Abstract
This study investigated the inhibitory effect of Lactobacillus reuteri with glycerol inoculated at different production stages of white-brined cheese (WBC) immersed in different concentrations of brine solutions under different storage temperatures as a bio-preservative against Listeria monocytogenes. Additionally, the physicochemical properties of WBC and brine solution were evaluated. A cocktail of five L. reuteri strains (~6 log CFU/g) with the addition of 100 mM glycerol was inoculated at either (1) water used to make the brine, (2) directly to the brine solution, or (3) pasteurized milk used to prepare cheese. The effect of L. reuteri against a cocktail of five L. monocytogenes strains (~5 log CFU/g) in WBC and stored in 10% or 15% brine at 4, 10, or 24 °C for 91 days was investigated. The salt content, pH, and water activity (aw) of WBC were also evaluated. L. reuteri inoculated in brine solution reduced the numbers of L. monocytogenes by 0.7–1.4 and 0.4–1.6 log CFU/g, in WBC and brines (10–15%), respectively, stored at different storage temperatures for 91 days compared to L. monocytogenes numbers in the absence of L. reuteri (control). When L. reuteri and glycerol were added to pasteurized milk during the production of WBC, the L. monocytogenes counts decreased by 1.2–2.9 and 1.4–2.5 log CFU/g in cheese and brines, respectively. However, the addition of L. reuteri and glycerol to water used in the preparation of brines reduced L. monocytogenes by 1.2–2.6 and 1.2–2.2 log CFU/g in cheese and brines, respectively. The highest inhibitory effect of L. reuteri was observed against L. monocytogenes in both cheese and brine with 10% NaCl and high temperatures (10–24 °C). The addition of L. reuteri with glycerol has the potential to reduce the risk of L. monocytogenes without negatively affecting the physicochemical characteristics of the cheese.
1. Introduction
White-brined cheese (WBC) is a common type of cheese in different Eastern Mediterranean countries and neighboring regions. WBC is a typically fresh unripened, rennet-coagulated cheese, with a soft to semi-hard texture [1]. It is considered a ready-to-eat (RTE) product that can be consumed fresh or after storage in a brine solution containing a high salt content (10–24% NaCl), most often kept refrigerated [2]. However, sometimes WBC is stored at room temperature [3], and produced seasonally from goat or ewe milk, or their combination, in un-mechanized conditions at various phases of production [4]. WBC could be contaminated at any stage of production with different foodborne pathogens, including Escherichia coli O157:H7, Salmonella spp., Staphylococcus aureus, and Listeria monocytogenes [5,6].
L. monocytogenes is a Gram-positive bacterium that is recognized as a ubiquitous pathogen and a causative agent of foodborne listeriosis, with a high mortality rate of nearly 20–30% [7], particularly in high-risk groups including pregnant women, infants, elderly, immunocompromised people, and, on rare occasions, people without any underlying conditions [8]. This pathogen is a cause of concern for different types of industries. In fact, Listeria spp. are widely spread in the environment [9] and may also colonize foods and food contact surfaces, such as polystyrene, stainless steel, and glass, at any point during food processing [10,11]. Listeria spp., mainly L. monocytogenes, have a remarkable tolerance to relatively extreme pH (can survive but not grow from 3.3 to 4.2), refrigerated temperatures (between 4 and 10 °C), and high salt concentrations (from 0.5 to 12%), and can form biofilms on surfaces waste water pipes, bends in pipes and rubber seals, as well as improperly sanitized equipment [12]. Consequently, these characteristics make L. monocytogenes a perilous hazard for a wide range of food products, including dairy products, particularly cheeses, which are highly perishable with a limited shelf life even after applying different preservative hurdles such as brine solution and refrigeration [13]. Since 1985, many listeriosis outbreaks linked to different types of cheese have been reported [14]. In addition, the presence of L. monocytogenes in cheeses causes a significant economic burden to the dairy industry in terms of product testing, product loss, and recalls [15], which requires particular attention to use different control strategies, including physical treatments such as high-pressure and irradiation, to enhance the microbial safety of cheese by controlling the growth and survival of foodborne pathogens, including L. monocytogenes, during processing and storage [16]. Although these techniques were considered effective in preventing the growth of foodborne pathogens in food, in the meantime, they also showed limitations in their application, such as the need for high capital investment or affecting the quality parameters of foods [12]. Therefore, there is a culminating interest in naturally occurring and safe antimicrobial strategies such as bio-preservatives in food processing and preservation, which have the potential to improve the overall quality and safety of food products [17].
The use of bacteriocins or bacteriocin-producing lactic acid bacteria (LAB) is a promising technique to enhance cheese safety by preventing the growth of foodborne pathogens, including L. monocytogenes [18]. Bacteriocins are generally recognized as safe (GRAS) for consumers, since they can be degraded by proteases in the gastrointestinal tract (GIT) [19]. These compounds can be incorporated in different forms, including direct addition to the food, immobilized form on the packaging, or inoculation of foods by bacteriocin-producing LAB [18]. Several studies have established that LAB promote microbial food safety based on various synergistic mechanisms. LAB synthesize a variety of natural antimicrobial compounds, e.g., organic acids (e.g., lactic, acetic, and propionic acids), that lower the environmental pH and compromise the structural integrity of microbial membranes. Bacteriocins such as nisin and plantaricins function by pore formation or inhibition of cell wall synthesis in target pathogenic bacteria. LAB also contend with the pathogens for the required nutrients and adhesion sites, thereby inhibiting their colonization and growth. There exist a few limitations, however, like strain dependency, variable efficacy on food matrix and storage conditions, and potential sensory effects. In addition, while bacteriocins have excellent potential as antimicrobials, their spectrum of activity may be narrow, and few compounds (e.g., nisin) are actually cleared for use in foods. These aspects require further research in order to optimize delivery systems and improve stability and effectiveness of LAB-based bio-preservation strategies [20,21,22].
Lactobacillus reuteri is an obligatory heterofermentative bacterium [23], often found in probiotic and fermented foods, including cheeses. It has an antimicrobial activity against molds, yeasts, parasites, and a wide range of pathogenic bacteria (including L. monocytogenes) due to the production of reuterin [2,24]. Reuterin is a water-soluble aldehyde with a high potential to be used as a food preservative due to its resistance to proteolytic and lipolytic enzymes and its ability to be active under a broad range of pH conditions [25]. Therefore, this study aimed to investigate the effect of L. reuteri as a bio-preservative combined with different salt concentrations on the inhibition of L. monocytogenes in WBC at different storage temperatures. Additionally, the effect of L. reuteri inoculation method (through pasteurized milk, distilled water, or brine solution) was investigated.
2. Materials and Methods
2.1. L. monocytogenes Strains and Culture Preparation
Five L. monocytogenes strains (Lis-2-138, Lis-2-243, GLM-1, GLM-3, and GLM-5) isolated from meat or dairy product plants, obtained from the culture collection of the Food Microbiology Laboratory at the Hashemite University, were used in this study. The activation of bacterial cultures was done by taking one loopful from the frozen cultures of each L. monocytogenes strain and streaking onto the surface of Tryptone Soy Agar (TSA, Oxoid, Basingstoke, UK) plates, incubating at 37 °C for 24 h, then a single colony from each L. monocytogenes strain was streaked on Listeria Selective Agar Base with Listeria Selective Supplement (LSA, Oxoid, Basingstoke, UK) and incubated aerobically at 37 °C for 48 h. Thereafter, a single colony of each L. monocytogenes strain was transferred into 10 mL of Brain Heart Infusion (BHI) broth (Oxoid Ltd., Basingstoke, UK) and incubated at 37 °C for 24 h. Thereafter, 0.1 mL of this culture was transferred to fresh BHI broth and incubated aerobically overnight at 37 °C. For experimental use, another final culture transfer was carried out in BHI broth.
2.2. L. reuteri Strains and Culture Preparation
Five L. reuteri strains (SS730, S3608, CF2, MM-2, and RC14) originally provided by the Department of Nutrition and Food Sciences, University of Manitoba, Canada, were used in this study. A loopful of each frozen culture was streaked onto MRS agar (Oxoid Ltd., Basingstoke, UK) and incubated at 37 °C for 24 h under anaerobic conditions using a CO2 incubator (Astec Co., Ltd., Fukui, Japan) with O2 less than 5% and 95% N2. A single colony of each L. reuteri strain was transferred into MRS broth for activation before experimental use. Then, L. reuteri strains were sub-cultured twice in MRS broth (Oxoid Ltd., Basingstoke, UK) anaerobically for 24 h at 37 °C.
2.3. Cocktail Preparation of L. monocytogenes and L. reuteri
To prepare the 10 mL bacterial cocktails, 2.0 mL of each strain of L. monocytogenes or L. reuteri was transferred into sterile empty tubes. The cocktails of L. monocytogenes or L. reuteri were separately mixed thoroughly and centrifuged at 3000× g for 18 min (Nüve, Istanbul, Turkey), and then the supernatants were discarded and 10 mL of 0.1% peptone water (Oxoid Ltd., Basingstoke, UK) was added to wash the pellets of each cocktail using a vortex mixer. The cultures were centrifuged and washed again, and the pellets of L. monocytogenes were finally collected in 10 mL of 0.1% peptone water to yield 8–9 log CFU/mL; the pellets of L. reuteri were collected in 1 mL of 0.1% peptone water to yield 9–10 log CFU/mL.
2.4. Cheese Production
The WBC production was prepared according to Al-Nabulsi et al. [2] at the dairy factory at the Jordan University of Science and Technology, Jordan. Each treatment was prepared using whole-fat raw bovine milk (15 L) obtained from a local supplier. The milk was pasteurized at 72 °C for 15 s and cooled to 36–37 °C. The pasteurized milk was screened for the presence of L. monocytogenes by taking 6 pasteurized milk samples plated on LSA (Oxoid, Basingstoke, UK) and incubated for 48 h at 37 °C. Afterward, the pasteurized milk was coagulated with diluted single-strength calf rennet (Dairy Connection, Inc., Madison, WI, USA) in sterile distilled water (1:10) and added to the milk for 30–40 min, to yield a milky texture that was cut with a knife to allow releasing whey. The curds were transferred in a sterilized cheesecloth to a perforated sterile steel mold (50 × 50 × 2 cm in length, width, and height, respectively) and pressed with a stainless-steel plate for 30 min. The cheese was manually cut with a sterile knife into 20–25 g pieces (5 × 5 × 2 cm in length, width, and height, respectively) that were immersed in either 10 or 15% (w/v) NaCl brine solutions in a ratio of 1:4 (cheese:brine).
The L. monocytogenes cocktail was inoculated into the brine solution to yield ~5.0 log CFU/g. The L. reuteri cocktail was inoculated with 100 mM glycerol (Panreac Química, Barcelona, Spain) into the WBC to yield ~6.0 log CFU/g at three different steps during the processing, including direct inoculation to the brine solution, to the distilled water used to prepare the brine solution and held for 2.5 h before adding the salt, or to the pasteurized milk that was held for 2.5 h before adding the rennet enzyme (Figure 1). Then, the cheese samples were stored at 4, 10, and 24 °C for 91 days (Table 1).
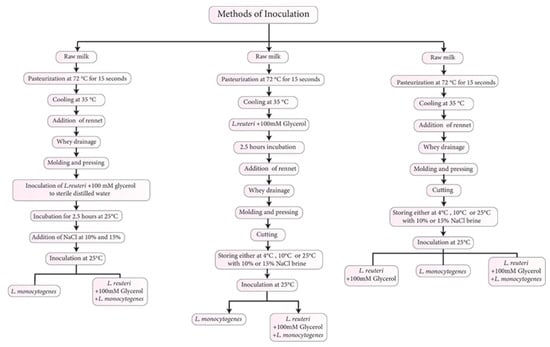
Figure 1.
Inoculation of L. reuteri and L. monocytogenes during different stages of white-brined cheese processing.

Table 1.
The experimental design used for survival studies of Listeria monocytogenes in white-brined cheese and brine solution.
2.5. Sampling and Microbiological Analysis
The viability of L. monocytogenes and L. reuteri in the cheese samples and brine solution was analyzed at specific intervals (0, 3, 7, 14, 21, 28, 49, 71, 91 days). A cross-sectioned 5 g piece of WBC sample was taken by using a sterile spoon, or 5 mL of brine was taken via micropipette under aseptic conditions and homogenized with 45 mL of 0.1% peptone water (Oxoid Ltd., Basingstoke, UK) by shaking for 2 min in a sterile stomacher bag using a stomacher (Easy Mix, AES Laboratories, Combourg, France).
A proper dilution of stomached cheese or brine samples was prepared by diluting the samples in 0.1% peptone water, and then 100 μL was plated on the surface of LSA (Oxoid, Basingstoke, UK) and incubated for 48 h at 37 °C for enumeration of L. monocytogenes, and Rogosa agar (Oxoid Ltd., Basingstoke, UK) for enumeration of L. reuteri, and incubated under anaerobic conditions in a CO2 incubator (ASTEC Ltd., Suita, Osaka, Japan) for 48 h at 37 °C.
2.6. Chemical Analysis
2.6.1. Water Activity (aw) Measurements
The aw of WBC at different day intervals was assessed using an aw meter (Novasina AG, Labmasters AW, Lachen, Switzerland) by analyzing ca. 2 g from a cross-section of a WBC sample at 21 °C for all of the day intervals at 4, 10, and 24 °C in 10% or 15% brine solutions.
2.6.2. Salt Determination
The NaCl concentration in WBC samples was determined by the AOAC method [26]. About 1.0–1.5 g of the cheese samples at different day intervals was taken and held in a muffle furnace (Barnstead Thermolyne, IA, USA) for 8 h at 550 °C. The ashed sample was mixed with 25 mL of distilled water. Then, the mixture was titrated using 0.05 N AgNO3 (Carlo Erba, Val de Reuil, France) after adding drops (0.5 mL) of 0.5 N potassium chromate (Alpha Chemika, Mumbai, India) as an indicator. The salt concentration was calculated by applying the following equation:
%Salt content = (titrated volume of AgNO3 mL × 0.00292) ÷ mass of sample (g) × 100.
2.6.3. pH Measurements
The pH values of the WBC and brine solution samples at the corresponding time intervals were determined using a pH meter (Adwa pH meter, AD 1000, Adwa, Nușfalău, Romania).
2.7. Statistical Analysis
Statistical analysis was carried out using the Statistical Package for Science (SPSS) software version 22.0 (2013, IBM Corp., Armonk, NY, USA). Each reported value was the mean of two experiments for each measurement, and the results were expressed as means ± SD. The data obtained were subject to analysis of variance (ANOVA) to test the effects of different treatment factors (absence or presence of L. monocytogenes and L. reuteri, brine concentration, and storage time and temperature), and Tukey’s HSD test was used to determine the statistical significance of the effects at a p-value of 0.05 among treatments or storage time.
3. Results and Discussion
3.1. pH of WBC and Cheese Brine
Table 2 and Table 3 show the pH values of the WBC and cheese brines, respectively, in the presence of L. monocytogenes only or with L. reuteri stored at 4, 10, and 24 °C for 91 d. The initial pH values of WBC samples in the presence of L. monocytogenes only or with L. reuteri stored in 10% and 15% NaCl solution ranged between 6.3 and 6.5 at day 0 and changed to 5.7–6.4, 5.2–6.3, and 4.9–5.7 after 91 d of storage at 4, 10, and 24 °C, respectively, regardless of the L. reuteri inoculation method (Table 2). The initial pH values of cheese brine samples of 10% and 15% NaCl in the presence of L. monocytogenes only or with L. reuteri ranged between 6.4 and 6.8 at day 0 and decreased to 5.7–6.4, 5.5–6.2, and 4.9–6.6, respectively, after 91 d of storage at 4, 10, and 24 °C, respectively, regardless of the L. reuteri inoculation method (Table 3).

Table 2.
pH values 1 of white-brined cheese inoculated with L. monocytogenes or L. reuteri and L. monocytogenes and stored in 10% or 15% brine solution at 4 °C, 10 °C, and 24 °C for 91 days.

Table 3.
pH values 1 of brine solution of white-brined cheese inoculated with L. monocytogenes or L. reuteri and L. monocytogenes and stored in 10% or 15% brine solution at 4 °C, 10 °C, and 24 °C for 91 days.
The highest reductions in pH values of cheese or brine were observed at the highest storage temperature (24 °C) at d 91 d, regardless of salt concentration and the presence of L. monocytogenes, L. reuteri, or both. This finding aligns with a study that analyzed the pH values of cheese samples stored under various storage conditions and found that the pH value declined from 5.8 to 5.2 after 29 d of storage at 5 °C, while the pH value at 24 °C decreased to 4.8 at day 14 [27]. Another study also indicated that pH value of soft white cheese significantly declined from 4.8 to 4.7 after 100 d of storage at 8 °C [28]. Al-Nabulsi et al. [2] indicated that lower pH values of WBC and brine samples were found in 10% brine compared to the 15% brine solution, where the pH values ranged from 4.7 to 6.1 and from 4.6 to 6.0, respectively, in 10% brine compared to 5.2–6.1 and 5.0–6.2, respectively, in 15% brine in the presence of E. coli O157:H7, L. reuteri, or both. In the current study, there were fluctuations in the pH values of cheese and brine at different salt concentrations, although it is expected that the numbers of LAB, proteolytic, and lipolytic activities during ripening and microbial lactose metabolism decrease as the level of NaCl increase [2,3]. Furthermore, the pH reduction may be due to the production of organic acids by inoculated and naturally occurring LAB [29].
3.2. Water Activity of White-Brined Cheese
Table 4 shows the aw values of all cheese samples inoculated with L. reuteri, L. monocytogenes, or both and stored in a 10% or 15% brine solution at 4, 10, or 24 °C. The initial aw values of cheese were 0.92–0.96 in a 10% or 15% brine solution. However, the final aw values of cheese samples remained constant and were not affected by storage temperature, NaCl concentration, or presence of L. monocytogenes, L. reuteri, or both after 91 d of storage, with a range of 0.90–0.95 (Table 4). Similar results were obtained by Al-Nabulsi et al. [2], who reported that the aw values of cheese stored in 10% or 15% brine solutions were 0.90–0.95 after 28 d of storage at 10 and 24 °C. Osaili et al. [5] also indicated that the aw in cheese stored in 15% brine at 10 or 21 °C ranged from 0.94 to 0.95 from day 1 to 0.93 to 0.94 at day 28. However, the aw of cheese stored in 15% brine was 0.92 on day 1 at both temperatures and decreased to 0.88–0.90 after 28 d of storage.

Table 4.
Water activity values 1 of white-brined cheese inoculated with L. monocytogenes or L. reuteri and L. monocytogenes stored in 10% or 15% brine solution at 4 °C for 91 days.
3.3. Salt Content of WBC
The salt concentration is a significant factor that affects the shape, texture, flavor, and quality of cheese [26]. Temperature and storage time significantly impacted the salt content of cheese [30]. In the current study, the salt contents of WBC samples were 4.8–6.1, 5.6–5.7, and 4.6–5.5% at 4, 10, and 24 °C, respectively, after 12 h of immersion in a 10% brine solution, and increased to reach 6.3–6.8, 6.3–6.9, and 6.0–6.7%, respectively, after 91 d of storage. While the salt contents of cheeses stored in 15% brine solution were 4.3–5.4, 4.9–5.2, and 4.8–5.2 at 4, 10, and 24 °C, respectively, after 12 h of immersion in the brine solution and increased to reach 6.6–6.8, 6.6–6.8, and 6.5–7.3%, respectively, after 91 d of storage (Table 5). This increase is due to the diffusion process that occurs during storage, where NaCl molecules move from the brine into the cheese and water diffuses out through the matrix, which increases the salt content and reduces the aw [2].

Table 5.
Salt content 1 of white-brined cheese in the presence of L. monocytogenes or L. reuteri and L. monocytogenes and stored in 10% or 15% brine solution at 4 10, or 24 °C for 91 days.
It was evident that the salt content of WBC increased as the brine solution and temperature increased, with higher levels of salt observed in cheeses stored in a 15% brine solution at 10 and 24 °C. Similarly, Setyawardani et al. [30] reported that the refrigerated artisanal goat cheese samples had a higher salt content than the frozen cheeses, and the salt content increased as the storage time increased. Kaya [31] also reported that increasing salt content in the brine has a substantial effect on the salt contents of Gaziantep cheese, where higher brine concentrations resulted in greater salt uptake by the cheese during storage. Moreover, Al- Nabulsi et al. [2] pointed out that the salt content of WBC stored in 15% brine at 4 and 25 °C was higher than samples stored in 10% brine solution. The salt levels in cheese stored in 15% brine solution were 5.3–7.7% after 28 d, while the salt content of cheese stored in a 10% brine solution was 4.5–6.7% after 28 d.
3.4. Behavior of L. reuteri in White-Brined Cheese Inoculated at Different Stages of Processing in the Presence of L. monocytogenes at Different Storage Temperatures
The behavior of L. reuteri in cheese stored in 10% or 15% NaCl brine inoculated with L. monocytogenes at 4, 10, or 24 °C for 91 d was investigated. The initial numbers of L. reuteri (6.1–6.5 log CFU/g) significantly (p < 0.05) increased to 7.3, 6.7, and 6.6 log CFU/g in cheese made with L. monocytogenes after 91 d of storage in 10% NaCl at 4, 10, and 24 °C, respectively (Table 6). The growth of L. reuteri in cheese at 4 or 10 °C is explained by the production of cold shock proteins that enable microorganisms to overcome the environmental stresses. In another study, Chen et al. [32] found that L. reuteri survived and remained at steady levels in drinkable yogurt, with numbers of approximately 4.0 log CFU/g in thin yogurt stored at 37 °C for 4 weeks and 5.0 log CFU/g in thick yogurt stored at 28 °C for 5 months.

Table 6.
Survival of L. reuteri (log CFU/g) 1 in WBC stored in 10% or 15% NaCl brine in the presence of L. monocytogenes at 4, 10, and 24 °C for 91 days.
Meanwhile, the initial L. reuteri numbers (6.1–6.7 log CFU/g) decreased in WBC stored in 15% NaCl and reached to 5.3–5.5 log CFU/g at 4, 10, and 24 °C by the end of the storage period. This inhibitory effect could be attributed to the high salt content, which may have led to osmotic stress [33]. Moreover, Al-Nabulsi et al. [2] reported that for L. reuteri, the initial numbers were significantly reduced in cheese made without E. coli O157:H7 in 10% brine at 10 °C, from 6.2 to 5.1 log CFU/g after 28 d. In 10% brine at 25 °C and in 15% NaCl at 10 °C, the initial numbers of L. reuteri in cheese made without E. coli O157:H7 decreased from 5.9 to 5.1 log CFU/g after 28 d.
It was evident that L. reuteri inoculated into WBC at different preparation stages was not affected by the presence of L. monocytogenes. In contrast, Langa et al. [34] indicated that L. reuteri decreased by ~1.5 log CFU/g when co-cultured with E. coli O157:H7 and L. monocytogenes in semi-hard cheese stored at 12 °C for 30 d and found that the survival of L. reuteri plus glycerol decreased from 6.8 at day 1 to 2.6 after 30 d of storage. Al-Nabulsi et al. [2] reported that the numbers of L. reuteri increased from 6.2 to 8.3 and 7.8 log CFU/g in WBC made with E. coli O157:H7 and stored in 10% or 15% brine, respectively, at 25 °C after 28 d; in 15% brine at 10 °C, the numbers of L. reuteri in WBC made with E. coli O157:H7 significantly decreased from 6.2 to 5.2 log CFU/g.
3.5. Behavior of L. reuteri in Cheese Brine Inoculated at Different Stages of Processing in the Presence of L. monocytogenes at Different Storage Temperatures
The behavior of L. reuteri in cheese brine at 10% or 15% NaCl inoculated with L. monocytogenes and stored at 4, 10, or 24 °C for 91 d was investigated (Table 7). The initial number of L. reuteri (5.5–6.4 log CFU/g) slightly increased in 10% cheese brine stored at 4 (7.1 log CFU/g), 10 °C (7.3 log CFU/g), and 24 °C (7.5 log CFU/g). However, the numbers changed to 5.6–6.4 log CFU/g in a 15% brine solution at different storage temperatures. On the other hand, the inoculation of L. reuteri into milk used in cheese processing or distilled water used in brine preparation with glycerol in the presence of L. monocytogenes showed lower numbers at the end of the storage period, and reached 6.2–6.8 log CFU/g in 10% brine and 5.4–5.6 log CFU/g in 15% brine.

Table 7.
Survival of L. reuteri (log CFU/g) 1 in cheese brine stored in 10% or 15% NaCl brine in the presence of L. monocytogenes at 4, 10, and 24 °C for 91 days.
Similar results were obtained by Al-Nabulsi et al. [2], who reported that L. reuteri numbers significantly increased from 5.0 to 5.3 log CFU/mL at d 1 to reach 5.7 to 7.8 log CFU/mL after 28 d in the presence of E. coli O157:H7 in 10% or 15% NaCl brine concentrations at 25 °C. The increased presence of L. reuteri could be due to the cell surface proteins, as reported by Singh et al. [35], who reported that different strains of L. reuteri were resistant to the highly acidic environment compared to the strains treated in 5 M LiCl for the removal of cell surface proteins, which are a protective sheath against hostile environmental agents such as acidic and high-NaCl environments. A study by Rasch [36] reported that the different environmental parameters, including pH (4.5–6.5) and NaCl (0.5–3%), did not interfere with the efficiency of reuterin against E. coli K12.
3.6. Inhibitory Effect of L. reuteri Inoculated at Different Stages of White-Brined Cheese Processing Against L. monocytogenes in Cheese at Different Storage Temperatures
WBC has high nutrient content, high water activity, and high buffering capacity; therefore, it supports the growth of spoilage and foodborne pathogens, such as L. monocytogenes [37]. This was confirmed by the findings of this study, which investigated the behavior of L. monocytogenes in WBC made with or without L. reuteri and stored in 10% or 15% NaCl at 4, 10, or 24 °C for 91 d (Table 8). The initial numbers of L. monocytogenes (4.6–4.8 log CFU/g) in cheese made without L. reuteri at 4, 10, and 24 °C significantly increased (p < 0.05) during the 91 d storage time and reached to 6.9, 6.4, and 6.7 log CFU/g, respectively, in cheese stored with 10% brine and to 6.2, 6.3, and 6.0 log CFU/g, respectively, in cheese stored with 15% NaCl. Although factors including temperature, pH, and salt content affect the growth of L. monocytogenes in cheese, the microbe still has the ability to adapt and grow in the presence of highly osmotic environments [38]. In the current study, the salt contents of WBC samples stored in 10% and 15% brine solutions reached 6.0–6.9% and 6.5–7.3%, respectively, by the end of the 91 d storage period (Table 5). Further, the initial pH values of cheese were 6.3–6.5 and reduced to 4.9–6.4 (Table 2), with a final aw of 0.9–0.95 (Table 4) after 91 d at different storage temperatures.

Table 8.
Survival of L. monocytogenes (log CFU/g) 1 in white-brined cheese stored in 10% or 15% NaCl brine at 4, 10, or 24 °C for 91 days in the absence or presence of L. reuteri inoculated into milk, brine solution, or distilled water.
It has been reported that when L. monocytogenes was exposed to sub-lethal environmental conditions, it may provide cross-protection against other factors such as salt, acid, alkaline pH, ethanol, heat, and oxidative stress [39]. For example, a study by Ilhak et al. [40] indicated that the survivability of L. monocytogenes in Turkish white cheese prepared from raw milk that was ripened in high-salt brine (6.7%) for 15 d at 4 °C was improved when the pathogen was pre-exposed to a sub-lethal acidic environment (pH 4.6–4.8). Faleiro et al. [41] noted that foods with a low pH (5.0), such as cheese, may induce an acid tolerance response in L. monocytogenes. Additionally, Cataldo et al. [42] indicated that acid-adapted L. monocytogenes survived significantly better compared to un-adapted cells in Crescenza cheese, a soft cheese with 4–10% NaCl and pH 5.0–5.6, after 14 d of storage at 4 °C. Additionally, Kapetanakou et al. [43] reported that L. monocytogenes persisted at the levels > of 2 log CFU/g in Cottage cheese with pH 5.0 during the entire product shelf life.
Numerous studies have demonstrated that some bacteria, including L. monocytogenes, adapt to high osmolality by synthesizing or uptaking suitable solutes such as proline and carnitine to maintain equilibrium between the intracellular and extracellular environments [44]. These solutes are extremely soluble substances, known as protective osmolytes, that play a significant role in enabling the cells to restore their osmotic balance without negatively influencing the cell structure or function [45]. Likewise, L. monocytogenes survived in different types of cheese, such as curd and soft cheeses, for long periods at refrigeration and room temperature, with high survival rates at lower temperatures [46]. It was also reported that L. monocytogenes was more resistant to salt in cheese at low temperatures (8 °C) [41].
Salting is widely used in the food industry for food preservation as it reduces aw, thus allowing a considerable increase in the storage time of food products. It is worth mentioning that the levels of salt, pH, and aw used in the current study did not prevent the growth of L. monocytogenes at 4, 10, and 24 °C. Furthermore, typical cheese preservation procedures may not only fail to prevent the growth or survival of L. monocytogenes, but may markedly improve their virulence [47]. However, the addition of other factors, such as bacteriocins or LAB cultures producing bacteriocins, to WBC may substantially reduce the numbers of L. monocytogenes in combination with other factors such as salt and low pH. On the other hand, the addition of L. reuteri to WBC at different stages of processing significantly (p ˂ 0.05) affected the survivability of L. monocytogenes at different storage temperatures. At the end of the 91 d storage time, the L. monocytogenes count for the control (cheese un-inoculated with L. reuteri), regardless of the brine concentration or the storage temperature, was 6.0–6.9 log CFU/g. However, L. monocytogenes counts decreased significantly (p ˂ 0.05) to 4.9–5.9 log CFU/g when L. reuteri was added with glycerol to the cheese brine, to 4.1–5.0 log CFU/g when L. reuteri was added with glycerol to the distilled water used to prepare cheese brine, or to 3.5–5.0 log CFU/g when L. reuteri was added to the pasteurized milk during processing (Table 6). It is worth mentioning that the addition of L. reuteri to pasteurized milk showed the most inhibitory effect against L. monocytogenes in cheese stored in 10% NaCl at 10 °C. Further, the addition of L. reuteri to pasteurized milk during processing or sterile water used for brine preparation was more effective against L. monocytogenes in cheese compared to its inoculation in cheese brine.
The decrease in L monocytogenes counts could be attributed to the inhibitory effects of L. reuteri and its bacteriocin, reuterin. Similar results were obtained by Al-Nabulsi et al. [2], who reported that an L. reuteri cocktail reduced the numbers of E. coli O157:H7 in WBC by 2.6 log CFU/g after 28 d of storage in 10% NaCl brine at 25 °C. In the current study, it is evident that L. reuteri has a significant role in the reduction of L. monocytogenes due to its capability to produce antimicrobial molecules including organic acids, inhibitory enzymes, hydrogen peroxide, and reuterin [48].
Similarly, Langa et al. [34] reported that L. reuteri INIA P572 generated reuterin in the presence of 100 mM glycerol during semi-hard cheese production and ripening to quantities that showed a bactericidal effect against L. monocytogenes, which was not detected after 7 d of storage. Furthermore, L. monocytogenes and E. coli O157:H7 were not detected in cheese treated with 5.30 mM/g of reuterin by 7 d at 12 °C [34].
The current study is further proof that L. reuteri has a potent antibacterial activity against L. monocytogenes in both 10 and 15% brine at different storage temperatures (4, 10, and 24 °C). This could be related to the quorum-sensing mechanism of L. reuteri, which promotes reuterin production during growth [49]. Reuterin is known to be an analog of D-ribose, which inhibits ribonucleotide reductases, and are necessary for the de novo synthesis of deoxyribonucleotides needed for DNA synthesis [50]. This could explain the considerable reduction in L. monocytogenes populations in cheeses made with L. reuteri with glycerol after 91 days at different levels of brine and storage temperatures. L. reuteri survival increased with decreasing salt concentrations and increasing temperature (Table 6 and Table 7), which may enhance production and produce an even higher quantity of reuterin compared to when using high brine concentrations, which may cause bacterial cell dehydration and osmotic stress and decrease the reuterin yield [32].
Apparently, reuterin and salt showed synergistic effects against L. monocytogenes. Similarly, previous studies showed that increasing salt content enhanced the antimicrobial activity of reuterin against L. monocytogenes and E. coli O157:H7 [50]. The addition of 3-HPA at 2 AU/mL with nisin (100 IU/mL) and lactoperoxidase system (0.2 ABTSU/mL) synergistically inactivated L. monocytogenes and Staphylococcus aureus in Cuajada cheese (curdled milk) after 12 d of storage at 10 °C [51]. In another study, E. coli O157:H7, Salmonella enteritidis, and L. monocytogenes were inhibited in acidified milk at pH 5.0 by adding 1 AU/mL 3-HPA with 100 mg/kg diacetyl [52]. Furthermore, the application of high-pressure processing at 450 MPa/5 min with the addition of 16 mM of 3-HPA reduced the numbers of L. monocytogenes in cooked ham by 1.7 and 2.6 log CFU/g at 4 and 10 °C, respectively, by 35 d of storage and reduced S. enterica numbers by 2 and 1 log CFU/g at 4 and 10 °C, respectively, compared to control samples [53]. Reuterin treatment of cold-smoked salmon at 10 AU/g effectively reduced L. monocytogenes by 2.0 log CFU/g by 15 d of storage at 8 °C or by 1.4 log CFU/g by day 1 at 30 °C, compared to control samples [54]. In another study, the addition of reuterin to creamed cottage cheese at 50 AU/g decreased L. monocytogenes numbers by 1.5 log CFU/g by 21 d of storage at 7 °C [55].
3.7. Inhibitory Effect of L. reuteri Inoculated at Different Stages of White-Brined Cheese Processing Against L. monocytogenes in Cheese Brine at Different Storage Temperatures
The brine could be a possible cause of contamination in cheeses via transferring foodborne pathogens such as L. monocytogenes from brine to cheese. Commercial cheese brines are frequently used in WBC, generating a brine that is rich in nutrients that are released from cheese and perhaps encouraging the growth of pathogenic microorganisms [3]. This may explain the capability of L. monocytogenes to survive and grow in 10% or 15% brine of cheese made without or with the addition of L. reuteri at 4, 10, or 24 °C (Table 9).

Table 9.
Survival of L. monocytogenes (log CFU/mL) 1 in cheese brine at 10% or 15% NaCl at 4, 10, or 24 °C for 91 days in the absence or presence of L. reuteri inoculated into milk, brine solution, or distilled water.
It was clear that the minimum growth rate of the L. monocytogenes population was at the higher salt concentration at all storage temperatures. The number of L. monocytogenes at day 0 was 5.5–5.8 log CFU/mL, and the relatively low number could be due to the high salt concentration and absence of protective matrix in the brine, such as fat and protein [5].
L. monocytogenes numbers in both 10% and 15% brine un-inoculated with L. reuteri at 4, 10, or 24 °C significantly (p ˂ 0.05) increased to 6.1–6.8 log CFU/mL by the end of 91 d storage period (Table 7). Barancelli et al. [56] reported that L. monocytogenes was isolated from brine samples from different cheese factories. Further, Boyer et al. [57] reported that no significant decline in L. monocytogenes numbers was obtained in chill brines with salt levels of 7.9–13.2% after 10 d of storage at 4 and 12 °C.
However, the initial L. monocytogenes number (5.3–5.8 log CFU/mL) decreased to 4.4–5.9, 3.6–4.6, and 4.9–6.4 log CFU/mL in 10% NaCl brine at 4, 10, and 24 °C, respectively, and to 4.1–5.4, 4.2–5.5, and 5.1–5.5 log CFU/mL in 15% NaCl brine at 4, 10, and 24 °C, respectively, when L. reuteri and glycerol were added at different stages of cheese processing by 91 d of storage. It was evident that adding L. reuteri to brine was the least effective L. reuteri delivery method against L. monocytogenes in cheese brine, and this could be due to the inhibitory effect of salt against L. reuteri and the decrease in reuterin yield. On the other hand, inoculation of L. reuteri into the milk used in the processing of WBC yielded the most inhibitory effect against L. monocytogenes in cheese brine, and this could be due to the presence of nutrients that support the growth of L. reuteri and enhanced production of reuterin. Al- Nabulsi et al. [2] reported that the numbers of E. coli O157:H7 (6.9 log CFU/mL) in 15% NaCl brine containing L. reuteri was reduced to 4.4 CFU/mL after 28 d of storage of WBC. Langa et al. [52] reported that adding reuterin (1 AU/mL) with Diacetyl (100 mg/kg) in acidic milk (pH = 5) was effective in decreasing L. monocytogenes from an initial level of ca. 6.0 log CFU/mL to 3.6 log CFU/g after 24 h; with normal pH milk (pH = 6.8), the count increased to ca. 6.9 log CFU/mL.
4. Conclusions
Using L. reuteri as an adjunct culture to cheese may pose a considerable antimicrobial activity against L. monocytogenes. This is potentially due to the production of antimicrobial substances such as reuterin. The findings of this study confirmed the protective effect of reuterin-producing L. reuteri with 100 mM glycerol in the production of cheese contaminated with L. monocytogenes. L. monocytogenes was capable of surviving in WBC (immersed into 10 or 15% brine concentrations) at different storage temperatures (4, 10, or 24 °C for 91 d). In the absence of L. reuteri, numbers of L. monocytogenes increased by ca. 2 logs after 3 d of storage; this increase persisted till the end of the 91 d of storage at the different storage temperatures, and the growth was more apparent in the WBC immersed into the 10% brine compared to the 15% brine solutions. Using glycerol (100 mM) in the different L. reuteri delivery methods significantly decreased the numbers of L. monocytogenes in both cheese and brine solutions compared to the control. Delivering L. reuteri along with glycerol to pasteurized milk used in cheese processing was the most effective inoculation method in inhibiting the growth of L. monocytogenes in BWC compared to inoculating L. reuteri into distilled water or brine solution. L. reuteri was able to grow in cheese immersed into 10 or 15% brine solutions under the different storage temperatures. Yet, L. reuteri growth was more prominent when using 10% brine solution at 24 °C. These conditions may enhance the production of reuterin and other metabolites by L. reuteri, which may entail higher inhibitory activity against L. monocytogenes in cheese.
The future prospects for L. reuteri and glycerol in food products are positive, with chances to improve food safety, increase shelf life, provide functional advantages, and match customer demands for natural ingredients. However, realizing this potential will require further research, such as investigating the antagonistic of L. reuteri against other foodborne pathogens in WBC and investigating the antagonistic of L. reuteri against L. monocytogenes and other foodborne pathogens in other fermented products. Further, the allied combinations of L. reuteri and glycerol with other antibacterial agents or food preservatives like essential oils or organic acids could be investigated.
Author Contributions
Conceptualization, A.N.O., F.A.-a. and M.A.-H.; methodology, A.N.O., F.A.-a., R.H. and M.A.-H.; software, F.A.-a.; validation, A.A.-N., H.A.-Q. and T.O.; formal analysis, A.N.O. and F.A.-a.; investigation, F.A.-a. and R.H.; resources, A.N.O., A.A.-N., T.O., H.A.-Q., M.A. and M.A.-H.; data curation, A.N.O., F.A.-a. and M.A.-H.; writing—original draft preparation, A.N.O., F.A.-a. and M.A.-H.; writing—review and editing, A.N.O., F.A.-a., R.H., M.A.-H., A.A.-N., T.O., H.A.-Q. and M.A.; visualization, A.N.O., F.A.-a., R.H., M.A.-H., A.A.-N., T.O., H.A.-Q. and M.A.; supervision, A.N.O. and M.A.-H.; project administration, A.N.O.; funding acquisition, A.N.O. and F.A.-a. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Hashemite University, grant number 14/2020; the APC will be covered by the authors of the paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Holy, M.A.; Al-Nabulsi, A.; Osaili, T.M.; Ayyash, M.M.; Shaker, R.R. Inactivation of Listeria innocua in brined white cheese by a combination of nisin and heat. Food Control 2012, 23, 48–53. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Oqdeh, S.B.; Olaimat, A.N.; Jaradat, Z.W.; Ayyash, M.; Holley, R.A. Antagonistic effects of Lactobacillus reuteri against Escherichia coli O157:H7 in white-brined cheese under different storage conditions. J. Dairy Sci. 2021, 104, 2719–2734. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Taha, M.H.; Al-Holy, M.A.; Alaboudi, A.R.; Al-Rousan, W.M.; Shaker, R.R. Occurrence and antimicrobial susceptibility of Listeria monocytogenes isolated from brined white cheese in Jordan. J. Food Sci. 2012, 77, M528–M532. [Google Scholar] [CrossRef] [PubMed]
- Kursun, O.; Kirdar, S.S.; Keyvan, E.; Guner, A. Microbiological quality of white pickled cheese produced in small plants in Burdur, Turkey. J. Food Agric. Environ. 2011, 9, 110–112. [Google Scholar]
- Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Shaker, R.R.; Taha, M.; Holley, R.A. Survival of Escherichia coli O157:H7 during manufacture and storage of white brined cheese. J. Food Sci. 2014, 79, M1750–M1755. [Google Scholar] [CrossRef]
- Kahraman, T.; Ozmen, G.; Ozinan, B.; Omer Goksoy, E. Prevalence of Salmonella spp. and Listeria monocytogenes in different cheese types produced in Turkey. Br. Food J. 2010, 112, 1230–1236. [Google Scholar] [CrossRef]
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Torres-Vitela, M.R.; Mendoza-Bernardo, M.; Castro-Rosas, J.; Gomez-Aldapa, C.A.; Garay-Martinez, L.E.; Navarro-Hidalgo, V.; Villarruel-López, A. Incidence of Salmonella, Listeria monocytogenes, Escherichia coli O157:H7, and Staphylococcal enterotoxin in two types of Mexican fresh cheeses. J. Food Prot. 2012, 75, 79–84. [Google Scholar] [CrossRef]
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Bittencourt, G.M.; Barancelli, G.V.; Kamimura, E.S.; Lee, S.H.I.; Oliveira, C.A.F. Listeria monocytogenes in Brazilian foods: Occurrence, risks to human health and their prevention. Curr. Res. Nutr. Food Sci. J. 2019, 7, 320–330. [Google Scholar] [CrossRef]
- Pang, X.; Wong, C.; Chung, H.J.; Yuk, H.G. Biofilm formation of Listeria monocytogenes and its resistance to quaternary ammonium compounds in a simulated salmon processing environment. Food Control 2019, 98, 200–208. [Google Scholar] [CrossRef]
- Morandi, S.; Silvetti, T.; Vezzini, V.; Morozzo, E.; Brasca, M. How we can improve the antimicrobial performances of lactic acid bacteria? A new strategy to control Listeria monocytogenes in Gorgonzola cheese. Food Microbiol. 2020, 90, 103488. [Google Scholar] [CrossRef] [PubMed]
- Kevenk, T.O.; Terzi Gulel, G. Prevalence, antimicrobial resistance and serotype distribution of Listeria monocytogenes isolated from raw milk and dairy products. J. Food Saf. 2016, 36, 11–18. [Google Scholar] [CrossRef]
- Panebianco, F.; Giarratana, F.; Caridi, A.; Sidari, R.; De Bruno, A.; Giuffrida, A. Lactic acid bacteria isolated from traditional Italian dairy products: Activity against Listeria monocytogenes and modelling of microbial competition in soft cheese. LWT—Food Sci. Technol. 2021, 137, 110446. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Ibarra-Sánchez, L.A.; Hoepker, G.P.; Miller, M.J. Hot topic: Antilisterial activity by endolysin PlyP100 in fresh cheese. J. Dairy Sci. 2017, 100, 2482–2487. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Viale, S.; Deplano, M.; Mereu, F.; Blažić, M.; Cosentino, S. Inhibitory effect of Lactiplantibacillus plantarum and Lactococcus lactis autochthonous strains against Listeria monocytogenes in a laboratory cheese model. Foods 2022, 11, 715. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Coelho, M.C.; Silva, C.C.; Ribeiro, S.C.; Dapkevicius, M.L.; Rosa, H.J. Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Int. J. Food Microbiol. 2014, 191, 53–59. [Google Scholar] [CrossRef]
- da Costa, R.J.; Voloski, F.L.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; Capozzi, V.; Drider, D.; Spano, G.; Fiocco, D. Bioprospecting antimicrobials from Lactiplantibacillus plantarum: Key factors underlying its probiotic action. Int. J. Molecul. Sci. 2021, 22, 12076. [Google Scholar] [CrossRef] [PubMed]
- Scatassa, M.L.; Gaglio, R.; Cardamone, C.; Macaluso, G.; Arcuri, L.; Todaro, M.; Mancuso, I. Anti-Listeria activity of lactic acid bacteria in two traditional Sicilian cheeses. Ital. J. Food Saf. 2017, 6, 6191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dastmalchi, F.; Razavi, S.H.; Faraji, M.; Labbafi, M. Effect of Lactobacillus casei-casei and Lactobacillus reuteri on acrylamide formation in flat bread and bread roll. J. Food Sci. Technol. 2016, 53, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Abu Ershaid, J.M.; Abudoleh, S.M.; Lafi, D.N. Freeze-dried erythromycin nanocrystals: Preparation, characterisation, antimicrobial activity, and aerodynamic properties. Pharmacia. 2024, 71, 1–10. [Google Scholar] [CrossRef]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef]
- AOAC. Chloride in Cheese. Potentiometric Method. In Official Methods of Analysis of AOAC International, 17th ed.; Method 983.14; AOAC International: Gaithersburg, MD, USA, 1997; Volume II. [Google Scholar]
- Eljagmani, S.; Altuner, E.M. Effect of storage temperature on the chemical and microbiological properties of white cheese from Kastamonu, Turkey. Cogent Food Agric. 2020, 6, 1829270. [Google Scholar] [CrossRef]
- Memiši, N.R.; Vesković-Moračanin, S.M.; Škrinjar, M.M.; Iličić, M.D.; Ač, M.Đ. Storage temperature: A factor of shelf life of dairy products. Acta Period. Technol. 2014, 45, 55–66. [Google Scholar] [CrossRef]
- McMahon, D.J.; Oberg, C.J.; Drake, M.A.; Farkye, N.; Moyes, L.V.; Arnold, M.R.; Ganesan, B.; Steele, J.; Broadbent, J.R. Effect of sodium, potassium, magnesium, and calcium salt cations on pH, proteolysis, organic acids, and microbial populations during storage of full-fat Cheddar cheese. J. Dairy Sci. 2014, 97, 4780–4798. [Google Scholar] [CrossRef]
- Setyawardani, T.; Sumarmono, J.; Widayaka, K. Effect of cold and frozen temperatures on artisanal goat cheese containing probiotic lactic acid bacteria isolates (Lactobacillus plantarum TW14 and Lactobacillus rhamnosus TW2). Vet. World 2019, 12, 409–417. [Google Scholar] [CrossRef]
- Kaya, S. Effect of salt on hardness and whiteness of Gaziantep cheese during short-term brining. J. Food Eng. 2002, 52, 155–159. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Q.; Li, P.; Chen, S.; Li, Y. Genomic analysis of Lactobacillus reuteri WHH1689 reveals its probiotic properties and stress resistance. Food Sci. Nutr. 2019, 7, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Chen, L.; Ren, X.; Ge, H.; Li, B.; Ma, G.; Ke, X.; Zhu, J.; Li, L.; et al. Potential Probiotic Characterization of Lactobacillus reuteri from Traditional Chinese Highland Barley Wine and Application for Room-Temperature-Storage Drinkable Yogurt. J. Dairy Sci. 2018, 101, 5780–5788. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Peirotén, Á.; Arqués, J.L.; Medina, M. Protective Effect of Reuterin-Producing Lactobacillus reuteri against Listeria monocytogenes and Escherichia coli O157:H7 in Semi-Hard Cheese. Food Control 2018, 84, 284–289. [Google Scholar] [CrossRef]
- Singh, T.P.; Malik, R.K.; Kaur, G. Cell Surface Proteins Play an Important Role in Probiotic Activities of Lactobacillus reuteri. Nutrients 2016, 41, 5. [Google Scholar] [CrossRef]
- Rasch, M.; Barker, G.C.; Sachau, K.; Jakobsen, M.; Arneborg, N. Characterisation and Modelling of Oscillatory Behaviour Related to Reuterin Production by Lactobacillus reuteri. Int. J. Food Microbiol. 2002, 73, 383–394. [Google Scholar] [CrossRef]
- Tavşanlı, H.; İrkin, R.; Kısadere, İ. The Effects of Different Organic Acid Treatments on Some Microflora and Pathogen Listeria monocytogenes of White Brine Cheese. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 201–207. [Google Scholar]
- Ozer, H.; Uraz, G.; Beyzi-Yilmaz, E.; Ferit Atasoy, A. The Effects of Brine Concentration and Scalding on Survival of Some Pathogens in Urfa Cheese: A Traditional White-Brined Turkish Cheese. Int. J. Food Sci. Technol. 2004, 39, 727–735. [Google Scholar] [CrossRef]
- Adrião, A.; Vieira, M.; Fernandes, I.; Barbosa, M.; Sol, M.; Tenreiro, R.P.; Chambel, L.; Barata, B.; Zilhao, I.; Shama, G.; et al. Marked Intra-Strain Variation in Response of Listeria monocytogenes Dairy Isolates to Acid or Salt Stress and the Effect of Adaptation on Adherence to Abiotic Surfaces. Int. J. Food Microbiol. 2008, 123, 142–150. [Google Scholar] [CrossRef]
- Ilhak, O.I.; Oksuztepe, G.; Calicioglu, M.; Patir, B. Effect of Acid Adaptation and Different Salt Concentrations on Survival of Listeria monocytogenes in Turkish White Cheese. J. Food Qual. 2011, 34, 379–385. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Andrew, P.W.; Power, D. Stress Response of Listeria monocytogenes Isolated from Cheese and Other Foods. Int. J. Food Microbiol. 2003, 84, 207–216. [Google Scholar] [CrossRef]
- Cataldo, G.; Conte, M.P.; Chiarini, F.; Seganti, L.; Ammendolia, M.G.; Superti, F.; Longhi, C. Acid Adaptation and Survival of Listeria monocytogenes in Italian-Style Soft Cheeses. J. Appl. Microbiol. 2007, 103, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakou, A.E.; Gkerekou, M.A.; Vitzilaiou, E.S.; Skandamis, P.N. Assessing the Capacity of Growth, Survival, and Acid Adaptive Response of Listeria monocytogenes during Storage of Various Cheeses and Simulated Gastric Digestion. Int. J. Food Microbiol. 2017, 246, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Gardan, R.; Cossart, P.; Labadie, J.; European Listeria Genome Consortium. Identification of Listeria monocytogenes Genes Involved in Salt and Alkaline-pH Tolerance. Appl. Environ. Microbiol. 2003, 69, 3137–3143. [Google Scholar] [CrossRef] [PubMed]
- Wonderling, L.D.; Wilkinson, B.J.; Bayles, D.O. The htrA (degP) Gene of Listeria monocytogenes 10403S Is Essential for Optimal Growth under Stress Conditions. Appl. Environ. Microbiol. 2004, 70, 1935–1943. [Google Scholar] [CrossRef]
- Possas, A.; Hernández, M.; Esteban-Carbonero, Ó.; Valero, A.; Rodríguez-Lázaro, D. Listeria monocytogenes Survives Better at Lower Storage Temperatures in Regular and Low-Salt Soft and Cured Cheeses. Food Microbiol. 2022, 104, 103979. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Baca-Castañón, M.L.; De la Garza-Ramos, M.A.; Alcázar-Pizaña, A.G.; Grondin, Y.; Coronado-Mendoza, Y.; Sanchez-Najera, R.I.; Cardenas-Estrada, E.; Medina-De la Garza, C.E.; Escamilla-Garcia, E. Antimicrobial Effect of Lactobacillus reuteri on Cariogenic Bacteria Streptococcus gordonii, Streptococcus mutans, and Periodontal Diseases Actinomyces naeslundii and Tannerella forsythia. Probiotics Antimicrob. Proteins 2015, 7, 1–8. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Holley, R.A. Survival of Escherichia coli O157:H7 in Dry Fermented Sausages Containing Micro-Encapsulated Probiotic Lactic Acid Bacteria. Food Microbiol. 2007, 24, 82–88. [Google Scholar] [CrossRef]
- Talarico, T.L.; Dobrogosz, W.J. Chemical Characterization of an Antimicrobial Substance Produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989, 33, 674–679. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Antimicrobial Activity of Nisin, Reuterin, and the Lactoperoxidase System on Listeria monocytogenes and Staphylococcus aureus in Cuajada. J. Dairy Sci. 2008, 91, 70–75. [Google Scholar] [CrossRef]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Landete, J.M.; Medina, M.; Arqués, J.L. Short Communication: Combined Antimicrobial Activity of Reuterin and Diacetyl against Foodborne Pathogens. J. Dairy Sci. 2014, 97, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Montiel, R.; Martín-Cabrejas, I.; Medina, M. Reuterin, Lactoperoxidase, Lactoferrin and High Hydrostatic Pressure on the Inactivation of Food-Borne Pathogens in Cooked Ham. Food Control 2015, 51, 122–128. [Google Scholar] [CrossRef]
- Montiel, R.; Martín-Cabrejas, I.; Langa, S.; El Aouad, N.; Arqués, J.L.; Reyes, F.; Medina, M. Antimicrobial Activity of Reuterin Produced by Lactobacillus reuteri on Listeria monocytogenes in Cold-Smoked Salmon. Food Microbiol. 2014, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- EL-Ziney, M.G.; Debevere, J.M. The Effect of Reuterin on Listeria monocytogenes and Escherichia coli O157:H7 in Milk and Cottage Cheese. J. Food Prot. 1998, 61, 1275–1280. [Google Scholar] [CrossRef]
- Barancelli, G.; Camargo, T.; Gagliardi, N.; Porto, E.; Souza, R.; Campioni, F.; Falcao, J.P.; Hofer, E.; Cruz, A.G.; Oliveira, C.A. Pulsed-Field Gel Electrophoresis Characterization of Listeria monocytogenes Isolates from Cheese Manufacturing Plants in São Paulo, Brazil. Int. J. Food Microbiol. 2014, 173, 21–29. [Google Scholar] [CrossRef]
- Boyer, R.; Matak, K.; Sumner, S.; Meadows, B.; Williams, R.; Eifert, J.; Birbari, W. Survival of Listeria monocytogenes, Listeria innocua, and Lactic Acid Bacteria in Chill Brines. J. Food Sci. 2009, 74, 219–223. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).