Abstract
This study evaluated the nutritional, functional, biological, and sensory potential of proteins derived from Yarrowia lipolytica biomass and their enzymatic hydrolysates for food applications. Three strains were cultivated under bioreactor conditions, with strain JII1c selected for its superior biomass yield and protein content. Its amino acid composition was rich in lysine and branched-chain amino acids, with protein quality indices (CS = 37.8%, EAAI = 36.17%) confirming value in plant-based diets. Proteins were isolated and hydrolysed using a non-commercial serine protease from Cucurbita ficifolia, which enhanced solubility (NSI: 19.4 → 49.2%), water and oil absorption, and emulsion stability. Hydrolysates showed notable biological activities, including ACE (71.8%), DPP-IV (52.3%), and α-glucosidase (67.4%) inhibition, indicating potential metabolic benefits. Sensory evaluation of extrudates confirmed improvements in aroma, texture, and flavour when hydrolysates were incorporated. The use of a plant-derived protease demonstrates a sustainable approach to producing bioactive peptides. Y. lipolytica hydrolysates emerge as promising clean-label ingredients that combine nutritional quality with techno-functional performance, supporting their integration into health-oriented and sustainable food products.
1. Introduction
The global demand for high-quality protein is rising due to protein–energy malnutrition, amino acid imbalance, climate change, and shifting dietary preferences. Conventional protein sources such as meat, dairy, and fish, though rich in essential amino acids, contribute to environmental degradation through greenhouse gas emissions, land use, and water consumption [1,2]. Despite overall growth in food output, many populations still lack access to adequate diets, reflecting deficiencies of food systems that prioritize yield and cost-efficiency over nutritional quality [3]. Meanwhile, vegetarian, vegan and flexitarian trends are driving demand for novel protein ingredients that are both sustainable and nutritionally balanced. In the United States, plant-based foods reached 61% household penetration in 2021, with 19% buying meat substitutes [2,4,5,6,7].
Alternative proteins from plants, insects, microalgae and fungi are gaining attention for their environmental and functional potential [8,9]. Among microbial sources, Yarrowia lipolytica is particularly promising, offering up to 60% protein per dry weight when cultivated under optimised bioreactor conditions using agro-industrial by-products [10]. Its proteins exhibit a complete amino acid profile, with high levels of lysine and leucine, surpassing even egg protein in lysine content [5,11]. Y. lipolytica has been recognized as safe for food and feed applications, holding GRAS status in the United States and approval as a novel food by the European Food Safety Authority (EFSA, 2019), confirming its safety, metabolic versatility, and high productivity [12]. Additionally, its cell wall provides bioactive polysaccharides and B vitamins such as riboflavin and B12, aligning with clean-label and sustainability trends [5,13,14].
This yeast also supports circular economy strategies by valorising waste streams, including bread, brewer’s spent grain and lignocellulosic residues, without extensive pretreatment [15,16,17]. Such processes reduce environmental burden while producing protein-rich biomass for food and feed applications.
Protein hydrolysis is an effective approach to enhance the biofunctionality of yeast proteins, releasing bioactive peptides (BAPs) with antioxidant, antihypertensive, antimicrobial and anti-inflammatory effects [18]. Functional properties of hydrolysates depend on substrate composition and processing conditions [19]. Plant-derived enzymes are particularly attractive for sustainable applications. A serine protease from Cucurbita ficifolia (Asian pumpkin) has been shown to generate peptides with strong antioxidant and functional properties and has been successfully applied to substrates including casein and insect protein [20,21,22,23]. Recent studies confirm that Y. lipolytica hydrolysates obtained via enzymatic treatment display potent antioxidant activity and functionality as flavour enhancers, emulsifiers and foaming agents. For example, Gottardi et al. (2022) demonstrated that hydrolysates obtained with Y. lipolytica strain YL2 showed strong DPPH radical scavenging activity, reaching 86.4% after 72 h of incubation, confirming the high bioactive potential of peptides derived from this yeast [5,14,24,25].
The aim of this study was to evaluate the potential of Y. lipolytica proteins and their enzymatic hydrolysates in food applications. The research focused on optimising cultivation and biomass processing for high protein yield, followed by enzymatic hydrolysis to generate bioactive peptide fractions. The nutritional, functional and sensory properties of both protein isolates and hydrolysates were assessed to explore their value in plant-based formulations.
2. Materials and Methods
2.1. Substrates
The material used for the study was the biomass of Yarrowia lipolytica yeast, which was obtained from bioreactor cultures (Yarrowia lipolytica yeast was obtained from the cultures collection of the Department of Biotechnology and Food Microbiology, Wroclaw University of Environmental and Life Sciences). The protein was then isolated from this biomass. Three distinct yeast strains were utilised for the culture of Y. lipolitica: JII1c, JII1a, and PII6a. The medium used for the reactor cultures was of plant origin, i.e., it was a vegan product.
2.2. Bioreactor Culture
Cultures were grown in a BioFlow 310 bioreactor (New Brunswick Scientific GmbH, Nurtingen, Germany) with a total volume of 7 L and a working volume of 4 L at a temperature of 28 °C. Aeration was maintained at 1 vvm, and agitation was set at 500 rpm for 48–72 h. The culture medium consisted of (g/L): yeast extract (1.7), casein (4.0), refined fatty acids (10.0), KH2PO4 (0.5), MgSO4·7H2O (0.25) and NH4Cl (1.0). Inoculum propagation was conducted sequentially in three bioreactors of increasing volume. In the first stage, a 7 L BioFlow 310 bioreactor (working volume: 4 L) was operated under the same temperature, aeration (1 vvm), and agitation (500 rpm) conditions for 48 h. The second stage involved a 50 L MPPF bioreactor (New Brunswick Scientific Co., Inc., Edison, NJ, USA) with a working volume of 4 L, maintained at identical operating conditions. In the third stage, cultivation was continued in the same 50 L bioreactor with an increased working volume, while aeration was reduced to 0.6 vvm and agitation adjusted to 350 rpm. The resulting concentrate was subjected to spray-drying with the addition of 3% (w/w) methylcellulose as a carrier. Spray-drying was performed at an inlet air temperature of 140 °C and an outlet air temperature of 88 °C, with a feed flow rate of 2.8 L/h and an air pressure of 1.1 bar. Biomass was harvested based on optical density. Optical density was reported based on the standard curve, for which extinction was measured for a specific amount expressed as [log10 CFU/mL]. Three replicates were performed for each strain.
2.3. Nutritional Quality Assessment of Biomasses
The obtained biomasses were analysed for their content of dry matter, protein, carbohydrates (including dietary fibre and sugars), fat and fatty acids, and both macro- and microelements [26,27,28,29,30,31,32]. B vitamins were determined by HPLC after enzymatic digestion and saponification, using a C18 column with UV/fluorescence detection [33,34,35,36,37,38]. Determination of the content of sodium was carried out in an acetylene/air flame by atomic emission spectrometry using the SpectraAA atomic absorption spectrometer with the flame attachment AA240FS (Varian). The methods were validated using certified reference material; the measurement uncertainty was estimated at 5%. Mineralization was performed in accordance with the Polish Standard PN-EN 13805:2003 “Foodstuffs. Determination of trace elements. Pressure mineralization” [39].
The amino acid content was analysed by reversed-phase high-performance liquid chromatography (RP-HPLC). Samples (500 μL) were solubilised in Tris/HCl buffer with urea and 2-mercaptoethanol, loaded on a Zorbax XDB-C18 column (250 × 4.5 mm, Agilent Technologies, Inc., Santa Clara, CA, USA), and separated at 1 mL/min, 30 °C, with gradient elution (0–100% acetonitrile/TFA); detection at 230 nm [40].
To assess the nutritional quality of the yeast biomasses, the method described by Liang et al. was applied [41]. Essential amino acid (EAA) profiles were analysed and compared to a reference protein (whole egg). Two parameters were calculated: the Chemical Score (CS) and the Essential Amino Acid Index (EAAI).
The CS was determined as the ratio of each essential amino acid present in the protein sample to its corresponding content in whole egg protein, multiplied by 100. The final CS was expressed as the geometric mean of these individual ratios, according to the equation:
The essential amino acid index (EAAI) reflects the overall balance of essential amino acids relative to the reference protein and provides a holistic metric for protein quality. The reference amino acid pattern of whole egg protein was adopted from FAO/WHO/UNU (1985) [42]. The EAAI was calculated as the geometric mean of the ratios of each essential amino acid in the sample to its corresponding content in whole egg protein. The calculation followed the formula:
where
p—refers to the protein sample;
s—refers to the standard (whole egg protein);
n—is the number of essential amino acids considered (with Met + Cys and Phe + Tyr treated as combined pairs).
The energy value of biomass was calculated using the Atwater general factors (4 kcal/g for protein, 9 kcal/g for fat, and 4 kcal/g for carbohydrates), based on the measured contents of macronutrients, according to FAO/WHO recommendations and Codex Alimentarius guidelines [43].
2.4. Microbiological Analysis
The microbiological safety of the dried Yarrowia lipolytica biomass was evaluated according to international standards. Total aerobic mesophilic bacteria (TAMC) were determined using ISO 4833-1:2013 [44]. Total yeast and mould count (TYMC) was measured according to ISO 21527-2:2008 [45]. The presence of viable Yarrowia lipolytica cells was confirmed using YPD agar (yeast extract–peptone–dextrose) incubated at 28 °C for 48 h, with colony morphology verified microscopically and through Gram staining. Coliform bacteria were detected based on ISO 4832:2006 [46]. Salmonella spp. detection followed ISO 6579-1:2017 [47]. Results were expressed as presence or absence in 25 g of sample.
2.5. Protein Extraction from JII1c Biomass
The protein isolates (PI) were obtained from Yarrowia lipolytica biomass JII1c using a two-step procedure. Initially, the yeast biomass, suspended in water, was subjected to acid hydrolysis to disrupt the cell walls and release intracellular components. In the first step, NaOH was used at a concentration of 1 M (t = 60 min), and in the second step, the 1 M HCl was used (t = 15 min). Following cell disruption, proteins were extracted under alkaline conditions (pH = 12) to increase their solubility. Subsequently, protein precipitation was induced by adjusting the pH to the isoelectric point (pH = 4.5), as described by Shetty K. J. & Kinsella (1979) [48]. During both steps, mechanical stirring was used. The protein content was determined post-precipitation by the Kjeldahl method. The efficiency of the entire process was estimated at approximately 55–60%.
2.6. Enzymatic Hydrolysis (EH)
2.6.1. Enzyme Applied in the Study
Serine proteinase was isolated from Asian pumpkin (Curcubita ficifolia) by the method according to Dryjanski et al., 1990 [49] Proteolytic activity was determined using 2% casein in Tris/HCl (pH 8.6), incubated for 10 min at 35.5 °C. The reaction was stopped with 5% TCA, centrifuged at 5500× g (RCF) for 10 min, and absorbance was measured at λ = 280 nm. One unit was defined as a 0.1 increase in absorbance.
2.6.2. Hydrolysis Procedure
Protein isolates (PI) from Yarrowia lipolytica JII1c biomass were subjected to enzymatic hydrolysis (EH) using pepsin 107,192 (Sigma-Aldrich, Poznań, Poland) and and non-commercial serine protease extracted from Cucurbita ficifolia. Enzymes were added at 150 enzyme units (U) per mg of protein to 1% substrate protein solution.
Hydrolysis was conducted at 37 °C, and samples as protein hydrolysates (PHs) were collected at 0, 5, 12, and 24 h for further analyses. The enzyme was thermally inactivated at 100 °C. After inactivation, the hydrolysates were centrifuged (5000× g; 20 min) and then frozen. The hydrolysates were captured at −18 °C and resuspended at room temperature before analysis.
The control consisted of protein isolates (PIs) not treated with enzymes but subjected to the same thermal conditions and incubation times as the enzymatic samples. The hydrolysis conditions were established based on previously published studies describing the catalytic characteristics of Cucurbita ficifolia serine protease [20,21,22,23].
2.6.3. Degree of Hydrolysis (DH) [%]
The degree of hydrolysis (DH) was determined according to the method of Silvestre (1996), based on the concentration of peptides soluble in 5% trichloroacetic acid (TCA) [50]. Briefly, 1 mL of hydrolysate was mixed with 1 mL of 10% TCA and allowed to stand for 1 h to precipitate unhydrolyzed proteins. The samples were then centrifuged at 5000× g for 15 min at 5 °C. Protein concentration in the obtained supernatant was measured spectrophotometrically at wavelengths of λ = 280 and λ = 235 nm. The degree of hydrolysis was expressed as the relative increase in soluble peptide content after enzymatic treatment compared to the control sample.
2.7. Determination of Antioxidant Activity
2.7.1. DPPH
Antioxidant activity was determined by the modified Yen & Chen (1995) method by adding 1 mL of 96% ethanol and 500 μL of a 0.3 mM ethanolic DPPH (2,2-di(4-tertoctylphenyl)-1-picrylhydrazyl) radical solution to 1 mL of peptide solution in Tris-HCl buffer at pH 7.0, with mixing [51]. Absorbance was measured at λ = 517 nm after 30 min of incubation. The antioxidant activity of a 1 mg/mL peptide solution was determined on the basis of a standard curve prepared for Trolox and expressed as [μM Trolox/mg].
2.7.2. Ferric Reducing Antioxidant Power Assay (FRAP)
Antioxidant activity was determined as the ability of the hydrolysate to reduce the Fe(III) to Fe(II) ions in reaction with TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine) [52]. To 1 mL of an aqueous peptide solution was added 3 mL of a working solution containing 1A:1B:10C, where A (10 mM TPTZ in 40 mM HCl), B (20 mM FeCl3 × 6 H2O), C (0.3 M acetate buffer pH 3.6 (3.1 g C2H3NaO2 × 3H2O, 16 mL acetic acid/1 L H2O). The samples were incubated for 10 min at room temperature, and then the absorption was measured at λ = 593 nm. Antioxidant activity was expressed as the ability to reduce the oxidation state of iron ions—Fe(III) to Fe(II). The concentration of Fe(II) ions in the sample was calculated based on a standard curve prepared for specific concentrations of FeSO4 solution. The reducing capacity of enzymatic hydrolysates and peptides was calculated per 1 mg of protein. The results were expressed as [g Fe3+/mg].
2.7.3. Fe(II) Ion Chelation
Chelation of iron ions was determined by colorimetric measurement of Fe(II) not bound by hydrolysate in a reaction mixture using ferrozine (3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate) [53]. To 250 μL of appropriately diluted peptide solution was added 1250 μL of H2O and 110 μL of 1 mM FeCl2 (9.94 mg FeCl2 in 50 mL H2O). After 2 min of incubation, 1 mL of 0.5 mM ferrozine solution (12.31 mg ferrozine in 50 mL H2O) was added. The samples were kept for 10 min at room temperature, after which absorbance was measured at λ = 562 nm. Simultaneously, a blank sample was performed, in which 1000 μL of distilled H2O and a reagent sample (110 μL of 1 mM FeCl2, 1190 μL H2O, 1000 μL H2O) were added. Iron ion chelating activity was expressed as micrograms of bound FeCl2 per milligram of protein [μg Fe2+/mg], based on a standard curve prepared with FeCl2 as standard.
2.7.4. ACE Inhibitory Activity
The ACE inhibitory activity was determined according to the method of Hernández-Ledesma et al., with modifications made for the purpose of the study [54]. Briefly, 20 µL of each sample was added to 0.1 mL of 0.1 M potassium phosphate buffer (pH 8.3) containing 0.3 M NaCl and 5 mM Hip-His-Leu (HHL) as the substrate. After the addition of 5 milliunits (mU) of ACE (Angiotensin-Converting Enzyme, Sigma Aldrich, >2.0 U/mg protein), the reaction mixture was incubated at 37 °C for 30 min. The reaction was stopped by adding 0.1 mL of 1 M HCl. The hippuric acid formed was extracted with ethyl acetate, evaporated by heating at 95 °C for 10 min, redissolved in distilled water, and measured spectrophotometrically at λ = 228 nm. All assays were performed in triplicate. The inhibitory activity was expressed as the percentage of ACE inhibition at a given peptide nitrogen concentration, and the IC50 value (the concentration of peptides required to inhibit 50% of ACE activity) was calculated.
2.7.5. α-Glucosidase Inhibitory Activity
According to Yu et al., peptides were incubated with α-glucosidase and substrate (pNPG) [55]. To 610 µL of 0.1 M potassium phosphate buffer, pH 6.8, were added 10 µL of 3 mM reduced glutathione solution, 5 µL of α-glucosidase (10 U/mL), and 10 µL of peptide solution. The mixture was preincubated for 20 min at 37 °C, after which the reaction was initiated by adding 10 µL of the substrate p-NPG (p-nitrophenyl β-D-glucopyranoside) (10 mM). The reaction was continued for 30 min at 37 °C. After this time, 650 µL of 1 M Na2CO3 was added to terminate the process. The amount of released product, p-nitrophenol, was determined spectrophotometrically at λ = 410 nm. The enzyme inhibitory activity (IC50) was expressed as the amount of substance required to half-inhibit (IC50) the activity of α-glucosidase under the conditions described above.
2.7.6. DPP-IV Inhibitory Activity
Activity was determined according to Giovanni Tulipano’s method [56]. The test material was suspended in 0.1 M Tris-HCl buffer, pH 8.0. The sample (25 µL) was preincubated with the same volume of Gly-Pro-p-nitroanilide substrate (1.6 mM) at 37 °C for 10 min. Then, 50 µL of DPP-IV (0.01 U/mL, in 0.1 M Tris-HCl buffer, pH 8.0) was added to the mixture and incubated at 37 °C for 60 min. The reaction was stopped by adding 100 µL of 1 M acetate buffer, pH 4.0. The released p-nitroanilide hydrolysis product was measured at λ = 405 nm. The DPP-IV enzyme inhibitory activity (IC50) was expressed as the amount of substance required to half-inhibit the DPP-IV enzyme activity under the conditions described above.
2.8. Functional Properties Measurement
2.8.1. Water Absorption Capacity (WAC)
The method was based on Timilsena et al. (2016) [57]. Approximately 1 g of the protein preparation was weighed into a test tube and mixed with 20 mL of distilled water. The mixture was shaken using a laboratory shaker. After 15 min, it was shaken again for 60 s and then centrifuged at 4500× g (RCF) for 15 min (Rotofix 32A; Merazet, Poland). Unabsorbed water was carefully decanted, and remaining droplets were removed with blotting paper. The solid phase was dried at 50 °C for 30 min. Water binding capacity (W [g H2O/g]) was calculated as:
where
C—weight of tube with dried sediment (g);
B—weight of empty tube (g);
A—sample weight (g).
2.8.2. Oil Absorption Capacity (OAC)
The procedure followed that of Wu et al. (2009) [58]. One g of protein preparation was mixed with 15 mL of rapeseed oil and shaken. After 30 min, the mixture was at 4000× g (RCF) for 10 min. The oil binding capacity (X) was calculated as:
where
V—volume of oil used (mL);
L—volume of unabsorbed oil (mL);
a—weight of the sample, calculated per 100 g of dry matter.
2.8.3. Nitrogen Solubility Index (NSI)
According to Achouri et al. (2012) [59], 200 mg of protein preparation was mixed with 15 mL of distilled water. The pH was adjusted to 2, 4, 6, 8, 10, or 12 using 0.5 M NaOH or 0.5 M HCl. Samples were shaken at room temperature for 30 min, then centrifuged at 4500× g (RCF, ≈10,000 rpm) for 15 min (MPW-351 R centrifuge, MPW, Warsaw, Poland, 10,000 rpm). From the supernatant, 10 g was taken for total nitrogen determination using the Kjeldahl method. The total protein content was calculated using a nitrogen-to-protein conversion factor of 6.25, in triplicate. Protein solubility (PS) was calculated as:
where
A—protein content in supernatant (g);
B—protein content in 200 mg of preparation.
2.8.4. Emulsion Stability
Hydrolysates at 1%, 2%, and 3% concentrations were mixed with 10 mL of water and homogenized until uniform. Pre-emulsification was performed using a lab homogenizer for 2 min at 16,000 rpm (T10 basic ULTRA-TURRAX, IKA WerkeGmbH&Co., Baden-Wurttemberg, Germany). Subsequently, the homogenizer was operated for 20 s, during which a quantity of 10 mL of soybean oil was added. Homogenization continued until 2 min had passed from the first drop of oil (total time: 180 s). The emulsion was transferred to a 10 mL polypropylene tube (14 mm internal diameter). Phase separation (sedimentation) was observed at 30 min, 1 h, 2 h, and 3 h. Emulsion stability (SE) was calculated as:
where
a—height of emulsified layer [cm];
b—total emulsion height [cm].
2.8.5. Foam Stability
Into a 1 L beaker, 100 mL of fresh egg white was mixed with hydrolysate (1%, 2%, 3%). The mixture was whipped using a kitchen mixer at 800 rpm for 10 min. The liquid at the bottom was poured into a graduated cylinder. The beaker with foam was left at ~20 °C for 30 min. After 30 min, drainage liquid from the foam was collected and combined with the initial bottom phase for total drainage volume measurement. Foam stability was expressed in millilitres as the total drainage volume and as a percentage [%] of retained foam volume. The control sample contained only fresh egg white, without the addition of any test substances.
2.9. Protein Extrudates
2.9.1. Production
The protein isolates and their hydrolysates obtained from Yarrowia lipolytica JII1c biomass were processed using a laboratory twin-screw extruder (AEV 650, Brabender). The extrusion was conducted according to the methodology of Rytel, under controlled conditions, with a screw compression ratio of 4:1, a screw speed of 180 revolutions per minute, and a load range of 4.5 to 7 amperes [60]. A die with a diameter of 4 mm was used for shaping the extrudates. To assess the impact of processing temperature, the extrusion was performed at three different temperatures: 160 °C, 170 °C, and 180 °C. These parameters were selected to simulate industrial processing conditions for the development of high-protein, plant-based meat analogues.
2.9.2. Sensory Analysis
The sensory evaluation was conducted by a trained panel (n = 8) in accordance with ISO 13299:2016 guidelines [61]. The assessed attributes included taste, odour, texture, saltiness, and appearance. Evaluations were performed under controlled laboratory conditions (20 ± 1 °C, neutral lighting, individual booths). Samples of extrudates were coded with random three-digit numbers and presented in randomized order to avoid positional bias. Panelists rinsed their mouths with water between evaluations. Each attribute was rated on a 5-point hedonic scale, where 1 indicated “very poor” and 5 indicated “excellent.” Results were expressed as mean values. Bioethics approval for the study was granted by the Rector’s Committee for Ethics in Scientific Research, Wroclaw University of Environmental and Life Sciences (Resolution No. N0N00000.020.1.8.4.2024, dated 21 October 2024).
2.10. Statistical Analysis
The analysis was performed with the use of the “STATISTICA 13 PL” program by StatSoft Inc., Tulsa, OK, USA. All determinations were performed in triplicate and presented as average value (X) with standard deviation (SD). Assessment of the significant differences between average values in analysed groups was conducted by variance analysis (ANOVA), followed by a Duncan multiple range test. The level of statistical significance was set at p < 0.05.
3. Results
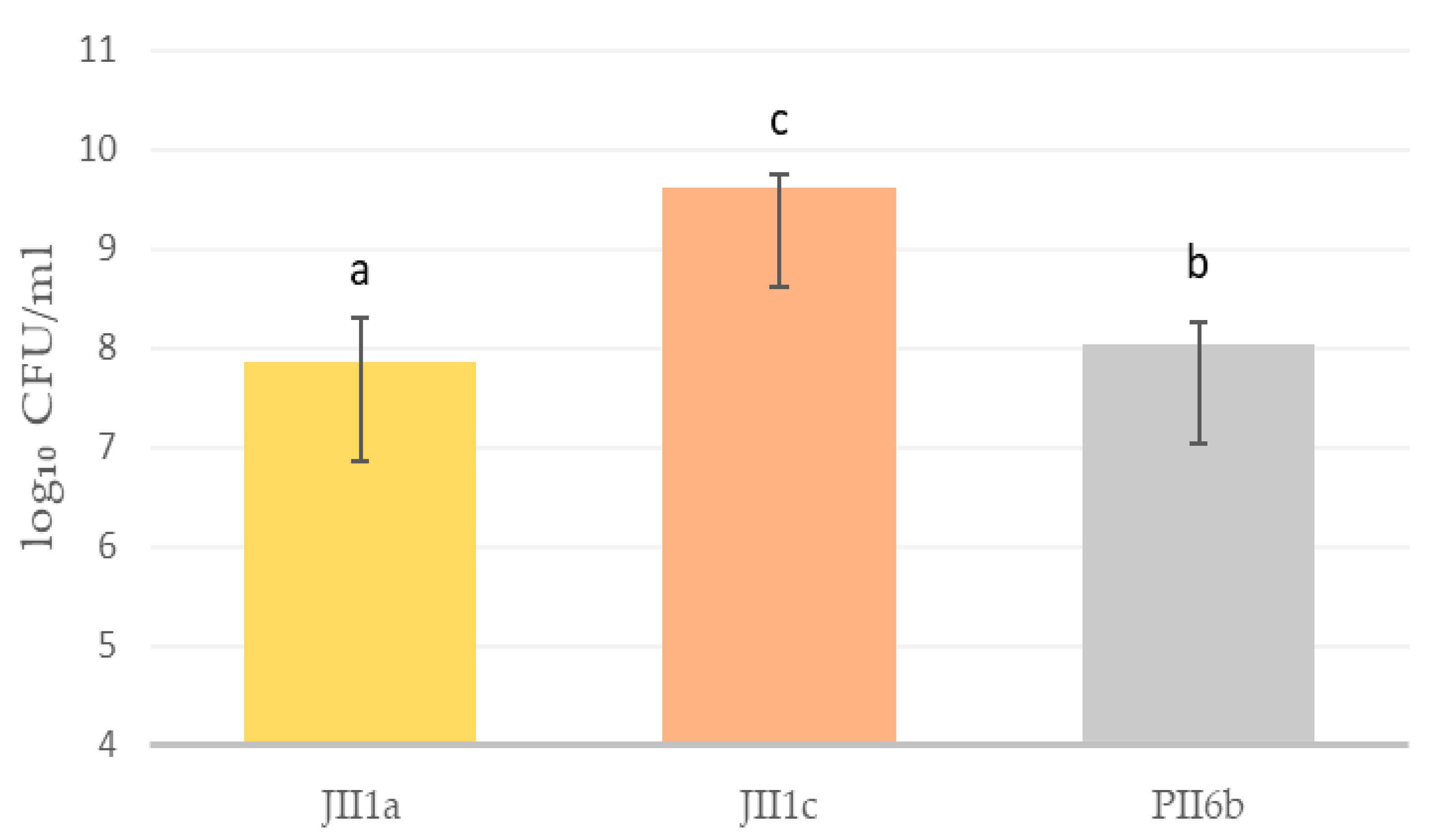
A significant productivity difference was observed between the three Yarrowia lipolytica strains cultivated under bioreactor conditions (Figure 1). The highest determined cell count was observed in strain JII1c, which reached 9.62 log10 CFU/mL, indicating its strong potential for high-yield biomass production. Conversely, strain JII1a exhibited the lowest productivity, measuring 7.86 log10 CFU/mL, while PII6b demonstrated an intermediate value of 8.04 log10 CFU/mL (1II1a vs. JII1c, p = 0.00011; 1II1a vs. PII6b, p = 0.04193; JII1c vs. PII6b, p = 0.00023). The observed differences in cell density were supported by standard deviation bars, suggesting biological variation across replicates. The markedly higher cell count of JII1c reinforces its selection for subsequent protein isolation, as it combines both high biomass accumulation and favourable compositional traits.
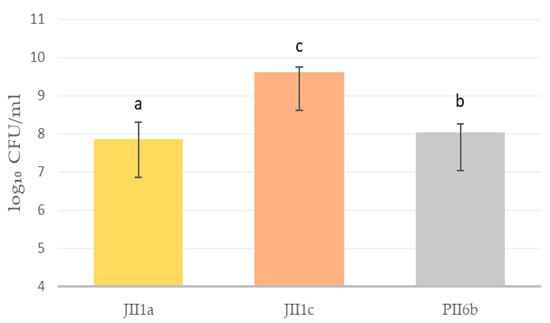
Figure 1.
Biomass productivity of selected yeast strains of Y. lipolytica in bioreactor cultures [log10 CFU/mL]. Small letters (a–c) indicate statistically significant values: a vs. b vs. c (p < 0.05), CFU—colony forming units.
3.1. Biomass Yield and Nutritional Composition
The dried biomass of Yarrowia lipolytica JII1c appeared as a light beige, free-flowing powder with a characteristic yeast-like odour. Microbiological analysis confirmed its safety, showing <10 CFU/mL viable cells, ≤5 × 103 CFU/mL total aerobic microorganisms, ≤102 CFU/mL yeasts and moulds, and the absence of coliform bacteria and Salmonella spp. in 25 g of the sample.
The Nutritional characteristic of dried biomass from Yarrowia lipolytica JII1c strain was presented in Table 1. The chemical composition revealed a high dry matter content (95.11%) and a substantial protein level (43.12%), thus confirming the strain’s potential as a protein-rich ingredient. The carbohydrate content constituted 32.34% of the total composition, with the predominant proportion being dietary fibre (32.32%), and negligible levels of sugars (<0.20%), thereby substantiating its compatibility for low glycaemic formulations. The fat content was moderate (7.03%) and primarily composed of monounsaturated (4.05%) and polyunsaturated (3.30%) fatty acids, with saturated fats present at minimal levels (0.50%). The ash content (11.00%) indicated an elevated mineral load, while the low moisture content (4.89%) ensured the desired shelf stability. The salt content was found to be 4.62 g/100 g.

Table 1.
Nutritional composition characteristic of dried biomass from Yarrowia lipolytica JII1c strain.
The biomass also proved to be a source of micronutrients relevant to human nutrition. It contained a complex of B-group vitamins, including riboflavin, biotin, folic acid, and vitamin B12—compounds of particular importance in plant-based diets. The mineral profile included considerable amounts of phosphorus, potassium, and sodium, alongside nutritionally relevant levels of iron, zinc, magnesium, and other trace elements.
The amino acid profile indicated the presence of all essential and non-essential amino acids, with lysine (31.1 g/kg), leucine (31.2 g/kg), and valine (25.1 g/kg). The content of methionine + cysteine (9.3 g/kg), tryptophan (5.1 g/kg), and histidine (8.7 g/kg) further emphasized the high nutritional quality of the protein. Furthermore, the presence of gamma-aminobutyric acid (11.0 g/kg) may contribute to functional properties. The fatty acid composition was dominated by oleic acid (48.20%) and linoleic acid (29.20%), with minor contributions from saturated fatty acids such as palmitic and stearic acid. Detailed comparative data on the chemical composition and amino acid profile of the three Y. lipolytica strains (JII1a, PII6b and JII1c) are provided in the Supplementary Materials (Table S1).
The protein quality indices indicated that the biomass exhibited high nutritional adequacy, with a chemical score (CS) of 37.80% and an essential amino acid index (EAAI) of 36.17%, calculated relative to whole egg protein. A thorough microbiological analysis was conducted to ascertain the safety of the product. The analysis yielded low levels of aerobic microorganisms, negligible yeast and mould counts, and an absence of coliform bacteria and Salmonella spp.
3.2. Protein Hydrolysis and Peptide Characterization
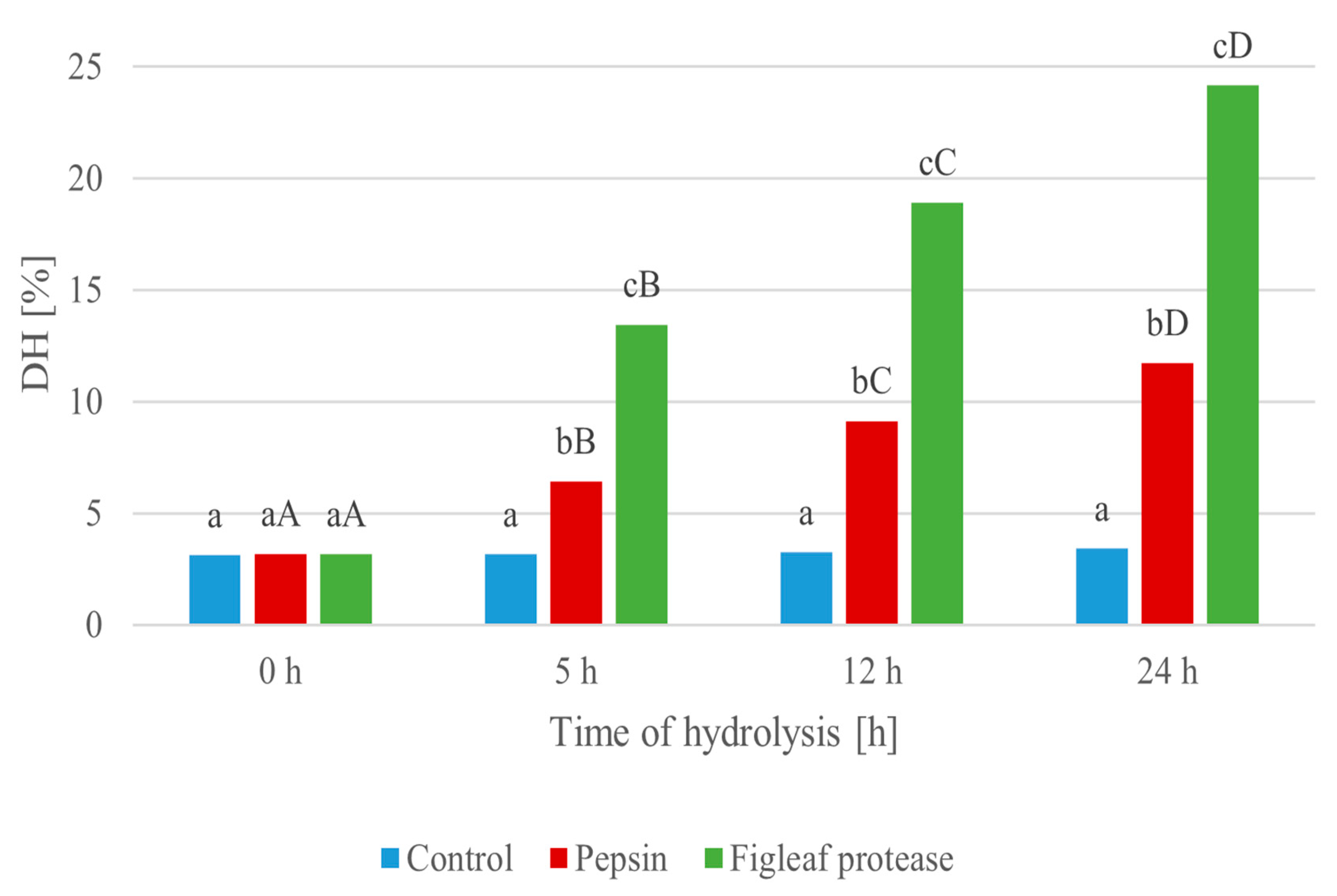
The progression of protein hydrolysis (Figure 2) was evaluated over a 24 h period using two enzymatic treatments: pepsin and a serine protease derived from figleaf pumpkin (Cucurbita ficifolia). As shown in Figure 2, minimal changes in DH values were observed in the control sample, ranging from 3.12% at 0 h to 3.41% at 24 h, indicating spontaneous, non-enzymatic breakdown of peptides [62]. A higher DH indicates more extensive protein breakdown, which can correlate with increased bioactivity and improved techno-functional properties of the hydrolysates.
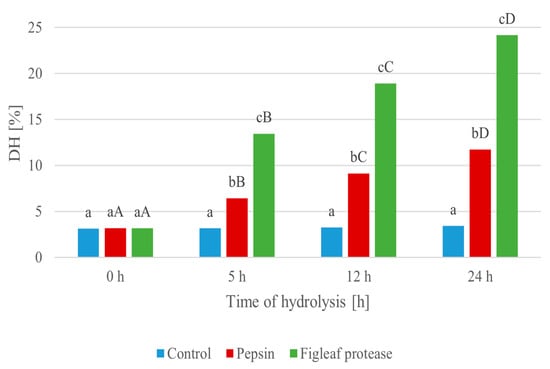
Figure 2.
Degree of hydrolysis (DH [%]) during enzymatic degradation of protein extracted from Y. lipolytica JII1c yeast biomass, carried out using pepsin and serine protease from figleaf pumpkin at different times of reaction. Small letters (a–c) indicate statistically significant differences (p < 0.05) between sampling times within the same enzyme treatment. Capital letters (A–D) indicate statistically significant differences (p < 0.05) between enzyme types (control, pepsin, figleaf protease) at the same time point.
The hydrolysis level statistically increased after enzymatic treatment with pepsin, reaching 11.72% after 24 h, with a clear stepwise progression at 5 h (6.42%) and 12 h (9.13%) (0 h vs. 24 h, p = 0.000077). Moreover, it is notable that the hydrolysis catalyzed by the figleaf protease yielded a statistically higher degree of hydrolysis (DH) over the course of the experiment, increasing from 3.16% at 0 h to 24.15% at 24 h (0 h vs. 24 h, p = 0.000077). The most pronounced increase occurred within the first 5 h (13.45%), indicating strong and rapid proteolytic activity.
Hydrolysate produced using figleaf protease exhibited the highest degree of hydrolysis, confirming the superior efficiency of this plant-derived enzyme in degrading Y. lipolytica protein. Consequently, this hydrolysate was selected for further investigation, including the assessment of its biological activities and techno-functional properties. The 24 h hydrolysate was selected for further analysis, including assessment of its biological activities and techno-functional properties.
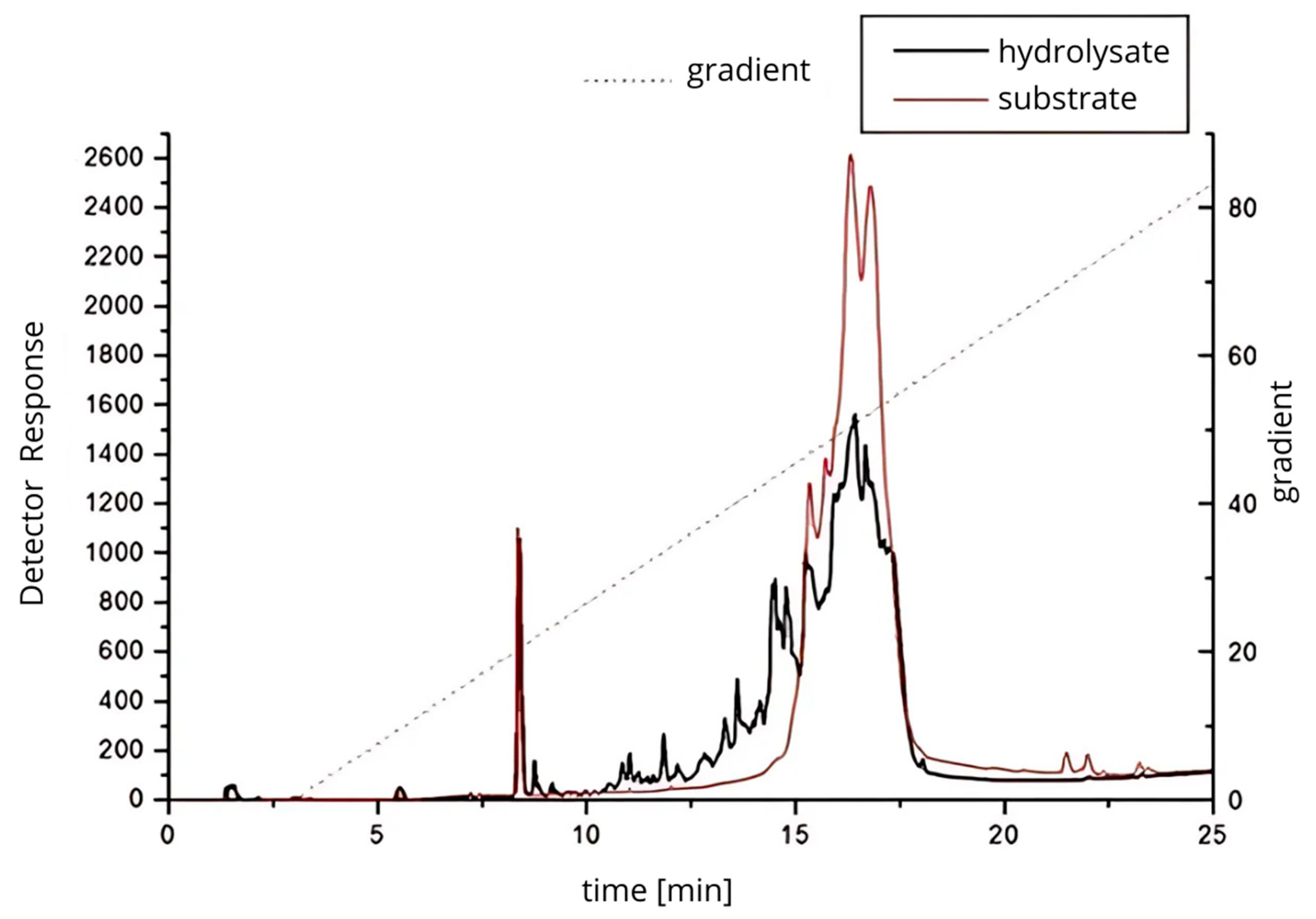
RP-HPLC analysis (Figure 3) demonstrated distinct differences in peptide profiles between the untreated protein isolate and the 24 h hydrolysate generated with Cucurbita ficifolia serine protease. The hydrolysate exhibited a broader distribution of peaks and increased peak complexity, particularly between 10 and 20 min of retention time, reflecting extensive proteolysis and the release of low molecular weight peptides. These chromatographic results are indicative of a more heterogeneous mixture of peptides, likely with varying molecular weights and bioactivities, and confirm the potent proteolytic activity of the figleaf pumpkin enzyme, capable of producing diverse peptide fractions.
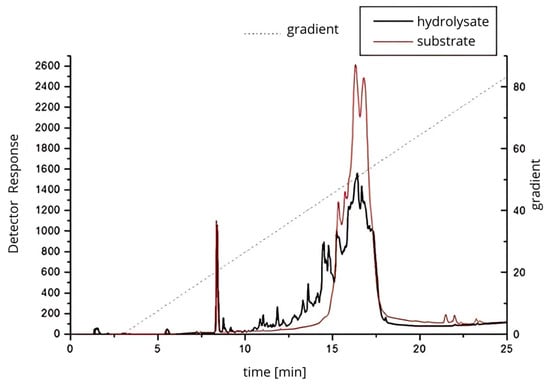
Figure 3.
Protein–peptide separation of 24 h protein hydrolysate from Y. lipolytica JII1c yeast biomass and digested with serine protease from figleaf pumpkin.
3.3. Biological Activities of Hydrolysates
The 24 h enzymatic hydrolysate of Yarrowia lipolytica JII1c protein exhibited significantly enhanced biological activity in comparison with the native protein isolate (Table 2). The antioxidant activity, measured by DPPH and FRAP assays, increased more than twofold (from 0.390 to 0.87 µM Trolox/mg; p = 0.000291) and nearly tenfold (from 4.03 to 39.12 µg Fe3+/mg; p = 0.000191), respectively. Concurrently, the Fe2+ ion chelating capacity exhibited a marked enhancement, rising from 275 to 1326 µg Fe2+/mg (p = 0.000272).

Table 2.
Level of biological activity in a 24 h hydrolysate solution of protein extracted from Y. lipolytica JII1c yeast biomass digested with serine protease from figleaf pumpkin.
In terms of enzyme inhibition, the hydrolysate demonstrated a substantial decrease in IC50 values, indicating enhanced inhibitory potency. ACE inhibitory activity improved nearly fivefold (from 37.43 to 8.20 mg/mL; p = 0.000291). Additionally, α-glucosidase and DPP-IV inhibitory activities increased approximately three- to fourfold, with IC50 values dropping from 12.23 to 4.17 mg/mL (p = 0.000261) and from 10.20 to 2.40 mg/mL (p = 0.000181), respectively.
3.4. Functional Properties of Protein Preparations
The water absorption capacity of the hydrolysate more than doubled compared to the native protein (from 4.34 g H2O/g to 9.12 g H2O/g; p = 0.000291) (Table 3). This indicates an improved exposure of hydrophilic groups and a higher degree of molecular unfolding after hydrolysis. This facilitates water binding, which is advantageous for plant-based formulations requiring moisture retention.

Table 3.
Functional properties of the protein isolate extracted from Y. lipolytica JII1c yeast biomass and its hydrolysate.
A similar trend was observed in the OAC, which increased from 2.35 g oil/g in the protein isolate to 7.23 g oil/g in the hydrolysate (p = 0.000391). The elevated OAC may be attributed to the generation of smaller peptides with exposed nonpolar residues that interact more effectively with lipids, thereby enhancing the emulsion or mouthfeel properties in fat-containing food matrices.
The analysis of the NSI, which reflects protein dispersibility and processing potential, revealed more than a twofold increase in the hydrolysate compared to the isolate (49.20% vs. 19.40%; p = 0.000291).
This enhancement can be attributed to the breakdown of high-molecular-weight proteins into smaller, more soluble peptide fragments, thereby improving the protein’s suitability for use in liquid or semi-liquid food systems.
The stability of egg white foam was found to be significantly influenced by the addition of yeast protein preparations from Yarrowia lipolytica JII1c biomass (Table 4). The incorporation of the protein isolate resulted in a notable enhancement of foam persistence, which was observed to be dose-dependent. At the highest concentration of yeast PI (3%), the leakage volume was reduced to 0 mL (100% of retained foam volume), indicating full foam stability, in contrast to 29.0 mL in the control sample without any additive (p = 0.000059). Lower doses of yeast protein isolate (1% and 2%) also demonstrated a statistically significant improvement in foam stability, in comparison to the control sample, with leakage reduced to 6.6 mL (77.2% of retained foam volume) and 1.9 mL (93.5% of retained foam volume), respectively.

Table 4.
Persistence of egg white foam and emulsion stability of yeast protein isolate Y. lipolitica JII1c and its hydrolysate.
In contrast, the 24 h hydrolysate from Y. lipolytica JII1c showed much lower effectiveness in stabilising the foam (Table 4). Even at the highest tested concentration of 3%, the leakage volume was 2.4 mL (91.7% of retained foam volume), while at 1% and 2% doses, the leakage remained relatively high at 28.0 mL (3.5% of retained foam volume) and 6.8 mL (76.6% of retained foam volume) (p = 0.000152), respectively.
The addition of yeast protein preparations from Yarrowia lipolytica JII1c resulted in a significant enhancement of emulsion stability in a model oil-in-water system (1:1 ratio). The control sample exhibited a gradual decline in stability over time, dropping from 64.87% at 0.5 h to 53.36% at 3 h. Both the protein isolate and its 24 h hydrolysate demonstrated markedly higher and more sustained emulsion stability.
For the isolate, emulsion stability improved with increasing dose. At 3%, emulsion stability remained above 95% throughout the 3 h period, reaching 97.41% at 0.5 h and 95.11% at 3 h. A similar trend was observed for the 1% and 2% doses, though with slightly lower stability than the highest dose. A notable observation was the superior performance of the hydrolysate in comparison to the isolate, particularly in the initial stages of the test. All concentrations of hydrolysate tested yielded consistent and high stability values, exceeding 96% throughout the entire testing period. The highest result was noted for the 3% dose at 0.5 h (96.86%) and 3 h (95.75%), indicating superior emulsifying properties.
3.5. Sensory Evaluation of Extrudates
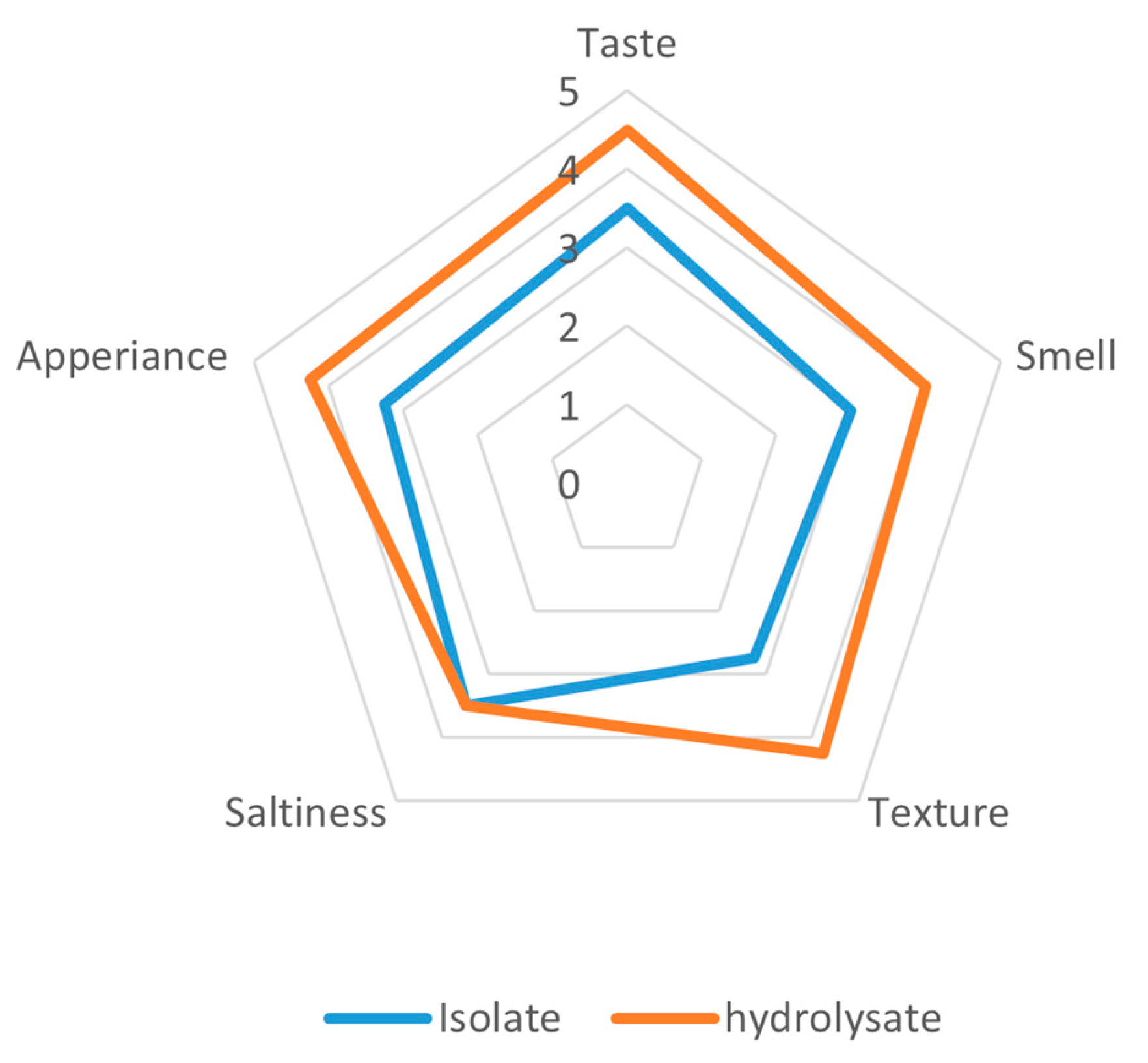
The organoleptic analysis of extrudates supplemented with yeast protein preparations from Yarrowia lipolytica JII1c revealed a clear preference for the hydrolysate over the isolate across most sensory attributes (Figure 4). The hydrolysate-enriched extrudates obtained higher scores in terms of taste (4.5 and 3.5), smell (4.0 and 3.0), texture (4.25 and 2.75), and appearance (4.25 and 3.25). Notably, saltiness perception was consistently unaltered for both samples at a rating of 3.5, indicating that the process of enzymatic hydrolysis did not affect the perception of saltiness.
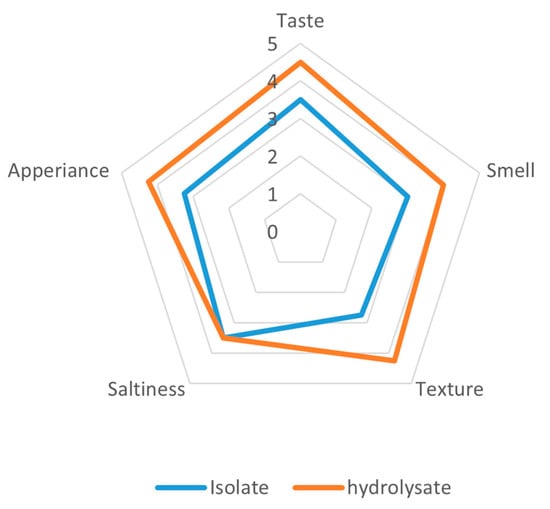
Figure 4.
Organoleptic evaluation of extrudates prepared from a protein isolate extracted from Y. lipolytica JII1c yeast biomass and its hydrolysate.
The most significant differences were observed in texture and aroma, suggesting that the hydrolysate contributed to more appealing sensory properties, such as mouthfeel and aroma profile, possibly due to the formation of flavour-active peptides during hydrolysis. These findings underscore the potential of protein hydrolysates from Y. lipolytica to enhance the sensory profile of extruded food products.
4. Discussion
The primary challenge in the utilisation of yeasts in food applications lies in the efficient release of intracellular components through cell wall disintegration. Another crucial aspect is the conversion of proteins into bioactive peptides while maintaining low processing costs. The findings of this study demonstrate that strain selection is a pivotal factor in determining techno-economic feasibility. The findings of this study demonstrate that strain selection is a pivotal factor influencing process efficiency and the potential applicability of yeast-derived proteins in food production [9,63,64,65,66]. Such gains directly support sustainability goals by reducing substrate input and enabling valorisation of industrial side streams.
The nutritional profile of JII1c, characterized by its high protein content and balanced essential amino acid (EAA) pattern, supports its role as an alternative to conventional proteins. This profile is consistent with the ranges reported for Y. lipolytica across various substrates and strains [16,67,68,69]. Although sulphur-containing amino acids continue to be a limiting factor, comprehensive protein quality indices suggest that the EAA composition is appropriate for incorporation into plant-based formulations, particularly due to its ability to complement cereal proteins, as reported by Michalik et al. (2014) [68]. In addition, the presence of dietary fibre and low residual sugars is conducive to a low glycaemic design, while the micronutrient package (iron, zinc, B vitamins including B12) contributes nutritional value relevant to vegan diets and reflects the species’ metabolic flexibility on biofuel-derived streams [25,70]. These characteristics are consistent with the regulatory framework of Y. lipolytica biomass as a novel food supplement in the EU, which stipulates explicit utilisation thresholds [12,71].
With regard to the processing strategy, enzymatic hydrolysis remains the most effective method of solubilising yeast matrices and tailoring protein functionality through controlled cleavage, exposure of reactive side chains, and modulation of molecular size without damaging the proteins [72,73]. In the present study, a plant-derived serine protease from Cucurbita ficifolia was found to exhibit superior proteolytic efficacy in comparison to pepsin, as evidenced by the distinct peptide distributions observed by RP-HPLC. This finding aligns with the current understanding that the selection of enzymes and the prevailing conditions of a reaction (time, pH, enzyme-to-substrate ratio) regulate the ratio of peptides to free amino acids, thereby influencing the balance between functionality and taste [74,75,76,77]. Elevated levels of hydrolysis can lead to an increase in free amino acids, accompanied by a reduction in longer peptides. Consequently, the optimisation process should be oriented towards application-specific endpoints rather than the pursuit of maximal DH alone [75].
Functionally, hydrolysis enhanced water and oil binding and markedly increased solubility, properties critical for moist textures and dispersibility in beverages or semi-liquid systems. Mechanistically, peptide fragmentation and unfolding expose hydrophilic and hydrophobic groups, thereby enabling improved interactions with both aqueous and lipid phases [78,79,80,81]. The observed trade-off between foaming and emulsion stability, with the former being reduced and the latter enhanced, aligns with interfacial principles. This is evidenced by the rapid migration of smaller peptides, which can form cohesive interfacial films that stabilise oil-in-water emulsions. A similar phenomenon has been reported for yeast-derived bioemulsifiers [81].
Beyond functionality, the bioactivities of the hydrolysate were substantially higher than those of the native isolate. This is in line with literature on yeast-derived antioxidant and enzyme inhibitory peptides relevant to cardiometabolic health [5,82,83,84]. Previous studies have demonstrated that the hydrolysate exhibits immunomodulatory, antimicrobial, and anti-inflammatory properties, indicating potential for broader health benefits [85,86,87]. The sensory improvements observed in extrudates are consistent with the enhanced release of amino nitrogen and small taste-active peptides contributing to umami, mouthfulness, and flavour continuity [88]. This relationship between the biochemical activities and sensory perception suggests that peptides responsible for antioxidant and enzyme inhibitory effects may also modulate taste responses, thereby linking nutritional and sensory functionality. Recent findings further indicate that yeast peptides may enhance saltiness perception through receptor interactions, offering a plausible method for reducing sodium levels without compromising flavour intensity [89].
The following section will address the practical implications of the aforementioned points. For manufacturers, Y. lipolytica JII1c provides a flexible platform for the generation of application-tuned peptide fractions. The benefits that can be derived from these emulsions and beverages include high solubility and emulsion stability. Meat analogues and baked systems, meanwhile, leverage water/oil binding. Finally, savoury snacks and extruded matrices gain from umami-active peptides and potential salt reduction. The utilisation of a plant protease has been demonstrated to enhance consumer acceptance of processing aids, thereby aligning with prevailing sustainability narratives.
Although the current results highlight the potential of Y. lipolytica proteins, there are still several challenges to overcome. The bioavailability and stability of the proteins during processing and storage, as well as their sensory thresholds in complex foods, require confirmation in both model and real systems. Future work should also address bitterness at higher degrees of hydrolysis and identify bioactive peptides. Finally, techno-economic analyses are required to benchmark enzyme costs and scaling-up scenarios.
5. Conclusions
Yarrowia lipolytica JII1c exhibited elevated levels of protein and significant biomass yield, in addition to high levels of dietary fibre and essential micronutrients (e.g., iron, zinc, B vitamins), indicating its potential as an alternative protein source.
The amino acid profile of the biomass demonstrated a balanced EAA composition, and although sulphur-containing amino acids remained limiting, the overall protein quality (CS and EAAI) supports its use in plant-based nutrition systems.
Enzymatic hydrolysis using serine protease from Cucurbita ficifolia markedly enhanced several techno-functional properties of the protein isolates, notably water absorption capacity, oil absorption capacity and nitrogen solubility, which are key attributes for developing novel food formulations. The process also generated bioactive peptides with increased antioxidant and enzyme inhibitory activities that, in addition to their physiological potential, enhanced the perception of umami and saltiness, demonstrating their promise as natural flavour enhancers for reduced-sodium and clean-label food products.
The study highlights the multifunctional character of Y. lipolytica, combining nutritional richness, functional versatility, and sensory benefits, which strongly supports its use as a natural, health-promoting ingredient in sustainable food production. The yeast’s natural origin, efficient bioprocess scalability, and ability to valorise agro-industrial by-products further strengthen its relevance to the circular bioeconomy model and to the growing demand for environmentally responsible protein sources.
In order to apply these results in a practical context, it is essential that future research focus on evaluating the bioavailability of the released peptides, their stability during food processing and storage, and comprehensive toxicological and safety assessments for human consumption. Furthermore, the investigation of sensory optimisation, digestive stability, and techno-economic feasibility under industrial conditions will provide a clearer path towards the commercial utilisation of Y. lipolytica-derived proteins and peptides in modern nutrition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14213801/s1, Table S1: Nutritional composition characteristic of dried biomass from Yarrowia lipolytica JII1a, JII1c, an PII6b strains.
Author Contributions
Conceptualization, M.S.; methodology, M.S. and A.D.; software, A.M.; validation, M.S.; formal analysis, M.M.; investigation, M.M.; resources, M.S.; data curation, M.M.; writing—original draft preparation, M.N.; writing—review and editing, A.D.; visualization, A.M.; supervision, A.D.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wrocław University of Environmental and Life Sciences (Poland), the Department of Research Administration (DRA) as part of the research project “The use of Yarrowia lipolytica yeast protein as a potential source of biologically active peptides and an ingredient useful in food processing”/no. N090/0011/23.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Rector’s Committee for Ethics in Scientific Research (No. N0N00000.020.1.8.4.2024, 21 October 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gorissen, S.H.M.; Witard, O.C. Characterising the Muscle Anabolic Potential of Dairy, Meat and Plant-Based Protein Sources in Older Adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, P.; Grochowicz, J.; Łusiak, P.; Żukiewicz-Sobczak, W. Development of Alternative Protein Sources in Terms of a Sustainable System. Sustainability 2023, 15, 12111. [Google Scholar] [CrossRef]
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.N. Current Global Food Production Is Sufficient to Meet Human Nutritional Needs in 2050 Provided There Is Radical Societal Adaptation. Elem. Sci. Anthr. 2018, 6, 52. [Google Scholar] [CrossRef]
- Li, X.; Cao, Q.; Liu, G. Advances, Applications, Challenges and Prospects of Alternative Proteins. J. Food Compos. Anal. 2025, 137, 106900. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Meng, D.; Zhou, Z.; Zhang, Y.; Yang, R. Yeast Proteins: The Novel and Sustainable Alternative Protein in Food Applications. Trends Food Sci. Technol. 2023, 135, 190–201. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-Based Food and Protein Trend from a Business Perspective: Markets, Consumers, and the Challenges and Opportunities in the Future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef]
- Abe-Inge, V.; Aidoo, R.; Moncada de la Fuente, M.; Kwofie, E.M. Plant-Based Dietary Shift: Current Trends, Barriers, and Carriers. Trends Food Sci. Technol. 2024, 143, 104292. [Google Scholar] [CrossRef]
- Fatima, N.; Emambux, M.N.; Olaimat, A.N.; Stratakos, A.C.; Nawaz, A.; Wahyono, A.; Gul, K.; Park, J.; Shahbaz, H.M. Recent Advances in Microalgae, Insects, and Cultured Meat as Sustainable Alternative Protein Sources. Food Humanit. 2023, 1, 731–741. [Google Scholar] [CrossRef]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A Critical Review of Fungal Proteins: Emerging Preparation Technology, Active Efficacy and Food Application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Schorer, R.; Arthur, W.; Drabold, E.; Rudar, M.; Higgins, B. Upcycling Nutrients from Poultry Slaughterhouse Wastewater through Cultivation of the Nutritional Yeast, Yarrowia lipolytica. J. Environ. Chem. Eng. 2025, 13, 115245. [Google Scholar] [CrossRef]
- Cao, X.; Liu, H.; Yang, M.; Mao, K.; Wang, X.; Chen, Z.; Ran, M.; Hao, L. Evaluation of the Nutritional Quality of Yeast Protein in Comparison to Animal and Plant Proteins Using Growing Rats and INFOGEST Model. Food Chem. 2025, 463, 141178. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica Yeast Biomass as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef] [PubMed]
- Mamaev, D.; Zvyagilskaya, R. Yarrowia lipolytica: A Multitalented Yeast Species of Ecological Significance. FEMS Yeast Res. 2021, 21, foab008. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, D.; Ciccone, M.; Siroli, L.; Lanciotti, R.; Patrignani, F. Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds. Fermentation 2022, 8, 708. [Google Scholar] [CrossRef]
- Carsanba, E.; Agirman, B.; Papanikolaou, S.; Fickers, P.; Erten, H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation 2023, 9, 687. [Google Scholar] [CrossRef]
- Drzymała, K.; Mirończuk, A.M.; Pietrzak, W.; Dobrowolski, A. Rye and Oat Agricultural Wastes as Substrate Candidates for Biomass Production of the Non-Conventional Yeast Yarrowia lipolytica. Sustainability 2020, 12, 7704. [Google Scholar] [CrossRef]
- Lalić, A.; Jagelavičiūtė, J.; Rezić, T.; Trivunović, Z.; Žadeikė, D.; Bašinskienė, L. From Bakery Leftovers to Brewing Sustainability: Fermentation of Spent Grain with Yarrowia lipolytica and Lactobacillus Acidophilus. Sustainability 2025, 17, 782. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Mora-Cortes, W.G.; Leandro-Roldan, M.M.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Ramírez-Suarez, J.C.; González-Córdova, A.F.; Vallejo-Córdoba, B. Production of Whey Protein Hydrolysates with Angiotensin-Converting Enzyme-Inhibitory Activity Using Three New Sources of Plant Proteases. Biocatal. Agric. Biotechnol. 2020, 28, 101724. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Szołtysik, M.; Babij, K.; Pokora, M.; Zambrowicz, A.; Chrzanowska, J. Application of Asian Pumpkin (Cucurbita ficifolia) Serine Proteinase for Production of Biologically Active Peptides from Casein. Acta Biochim. Pol. 2013, 60, 117–122. [Google Scholar] [CrossRef]
- Maciejewska, M.; Dąbrowska, A.; Cano-Lamadrid, M. Sustainable Protein Sources: Functional Analysis of Tenebrio Molitor Hydrolysates and Attitudes of Consumers in Poland and Spain Toward Insect-Based Foods. Foods 2025, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Eckert, E.; Pokora, M.; Bobak, Ł.; Dąbrowska, A.; Szołtysik, M.; Trziszka, T.; Chrzanowska, J. Antioxidant and Antidiabetic Activities of Peptides Isolated from a Hydrolysate of an Egg-Yolk Protein by-Product Prepared with a Proteinase from Asian Pumpkin (Cucurbita ficifolia). RSC Adv 2015, 5, 10460–10467. [Google Scholar] [CrossRef]
- Babij, K.; Bajzert, J.; Dąbrowska, A.; Szołtysik, M.; Zambrowicz, A.; Lubec, G.; Stefaniak, T.; Willak-Janc, E.; Chrzanowska, J. Hydrolysis with Cucurbita ficifolia Serine Protease Reduces Antigenic Response to Bovine Whey Protein Concentrate and As-Casein. Amino Acids 2015, 47, 2335–2343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Da Silva, R.M.; da Silva, C.N.; de Faria-Júnior, C.S.; Buarque, F.S.; Ribeiro, B.D.; Lemes, A.C.; Coelho, M.A.Z. Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis. Processes 2025, 13, 615. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- AOAC International. Official Method of Analysis for Dry Matter, 18th ed.; AOAC International: Arlington, TX, USA, 2007. [Google Scholar]
- AOAC International. AOAC Official Method 2011.25: Insoluble, Soluble and Total Dietary Fiber in Foods. Enzymatic-Gravimetric-Liquid Chromatography; AOAC International: Arlington, TX, USA, 2011. [Google Scholar]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- AOAC International. Method 996.06, Fat (Total, Saturated, and Monounsaturated) in Foods. Official Methods of Analysis; AOAC International: Arlington, TX, USA, 1995. [Google Scholar]
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- EN 14084:2003; Foodstuffs—Determination of Trace Elements—Determination of Calcium, Copper, Iron, Magnesium, Manganese, Sodium, Phosphorus, Potassium and Zinc by ICP-OES. European Committee for Standardization: Brussels, Belgium, 2003.
- López-Bascón, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar]
- AOAC. Official Method 944.12 Folic Acid in Vitamin Preparation, Microbiological Methods; AOAC: Gaithersburg, MD, USA, 1996. [Google Scholar]
- AOAC. Official Method 992.05 Folic Acid in Infant Formula, Microbiological Methods; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- AOAC. 985.32 Vitamin B6 (Pyridoxine, Pyridoxal, Pyridoxamine) in Ready-to-Feed Milk Based Infant Formula; AOAC: Gaithersburg, MD, USA, 1988. [Google Scholar]
- AOAC. 961.15 Vitamin B6 (Pyridoxine, Pyridoxal, Pyridoxamine) in Food Extracts, Microbiological Methods; AOAC: Gaithersburg, MD, USA, 1975. [Google Scholar]
- AOAC. Official Method 960.46. Vitamin Assays, Microbiological Method; AOAC: Arlington, TX, USA, 2006. [Google Scholar]
- AOAC. Official Method 940.33 Riboflavin (Vitamin B2) in Vitamin Preparations, Microbiological Methods; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- PN-EN 13805:2003; Foodstuffs. Determination of Trace Elements. Pressure Mineralization. Polish Committee for Standardization: Warsaw, Poland, 2004.
- Ardö, Y.; Gripon, J.-C. Comparative Study of Peptidolysis in Some Semi-Hard Round-Eyed Cheese Varieties with Different Fat Contents. J. Dairy Res. 1995, 62, 543–547. [Google Scholar] [CrossRef]
- Liang, X.; Qian, G.; Yang, H.; Chen, N.; Ai, Z.; Xing, Y.; Huang, W.; Xu, L.; Li, M.; Wang, Z.; et al. Evaluation of IgG/IgE-binding Capacity and Functional Properties of Enzymatic Hydrolysis in Skimmed Cow Milk System. J. Food Sci. 2023, 88, 2780–2795. [Google Scholar] [CrossRef]
- WHO. Energy and Protein Requirements. In Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 1985; Volume 724, pp. 1–206. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; FAO Food and Nutrition Paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. ISO: Geneva, Switzerland, 2008.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony Count Technique. ISO: Geneva, Switzerland, 2006.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- Shetty, K.J.; Kinsella, J.E. Preparation of Yeast Protein Isolate with Low Nucleic Acid by Succinylation. J. Food Sci. 1979, 44, 633–638. [Google Scholar] [CrossRef]
- Dryjanski, M.; Otlewski, J.; Polanowski, A.; Wilusz, T. Serine Proteinase from Cucurbita ficifolia Seed; Purification, Properties, Substrate Specificity and Action on Native Squash Trypsin Inhibitor (CMTI I). Biol. Chem. Hoppe Seyler 1990, 371, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.P.C. Review of Methods for the Analysis of Protein Hydrolysates. Food Chem. 1997, 60, 263–271. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Xu, X.; Katayama, S.; Mine, Y. Antioxidant Activity of Triptic Digest of Hen Egg Yolk Phosvitin. J. Sci. Food Agric. 2007, 87, 2604–2608. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Miralles, B.; Amigo, L.; Ramos, M.; Recio, I. Identification of Antioxidant and ACE-Inhibitory Peptides in Fermented Milk. J. Sci. Food Agric. 2005, 85, 1041–1048. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-Diabetic Activity Peptides from Albumin against α-Glucosidase and α-Amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey Proteins as Source of Dipeptidyl Dipeptidase IV (Dipeptidyl Peptidase-4) Inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Physicochemical and Functional Properties of Protein Isolate Produced from Australian Chia Seeds. Food Chem. 2016, 212, 648–656. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Ma, T.; Ren, J. Comparative Studies on the Functional Properties of Various Protein Concentrate Preparations of Peanut Protein. Food Res. Int. 2009, 42, 343–348. [Google Scholar] [CrossRef]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame Protein Isolate: Fractionation, Secondary Structure and Functional Properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- Rytel, E.; Kita, A.; Pęksa, A.; Tajner-Czopek, A.; Miedzianka, J. Wpływ Zastosowania Soli w Produkcji Chrupek Kukurydzianych Wzbogaconych Dodatkiem Niekonwencjonalnych Surowców Na Wybrane Cechy Jakościowe. Bromat. Chem. Toksykol. 2015, 48, 512–517. [Google Scholar]
- ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016.
- Butré, C.I.; Buhler, S.; Sforza, S.; Gruppen, H.; Wierenga, P.A. Spontaneous, Non-Enzymatic Breakdown of Peptides during Enzymatic Protein Hydrolysis. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2015, 1854, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.; Melcher, F.A.; Zoll, T.; Hackenschmidt, S.; Kabisch, J. Evaluation of a Yarrowia lipolytica Strain Collection for Its Lipid and Carotenoid Production Capabilities. Eur. J. Lipid Sci. Technol. 2020, 122, 1900172. [Google Scholar] [CrossRef]
- Liu, L.; Pan, A.; Spofford, C.; Zhou, N.; Alper, H.S. An Evolutionary Metabolic Engineering Approach for Enhancing Lipogenesis in Yarrowia lipolytica. Metab. Eng. 2015, 29, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tsirigka, A.; Theodosiou, E.; Patsios, S.I.; Tsoureki, A.; Andreadelli, A.; Papa, E.; Aggeli, A.; Karabelas, A.J.; Makris, A.M. Novel Evolved Yarrowia lipolytica Strains for Enhanced Growth and Lipid Content under High Concentrations of Crude Glycerol. Microb. Cell Fact. 2023, 22, 62. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Yu, K.; Xu, S.; Liu, M.; Sun, J.; Zheng, J.; Zhang, Y.; Yuan, W. Engineering of Yarrowia lipolytica for Producing Pyruvate from Glycerol. 3 Biotech. 2022, 12, 98. [Google Scholar] [CrossRef]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass Production by Novel Strains of Yarrowia lipolytica Using Raw Glycerol, Derived from Biodiesel Production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical Composition and Biological Value of Proteins of the Yeast Yarrowia lipolytica Growing on Industrial Glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef][Green Version]
- Patsios, S.I.; Dedousi, A.; Sossidou, E.Ν.; Zdragas, A. Sustainable Animal Feed Protein through the Cultivation of Yarrowia lipolytica on Agro-Industrial Wastes and by-Products. Sustainability 2020, 12, 1398. [Google Scholar] [CrossRef]
- Jach, M.E.; Sajnaga, E.; Janeczko, M.; Juda, M.; Kochanowicz, E.; Baj, T.; Malm, A. Production of Enriched in B Vitamins Biomass of Yarrowia lipolytica Grown in Biofuel Waste. Saudi J. Biol. Sci. 2021, 28, 2925–2932. [Google Scholar] [CrossRef]
- Timira, V.; Chen, X.; Zhou, P.; Wu, J.; Wang, T. Potential Use of Yeast Protein in Terms of Biorefinery, Functionality, and Sustainability in Food Industry. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13326. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic Hydrolysis of Brewers’ Spent Grain Proteins and Technofunctional Properties of the Resulting Hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of Protein-Rich Extracts from Spent Brewer’s Yeast (Saccharomyces cerevisiae): An Overview. Biomass Convers. Biorefin. 2025, 15, 1771–1793. [Google Scholar] [CrossRef]
- Della Rosa, F.; Tonin, A.; Rocha, B.; Santos, M.; Silveira, F.; Cardoso-Filho, L.; Ribeiro, V.; Meurer, E. Optimization of Hydrolysis and Identification of Bioactive Peptides in Brewery Yeast Residuals. J. Braz. Chem. Soc. 2024, 35, e20230146. [Google Scholar] [CrossRef]
- Min, J.H.; Lee, Y.J.; Kang, H.J.; Moon, N.R.; Park, Y.K.; Joo, S.-T.; Jung, Y.H. Characterization of Yeast Protein Hydrolysate for Potential Application as a Feed Additive. Food Sci. Anim. Resour. 2024, 44, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Lin, Q.; Johns, P.W. Estimation of Degree of Hydrolysis of Protein Hydrolysates by Size Exclusion Chromatography. Food Anal. Methods 2021, 14, 805–813. [Google Scholar] [CrossRef]
- Suh, H.J.; Shin, J.C.; Kim, J.H.; Jang, J.H.; Han, S.H. Optimal Enzyme Selection for Organic Whey Protein Hydrolysis. Korean Soc. Food Nutr. 2017, 30, 1359–1363. [Google Scholar]
- Dent, T.; LeMinh, A.; Maleky, F. Comparison of Colorimetric Methods for Measuring the Solubility of Legume Proteins. Gels 2024, 10, 551. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current Insights into Protein Solubility: A Review of Its Importance for Alternative Proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Louhasakul, Y.; Cheirsilp, B.; Intasit, R.; Maneerat, S.; Saimmai, A. Enhanced Valorization of Industrial Wastes for Biodiesel Feedstocks and Biocatalyst by Lipolytic Oleaginous Yeast and Biosurfactant-Producing Bacteria. Int. Biodeterior. Biodegrad. 2020, 148, 104911. [Google Scholar] [CrossRef]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and Characterization of a Bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Hang, Y.; Wang, J.; Hou, Y.; Hu, S.-Q. Production of Yeast Hydrolysates by Bacillus subtilis Derived Enzymes and Antihypertensive Activity in Spontaneously Hypertensive Rats. Food Biotechnol. 2020, 34, 262–281. [Google Scholar] [CrossRef]
- Guo, H.; Guo, S.; Liu, H. Antioxidant Activity and Inhibition of Ultraviolet Radiation-Induced Skin Damage of Selenium-Rich Peptide Fraction from Selenium-Rich Yeast Protein Hydrolysate. Bioorg. Chem. 2020, 105, 104431. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent Brewer’s Yeast (Saccharomyces cerevisiae) as a Potential Source of Bioactive Peptides: An Overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef]
- Amorim, M.M.; Pereira, J.O.; Monteiro, K.M.; Ruiz, A.L.; Carvalho, J.E.; Pinheiro, H.; Pintado, M. Antiulcer and Antiproliferative Properties of Spent Brewer’s Yeast Peptide Extracts for Incorporation into Foods. Food Funct. 2016, 7, 2331–2337. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Monteiro, M.; Almeida, M.G.; Caldeira, J.; Arneborg, N.; Prista, C.; Albergaria, H. Antimicrobial Properties and Death-Inducing Mechanisms of Saccharomycin, a Biocide Secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 159–171. [Google Scholar] [CrossRef]
- Caldeira, J.; Almeida, G.; Macedo, A.L.; Silva, J.P.M.; Albergaria, H. Saccharomycin, a Biocide from S. cerevisiae That Kill-off Other Yeasts. Ann. Med. 2024, 51, 94–95. [Google Scholar] [CrossRef]
- Cui, C.; Qian, Y.; Sun, W.; Zhao, H. Effects of High Solid Concentrations on the Efficacy of Enzymatic Hydrolysis of Yeast Cells and the Taste Characteristics of the Resulting Hydrolysates. Int. J. Food Sci. Technol. 2016, 51, 1298–1304. [Google Scholar] [CrossRef]
- Niu, Y.; Gu, Y.; Zhang, J.; Sun, B.; Wu, L.; Mao, X.; Liu, Z.; Zhang, Y.; Li, K.; Zhang, Y. Characteristics of Saltiness-Enhancing Peptides Derived from Yeast Proteins and Elucidation of Their Mechanism of Action by Molecular Docking. Food Chem. 2024, 449, 139216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).