity and specificity. It also demonstrates the utility of quantitative image analysis for differentiating high-pressure from low-pressure MSM based on selected parameters. Although this approach requires validation in other species, the results obtained in pork meat highlight its robustness. The method aligns with EFSA and FSA recommendations for reliable and robust MSM detection [3,10], while offering a cost-effective and practical solution suitable for integration into routine official controls.
The approach holds exceptional value in contexts where advanced analytical technologies, such as LC–MS/MS proteomics, hyperspectral imaging, or micro-CT, are unavailable due to cost or technical constraints. By enabling rapid screening of meat products, it can help reduce the incidence of undeclared MSM, thereby supporting compliance with EU Regulation (EC) No 853/2004 and enhancing consumer trust.
Author Contributions
Conceptualization, M.P. and E.B. (Elena Bozzetta); methodology, M.P., E.B. (Elena Biasibetti), F.P., G.E. and N.G., software, R.P.; validation, C.M.; formal analysis, C.M.; investigation, M.P.; resources, E.B. (Elena Bozzetta); data curation, C.M.; writing—original draft preparation, M.P.; writing—review and editing, F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Ministry of Health grant number J19I24000810006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Commission. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- FEFAC. DG AGRI. Feed and Food Statistical Yearbook 2024. Available online: https://fefac.eu/wp-content/uploads/2025/04/FF_2024_FINAL.pdf (accessed on 12 August 2025).
- Food Standards Agency (FSA). New Guidance on Mechanically Separated Meat. Available online: https://euagenda.eu/news/885421 (accessed on 12 August 2025).
- Costa, R.M.; Oliveira, A.G.; de Souza, A.M.; Torres, K.G.; Pereira, G.S.; Bezerra, I.W.L. Prevalence of consumption of mechanically separated meat, consumer profile, nutrient intake and food choices among manufacturing workers in Northeastern Brazil. Sci. Rep. 2024, 14, 13289. [Google Scholar] [CrossRef] [PubMed]
- Langkabel, N.; Meemken, D.; Li, T.T.; Sotiraki, S.; Anastasiadou, S.; Nesbakken, T.; Langforth, S. Use of Harmonised Epidemiological Indicators for E. coli Monitoring in MSM and Meat Preparations. Food Control 2023, 150, 109687. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e09106. [Google Scholar] [CrossRef] [PubMed]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-pressure processing—Effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef] [PubMed]
- German Agricultural Society (DLG). Detection Challenges for Mechanically Separated Meat. DLG Expert Rep. 2022, 4, 1–6. [Google Scholar]
- Wieja, K.; Kiełczyński, P.; Szymański, P.; Szalewski, M.; Balcerzak, A.; Ptasznik, S. Identification and investigation of mechanically separated meat with an innovative ultrasonic method. Food Chem. 2021, 348, 128907. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel. Scientific Opinion on Public Health Hazards Related to MSM Detection. EFSA J. 2013, 11, 3137. [Google Scholar] [CrossRef]
- Al-Sharify, Z.T.; Al-Najjar, S.Z.; Anumudu, C.K.; Hart, A.; Miri, T.; Onyeaka, H. Non-Thermal Technologies in Food Processing: Implications for Food Quality and Rheology. Appl. Sci. 2025, 15, 3049. [Google Scholar] [CrossRef]
- Sun, Q.; Yuan, Y.; Xu, B.; Gao, S.; Zhai, X.; Xu, F.; Shi, J. Innovative Technologies Reshaping Meat Industrialization: Challenges and Opportunities in the Intelligent Era. Foods 2025, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- Janardhanan, R.; Olarte, C.; Sanz, S.; Rota, C.; Beriain, M.J. Combined Effect of High Hydrostatic Pressure, Sous-Vide Cooking, and Carvacrol on the Quality of Veal, Plant-Based, and Hybrid Patties during Storage. Foods 2023, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Ghimpeteanu, G.; Rajani, H.; Quintana, J.; Garcia, R. Hyperspectral Imaging with Vision Transformers for Contaminant Detection on Pork Belly. arXiv 2025, arXiv:2503.16086. [Google Scholar]
- Pospiech, M.; Zikmund, T.; Javůrková, Z.; Kaiser, J.; Tremlová, B. An Innovative Detection of Mechanically Separated Meat in Meat Products. Food Anal. Methods 2019, 12, 652–657. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).