Abstract
To identify the underlying mechanisms by which H. rhamnoides fruit extract exerts regulatory effects on intestinal function, we investigated its chemical composition using UHPLC Q-Orbitrap HRMS and evaluated its biological effects on Aquaporin-3 (AQP-3) expression via Western blot in the intestinal epithelial cell line (HT-29). Moreover, fecal microbiota from healthy and constipated adults was employed to mimic the in vitro fermentation of the digested extract and evaluate its effects on gut microbiota functionality. Antioxidant capacity (i.e., Total Phenolic Contents (TPC), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and ferric reducing antioxidant power (FRAP) assays) was assessed prior to and after simulated digestion and fermentation processes. Short-chain fatty acids (SCFAs) were quantified using UHPLC-RID of the fermented samples. In the extract, 23 compounds belonging to a variety of classes (mainly polyphenols) were tentatively identified. The extract significantly upregulated AQP-3 expression in the absence of cytotoxicity. After in vitro fermentation with gut microbiota isolated from constipated subjects, ABTS and FRAP values significantly decreased, as well as TPC, suggesting a greater consumption of antioxidant compounds, consistent with the increased production of radical compounds associated with constipation. Fermentation with intestinal microbiota with healthy and constipated gut microbiota resulted in an increase in SCFA. These results provide preliminary insights into a non-pharmacological strategy for functional constipation.
1. Introduction
Constipation is characterized by a pattern of symptoms, including hard or lumpy stools, abdominal discomfort, infrequent or difficult bowel movements, and/or a sensation of incomplete evacuation. Functional constipation (FC), a distinct constipation subtype, usually occurs in the absence of anatomical abnormalities or underlying systemic disease and is primarily influenced by unsuitable dietary habits, psychological stress, and altered composition and function of intestinal microbiota (dysbiosis) [1]. Based on the Rome IV criteria [2], the global prevalence rate of chronic idiopathic constipation is estimated at ≈14% [3]. Current clinical practice primarily relies on a combination of dietary and lifestyle modifications alongside the use of laxatives as a first-choice approach to manage FC, with emphasis on increasing dietary fiber intake (up to 25–30 g/day) to exert prebiotic activity, stimulating the gut microbiota to produce short-chain fatty acids (SCFAs), and helping retain water in the colon, thereby increasing stool bulk [4,5,6,7]. However, despite the widespread availability of over-the-counter laxatives and dietary fiber food supplements, most individuals with FC report incomplete symptom relief. Moreover, high dietary fiber doses cause side effects, e.g., bloating, gas, and abdominal distension, which greatly contribute to poor treatment adherence [8]. Several botanical species exert potential benefits to improve FC symptoms [9]. In parallel, the use of traditional laxative plants, such as Senna alexandrina Mill., Aloe vera (L.) Burm.f., Frangula purshiana (DC.) A.Gray ex J.G.Cooper, is restricted in Europe due to safety concerns. Indeed, the EFSA (European Food Safety Authority) is currently monitoring the carcinogenic potential of hydroxyanthracene derivatives they contain [10]. This underscores the need to explore effective and safer alternatives [9].
Hippophae rhamnoides L., commonly known as sea buckthorn, has a long history of traditional use in both Europe and Asia for a wide range of health conditions. While due to their tannin content, H. rhamnoides leaf extracts are used to treat diarrhea [11], fruit extracts are used to treat jaundice, respiratory tract diseases (asthma), cardiovascular risk factors (hypercholesterolemia and hyperglycemia) and gastrointestinal disorders, including constipation [12,13,14]. This wide range of effects is linked to the fruit content of nutritional and bioactive components, e.g., carbohydrates, lipids (fatty acids and phytosterols), amino acids, dietary fiber (cellulose, hemicelluloses, pectin, lignin), fat-soluble vitamins (A, E and K), vitamin C, organic acids, polyphenols, and terpenes [15]. Recently, an H. rhamnoides fruit hydro-methanol extract was tested in ex vivo and in vivo model systems for its therapeutic effectiveness in constipation [16]. The results were consistent with a laxative and prokinetic effect of H. rhamnoides fruit extract and increased fecal production, only in part mediated by activation of muscarinic receptors. The authors’ conclusions suggested that the extract may exert its effects via uncharacterized and as-yet unexplored mechanisms of action [16].
Here, we have evaluated the metabolite profile of a hydroethanolic extract from H. rhamnoides fruits to improve our knowledge of its chemical composition and identify underlying mechanisms of action through which this extract exerts its regulating effect on intestinal function. The in vitro bio-accessibility following simulated oro-gastrointestinal digestion and colonic fermentation by means of intestinal microbiota isolated from both healthy and constipated subjects was also assessed. The main results indicate that, following digestion and fermentation with the microbiota isolated from constipated subjects in H. rhamnoides extract, there is greater consumption of compounds with antioxidant activity, in line with the greater production of radical compounds, probably induced by constipation. Furthermore, the extract globally improves the functionality of both healthy and constipated gut microbiota by increasing SCFAs. Finally, the increase in AQP-3 expression suggests an additional mechanism of action for this plant extract. These results provide valuable insights into a non-pharmacological strategy for functional constipation.
2. Materials and Methods
2.1. H. rhamnoides Extract
Three batches of commercial, dry, powdered hydroethanolic extract of H. rhamnoides extract (standardized to contain a minimum of 0.10% isorhamnetin from the fruits, HPLC method, ref. Eur. Ph. 10.0, Monograph 1827), were provided by EPO S.R.L. (Milan, Italy). The extract was prepared by hydroethanolic extraction (50% ethanol), followed by concentration and evaporation solvent removal to dryness to obtain the standardized crude extract.
2.2. UHPLC Q-Orbitrap HRMS Analysis of H. rhamnoides Extract
The Vanquish UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) was used for the chromatographic analysis. The system comprised a thermostated column oven (TCC-3100, Thermo Fisher Scientific, Waltham, MA, USA), a refrigerated autosampler (WPS-3000, Thermo Fisher Scientific, Waltham, MA, USA), an in-line degasser (GPL-3400RS, Thermo Fisher Scientific, Waltham, MA, USA), and a binary solvent delivery pump (HPG-3400RS, Thermo Fisher Scientific, Waltham, MA, USA). At 30 °C, separation was accomplished on a Kinetex Biphenyl column (100 × 2.1 mm, 2.6 µm; Phenomenex, Torrance, CA, USA). Solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in methanol) were used as the mobile phase, which had a steady flow rate of 0.4 mL/min. The overall runtime was 13 min. The gradient program was the following: 100% A was maintained for 0.5 min, then linearly decreased to 30% A from 0.5 to 1.5 min, further reduced to 15% A from 1.5 to 8.0 min (corresponding to an increase in solvent B—0.1% formic acid in methanol—to enhance elution strength), and turned back to 100% A from 8.0 to 11.0 min, and maintained at that level for 2 additional min to wash and re-equilibrate the column. The injection volume was 5 µL. A Q-Exactive Orbitrap instrument (Thermo Fisher Scientific, Waltham, MA, USA), operated in negative mode, was used to perform high-resolution mass spectrometry. Data acquisition included full-scan MS followed by all-ion fragmentation (AIF). In full MS mode, data were collected over a mass-to-charge (m/z) range of 80–1200 with a resolving power of 70,000 full width at half maximum (FWHM). The acquisition rate was two scans/second, the maximum injection time was 0.2 s, and the automatic gain control (AGC) target was set at 1 × 106. AIF acquisition was carried out with a resolution of 17,500 FWHM, an m/z range of 80–1200, and an AGC target of 1 × 105. The scan duration was 0.1 s, and the maximum injection time was again set to 0.2 s. Fragmentation was induced using stepped normalized collision energies ranging between 15 and 45 eV. An isolation window of 5 m/z and a retention time integration window of 30 s were applied for AIF data collection. The ionization source parameters were as follows: spray voltage at 2.8 kV, capillary temperature of 275 °C, S-lens RF level set at 50, sheath gas (nitrogen, purity > 95%) at 35 arbitrary units, auxiliary gas at 10 units, and auxiliary gas heater temperature maintained at 350 °C. Compound identification followed the Metabolomics Standards Initiative (MSI) guidelines. Metabolites were annotated based on accurate mass (±5 ppm), MS/MS fragmentation pattern matching with spectral libraries, and comparison with authentic standards when available. Identifications were classified as Level 1 when confirmed by retention time, accurate mass, and MS2 spectra of standards; Level 2 for putatively annotated compounds based on accurate mass and MS2 spectral similarity; and Level 3 for putatively characterized compound classes showing characteristic fragmentation behavior but lacking full structural confirmation. MS2 data were used to support structural elucidation and annotation confidence. Mass accuracy was controlled with a tolerance of 5 ppm to ensure precise identification of analytes. Routine external calibration was performed before each analytical batch to ensure mass accuracy within ±5 ppm. Data processing and analysis were carried out using Xcalibur software (version 3.1.66.19, Thermo Fisher Scientific, Waltham, MA, USA) [17].
2.3. Evaluation of AQP-3 Expression in HT-29 Cells
2.3.1. HT-29 (Intestinal Epithelial Cell Line) Culture Conditions
The human colorectal adenocarcinoma adherent epithelial cell line, HT-29, was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were maintained in a humidified atmosphere with 5% CO2 at 37 °C, using Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin.
2.3.2. Cell Viability Determination
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was employed to assess cell viability. A total of 2.5 × 104 HT-29 cells per well were plated in a 96-well plate. The following day, cells were treated with increasing concentrations of H. rhamnoides fruit extract (2.1–210 ug/mL) and H. rhamnoides—maltodextrin combination (3–300 ug/mL). After 24 h, 25 µL of MTT solution (5 mg/mL in saline; Sigma-Aldrich, Milan, Italy) was added to each well, and the plate was incubated at 37 °C for 1.5 h. The resulting blue formazan crystals were dissolved in dimethyl sulfoxide (DMSO), and absorbance was measured at 490 nm using a microplate spectrophotometer (Multiskan FC, Thermo Scientific™, Waltham, MA, USA).
2.3.3. Protein Extraction and Western Blot
Collected cells (5 × 105/well) were lysed using RIPA (Thermo Fisher Scientific, Waltham, MA, USA) 1X lysis buffer 1X supplemented with 10 μL/mL phenylmethylsulfonyl fluoride (PMSF), 10 μL/mL sodium orthovanadate, and 10 μL/mL protease inhibitor and centrifuged at 14,000× g for 5 min at 4 °C. Protein concentration in the supernatant was determined by Bio-Rad protein assay (BioRad, Richmond, CA, USA), with bovine serum albumin (BSA) as the standard.
The 35 μg samples were loaded onto 8% SDS polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad Laboratories Inc.). The membranes were then blocked with 5% non-fat dry milk for 1 h at room temperature and incubated overnight at 4 °C with primary antibody: anti-Aquaporin 3 (ab125219, Abcam (Cambridge, UK), 1:1000) and anti-Alpha Tubulin (11224-1-AP, Proteintech (Rosemont, IL, USA), 1:2000). Following primary antibody incubation, membranes were rinsed with PBS Tween 0.05% and incubated with secondary antibody, anti-rabbit (HAF008, R&D System, Minneapolis, MN, USA, 1:1000), for 2.5 h. After washing with PBS Tween, membranes were exposed to enhanced chemiluminescence (ECL) detection and imaged using the Chemidoc imaging system (Bio-Rad Laboratories Inc.). Image quantification was performed using Image Tool software 3.0.
2.4. In Vitro Digestion
H. rhamnoides fruit extract underwent in vitro gastrointestinal digestion to simulate physiological human intestinal processes, according to Minekus et al. [18], with minor changes [19]. Briefly, 5 g of extract was mixed with 5 mL of simulated salivary fluid (SSF), followed by the addition of 0.5 mL of fresh α-amylase solution (150 U/mL). Following incubation for 2 min at 37 °C in a shaking water bath, 10 mL of simulated gastric fluid (SGF) containing pepsin (4000 U/mL; pH 3.00) was added. The final volume was adjusted to 20 mL, and the mixture was allowed to incubate for 2 h at 37 °C in a shaking water bath. Finally, gastric chyme was mixed with 5 mL of freshly prepared pancreatin (800 U/mL) and 2.5 mL. Gastric chyme was mixed with 20 mL of simulated intestinal fluid containing freshly prepared pancreatin (200 U/mL) and bile salts (20 mM). The final volume was adjusted to 40 mL (pH 7.00), and the mixture was incubated for 2 hrs at 37 °C in a shaking water bath. Enzyme activity was stopped at the end of each step by immersion of tubes in ice for 15 min. The supernatant at each digestion step was collected and stored at −20 °C for subsequent analysis of the antioxidant capacity, while the pellet (i.e., the undigested fraction) was directly used for in vitro fermentation. Three independent in vitro digestions were performed on separate aliquots of the extract (biological replicates, n = 3). Antioxidant assays on digested supernatants were measured in technical triplicate for each biological replicate.
2.5. In Vitro Fermentation
In vitro fermentation of the digested H. rhamnoides fruit extract was carried out according to Pérez-Burillo et al. [20]. Adult donors were healthy Caucasian and constipated subjects. Since samples collected from human subjects were used, the study adhered to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Granada (protocol code 1080/CEIH/2020) for the adult participants. None of the donors had taken antibiotics within the previous three months. For each arm (healthy and constipated), fecal material from three donors was pooled to prepare a single standardized inoculum (the donor is not the unit of replication). Using this pooled inoculum, we carried out three independent batch fermentations per arm (biological replicates, n = 3). Centrifuge tubes containing 10% of the final digestion volume and 500 mg of the solid digestion residue were supplemented with 7.5 mL fermentation growth medium (peptone (14 g/L), cysteine (312 mg/L), sodium sulfide (312 mg/L), and resazurin (0.1% v/v)) and 2 mL fecal inoculum, which was prepared by suspending the fecal material in phosphate-buffered saline, 33% w/v. Nitrogen was bubbled into the tubes to maintain anerobic conditions. The tubes were incubated under oscillation for 20 h at 37 °C, and finally placed on ice for 15 min to stop microbial activity. Sample centrifugation (6000× g for 10 min) allowed separation and collection of supernatant, which was stored at −20 °C for analysis of antioxidant capacity and SCFAs (technical triplicates per biological replicate) and SCFA analysis.
2.6. Antioxidant Capacity Profiling
2.6.1. TPC Determination
The Folin–Ciocalteu assay for the determination of the TPC was carried out according to Moreno-Montoro et al. [21]. Briefly, 30 µL of the digestion (or fermentation supernatant) was mixed with 15 µL of Folin–Ciocalteu reagent, 60 µL of sodium carbonate (10% w/v), and 195 µL of ultra-pure water in a 96-well plate (Cytation, Agilent Technologies Inc., Santa Clara, CA, USA). The mixture was incubated for 60 min at 37 °C. Gallic acid (0.01 to 1.00 mg/mL) was used as a standard to draw a calibration curve. The results were expressed as mg gallic acid/100 g (mg GAE/100 g) of the H. rhamnoides extract. Although the Folin–Ciocalteu assay is not fully specific for phenolic compounds and may also respond to other reducing agents present in digested/fermented matrices, it was selected here because it is the most widely adopted colorimetric method in this research area and therefore allows direct comparability of our data with previously published studies.
2.6.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
The DPPH assay of the digested and fermented samples was performed according to Yen and Chen [22]. Supernatants of digested or fermented samples were mixed with the 280 µL DPPH reagent, prepared freshly by dissolving 74 mg DPPH in 1 L methanol. The antioxidant reaction was monitored for 1 h at 37 °C using a Cytation 5 plate reader (Agilent Technologies Inc., Santa Clara, CA, USA). Trolox (0.01–0.4 mg/mL) was used as a standard for the calibration curve. Results were expressed as mmol Trolox equivalents/kg (mmol TE/kg) of H. rhamnoides extract.
2.6.3. 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Assay
The ABTS+ assay of the digested and fermented samples was carried out according to Re et al. [23] with slight modifications. A 7 mM ABTS+ stock solution was mixed with 2.45 mM potassium persulfate to prepare the ABTS+ solution, which was allowed to stand in the dark at room temperature for 16 h. Before use, the ABTS+ solution was diluted with 5 mM phosphate-buffered saline (pH 7.4) before the assay to achieve an absorbance of 0.70 + 0.02 at 730 nm. For the assay, 10 µL of the supernatant from digested or fermented samples was added to 4 mL of diluted ABTS+ solution and incubated for 20 min. The reading absorbance was at 730 nm. The calibration curve was prepared using the Trolox stock solution, and the results were expressed as mmol TE/kg of H. rhamnoides extract.
2.6.4. FRAP Assay
The FRAP assay of the digested and fermented samples was performed using protocols set by Benzie and Strain [24], with slight modifications. Samples (20 µL) were mixed with freshly prepared FRAP reagent (280 µL) in a 96-well plate. The FRAP reagent was prepared by mixing 40 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) solution, ferric chloride solution, and 0.3 M sodium acetate buffer (pH 3.6) in a ratio of 1:1:10. The assay was performed at 37 °C and the absorbance was measured at 595 nm every 30 s for 30 min. Trolox (0.01–0.4 mg/mL) was used as a standard to prepare a calibration curve. Results were expressed as mmol TE/kg of H. rhamnoides extract.
2.7. SCFA Analysis
SCFAs were analyzed in supernatants from fermentation samples according to Panzella et al. [25]. For chromatographic analysis, the samples were centrifuged at 13,300 rpm for 5 min, filtered through a 0.22 µm filter and diluted with 1 M HCl acid in a 1:10 ratio. The analysis was performed under isocratic elution (at 0.5 mL/min flow rate) using an Agilent Poroshell 120 SB-Aq column (3 × 150 mm, 2.7 µm). Sulfuric acid (5 mM) was used as a mobile phase, and a 5 µL sample volume was injected. The temperature of both the column and the refractive index detector was maintained at 35 °C. External standards (propionic, n-butyric, isobutyric acids, lactic acid, and succinic acid) were used to prepare a calibration curve to quantify SCFAs. n-butyrate and isobutyrate were quantified together, while total SCFA was calculated as the sum of the acetate, propionate, and butyrate. SCFAs were quantified from the three fermentation supernatants per arm (biological n = 3). Each sample was injected in duplicate, and duplicates were averaged prior to statistical analysis. Results were expressed as mmol of each acid/liter of the fermented sample.
2.8. Statistical Analysis
Statistical comparison between the groups was carried out as follows. First, the antioxidant capacity (TPC, TEACABTS, TEACFRAP, and TEACDPPH) of the H. rhamnoides fruit extract was evaluated by comparing non-digested samples with those subjected to oro-gastroduodenal digestion. Samples fermented with fecal microbiota (isolated from constipated adult subjects) were compared with those from healthy adults to assess changes in TPC values and antioxidant capacity under healthy and poor conditions. These comparisons were also carried out for samples exposed to duodenal digestion. The increase in SCFAs was assessed by comparing each metabolite produced during fermentation of H. rhamnoides fruit extract by healthy versus constipated microbiota. For these analyses, unpaired t-tests were applied; when the assumption of homogeneity of variance was not met, Welch’s correction was used. To minimize the risk of type I errors due to multiple testing, Bonferroni’s correction was applied.
The significance of the differences between treated samples and untreated cells in the cell viability assay and Western blot analysis was determined using one-way ANOVA followed by Dunnett’s test, with GraphPad Software Prism v9.0 (San Diego, CA, USA).
3. Results
3.1. Metabolite Profile of H. rhamnoides Fruit Extract
The first step was the evaluation of the metabolite profiling of the commercial hydroethanolic H. rhamnoides fruit extract. Both positive and negative electrospray ionization modes during method development were evaluated. However, the compounds of interest in H. rhamnoides extract—primarily phenolic acids and flavonoid—exhibited significantly better ionization efficiency and signal intensity in the negative ion mode, predominantly forming [M–H]− ions. In contrast, ionization in the positive mode resulted in weaker signals and lower sensitivity for most detected metabolites. Hence, we selected negative mode for the final LC–MS analysis to ensure optimal detection and coverage of the characteristic metabolite profile. The isolation window of 5 m/z was selected as a compromise between selectivity and sensitivity, in line with the resolution settings of the instrument and the complexity of the extract matrix. The main classes are flavonoids (Table 1 flavonols, particularly isorhamnetin and its glycosides, as well as quercetin derivatives; flavones, apigenin and glycosides; a flavanol, epicatechin; and a flavanone, naringenin) and phenolic acids (hydroxybenzoic and hydroxycinnamic acids: protocatechuic, syringic, caffeic, and p-coumaric), together with ellagic acid. In terms of abundance (peak area), quinic acid is the most abundant overall; among polyphenols, protocatechuic acid and isorhamnetin glycosides predominate.

Table 1.
Tentatively identified compounds in the hydroethanolic fruit extract by UHPLC Q-Orbitrap HRMS (negative ion mode) and their corresponding analytical parameters (n = 23). * columns report no.; compound name; RT (min); molecular formula; adduct/ion; theoretical m/z; measured m/z; mass accuracy (Δ, ppm); MSI level (1 = confirmed with authentic standard, 2 = putatively annotated by MS/MS spectral match).
Table 1.
Tentatively identified compounds in the hydroethanolic fruit extract by UHPLC Q-Orbitrap HRMS (negative ion mode) and their corresponding analytical parameters (n = 23). * columns report no.; compound name; RT (min); molecular formula; adduct/ion; theoretical m/z; measured m/z; mass accuracy (Δ, ppm); MSI level (1 = confirmed with authentic standard, 2 = putatively annotated by MS/MS spectral match).
| No. | Compound | Retention Time (RT) | Molecular Formula | Adduct/ Ion | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (Δ) | MSI Level |
|---|---|---|---|---|---|---|---|---|
| 1 | Apigenin | 5.28 | C15H10O5 | [M-H]− | 269.04555 | 269.0457 | 0.3717 | 1 |
| 2 | Apigenin 7-glucoside | 5.03 | C21H20O10 | [M-H]− | 431.09837 | 431.0985 | 0.2552 | 1 |
| 3 | Caffeic acid | 4.61 | C9H8O4 | [M-H]− | 179.03403 | 179.0342 | 1.0054 | 1 |
| 4 | Ellagic acid | 4.57 | C14H6O8 | [M-H]− | 300.99899 | 300.9991 | 0.3987 | 1 |
| 5 | Epicatechin | 4.16 | C15H14O6 | [M-H]− | 289.07176 | 289.0723 | 1.7643 | 1 |
| 6 | Isorhamnetin-3-rutinoside | 4.59 | C28O32O16 | [M-H]− | 623.16176 | 623.1622 | 0.6579 | 1 |
| 7 | Kaempferol-3-O-glucoside | 4.61 | C21H20O11 | [M-H]− | 447.09328 | 447.0936 | 0.7157 | 1 |
| 8 | Kaempferol | 5.12 | C15H10O6 | [M-H]− | 285.04046 | 285.0404 | −0.0702 | 1 |
| 9 | Luteolin-7-glucoside | 4.78 | C21H20O11 | [M-H]− | 447.09328 | 447.0938 | 1.1183 | 1 |
| 10 | Naringenin | 5.22 | C15H12O5 | [M-H]− | 271.06119 | 271.0612 | 1.3650 | 1 |
| 11 | p-coumaric acid | 4.36 | C9H8O3 | [M-H]− | 163.04006 | 163.0391 | −2.7601 | 1 |
| 12 | Protocatechuic acid | 2.83 | C7H6O4 | [M-H]− | 153.01933 | 153.0182 | −0.7189 | 1 |
| 13 | Quercetin | 4.85 | C15H10O7 | [M-H]− | 301.03537 | 301.0359 | 1.7274 | 1 |
| 14 | Quercetin 3b glucoside | 4.46 | C21H20O12 | [M-H]− | 463.08819 | 463.0889 | 1.4684 | 1 |
| 15 | Quinic acid | 0.65 | C7H12O6 | [M-H]− | 191.05528 | 191.0562 | 4.9200 | 1 |
| 16 | Rutin | 4.40 | C27H30O16 | [M-H]− | 609.14610 | 609.1461 | 0.0657 | 1 |
| 17 | Syringic acid | 4.30 | C9H10O5 | [M-H]− | 197.04510 | 197.04490 | −1.2180 | 1 |
| 18 | Isorhamnetin | 5.29 | C16H12O7 | [M-H]− | 315.05103 | 315.05140 | 1.0474 | 2 |
| 19 | Isorhamnetin 3-rhamnoside | 5.20 | C22H22O11 | [M-H]− | 461.10893 | 461.10920 | 0.6289 | 2 |
| 20 | Isorhamnetin O-hexoside | 4.65 | C22H22O12 | [M-H]− | 477.10384 | 477.10420 | 0.7336 | 2 |
| 21 | Apigenin-O-hexosy-l-6-C-hexoside | 4.55 | C27H30O15 | [M-H]− | 593.15119 | 593.15230 | 1.9388 | 2 |
| 22 | Quercetin 3-glucoside-7-rhamnoside | 4.26 | C27H30O16 | [M-H]− | 609.14610 | 609.14700 | 1.4282 | 2 |
| 23 | Isorhamnetin-3-rutinoside isomer 2 | 4.39 | C28H32O16 | [M-H]− | 623.16176 | 623.16230 | 0.8505 | 2 |
MS/MS fragmentation data (MS2) were used for the structural elucidation and to support the annotations, particularly for Level 1 and Level 2 identifications. Where available, authentic standards were employed to validate compound identity.
3.2. Bioaccessibility of H. rhamnoides Fruit Extract After In Vitro Simulated Oro-Gastroduodenal Digestion and Fermentation Processes
3.2.1. Impact on H. rhamnoides Polyphenol Stability
The extract was subjected to in vitro simulated oro-gastroduodenal digestion and fermentation using the gut microbiota isolated from healthy and constipated adults to test the effect of digestion and microbial fermentation on individual and total polyphenols. The extract was then analyzed using UHPLC Q-Orbitrap HRMS before and after digestion and fermentation. Table 2 shows the total and % areas of the chromatographic peaks of the identified compounds occurring in the extract after oro-gastroduodenal digestion before (OGDd) and after digestion and fermentation with gut microbiota isolated from healthy (OGDd + healthy fermentation) and constipated (OGDd + constipated fermentation) adults. All in all, oro-gastroduodenal digestion induced a 10% increase in polyphenol total peak areas, the highest % increase being found in apigenin aglycone, maybe due to the hydrolysis of glycosidic bonds of apigenin glycoside derivatives, and p-coumaric acid, likely released as a free polyphenol by the food matrix during digestion. Microbial fermentation also showed a 10% and 70% loss of polyphenol total peak areas in healthy and constipated groups, respectively, suggesting a different metabolic end for the polyphenols when interacting with the gut microbiota isolated from the fecal material of constipated and healthy subjects. These results were confirmed by the TPC measurement (Table 3). The decrease in TPC of the extract (982.6 ± 19.9 mg GAE/100 g after digestion and fermentation using gut microbiota from healthy subjects and 587.3 ± 46.6 mg GAE/100 g, p < 0.01, after digestion and fermentation with gut microbiota from constipated subjects) argues for higher than normal microbial metabolism of polyphenols in constipation.

Table 2.
Impact of simulated oro-gastroduodenal digestion and microbial fermentation with gut microbiota isolated from the fecal materials of healthy and constipated adults on the composition of H. rhamnoides extract determined by UHPLC–HRMS. Extract = undigested extract; OGDd = after oro-gastroduodenal digestion; OGDd + healthy fermentation and OGDd + constipated fermentation = after subsequent batch fermentation with fecal microbiota from healthy or constipated adults. Peak area is reported in arbitrary units. % columns indicate the percentage of each compound’s peak area relative to the corresponding condition’s total peak area (* % after digestion; ** % after digestion + fermentation). Totals at the bottom give the sum of peak areas and % of total area vs. the undigested extract.

Table 3.
Total polyphenol content (TPC) and antioxidant capacity of H. rhamnoides before and after in vitro oro-gastroduodenal digestion and microbial fermentation with gut microbiota of healthy and constipated adults. TPC is expressed as mg gallic acid equivalents per g of extract (mg GAE/100 g); TEAC values from DPPH, ABTS, and FRAP assays are expressed as mmol Trolox equivalents per kg of extract (mmol TE/kg). Values are mean ± SD. Statistics: different letters indicate p < 0.05 for the digestion comparison; ns = not significant; symbol codes.
3.2.2. Antioxidant Properties of H. rhamnoides Fruit Extract, Before and After Digestion and Fermentation
Determination of the residual antioxidant properties of the extract provides an estimate of the radical scavenging and reducing capacity of the extract during the digestion and fermentation processes, with the gastrointestinal tract being a major site of reactive oxygen species (ROS) formation. We selected the DPPH, ABTS, and FRAP assays to comprehensively evaluate the antioxidant capacity of the H. rhamnoides extract, as these methods provide complementary information regarding different antioxidant mechanisms. DPPH and ABTS assays both assess radical scavenging capacity (DPPH primarily captures activity of lipophilic antioxidants, while ABTS also encompasses hydrophilic compounds). In contrast, FRAP measures the ability of antioxidants to reduce ferric (Fe3+) to ferrous (Fe2+) ions, reflecting the reductive potential of the sample [26]. The combined use of DPPH, ABTS, and FRAP provides methodological advantages, as it offsets the limitations associated with any single assay and ensures sensitivity to a wider range of antioxidant molecules present in complex botanical extracts. DPPH is sensitive to smaller, nonpolar antioxidants; ABTS detects both hydrophilic and lipophilic compounds; and FRAP assesses the electron-donating capacity relevant to physiological oxidation-reduction reactions. Statistically significant differences between treatment conditions were obtained (Table 3).
In terms of the radical scavenging activity, digestion processes did not modify the hydrogen donating ability of the H. rhamnoides extract, as shown by statistically unchanged DPPH values before and after digestion (201.8 ± 5.3 vs. 202.5 ± 6.6 mmol TE/kg; Table 3). Following fermentation, DPPH values decreased both in healthy (30.6 ± 1.7 mmol TE/kg) and constipated (45.9 ± 4.9 mmol TE/kg) groups, with differences across the groups being not statistically significant. After oro-gastroduodenal digestion, ABTS+ values were increased significantly (from 35.5 ± 2.7 to 59.3 ± 0.7 mmol TE/kg, p < 0.01; Table 3). This suggests the release of bound antioxidant compounds from the food matrix (or the conversion of polyphenols into more active forms during digestion processes). ABTS+ activity was decreased post-fermentation (21.2 ± 1.1 mmol TE/kg and 15.9 ± 0.6 mmol TE/kg in healthy and constipated groups, respectively). However, consistent with diversity in gut microbiota and microbial metabolism of constipated subjects, the healthy microbiota group had higher residual ABTS+ activity, while the constipated microbiota showed greater use of radical scavengers. On the other hand, consistent with a partial loss of reducing power of H. rhamnoides fruit extract following digestion, FRAP values were decreased to 145.4 ± 3.8 mmol TE/kg after digestion (values before digestion being 194.6 ± 30.8 mmol TE/kg, p < 0.05; Table 3). Following fermentation, FRAP activity was decreased to 2.94 ± 0 mmol TE/kg in healthy adults and was not detected in constipated adults (p < 0.0001), indicating near-to-complete loss of ferric reducing potential.
3.3. SCFA Analysis
SCFAs are the primary metabolites of intestinal microbiota. They are important for the homeostasis of the intestinal environment and promote normal intestinal regularity, being able to stimulate water and electrolyte absorption and to affect gastrointestinal motility. SCFA concentration was determined by UHPLC-RID analysis to evaluate the impact of digested H. rhamnoides fruit extract on the functionality of gut microbiota isolated from healthy and constipated donors. The results (Figure 1) showed a higher concentration of SCFAs produced in response to H. rhamnoides fruit extract in the healthy group (56.02 mM) than in the constipated group (48.57 mM). In particular, lactate, propionate, and butyrate were produced in higher concentrations by the healthy microbiota, whereas acetate showed higher production by the microbiota isolated from constipated donors.
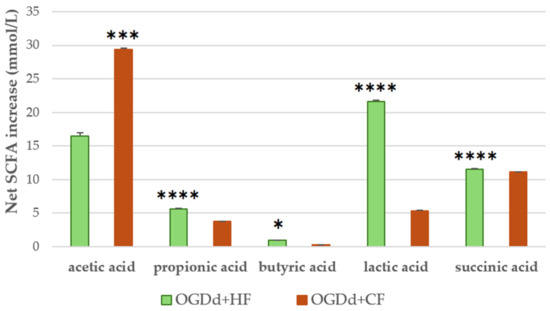
Figure 1.
SCFAs produced by gut microbiota from healthy and constipated adults after fermentation of the digested H. rhamnoides fruit extract. The y-axis shows the net increase in SCFAs, calculated as the sample minus its blank (fecal fermentation without extract). The values are expressed in mmol/L, determined by subtracting the SCFA values of the blank controls (fecal samples fermented without the H. rhamnoides extract) from those of the H. rhamnoides-containing samples. Data are presented as the means ± SD. Statistics between group comparisons were performed by unpaired t-tests (Welch’s correction when needed) with Bonferroni adjustment for multiple testing; * p < 0.05, *** p < 0.001, and **** p < 0.0001.
3.4. Intestinal Stimulatory Effect of H. rhamnoides Fruit Extract on Aquaporin-3 Expression
Aquaporins (AQPs) play an important role in the water transport system of the human body. AQP-3 is predominantly expressed in the colon, ultimately controlling water transport. Several products exhibit a laxative effect by changing the expression level of AQP-3 in the colon. Based on these reports, AQP-3 is hypothesized to play a key role in water transport in the colon. We evaluated the effect of the treatment of HT-29 cells with non-cytotoxic concentrations of H. rhamnoides fruit extract on AQP-3 expression.
3.4.1. Effect of H. rhamnoides Fruit Extract on Cell Viability
As shown in Figure 2, at concentrations ranging from 2.1 to 210 µg/mL, the treatment with H. rhamnoides fruit extract had no significant effects on the viability of HT-29 cells when compared to the vehicle control (10% methanol/water). Hence, H. rhamnoides fruit extract is non-cytotoxic to HT-29 cells in these conditions.
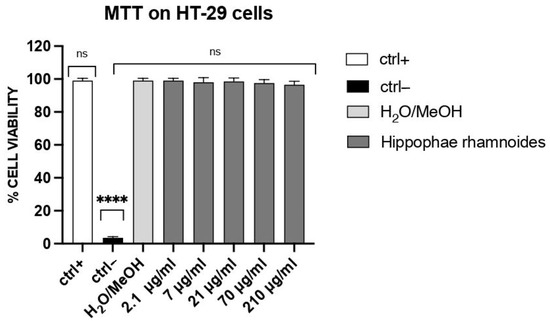
Figure 2.
Effect of H. rhamnoides fruit extract on HT-29 cell viability. Cells were treated for 24 h with the hydroethanolic extract (2.1–210 μg/mL) or vehicle (10/90 methanol/water). Cell viability was evaluated by the mitochondrial-dependent reduction in MTT to formazan. Cell viability was assessed by the mitochondrial-dependent reduction in MTT to formazan. The y-axis reports show % cell viability (mean ± SD, n = 3 independent experiments) calculated from MTT absorbance at 490 nm and normalized to Ctrl+ (set to 100%). Groups: Ctrl+ = untreated cells (no solvent, no extract); Ctrl− = 100% DMSO (cytotoxic control). Statistics: one-way ANOVA followed by Dunnett’s test vs. ctrl+ (biological replicates n = 3; technical triplicates averaged); **** p < 0.0001; ns: not significant.
3.4.2. Effect of H. rhamnoides Fruit Extract on AQP-3 Expression
As shown in Figure 3 and Supplementary Figure S1, treatment of HT-29 cells with H. rhamnoides fruit extract at concentrations ranging from 7 to 70 µg/mL for 24 h resulted in a significant upregulation of AQP-3 expression compared to the vehicle control (10% methanol/water). These results suggest that H. rhamnoides extracts enhance AQP-3 expression in HT-29 cells, indicating their potential role in modulating AQP-3-related cellular functions.
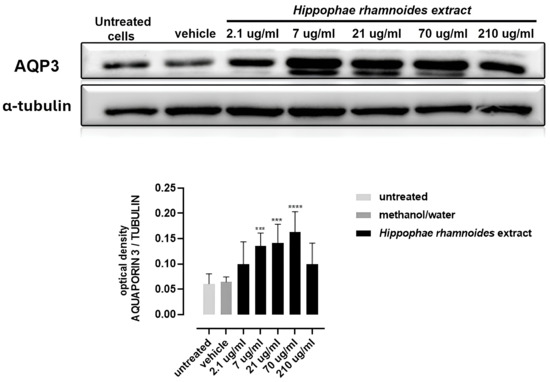
Figure 3.
AQP-3 expression in HT-29 cells after 24 h treatment with H. rhamnoides fruit extract (2.1–210 μg/mL) or vehicle (10/90 methanol/water). Representative Western blots are shown; quantification is reported as the AQP-3/α-tubulin ratio. Statistics: one-way ANOVA with Dunnett’s post-hoc vs. vehicle; *** p < 0.001, **** p < 0.0001.
4. Discussion
Oxidative stress is an important risk factor for colonic dysmotility, which can lead to constipation [27]. Polyphenols exert antioxidant and radical scavenging activities. Since the small intestine is known to absorb polyphenols poorly [28], a large quantity of dietary polyphenols reaches the colon, where they are metabolized by the colonic microbiota [29], with the potential to confer health benefits via modulation of the gut micro-ecology [30]. Its disruption (dysbiosis) can lead to functional gastrointestinal disorders and, in turn, constipation [31]. Thus, prior to assessing bioaccessibility and the biological effects of H. rhamnoides fruit extract, this study was aimed at evaluating its polyphenol profiling using UHPLC Q-Orbitrap HRMS analysis. A rich profile of polyphenols, including both aglycones and glycosides, was revealed by the present study. Among the phenolic acids, protocatechuic acid and caffeic acid exhibit higher peak areas, indicating their predominance. Notably, caffeic acid maintains considerable levels after digestion and fermentation, reflecting its stability and potential bioactivity. Within the flavonol class, the extract revealed high concentrations of isorhamnetin and its derivatives (isorhamnetin-3-rutinoside, isorhamnetin 3-rhamnoside, and isorhamnetin O-hexoside). These products persist relatively abundantly following digestion and fermentation. Quercetin and its glucoside are also present in substantial amounts, although their levels somehow decrease post-fermentation, suggesting moderate stability. Other significant flavonoids include apigenin 7-glucoside and luteolin-7-glucoside, further confirming the richness of flavones in the extract. Our findings align with a comprehensive review that documented the presence of polyphenols from different chemical classes in H. rhamnoides [12]. Another study in which UPLC-PDA-Q/TOF-MS was employed confirmed the presence of phenolic acids, catechins, and flavonol derivatives in an H. rhamnoides fruit extracts. [32].
Although being beneficial for GI health, ROS are potentially involved in the pathogenesis of intestinal disorders, including constipation, due to the oxidative stress generated by an imbalance between their production and catabolism. Thus, we evaluated the antioxidant properties of H. rhamnoides fruit extract before and after the in vitro simulated digestion process and fermentation by fecal microbiota through the determination of the total polyphenol content, DPPH and ABTS radical scavenging activity and reducing capacity. Our results are at variance with most available literature. Li et al. reported a slightly higher (from 32.20 to 33.51 mg GAE/g TPC) fruit extract for H. rhamnoides [32]. Interestingly, our TPC values are higher than those by Ursache et al. with respect to nine different H. rhamnoides cultivars from Slovakia, where TPC ranged from 5.19 to 23.97 mg GAE/g [33]. Other studies have reported significantly higher TPC in different parts of the H. rhamnoides plant, with values ranging from 84.94 to 302.72 mg GAE/g [34,35]. The DPPH, ABTS, and FRAP values in our study were also lower than those reported in the literature. He et al. [36] tested different H. rhamnoides leaf tea extracts and reported DPPH, ABTS, and FRAP values of 20.69–50.21, 0.81–9.84, and 12–17.94 mmol Trolox equivalent/g, respectively. This suggests that leaves may possess a more potent antioxidant profile compared to fruits in this setting. The possibility is now under investigation that differences are likely due to differing extraction procedures, geographical location, or genetic backgrounds of the plants.
In vitro digestion of the H. rhamnoides fruit extract led to a non-significant decrease in its TPC (from 2924.2 mg GAE/100 g to 2235.7 mg GAE/100 g) and a slight increase in the total chromatographic peak area of the selected polyphenols. The DPPH radical scavenging activity persisted stable after digestion (201.8 mmol Trolox equivalent/kg before vs. 202.5 mmol Trolox equivalent/kg after digestion). ABTS values increased significantly after digestion (p < 0.05), vis-à-vis the significant decrease (p < 0.05) of the reducing potential. The data on the content of polyphenols show that some compounds are unstable in the gastric and intestinal environments. Pancreatic enzymes and bile salts can hydrolyze glycosidic bonds, releasing aglycones, which are more susceptible to degradation by digestive enzymes and pH changes [37,38,39,40]. However, digestion can also release polyphenols from their food matrix, thus partially offsetting the loss [41]. The release of these bound polyphenols and the hydrolysis of glycosides into aglycones likely explain why DPPH and ABTS values were not as affected as the TPC. Since aglycones are potent hydrogen donors, the DPPH values were unchanged [42]. Where DPPH is more responsive to lipophilic compounds, ABTS can react with both hydrophilic and lipophilic phenols [23,43].
Fermentation of the digested H. rhamnoides fruit extract was performed using fecal samples from three donors. The pooling of fecal samples from three donors per group is a common practice to average inter-individual variability in in vitro fermentation studies. However, the main limitation of this choice, i.e., the loss of information on individual variation in microbial response, should be acknowledged. The fermentation resulted in a significant loss of total polyphenols, as measured both spectrophotometrically and chromatographically, as well as antioxidant capacity. This effect was more pronounced when fermentation was induced by the gut microbiota isolated from the fecal materials of constipated subjects. Evidence suggests that the gut microbiota degrades complex polyphenols into simpler undetectable metabolites [44,45]. This polyphenol loss directly reduced the antioxidant capacity, as measured by DPPH, ABTS, and reducing power. Additionally, gut microbiota consumes hydrogen donors as metabolic substrates during fermentation and, in turn, decreases DPPH activity [46,47]. While a decreased DPPH activity was found using samples from both healthy and constipated subjects, the effect was more pronounced in the samples from the healthy individuals. This is likely related to a higher metabolic conversion due to a diverse, healthy intestinal microbiota in these settings. The ABTS assay argued for a slightly greater preserving effect in fecal samples from healthy subjects, suggesting that the microbiota in constipated individuals transform radical scavengers into less active forms and/or deplete them more rapidly. The complete loss of FRAP activity is likely due to the extensive degradation of electron-rich polyphenols, e.g., quercetin and catechins, during fermentation, with smaller compounds with negligible reducing power persisting [48,49].
In addition to the direct radical scavenging activity of the H. rhamnoides fruit extract, the effect of the extract following digestion and fermentation with fecal bacteria isolated from healthy and constipated adults on SCFA production was investigated to unravel its impact on gut microbiota functionality. Some investigations have documented that the microbiota exerts a crucial influence on gut motility mediated primarily by SCFA metabolites [50,51,52]. We show that the levels of acetate, butyrate, lactate, succinate, and propionate variably increase in the presence of gut microbiota isolated from both healthy and constipated subjects after exposure to H. rhamnoides fruit extract. All in all, SCFA concentrations in samples from healthy individuals were higher (56.0 mM) than those from constipated subjects (48.6 mM). These results are interesting, as SCFA concentrations are low in subjects with constipation [53]. Some studies suggest that propionic and butyric acid affect intestinal motility by directly acting on colonic smooth muscles [54], and that SCFA can reduce the intestinal transit time by increasing the concentration of serotonin (5-HT) in the gut [55]. Specifically, by stimulating 5-HT release [56], butyrate reduces constipation by increasing intestinal motility. In keeping with this, 5-HT levels in constipated subjects are often lower than normal subjects, causing slowed transit and hardened stools [57,58]. SCFA concentration increases suggest improved functionality of the gut microbiota as well as of its composition in support of microbial species producing SCFA. In this study, a potential reduction in the diversity of lactate- and butyrate-producing bacteria may have shifted fermentation pathways toward acetate as the predominant SCFA present when fermentation is induced by fecal microbiota isolated from constipated adults. Growing evidence suggests that an abnormal gut microbe (dysbiosis) contributes to functional constipation by modifying colonic motility, secretion, and absorption via microbial metabolites such as methane, 5-hydroxytryptamine, bile acids, and SCFA [59]. Dysbiosis in constipation is often characterized by a high concentration of methanogens and a low presence of lactate- and butyrate-producing bacteria [60]. Zoppi et al. [61] found that constipated children had higher levels of Bifidobacteria and Clostridium compared to healthy controls. Khalif et al. observed a similar trend in adults [62]. Using quantitative real-time PCR, Kim et al. showed that feces from patients with functional constipation had abnormally low species of Bacteroides and Bifidobacterium [63]. Using whole-genome and 16S rRNA sequencing, the gut microbial composition of adults with functional constipation was consistent with lower SCFA synthesis [64]. Acetate is physiologically consumed by butyrate-producing bacteria. The lack of these cross-feeding bacteria in constipation might accumulate acetate [65].
Recent studies have proposed AQPs as a therapeutic target in both diarrhea and constipation [66]. AQPs are specialized membrane proteins acting as channels to facilitate water and small, neutral solute transport across cell membranes. Of the 13 known types (AQP0-AQP12), AQP-3 is highly expressed in the colon and plays a key role in water transport, making it a key target for handling constipation [66]. Modulating AQP-3 expression could relieve constipation by enhancing stool hydration. We find that the H. rhamnoides fruit extract (at 7–70 µg/mL concentrations) upregulates AQP-3 expression in HT-29 cells. The regulation of AQP-3 expression by plant and food extracts is predicted to relieve constipation. Soluble fibers from hawthorn (Crataegus monogyna Jacq.) alleviate loperamide-induced constipation in mice by improving gut microbiome and modulating AQP expression. [67]. Likewise, naringenin alleviates loperamide-induced constipation in mice by upregulating AQP-3 expression [68]. On the other hand, consistent with the possibility that pathways other than AQP-3 modulation are involved in constipation relief, a fermented rice extract alleviates loperamide-induced constipation while reducing AQP-3 expression and oxidative stress and suppressing inflammation by altering MAPK phosphorylation [69].
5. Conclusions
The H. rhamnoides fruit extract examined in this study emerged as a rich source of bioactive compounds able to modulate the gut microbiota and AQP-3 membrane protein channel expression. Following in vitro digestion, our study found that the polyphenol content and DPPH radical scavenging activity remained unchanged, the reducing capacity of the extract decreased, and its ABTS values increased. This is tentatively due to the different sensitivity of different antioxidant compounds to different assays and the balance between the degradation of polyphenols, caused by the gastric and intestinal environment and the action of digestive enzymes and bile salts (offset by the release of some polyphenols from the vegetable matrix). When the digested extract was fermented with fecal samples, a key finding was the variability in total polyphenols and antioxidant capacity between samples from healthy and constipated subjects. This difference is accounted for by the diversity in gut microbiota and microbial metabolism of the constipated group. The modulation of SCFA synthesis and AQP-3 expression by the H. rhamnoides fruit extract suggests a plausible direction to be pursued to understand the mechanisms underlying the traditional use of sea buckthorn as a constipation remedy.
This study has several limitations. The simplified in vitro digestion model, while useful, cannot fully replicate the complex digestive and absorptive processes in humans. The practice of pooling fecal samples masks individual differences in microbial response. The biological effects observed lack in vivo validation. Accordingly, these findings remain preliminary, and human studies are mandatory to account for factors like intestinal motility, osmotic gradients, and hormonal effects. To address these issues and to validate our preliminary preclinical results and provide a more comprehensive understanding of the extract’s effects, a randomized, double-blind, placebo-controlled, parallel-arm clinical trial is in progress in subjects with functional constipation diagnosed with Rome IV criteria (https://clinicaltrials.gov/search?spons=epo%20, accessed on 15 October 2025).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/foods14213800/s1, Expression of AQP3 in HT-29 cells treated with Hippophae rhamnoides extract.
Author Contributions
Conceptualization, M.D., J.Á.R.H. and A.I.; investigation, L.F.D.L., Á.T.-M., M.N.-M., D.G.B., A.D.-O., E.C., G.M., M.V.M. and A.P.; data curation, D.G.B., Á.T.-M., M.N.-M., A.D.-O., L.I., E.C. and G.M.; methodology, L.F.D.L., A.D.-O. and L.I.; writing—original draft preparation, A.D.M., H.U., M.V.M. and M.D.; formal analysis, Á.T.-M., M.N.-M., A.D.-O. and L.F.D.L.; visualization, D.G.B.; writing—review and editing, J.Á.R.H., M.V.M., A.D.F., M.G. and M.D.; project administration, M.D.; supervision, M.D., A.I. and J.Á.R.H.; funding acquisition, M.D. and J.Á.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by II Bando Ricerca & Innova, PR FESR 2021–2027 Lombardia—Azione 1.1.1. “Sostegno agli Investimenti in Ricerca, Sviluppo e Innovazione” (Project ID: 5097130) and the “Plan propio de Investigación y Transferencia” of the University of Granada under the program “Intensificación de la Investigación, modalidad B”, granted to José Á. Rufián-Henares.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Granada (protocol code 1080/CEIH/2020, approved date 18 December 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank EPO srl for providing the three batches of the H. rhamnoides fruit extract samples tested in this investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FRAP | Ferric Reducing Antioxidant Power (assay) |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (assay) |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl (assay) |
| TPC | Total Polyphenol Content |
| SCFAs | Short-Chain Fatty Acid(s) |
| AQP3 | Aquaporin-3 |
| FC | Functional Constipation |
| UHPLC-RID | Ultra-High Performance Liquid Chromatography |
| HESI | Heated Electrospray Ionization |
| AIF | All Ion Fragmentation |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| PMSF | Phenyl-Methyl-Sulfonyl-Fluoride |
| BSA | Bovine Serum Albumin |
| SDS | Sodium Dodecyl Sulfate |
| PBS | Phosphate-Buffered Saline |
| OGD | Oro-Gastroduodenal |
| ROS | Reactive Oxygen Species |
| RID | Ultra-High Performance Liquid Chromatography with Refractive Index Detector |
References
- Vriesman, M.H.; Koppen, I.J.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Arco, S.; Saldaña, E.; Serra-Prat, M.; Palomera, E.; Ribas, Y.; Font, S.; Clavé, P.; Mundet, L. Functional constipation in older adults: Prevalence, clinical symptoms and subtypes, association with frailty, and impact on quality of life. Gerontology 2022, 68, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Suares, N.C.; Ford, A.C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Park, K.S.; Nam, K. Chronic functional constipation. Korean J. Gastroenterol. 2019, 73, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Yu, S.; Fedewa, A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 41, 1256–1270. [Google Scholar] [CrossRef]
- Suares, N.C.; Ford, A.C. Systematic review: The effects of fibre in the management of chronic idiopathic constipation. Aliment. Pharmacol. Ther. 2011, 33, 895–901. [Google Scholar] [CrossRef]
- Ashraf, W.; Park, F.; Lof, J.; Quigley, E.M.M. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment. Pharmacol. Ther. 1995, 9, 639–647. [Google Scholar] [CrossRef]
- Pannemans, J.; Masuy, I.; Tack, J. Functional constipation: Individualising assessment and treatment. Drugs 2020, 80, 947–963. [Google Scholar] [CrossRef]
- Liu, T.; Asif, I.M.; Bai, C.; Huang, Y.; Li, B.; Wang, L. The effectiveness and safety of natural food and food-derived extract supplements for treating functional gastrointestinal disorders—Current perspectives. Nutr. Rev. 2024, 83, nuae047. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Safety of hydroxyanthracene derivatives for use in food. EFSA J. 2018, 16, e05090. [Google Scholar] [CrossRef]
- Wani, T.A.; Wani, S.M.; Ahmad, M.; Ahmad, M.; Gani, A.; Masoodi, F.A. Bioactive profile, health benefits and safety evaluation of sea buckthorn (Hippophae rhamnoides L.): A review. Cogent Food Agric. 2016, 2, 1128519. [Google Scholar] [CrossRef]
- Ma, Q.G.; He, N.X.; Huang, H.L.; Fu, X.M.; Zhang, Z.L.; Shu, J.C.; Wang, Q.Y.; Chen, J.; Wu, G.; Zhu, M.N.; et al. Hippophae rhamnoides L.: A comprehensive review on the botany, traditional uses, phytonutrients, health benefits, quality markers, and applications. J. Agric. Food Chem. 2023, 71, 4769–4788. [Google Scholar] [CrossRef] [PubMed]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef]
- Gâtlan, A.M.; Gutt, G. Sea buckthorn in plant based diets. An analytical approach of sea buckthorn fruits composition: Nutritional value, applications, and health benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Hanif, M.; Mehmood, M.H.; Ishrat, G.; Abdullah, A.; Sohail, S.; Ahmed, M.; Gilani, A.H. Evaluation of prokinetic and laxative effects of Hippophae rhamnoides in rodents. Pak. J. Pharm. Sci. 2019, 32, 2527–2533. [Google Scholar]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and antioxidant capacity of bioactive compounds from various typologies of canned tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.R.S.T.E.N.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Buccato, D.G.; Delgado-Osorio, A.; De Lellis, L.F.; Morone, M.V.; Ullah, H.; Izzo, L.; Lombardi, S.; Di Minno, A.; Riccioni, C.V.; Moriki, D.; et al. Exploring the influence of a pomegranate extract on the functionality of healthy and diseased human gut microbiota: An in vitro study. Molecules 2025, 30, 1634. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar] [CrossRef]
- Yen, G.; Chen, H. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.Á.; Cerruti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.Á.; Napolitano, A.; d’Ischia, M. High Antioxidant action and prebiotic activity of hydrolyzed spent coffee grounds (HSCG) in a simulated digestion–fermentation model: Toward the development of a novel food supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef]
- Chen, K.D.; Wang, K.L.; Chen, C.; Zhu, Y.J.; Tang, W.W.; Wang, Y.J.; Chen, Z.P.; He, L.H.; Chen, Y.G.; Zhang, W. Hydrogen-rich water alleviates constipation by attenuating oxidative stress through the sirtuin1/nuclear factor-erythroid-2-related factor 2/heme oxygenase-1 signaling pathway. World J. Gastroenterol. 2024, 30, 2709–2725. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Bosscher, D.; Breynaert, A.; Pieters, L.; Hermans, N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 5–11. [Google Scholar]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Yang, K.; He, Q.; Wang, Y.; Sun, Y.; He, C.; Xiao, P. Impact of drying methods on phenolic components and antioxidant activity of sea buckthorn (Hippophae rhamnoides L.) berries from different varieties in China. Molecules 2021, 26, 7189. [Google Scholar] [CrossRef] [PubMed]
- Ursache, F.M.; Ghinea, I.O.; Turturică, M.; Aprodu, I.; Râpeanu, G.; Stănciuc, N. Phytochemicals content and antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) as affected by heat treatment–Quantitative spectroscopic and kinetic approaches. Food Chem. 2017, 233, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Sne, E.; Seglina, D.; Galoburda, R.; Krasnova, I. Content of phenolic compounds in various sea buckthorn parts. Proc. Latv. Acad. Sci. Sect. B 2013, 67, 411–415. [Google Scholar]
- Kant, V.; Mehta, M.; Varshneya, C. Antioxidant potential and total phenolic contents of seabuckthorn (Hippophae rhamnoides) pomace. Free. Radic. Antioxid. 2012, 2, 79–86. [Google Scholar] [CrossRef]
- He, Q.; Yang, K.; Wu, X.; Zhang, C.; He, C.; Xiao, P. Phenolic compounds, antioxidant activity and sensory evaluation of sea buckthorn (Hippophae rhamnoides L.) leaf tea. Food Sci. Nutr. 2023, 11, 1212–1222. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, J.; Cui, J.; Fan, Y.; Li, N.; Wang, C.; Liu, Y.; Dong, Y. Stability and mechanism of phenolic compounds from raspberry extract under in vitro gastrointestinal digestion. LWT 2021, 139, 110552. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal stability and bioavailability of (poly) phenolic compounds following ingestion of Concord grape juice by humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.L.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Alcaraz, O.; Acosta, M.; Arnao, M.B. On-line antioxidant activity determination: Comparison of hydrophilic and lipophilic antioxidant activity using the ABTS•+ assay. Redox Rep. 2002, 7, 103–109. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut 1993, 34, 437. [Google Scholar] [CrossRef]
- Benoit, S.L.; Maier, R.J.; Sawers, R.G.; Greening, C. Molecular hydrogen metabolism: A widespread trait of pathogenic bacteria and protists. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128. [Google Scholar] [CrossRef]
- Abudureheman, B.; Yu, X.; Fang, D.; Zhang, H. Enzymatic oxidation of tea catechins and its mechanism. Molecules 2022, 27, 942. [Google Scholar] [CrossRef]
- Di Pede, G.; Bresciani, L.; Calani, L.; Petrangolini, G.; Riva, A.; Allegrini, P.; Del Rio, D.; Mena, P. The human microbial metabolism of quercetin in different formulations: An in vitro evaluation. Foods 2020, 9, 1121. [Google Scholar] [CrossRef]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100170. [Google Scholar] [CrossRef]
- Terrapon, N.; Henrissat, B. How do gut microbes break down dietary fiber? Trends Biochem. Sci. 2014, 39, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Rondeau, M.P.; Meltzer, K.; Michel, K.E.; McManus, C.M.; Washabau, R.J. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J. Feline Med. Surg. 2003, 5, 167–173. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543. [Google Scholar]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Dimidi, E.; Scott, S.M.; Whelan, K. Probiotics and constipation: Mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals. Proc. Nutr. Soc. 2020, 79, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, G.; Cinquetti, M.; Luciano, A.; Benini, A.; Muner, A.; Minelli, E.B. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998, 87, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.J.; Constipation Research Group of the Korean Society of Neurogastroenterology and Motility. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Mangifesta, M.; Viappiani, A.; Ticinesi, A.; Nouvenne, A.; Meschi, T.; van Sinderen, D.; et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci. Rep. 2017, 7, 9879. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 9, 915–923. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef]
- Zhang, H.; Zu, Q.; Zhang, J.; Liu, S.; Zhang, G.; Chang, X.; Li, X. Soluble dietary fiber of hawthorn relieves constipation induced by loperamide hydrochloride by improving intestinal flora and inflammation, thereby regulating the aquaporin ion pathway in mice. Foods 2024, 13, 2220. [Google Scholar] [CrossRef]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hossain, M.K.; Kim, S.H.; Jeong, P.Y.; Lee, G.H.; Kim, D.S.; Chung, M.J.; Chae, H.J. Improving intestinal health and mitigating Loperamide-Induced constipation through the modulation of Aquaporin-3 expression, reduction of oxidative stress, and suppression of inflammatory response by fermented rice extract. J. Funct. Foods 2024, 121, 106444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).