Abstract
Total Soluble Solids (TSS) in tomatoes is a core indicator for evaluating fruit quality and processing characteristics. Its composition mainly consists of soluble sugars (such as fructose and glucose) and organic acids (such as citric acid and malic acid). The contents of sugars and acids and their ratio directly affect the flavor and nutritional value. Cultivated tomatoes have a TSS of 4–6%, compared with 10–15% in wild varieties. In recent years, with the advancement of molecular biology and genomics technologies, significant progress has been made in the research on the regulatory mechanisms of tomato fruit TSS and major sugars and acids, including the identification of major quantitative trait locus (QTLs) (Lin5, SlALMT9), functional characterization via CRISPR/Cas9 and elucidation of the transporter network. Breaking the negative correlation between TSS and yield remains a major bottleneck in breeding. Analyzing the mechanism by which environmental factors regulate the TSS and optimizing cultivation measures are crucial for increasing the TSS content in tomatoes. The deep integration of cutting-edge technologies (such as Genome-wide association studies (GWAS), metabolome-wide association studies (mGWAS), Genomic selection (GS), genome editing, and crop modeling) with design breeding is expected to accelerate the development of high-TSS tomato varieties. This paper reviews the current research status from the following four aspects: QTL mapping related to tomato TSS and mining of major genes, metabolic and transport mechanisms of major sugars and acids and key genes, the influence of environmental factors on TSS, and application of genetic improvement strategies and technologies.
1. Introduction
Tomato (Solanum lycopersicum) occupies a central role in the horticultural industry. From wild species to cherry tomatoes and subsequently to large-fruited cultivars, the processes of domestication and varietal improvement have been continuous [1]. For decades, breeding programs have mainly emphasized high yield and stress resistance, while fruit quality has been consistently neglected [2]. In the past decade, people’s consumption has been accelerating the shift from “eating enough” to “eating well” and a “nutritious and healthy diet”. Consumers’ demand for high—quality fruits has been increasing day by day, and fruit quality has become the focus of breeding [3]. In tomato, TSS, consisting mainly of soluble sugars and organic acids, are one of the most important indicators of fruit quality. The distinctive flavor of tomatoes is derived from the combined action of soluble sugars, organic acids, and volatile organic compounds, with the contents and ratios of sugars and acids exerting direct control over fruit taste [4,5]. In tomato raw material processing, TSS is a key indicator determining quality and competitiveness [6]. Based on a 5% TSS threshold in tomato, for every 1% increase, it is equivalent to a 25% reduction in raw material consumption [7]. Consequently, the processing tomato market has clear quantitative minimum requirements for TSS, aiming to pursue extremely high TSS to optimize production costs. In contrast, the fresh—market tomato market focuses more on the coordination between TSS and acidity, striving to provide consumers with the best flavor experience.
Tomato TSS represents a complex quantitative trait controlled by both genetic and environmental factors, and its genetic basis has been extensively investigated. QTL mapping widely employed in tomato breeding has provided support for dissecting the genetic architecture of complex traits, facilitating marker-assisted selection and revealing molecular mechanisms. GWAS have been recognized as an approach based on linkage disequilibrium and have recently provided effective strategies for rapidly identifying QTLs that regulate TSS [8]. Wild tomato species generally exhibit TSS values of 10–15%, approximately two to three times higher than those of cultivated varieties, rendering them valuable germplasms for breeding high-TSS cultivars and mapping TSS-related QTLs [9].
TSS is primarily composed of soluble sugars and organic acids, which constitute approximately 60% of tomato dry weight. The soluble sugars mainly include glucose, fructose, and sucrose, whereas the predominant organic acids are citric acid (CA) and malic acid (MA) [10]. Sugar accumulation and acid balance are regulated by complex interactions between genetic and environmental factors. Sucrose metabolism plays a central role in sugar accumulation, with sucrose phosphate synthase (SPS), sucrose synthase (SuSy), and invertase (INV) acting as key enzymes [11,12,13]. MA and CA participate in multiple metabolic pathways, including respiration, the tricarboxylic acid (TCA) cycle, and the glyoxylate cycle, and can also undergo transformation in mitochondria through the TCA cycle [14,15]. Owing to the involvement of multiple enzymes and transcription factors, sugar-acid metabolism remains difficult to predict and regulate. With the detailed functional analysis of key genes, elucidation of molecular regulatory networks, and construction of regulatory models, it becomes possible not only to enhance sugar and acid accumulation but also to adjust their ratios. Such advances have created the potential for breeding high-quality tomato varieties with elevated TSS and improved fruit flavors. Beyond genetic factors, environmental conditions, including temperature, light, water, fertilizer application, and air composition, exert major effects on TSS. Hence, the optimization of cultivation practices is critical for enhancing tomato TSS.
Cross-breeding in tomato improvement is significantly hindered by the polygenic nature of TSS, which leads to long breeding cycles, low efficiency, and the persistent challenge of linkage drag [16]. Decades of breeding have only achieved marginal TSS benefits while sacrificing the yield. In addition, the loci controlling TSS are located in close proximity to those regulating fruit size, and a negative correlation exists between these two traits [17]. Therefore, strategies for increasing tomato TSS without substantially reducing fruit size remain a critical focus in high-quality breeding programs. Modern biotechnological approaches have provided new opportunities. Based on the identification of TSS-associated markers such as simple sequence repeats (SSR) and single nucleotide polymorphisms (SNP), molecular marker-assisted selection (MAS) has accelerated breeding efficiency [18]. GS further expands the potential of molecular markers by employing genome-wide marker data to construct predictive models, thereby enabling more accurate evaluation of breeding values, enhancing selection efficiency, and accelerating genetic gains [19]. The development of genome editing technologies, including CRISPR/Cas9, has enabled precise gene knockout, knock-in, and base editing in the tomato genome. The first commercialized genome-edited crop, the “Sicilian Rouge High GABA” tomato, has significantly increased the γ-aminobutyric acid (GABA) content in its fruits by editing the SlGAD2/3 genes [20]. In addition, editing the promoter allows for the precise regulation of gene expression levels, providing a more powerful tool for crop breeding. These tools facilitate the creation of novel germplasms, shorten breeding cycles, and overcome interspecific hybridization barriers. On this basis, design-based breeding systematically integrates genomics, bioinformatics, and genetic design principles to achieve the comprehensive analysis and precise assembly of breeding objectives, promoting the shift from “empirical screening” to “directed design” and improving both the predictability and efficiency in tomato breeding.
Meanwhile, the application of high-throughput phenomics and artificial intelligence technologies has provided strong support for the optimization of cultivation management. By integrating multidimensional environmental data with plant phenotypic information, association models linking growth, environment, and traits can be established, offering a precise decision-making basis for water and fertilizer regulation as well as light and temperature management. These approaches maximize the TSS potential of tomato varieties and enable full-process optimization from genetic regulation to field management.
In recent years, the significance of tomato fruit quality has gained widespread recognition. However, a comprehensive review of TSS, a crucial determinant of tomato fruit quality, remains lacking. Therefore, this review article provides a comprehensive overview of research on tomato TSS. We first explore QTL mapping and gene identification, and subsequently summarize the metabolic and transport mechanisms of major sugars and acids along with their key genes. In addition, the impact of environmental factors on TSS is introduced. The article concludes by projecting breeding strategies enhanced by advanced technologies, thus offering a theoretical framework for targeted breeding of high-quality tomatoes.
2. Mapping and Identification of QTLs Associated with Tomato TSS
2.1. QTL Mapping and Identification Based on Hybrid Population
Over the past three decades, researchers have mapped more than 50 TSS QTLs on over 12 tomato chromosomes using different parental populations. The major loci are located on chromosomes 2, 3, 5, and 9 [10,21,22,23,24]. Paterson et al. mapped seven QTLs associated with fruit TSS in progeny derived from a cross between cultivated tomato (Lycopersicon esculentum Mill.) and its wild relative Lycopersicon cheesmanii [25]. Tanksley et al. identified three TSS-related QTLs, ssc5.1, ssc5.2, and ssc5.3, on chromosome 5 in approximately 170 backcross second-generation (BC2) plants derived from a cross between an elite processing inbred line and the wild species Lycopersicon pimpinellifolium (LA1589) [26]. Fulton et al. reported the detection of two TSS-related QTLs in a backcross population generated from cultivated tomato (Lycopersicon esculentum cv. ‘E6203’) and its wild relative Lycopersicon peruvianum [27].
Goldman et al. identified 12 TSS-related QTLs in a recombinant inbred line (RIL) population developed from a cross between cultivated tomato Lycopersicon esculentum and related wild tomato Lycopersicon cheesmanii [28]. Bernacchi et al. mapped seven QTLs associated with TSS in a near-isogenic line (NIL) population constructed using Lycopersicon hirsutum (LA1777) and Lycopersicon pimpinellifolium (LA1589) [29]. Furthermore, Bernacchi et al. identified five TSS-related QTLs located on chromosomes 3, 5, 6, and 9 in a Lycopersicon hirsutum introgression line population [30]. Causse et al. [23] employed a population of 75 introgression lines (ILs) with the genetic background of cultivated tomato (Lycopersicon esculentum, M82), each line containing a chromosomal segment introgressed from the wild species Lycopersicon pennellii (LA0716). They mapped multiple QTLs associated with sugar and organic acid content [23].
Among the identified QTLs, several key genes have been cloned and functionally characterized. In 1988, a comparison of carbohydrate changes and related enzyme activities during fruit development between Lycopersicon chmielewskii (LA1028) and cultivated tomato UC-82B revealed that LA1028 fruit accumulated sucrose [31]. Sucrose accumulation is primarily associated with reduced acid invertase (AI) activity [32]. Through gene mapping with restriction fragment length polymorphism (RFLP) and isozyme markers, a major QTL, sucrose accumulator (Sucr), was identified on chromosome 3, co-segregating with the TG102 marker and encoding vacuolar invertase [33]. Levin et al. discovered another QTL, Fgr on chromosome 4 of Lycopersicon hirsutum, which increased fructose content and reduced the glucose content in mature fruits without affecting the total hexose concentration [34]. To further explore the trait differences between cultivated and wild tomatoes, Eshed et al. developed an introgression line IL9-2-5 with high TSS by crossing Lycopersicon pennellii (LA0716) with cultivated tomato M82 [35]. Subsequently, the major QTL for TSS, Brix9-2-5 was identified, encoding the extracellular invertase Lin5 [36]. Further research indicates that the polymorphism near the catalytic site of Lin5 enhances invertase activity, resulting in an 11–25% increase in sugar content without reducing the total yield [37]. In the red fruits of the Lin5-silenced lines, the glucose content decreased by approximately 30–33% and the fructose content decreased by about 17–26% compared to the wild type [38], whereas Knocking out its invertase inhibitor gene can enhance invertase activity. The glucose content in CRISPR-invinh1 mutants increased by 36.39–40.82%, the fructose content increased by 35.69–42.76%, and TSS increased by 30.17–32.76% compared with the wild type [39].
In recent years, QTLs related to MA and CA contents in tomato fruits have been successively identified. Researchers have evaluated the main sugars and organic acids influencing tomato flavor in Solanum pimpinellifolium and its backcross inbred lines. The study revealed that Solanum pimpinellifolium harbored alleles that increased glucose and fructose contents and regulated acidity through changes in MA and CA levels. Compared with RFLPs/isoenzymes, SNPs have become the cornerstone of modern genetic research by virtue of their absolute advantages of high density, high throughput, and digitization. Using SNP markers, 71 QTLs related to organic acids have been identified [40]. For MA, loci ma1.2, ma1.3, ma2.1, and ma7.1 were consistently detected across populations, indicating the presence of major genetic variations related to MA content. QTLs cca2.1, ca-5E, cca6.3, cca7.1, cca8.1, ca-9F, and ca-10B may represent the major determinants of CA accumulation [41]. Tian et al. [42] used a recombinant inbred line population constructed from the cultivated tomato (Solanum lycopersicum ‘Moneymaker’) and the wild currant tomato (Solanum pimpinellifolium ‘PI365967’). Through bulked segregant analysis—resequencing, six QTLs were identified. Among them, the major—effect locus qCA6.1 (located on chromosome 6) accounted for 19.28% of the phenotypic variation [42]. Notably, ma1.1, ma3.2, ma3.3, and cca3.3 loci overlapped with those identified by Tieman et al. in heirloom tomatoes. In addition, QTLs controlling MA and CA were co-localized on chromosomes 1, 3, 6, and 7 [43].
For a single major QTL, the heritability of TSS it explains can reach up to approximately 30%, but a more common range is 5% to 15%. For the sum of all known QTLs, they may explain more than 50% of the heritability. There are epistatic interactions among different QTLs controlling the TSS content. This intergenic interaction effect is an important genetic basis for the complex quantitative trait of tomato TSS. A study on processing tomatoes found that there is a positive epistatic effect between the high-TSS loci in the introgression lines IL7-3 and IL9-2-5 derived from Solanum pennellii. When these two loci are present simultaneously, they can significantly increase the TSS content of hybrid offspring, showing a synergistic effect [7].
2.2. QTL Mapping and Identification Based on GWAS
GWAS combined with high-throughput genotyping technologies have become a key to dissecting the genetic basis of complex traits in plants [8]. The development of next-generation sequencing technologies and the reduction in sequencing costs have jointly transformed GWAS from an expensive technique into a powerful and standardized research tool capable of systematically dissecting the genetic basis of complex traits by promoting the expansion of sample size, the increase in marker density, the extension of research scope, and the enhancement of statistical power [44]. Newly developed mGWAS have further expanded this approach, providing an efficient forward genetics strategy to reveal the genetic and biochemical mechanisms of plant metabolism [45,46,47,48,49,50]. Sauvage et al. conducted an association analysis of metabolites influencing tomato quality using 5995 SNP markers in a natural population of 163 tomato accessions. A total of 8 loci associated with TSS were identified, distributed across chromosomes 2, 3, 6, 7, 8, 9, 11, and 12; 4 loci related to fructose were identified on chromosomes 3, 4, 5, and 6; 3 loci associated with sucrose were mapped on chromosomes 2, 4, and 5; and 2 loci associated with malate were mapped on chromosomes 2 and 6 [51]. Another study precisely identified two key loci controlling glucose and fructose contents: Lin5 at approximately 31.8 Mb on chromosome 9 (version SL4.0) and SSC11.1 on chromosome 11, as well as multiple loci controlling organic acids. Notably, a specific Asn366Asp mutation in Lin5 (which encodes an extracellular invertase) was further highlighted to significantly increase the hexose content in fruits [43]. A GWAS of 92 primary metabolites across 302 tomato accessions identified multiple loci associated with key fruit quality traits, including TSS, fructose, glucose, malate, and citrate. Notably, the major-effect locus TFM6 (tomato fruit malate 6) on chromosome 6, encoding the aluminum-activated malate transporter 9 (Sl-ALMT9 in tomato), was pinpointed as the primary QTL governing malate variation among genotypes [52]. A GWAS of TSS in red-ripe fruits of 481 tomato germplasm accessions further identified 5 significantly associated QTLs on chromosomes 2, 5, 6, 8, and 9. A sugar transporter, STP1, was identified on chromosome 2 as a regulator of TSS [53].
In summary, these GWAS collectively paint a “panoramic view” of the genetic basis of tomato sugar content: it is a complex network controlled by multiple genes, where chromosomes 2, 3, 5 and 9 are the major-effect regions influencing TSS, and several key functional genes have been precisely mapped. Major loci such as Lin5 and Sl-ALMT9 have been precisely localized in numerous independent studies. This provides a solid theoretical foundation and valuable target resources for subsequent flavor improvement breeding through molecular marker-assisted selection or gene editing.
3. Metabolic and Transport Mechanisms of Main Sugars and Acids in Tomato TSS and Related Genes
3.1. Fruit Development and Overview of TSS Composition
The development and ripening of tomato fruits proceeds through three stages: the cell division stage (2–10 d after flowering), during which cells proliferate rapidly and photosynthetic products are mainly allocated to cell division; the cell expansion stage (10–30 d after flowering), during which cell volume increases while soluble sugar content rises slowly and organic acid accumulation peaks; and the ripening stage (from 40 d after flowering onward), during which soluble sugars accumulate rapidly, organic acids are consumed, and TSS content increases significantly [9].
TSS in tomatoes constitutes approximately 60% of their dry weight and is primarily composed of soluble sugars (including glucose, fructose, and sucrose) and organic acids (mainly CA and MA). Among these, fructose and glucose account for 55–65% of TSS [54], while MA and CA together represent approximately 90% of the total acid content [55]. Therefore, the biosynthesis, degradation, and transport of major sugars and acids in tomatoes are of great importance for TSS improvement. Accumulation of TSS involves complex molecular regulatory mechanisms for sugars and organic acids. Studies have indicated that key enzymes in the glycolytic pathway (such as phosphofructokinase) are genetically associated with the levels of CA and MA, indicating that carbon allocation to organic acids directly influences TSS accumulation [56]. Moreover, the MdNADP-ME gene has been shown to regulate the coordinated accumulation of both acids and sugars [41]. In modern flavor-oriented breeding, an increase in TSS and sugar content does not necessarily lead to a reduction in acid levels. By precisely selecting and pyramiding specific superior alleles (such as Lin5), it is entirely feasible to develop tomato varieties that exhibit both high sugar and high acid content, resulting in a rich and well-balanced flavor profile.
3.2. Metabolic and Transport Mechanisms of Sucrose, Glucose, and Fructose and Related Genes
The contents of sucrose, fructose, and glucose in tomato fruits largely determine TSS, which is critical for fruit quality. Wild germplasm provides valuable resources for breeding tomato varieties with high TSS. According to sugar accumulation type, tomatoes can be classified into two categories: hexose-accumulating and sucrose-accumulating. Green-fruited wild tomato species such as Solanum chmielewskii, Solanum hirsutum and Solanum hirsutum f. glabratum, Solanum peruvianum, and Solanum neorickii accumulate sucrose in fruits, whereas cultivated tomato varieties primarily accumulate reducing sugars such as glucose and fructose, along with smaller amounts of sucrose [57].
In higher plants, sucrose synthesized in leaves (sources) via photosynthesis is transported to sink organs through the phloem. In sink organs, sucrose is enzymatically degraded into hexoses (Figure 1), supporting organ growth and development [58]. The accumulation of soluble sugars is regulated by a complex network of sugar metabolism, including sugar synthesis, transport, decomposition, and signal transduction. Current research has mainly focused on key enzymes in this network, such as sucrose synthase, sucrose phosphate synthase, and invertase, as well as sugar transporters [59] and some transcriptional regulators, which have significantly advanced the understanding of molecular mechanisms underlying sugar accumulation.
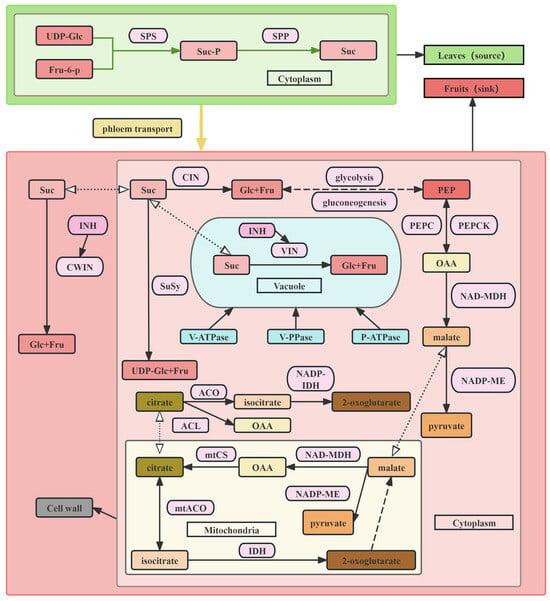
Figure 1.
Metabolism and transport of major sugars and acids in tomatoes.
3.2.1. Metabolic and Transport Mechanisms of Sucrose, Glucose, and Fructose
Sucrose metabolism is a crucial component of sugar accumulation, in which SPS, SuSy, and INV play essential roles. SPS plays a crucial role in sucrose accumulation. In many fruits, sucrose accumulation during ripening is closely associated with increased SPS activity. SPS catalyzes the synthesis of sucrose-6-phosphate using uridine diphosphate glucose as the donor and fructose-6-phosphate as the acceptor. Sucrose-6-phosphate is subsequently hydrolyzed into sucrose and phosphate ions by sucrose-6-phosphate phosphohydrolase (SPP). As SPS and SPP exist as a complex in plants, the reaction catalyzed by SPS to produce sucrose is irreversible [11]. SuSy catalyzes the reversible reaction of sucrose metabolism and exerts significant regulatory effects on fruit quality formation and signal transduction [60]. SuSy and INV hydrolyze sucrose into hexoses, and their activities are regulated by invertase inhibitors (INVINH) [61]. INV catalyzes the decomposition of sucrose into glucose and fructose, contributing to processes such as sugar synthesis, degradation, and transport [13]. In cultivated tomato (Solanum esculentum), high INV activity results in substantial accumulation of glucose and fructose in vacuoles, while the sucrose content remains low. Conversely, in Solanum peruvianum and Solanum chmielewskii, vacuolar INV activity is low, leading to higher sucrose accumulation and reduced glucose and fructose contents. Based on pH conditions, INV is divided into AI and neutral invertase (NI). Cell-wall invertase (CWIN) and vacuolar invertase (VIN) belong to AI. The CWIN encoded by the Lin5 gene on chromosome 9 functions in the intercellular space and cell wall and participates in sucrose decomposition during extracellular unloading from the phloem [36]. VIN, encoded by the Sucr gene on chromosome 3, regulates sucrose utilization within vacuoles, affecting sucrose levels, total sugar, and TSS content in fruits [33]. Cytoplasmic invertase (CIN), classified as a neutral invertase [62], functions in cooperation with SuSy to catalyze the hydrolysis of sucrose into glucose and fructose [58].
To gain an in-depth understanding of the regulation of soluble sugar transport in tomato fruits, two homozygous cherry tomato inbred lines, such as “TB0023” (hexose-accumulating) and “TB0278” (sucrose-accumulating), can be analyzed to characterize sugar transport mechanisms. In the phloem unloading pathway, transport may shift from the symplast to the apoplast or proceed through a mixed symplastic–apoplastic route. The high activities of AI, SPS, SuSy, and the sugar transporters LeSUT1, SlSWEET2a, and SlSWEET12c are critical for promoting hexose accumulation, whereas in sucrose-accumulating types, LeSUT2, SPS, SS, and SlSWEET1b/5b/11b/7a/14 play predominant roles [63]. Silencing of acid invertase TIV1 altered the composition of fruit sugars, increasing sucrose content and reducing fructose content [64]. The sucrose transporter (SUT) also regulates phloem unloading capacity. Inhibition of SUT2 expression can reduce glucose, fructose, and sucrose levels in tomato fruits [65]. Silencing of the plasma membrane-localized proteins SlSWEET7a and SlSWEET14, which mediate sugar transport, increases fruit sugar content, promotes taller plant growth, and produces larger fruits in SlSWEET7a-silenced lines [66]. In contrast, overexpression of the SlFgr gene from the SWEET family can reduce glucose content and increase fructose content [67]. The heterologous expression of the apple hexose transporter MdHT2.2 in tomato significantly elevates the glucose, fructose, and TSS contents in mature fruits, while decreasing sucrose concentration [68]. Sugar transporters, such as the tonoplast sugar transporter (TST) and the vacuolar glucose transporter (VGT), mediate sugar transfer to vacuoles [69]. The apple cytoplasmic H+/glucose symporter MdERDL6-1 promotes glucose efflux and upregulates the H+/sugar antiporter genes TST1 and TST2, thereby increasing glucose, fructose, and sucrose accumulation in tomato fruits. In contrast, double mutation of SlTST1/2 markedly reduces the soluble sugar content [70]. STPs generally function in glucose and fructose transport. CRISPR/Cas9 knockout of STP1 significantly reduces TSS and soluble sugar content in tomato fruits. Mechanistically, the zinc-finger protein ZAT10-LIKE specifically binds to the insertion-type promoter to enhance expression, whereas this binding capacity is absent in the deletion-type promoter, which explains the genetic basis for decreased TSS in modern cultivated tomatoes [53].
3.2.2. Key Genes and Transcription Factors of Sucrose, Glucose, and Fructose
In recent years, multiple studies have identified key genes involved in sugar metabolism (Table 1). The AGPase L1 allele of ADP-glucose pyrophosphorylase increases soluble sugar content by enhancing transient starch accumulation [71]. The upregulation of AGPase expression in tomatoes enhances the starch and TSS contents, while the reduced expression leads to decreased starch synthesis, delayed flowering, and lower yield [72]. VIFs regulate INV activity by forming inactive complexes. In tomatoes, overexpression of SlVIF inhibits sucrose hydrolysis and reduces hexose accumulation, whereas suppression of SlVIF promotes sucrose hydrolysis and increases hexose content [73]. As a key gene encoding CWIN, Lin5 regulates sugar and fructose accumulation in tomato fruits and is controlled by multiple factors. Silencing of Lin5 markedly reduces glucose and fructose levels [38], whereas INVINH1 can specifically suppress Lin5 activity, and the vacuolar-processing enzyme SlVPE5 negatively regulates sugar accumulation [74]. CRISPR/Cas9 editing has provided further insights into gene functions and interactions. Wang et al. suggested that silencing either SlINVINH1 or SlVPE5 increased the glucose, fructose, and TSS content, while double knockouts exerted stronger effects, indicating the synergistic regulation [39]. Kawaguchi et al. successfully knocked out SlINVINH1 using CRISPR/Cas9 and Target-AID technologies, generating high-sugar tomato lines without adverse effects such as reduced fruit size or poor plant growth, thereby confirming the potential of this gene for quality improvement [75]. Studies have shown that knockout of the ripening-specific vacuolar invertase SlVI induces sucrose accumulation, which suppresses the expression of key starch degradation genes BAM3 and AMY2 through a negative feedback mechanism, thereby significantly increasing sucrose and TSS contents while enhancing fruit firmness and post-harvest disease resistance [76]. In addition, the calcium-dependent protein kinases SlCDPK27/26 function as the “sugar brake” by phosphorylating SuSy to promote its degradation. The knockout of these kinases can increase glucose and fructose contents by 30%, enhancing sweetness perception without affecting yield [77].

Table 1.
Functions of genes and transcription factors regulating the main sugar and acid components in the formation mechanism of tomato TSS.
Additionally, several transcription factors have been implicated in the regulation of sugar accumulation (Table 1). The MADS-box transcription factor RIN modulates sucrose metabolism and fruit ripening by directly binding to and regulating the expression of SlVI and SlVIF [73]. The bZIP transcription factors SlbZIP1 and SlbZIP2 participate in sucrose-induced translational silencing (SIRT), thereby affecting sugar content in tomato fruits [87]. A dual-gRNA CRISPR/Cas9 system targeting the upstream open reading frame (uORF) of SlbZIP1 enhances the transcriptional activity of SlbZIP1 and related genes, leading to significant increases in sugar and TSS contents, with edited lines exhibiting improved fruit traits and normal growth [80]. The NAC transcription factor SlNAP2 responds to ABA signaling and regulates ABA homeostasis through feedback, delaying leaf senescence and enhancing soluble sugar and TSS accumulation [78]. The auxin response factor SlARF10 positively regulates starch and sucrose accumulation by modulating the expression of AGPase, a key gene in starch biosynthesis [79]. A recent study has reported that heterologous overexpression in tomato reveals that the nuclear-localized transcription factor MdWRKY126 can coordinately upregulate sucrose synthesis and transport genes (SPS and SUT) and suppress sucrose decomposition genes (CWINV and SuSy) as well as hexose transport genes (HTs and TSTs) [84]. The tomato C2H2-type zinc finger transcription factor SlC2H2-71 is highly expressed in low-TSS fruits. The knockout of SlC2H2-71 resulted in increased TSS, fructose, glucose, MA, and CA contents in red-ripe tomato fruits, accompanied by a reduction in sucrose content. Further analysis demonstrates that SlC2H2-71 can negatively regulate soluble solid accumulation in red ripe fruits by directly binding to the SlLIN5 promoter and repressing its expression [82].
3.3. Metabolic and Transport Mechanisms of Malic Acid and Citric Acid and Related Genes
3.3.1. Metabolic and Transport Mechanisms of Malic Acid and Citric Acid
The biosynthesis and degradation of MA are associated with multiple metabolic pathways, including the respiratory process, TCA cycle, and glyoxylate cycle. During anaerobic glycolysis, oxaloacetate (OAA), generated by the carboxylation of phosphoenolpyruvate (PEP) in the cytoplasm via phosphoenolpyruvate carboxylase (PEPC), is reduced to MA [88]. Conversely, NAD-dependent malate dehydrogenase (NAD-MDH) oxidizes MA to OAA, which is subsequently decarboxylated by phosphoenolpyruvate carboxykinase (PEPCK) to regenerate PEP. This process is associated with gluconeogenesis, that is, the conversion of organic acids to sugars. In addition, MA can be decarboxylated to pyruvate by NADP-dependent malic enzyme (NADP-ME) (Figure 1) [89,90,91,92]. Malate produced in the cytosol can be transported into mitochondria to participate in the TCA cycle, where malate and citrate interconversion occurs. Mitochondrial citrate synthase (mtCS) directly regulates citrate synthesis in OAA. Citrate produced in mitochondria can be reversibly converted to isocitrate by mitochondrial aconitase (mtACO) and then degraded to α-ketoglutarate by isocitrate dehydrogenase (IDH) [14,15]. In the cytoplasm, ATP citrate lyase (ACL) cleaves citrate into OAA and acetyl-CoA, whereas cytosolic aconitase (cytACO) and NADP-dependent isocitrate dehydrogenase (NADP-cytIDH) catalyze α-ketoglutarate formation, which feeds into the GABA pathway (Figure 1) [14,93].
In addition to metabolism, the transport of organic acids into vacuoles is essential for the regulation of fruit acidity. Once malate and citrate are transported into vacuoles, protonation maintains electrochemical gradients of MA and CA across the tonoplast, driving their continuous accumulation [14]. This process relies on vacuolar pH and membrane potential, which can be generated by proton pumps that transport protons into vacuoles. Three types of proton pumps, including V-ATPase, V-PPase, and P-type ATPase, participate in organic acid accumulation [41]. The aluminum-activated transporter SlALMT9 is a key protein controlling MA accumulation in tomato fruits, and variation in its promoter region has been linked to disruption of WRKY transcriptional repressor binding, leading to elevated MA levels [85]. Polymorphisms near the SlALMT9 locus have also been associated with variations in the MA content [94]. In addition, an InDel located upstream of Solyc06g072840, which encodes hydrogen peroxide-induced protein 1, has been linked to the MA content [95].
3.3.2. Key Genes and Transcription Factors of Malic Acid and Citric Acid
Organic acid metabolism as a key determinant of tomato flavor quality is regulated by multiple genetic factors (Table 1). Association mapping in tomato has identified loci for MA, CA, total organic acids, and pH on nearly every chromosome [96,97,98]. GWAS on MA has identified numerous SNPs, including 15 high-confidence annotated genes based on linkage disequilibrium and lead SNPs [96]. In the GWAS based on SNPs, InDels, and structural variations (SVs), 11 candidate genes associated with citrate content were identified [99]. Furthermore, silencing of PEPCK decreased gene expression and enzyme activity, reduced starch synthesis, and altered metabolite levels, including decreased fructose and CA, along with increased MA [86]. Overexpression of the ABA response factor SlAREB1 can significantly enhance organic acid accumulation, with elevated CA and MA contents through the upregulation of genes such as citrate synthase, confirming the role of ABA-mediated regulation in organic acid metabolism [51].
4. Effects of Environmental Factors on the TSS Content of Tomato Fruits
The cultivation environment exerts a significant influence on the TSS content of tomatoes. Therefore, regulating growth conditions through various approaches is critical for enhancing TSS in tomato fruits.
4.1. Temperature and Light
Temperature and light in the cultivation environment have a significant influence on tomato quality. Reductions in temperature and light lead to decreases in vitamin C (VC), TSS, soluble protein, and lycopene, while organic acid content increases [100]. Light intensity directly affects TSS levels in tomato fruits, where daytime lighting increases TSS by 8.2% in summer and 24% in winter, and LED cross-illumination at night during winter enhances TSS by 20%, while no significant effect is observed in summer [101]. Appropriate combinations of light qualities promote tomato growth and development. Combined red, blue, and white light (R1W1B0.5) significantly enhances photosynthesis, biomass, and fruit quality by activating the photoreceptor module (SIPHBYB1, SlCRY1, SlHY5, SlLHCA/B and SlCYCB) [102]. Under 16 h photoperiod conditions, treatment with combined red-blue light (R1B0.8) compared with white light can markedly increase chlorophyll content and biomass. This light spectrum enhances light capture and electron transfer efficiency by upregulating genes encoding photosystem subunits (SlPsaA/B/C) and the light-harvesting complex (SlLHCB/A), thereby improving the photosynthetic rate and elevating fructose and glucose levels [103]. The light systemic signal ELONGATED HYPOCOTYL 5 (HY5) directly activates sucrose metabolism genes, including LIN5, LIN6, VI, SS1 and SS7, by binding to cis-acting promoter elements. Fructose, glucose, and TSS contents were significantly increased in fruits of the HY5 overexpression line OE-HY5 [83].
4.2. Water and Nutrients
Agronomic measures can effectively and coordinately improve tomato fruit flavor by regulating key physiological processes and expression of sugar- and acid-related genes. Water deficit (WD) irrigation has been reported to promote the accumulation of reducing sugars, total acids, vitamin C, and TSS in fruits, thereby enhancing sweetness and enriching flavor [104]. A total of 5% biochar combined with 60% crop evapotranspiration deficit irrigation was an effective strategy to optimize tomato planting management under saline irrigation (2.3 dS m−1). This strategy can not only save 20–40% irrigation water, but also significantly improve fruit quality (soluble solids and total sugar content up to 99.75%), and reduce fruit sodium content, thus achieving the dual goal of “water saving and quality improvement” [105]. Potassium application has also been shown to improve fruit quality by increasing concentrations of TSS, sucrose, fructose, glucose, CA and MA. This improvement was achieved by upregulating the expression of SlSWEETs and SlSUTs, thereby promoting sucrose metabolism through the activation of SlSPS1, SlSUSs, and SlTINVI, which ultimately increased soluble sugar accumulation [106]. As a macronutrient, Cl− plays an important role in plant growth. Treatment with 3–5 mM Cl− at the red-ripe stage increased glucose by 18.6%, fructose by 10.7%, and TSS by 9.4% in tomato fruits without affecting yield. Additionally, Cl− enhances the activities of α-amylase, sucrose phosphate synthase, and sucrose synthase, thereby strengthening photosynthesis [107].
4.3. CO2 and Exogenous Substances
Compared with 400 ppm ambient CO2, Micro-Tom tomato fruits exposed to 900 ppm CO2 significantly decreased sucrose content and significantly increased fructose and glucose content, with the most statistically significant increase in glucose. This suggests that high concentrations of CO2 alter the sugar composition of tomato fruit [108]. The study confirmed that composting by using crop residues and animal manure in greenhouses can be used as an effective and inexpensive method of CO2 enrichment, doubling the CO2 concentration in the greenhouse during the day, increasing the yield of cherry tomatoes by up to 38%, and significantly increasing the concentration of TSS and soluble sugar [109]. Furthermore, pre-harvest spraying with GABA markedly enhances tomato fruit quality through metabolic regulation. The effect was most pronounced 3 d after application. Exogenous GABA enters cells via the GABA transporter, induces endogenous GABA metabolism, and promotes the accumulation of organic acids while upregulating related genes. In addition, GABA treatment regulates sugar metabolism by inhibiting part of glycolysis, increasing trehalose 6-phosphate (Tre6P) content, and elevating glucose, fructose, and sucrose levels [81].
5. Approaches to Increase TSS in Tomato Fruits
5.1. Cross Breeding
Improving TSS is an important breeding objective for tomatoes. Cross breeding remains a key approach for enhancing TSS by crossing cultivated tomatoes with high-TSS germplasms. This method is simple, easy to apply, and widely accepted because it does not involve exogenous gene introduction. However, this approach has significant limitations for improving the complex quantitative traits of TSS. The selection of high-TSS phenotypes relies primarily on phenotypic evaluations, such as refractometer measurements, and can only be performed after fruit maturity. This results in prolonged breeding cycles, low efficiency [16], and limited accuracy, as outcomes are strongly influenced by environmental factors, thereby obscuring true genetic potential. Moreover, the introduction of favorable alleles for high TSS is often accompanied by the incorporation of unfavorable alleles linked to them, such as those associated with reduced fruit size.
5.2. MAS and GS
Forward genetics has advanced our understanding of the genetic basis of phenotypic traits and revealed natural genetic variation. MAS utilizes relevant DNA markers to track traits of interest, providing an effective strategy for early selection at the juvenile stage and accelerating breeding [110]. MAS requires the identification of loci associated with traits, which is typically achieved through QTL mapping or linkage analysis [18]. In tomato TSS research, representative markers have been developed, including the STP1 InDel_21 marker related to sugar transport [53] and the InDel-based cleaved amplified polymorphic sequence (CAPS) marker of SlALMT9 related to malate transport [111]. However, considerable work remains to be conducted from QTL discovery to the development of reliable markers and their application in MAS, including the validation of marker predictive power for traits. GS, which can be used to evaluate complex traits using whole-genome DNA markers, has proven to be more effective than MAS. Substantial reduction in genotyping costs has made GS an attractive breeding strategy. However, its low prediction accuracy remains a critical limitation. GS prediction accuracy in tomato breeding varies with trait types, population structures, and marker densities. For simple traits with high heritability (such as disease resistance), the GS prediction accuracy can usually reach 0.7–0.8; for complex quantitative traits (such as yield and soluble solid content), the accuracy is generally between 0.5 and 0.7. Improving accuracy through the development of advanced statistical machine learning models and optimization of training populations represents a promising yet challenging direction for enhancing genetic prediction and facilitating the broader application of GS in breeding programs [19]. The successful application of GS in the research on resistance to bacterial wilt in tomato [112], highlights its potential to facilitate early selection of tomato TSS traits and promote more efficient breeding.
5.3. Genome Editing
With the development of genome editing technologies, tools such as CRISPR/Cas9 can be employed to efficiently knock out, knock in, or perform base editing of specific genes in the tomato genome, thereby creating new germplasms, accelerating the breeding process, and overcoming interspecific hybridization barriers [113]. To address the yield reduction under the global warming and the shortage of heat-tolerant varieties (expected to intensify by 3–13%), researchers have proposed the climate-responsive carbon allocation sink optimization (CROCS) strategy. Using prime editing technology, a 10 bp heat shock element (HSE) was precisely inserted into the promoter of CWIN gene Lin5. Following this modification, CWINs exhibited heat-responsive upregulation under both experimental and field conditions, significantly enhancing carbon allocation to fruits. Tests have demonstrated that the yield per mu of tomato AC increases by 33%, and the fruit sugar content increases [114]. The first genome-edited crop to reach the market was a tomato enriched in GABA, named Sicilian Rouge High GABA [20]. In this variety, the calmodulin-binding domains of the glutamate decarboxylase (GAD) genes SlGAD2 and SlGAD3 can be knocked out to increase GAD activity, thereby enhancing the conversion of glutamate to GABA and improving the nutritional quality of tomato fruits [115]. On the basis of gene editing, design-based breeding integrates genomics, bioinformatics, and genetic design strategies to achieve systematic analysis and precise assembly of breeding targets. This approach promotes transformation of the breeding process from “empirical screening” to “targeted design”, thereby improving both the predictability and efficiency of breeding.
5.4. Model-Based Precision Cultivation
By establishing crop models, the interactions between environmental factors and the physiological and biochemical processes of tomato growth and development can be better investigated. Zhou et al. [116] developed an integrated model of tomato plant and fruit growth combined with tomato growth and fruit sugar metabolism (TGFS). Further scenario-based simulations indicated that reducing nitrogen fertilizer by 15–25% and irrigation by 10–20% relative to the current level would increase tomato fresh weight by 27.8–36.4% and soluble sugar concentration by 10% [117]. A process-based biophysical model of fleshy fruit development [116] combined with an enzyme-based kinetic model of sugar metabolism [118] predicted that tomatoes could be larger and sweeter if biophysical factors and transmembrane transport were optimized during fruit development [119].
In brief, hybrid breeding is widely applied but relies on phenotypic selection, with limitations such as long cycles, low efficiency, and linkage drag (e.g., smaller fruit size). MAS enables early selection using DNA markers, yet it depends on accurate prior QTL mapping. GS improves complex traits through whole-genome prediction, and its prediction accuracy is the current key bottleneck for application. Gene editing can precisely design genes and break linkage drag, and has successfully bred tomatoes with heat tolerance, increased yield, and high GABA content. Precision cultivation optimizes environmental management through growth models and can effectively improve TSS without changing the variety. Gene-edited crops are generally regarded as a new type of breeding technology, and their regulatory policies are still being continuously improved and clarified. It is necessary to closely monitor the latest relevant regulations issued by the Ministry of Agriculture and Rural Affairs to ensure the compliance of breeding methods. In summary, improving tomato TSS no longer relies on a single technology. The future direction lies in technology integration: combining the macroscopic prediction ability of GS with the precise design ability of gene editing, and using MAS to rapidly introgress known major genes. By integrating model simulation and optimized management, the genetic potential of varieties can be maximized.
6. Conclusions and Future Prospects
As a core quantitative trait for evaluating fruit flavor and quality, TSS in tomatoes can be regulated by multiple genes and is strongly influenced by environmental conditions. The extremely high TSS levels in wild tomato resources (approximately two to three times those of cultivated varieties) provide valuable genetic material for identifying key loci. Multiple major QTLs and key genes regulating TSS have been identified through linkage analysis using interspecific hybrid populations or GWAS with natural populations. A single major QTLs (Lin5, etc.) can explain up to ~30% of TSS heritability, though 5–15% is more typical. Collectively, known QTLs account for over 50% of heritability. With the widespread application of next-generation sequencing and genotyping technologies, additional regulatory genes and molecular markers associated with sugar and acid metabolism have been gradually revealed, laying the foundation for MAS and GS.
The pathways to enhance tomato TSS through environmental regulation are multiple and feasible. Modern cultivation management has changed from single factor control to multi-factor coordinated regulation, the core of which is to influence plant physiological process and gene expression network by regulating environmental signals, and finally realize coordinated and optimized accumulation of flavor substances such as sugar and acid.
In modern agriculture, alternative crop improvement strategies beyond hybridization have been proposed, including mutation breeding, genomic engineering, and genome editing [120]. Chemical mutagens and irradiation can induce random mutations [121], but the low frequency of beneficial variants and difficulty in identifying desirable individuals remain challenges [122]. Continuous advances in plant biotechnology aim to address these issues [123]. Currently, tomato breeding has shifted from cross breeding to precision design breeding, integrating genome editing and multi-omics approaches. The CRISPR/Cas system enables precise knockout, knock-in, or base editing of target genes, mimics natural variations without introducing exogenous DNA, and significantly improves breeding efficiency and safety. Studies have identified major genes (Lin5, Sl-ALMT9, etc.) that regulate the sugar and acid contents in tomatoes. Furthermore, by knocking out SlINVINH1 through gene editing, a successful approximately 30% increase in sugar content has been achieved while maintaining normal fruit size. The application of genome editing technology in the “de novo domestication” of wild tomatoes has been particularly notable [124,125,126]. To increase the TSS in tomato, the era of “combination breeding” has arrived. Future winners will not be determined by the mastery of a single technique but by the ability to seamlessly integrate technologies such as gene editing, marker-assisted selection, and genomic selection to establish a rapid breeding system spanning from the laboratory to the dinner table.
With the advancement of sequencing technologies and the construction of pangenome maps for more tomato germplasm resources, by integrating multi—dimensional datasets such as transcriptomics, metabolomics, and proteomics, and leveraging artificial intelligence analysis, pan-genomics can systematically decipher the complex regulatory networks of tomato TSS by uncovering the entire genetic diversity of the tomato species. This approach enables researchers to discover novel genes and pathways that were previously hidden and to identify key genetic nodes for precise breeding. High-throughput TSS measurement under phenomics automation serves as a bridge connecting genotypes and trait manifestations. It has revolutionized the way we perceive and screen fruit quality through the integration of spectroscopy, automated platforms, and data analysis. In future breeding or cultivation practices, the “beneficial microbial community conducive to the formation of high quality” can be considered as a potential screening indicator to select tomato varieties with higher symbiotic efficiency with beneficial microorganisms [127].
Future research should focus on four aspects: the application of genome editing and related technologies to break the negative genetic correlation between TSS and yield; an in-depth analysis of the sugar-acid metabolic network and its responses to environmental factors, thereby balancing TSS, stress resistance, and adaptability through multi-trait collaborative selection; the optimization of the sugar-acid ratio in line with consumer preferences, while enhancing volatile flavor substances to achieve improved tomato flavor; and the continuous integration of multiple technologies with mechanistic analysis to promote directional breeding and industrial application of tomato varieties with high TSS.
Author Contributions
M.X.: conceptualization and writing—original draft. S.J.: methodology. W.X. and S.L.: supervision, resources, and writing—review and editing. S.P. and Y.L.: validation; W.X. and S.P.: project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32360750; 32060685), the Scientific Research Foundation of Shihezi University (RCZK202336), the “Tianshan Yingcai” Cultivation Program–Young Scientific and Technological Innovative Talents (2024TSYCCX0117), Bingtuan Science and Technology Program (2025DA029).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACL | ATP citrate lyase |
| AI | acid invertase |
| CA | citric acid |
| CAPS | cleaved amplified polymorphic sequence |
| CIN | cytoplasmic invertase |
| CROCS | Climate-responsive optimization of carbon partitioning to sinks |
| CWIN | cell-wall invertase |
| cytACO | cytosolic aconitase |
| GABA | γ-aminobutyric acid |
| GAD | glutamate decarboxylase |
| GS | Genomic selection |
| GWAS | Genome-wide association studies |
| HSE | heat shock element |
| HY5 | ELONGATED HYPOCOTYL 5 |
| IDH | isocitrate dehydrogenase |
| INV | invertase |
| INVINH | invertase inhibitors |
| MA | malic acid |
| MAS | marker-assisted selection |
| mGWAS | metabolome-wide association studies |
| mtACO | mitochondrial aconitase |
| mtCS | mitochondrial citrate synthase |
| NAD-MDH | NAD-dependent malate dehydrogenase |
| NADP-ME | NADP-dependent malic enzyme |
| NI | neutral invertase |
| OAA | oxaloacetate |
| PEP | Phosphoenolpyruvate |
| PEPC | Phosphoenolpyruvate carboxylase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| QTL | quantitative trait locus |
| SIRT | sucrose-induced translational silencing |
| SNP | single nucleotide polymorphisms |
| SPP | sucrose-6-phosphate phosphohydrolase |
| SPS | sucrose phosphate synthase |
| SSR | simple sequence repeats |
| SuSy | synthase |
| SVs | structural variations |
| TCA cycle | tricarboxylic acid cycle |
| Tre6P | trehalose 6-phosphate |
| TSS | Total soluble solids |
| TST | Tonoplast sugar transporter |
| uORF | upstream open reading frame |
| VC | vitamin C |
| VGT | Vacuolar glucose transporter |
| VIN | vacuolar invertase |
References
- Zhang, T.; Wang, Y.; Munir, S.; Wang, T.; Ye, Z.; Zhang, J.; Zhang, Y. Cyclin Gene SlCycB1 Alters Plant Architecture in Association with Histone H3.2 in Tomato. Hortic. Plant J. 2022, 8, 341–350. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Jiang, J.; Zhao, T.; Xu, X.; Yang, H.; Li, J. Virus-Induced Gene Silencing of SlPYL4 Decreases the Drought Tolerance of Tomato. Hortic. Plant J. 2022, 8, 361–368. [Google Scholar] [CrossRef]
- Parisi, M.; Pentangelo, A.; D’Alessandro, A.; Festa, G.; Francese, G.; Navarro, A.; Sanajà, V.O.; Mennella, G. Grafting Effects on Bioactive Compounds, Chemical and Agronomic Traits of ‘Corbarino’ Tomato Grown under Greenhouse Healthy Conditions. Hortic. Plant J. 2023, 9, 273–284. [Google Scholar] [CrossRef]
- Erika, C.; Ulrich, D.; Naumann, M.; Smit, I.; Horneburg, B.; Pawelzik, E. Flavor and Other Quality Traits of Tomato Cultivars Bred for Diverse Production Systems as Revealed in Organic Low-Input Management. Front. Nutr. 2022, 9, 916642. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yuan, Y.; Wang, K.; Wang, H.; Huang, J.; Yu, H.; Cui, X. Rootstock-Scion Interactions Affect Fruit Flavor in Grafted Tomato. Hortic. Plant J. 2022, 8, 499–510. [Google Scholar] [CrossRef]
- Gur, A.; Zamir, D. Mendelizing All Components of a Pyramid of Three Yield QTL in Tomato. Front. Plant Sci. 2015, 6, 1096. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Zhang, Y.; Zhong, X.D.; Lu, X.; He, S.; Chen, D.; Li, Y.; Li, R.; Huang, Z. Genetic and Interaction Analysis of High Soluble Solid Content Loci in Processing Tomato. Sci. Agric. Sin. 2025, 58, 1816–1829. [Google Scholar] [CrossRef]
- Nordborg, M.; Weigel, D. Next-Generation Genetics in Plants. Nature 2008, 456, 720–723. [Google Scholar] [CrossRef]
- Beckles, D.M.; Hong, N.; Stamova, L.; Luengwilai, K. Biochemical Factors Contributing to Tomato Fruit Sugar Content: A Review. Fruits 2012, 67, 49–64. [Google Scholar] [CrossRef]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of Loci Affecting Flavour Volatile Emissions in Tomato Fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L. Role and regulation of sucrose-phosphate synthase in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Wang, W.; Du, L.-Q.; Xie, J.-H.; Yao, Y.-L.; Sun, G.-M. Expression Patterns, Activities and Carbohydrate-Metabolizing Regulation of Sucrose Phosphate Synthase, Sucrose Synthase and Neutral Invertase in Pineapple Fruit during Development and Ripening. Int. J. Mol. Sci. 2012, 13, 9460–9477. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What Controls Fleshy Fruit Acidity? A Review of Malate and Citrate Accumulation in Fruit Cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase Activity and Expression During the Development of Lemon Fruit. Physiol. Plant. 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Huang, S.; Weigel, D.; Beachy, R.N.; Li, J. A Proposed Regulatory Framework for Genome-Edited Crops. Nat. Genet. 2016, 48, 109–111. [Google Scholar] [CrossRef]
- Tanksley, S.D. The Genetic, Developmental, and Molecular Bases of Fruit Size and Shape Variation in Tomato. Plant Cell 2004, 16 (Suppl. S1), S181–S189. [Google Scholar] [CrossRef]
- Chagné, D.; Vanderzande, S.; Kirk, C.; Profitt, N.; Weskett, R.; Gardiner, S.E.; Peace, C.P.; Volz, R.K.; Bassil, N.V. Validation of SNP Markers for Fruit Quality and Disease Resistance Loci in Apple (Malus × Domestica Borkh.) Using the OpenArray Platform. Hortic. Res. 2019, 6, 30. [Google Scholar] [CrossRef]
- Alemu, A.; Åstrand, J.; Montesinos-López, O.A.; y Sánchez, J.I.; Fernández-Gónzalez, J.; Tadesse, W.; Vetukuri, R.R.; Carlsson, A.S.; Ceplitis, A.; Crossa, J.; et al. Genomic Selection in Plant Breeding: Key Factors Shaping Two Decades of Progress. Mol. Plant 2024, 17, 552–578. [Google Scholar] [CrossRef]
- Waltz, E. GABA-Enriched Tomato Is First CRISPR-Edited Food to Enter Market. Nat. Biotechnol. 2022, 40, 9–11. [Google Scholar] [CrossRef]
- Fulton, T.M.; Bucheli, P.; Voirol, E.; López, J.; Pétiard, V.; Tanksley, S.D. Quantitative Trait Loci (QTL) Affecting Sugars, Organic Acids and Other Biochemical Properties Possibly Contributing to Flavor, Identified in Four Advanced Backcross Populations of Tomato. Euphytica 2002, 127, 163–177. [Google Scholar] [CrossRef]
- Toubiana, D.; Semel, Y.; Tohge, T.; Beleggia, R.; Cattivelli, L.; Rosental, L.; Nikoloski, Z.; Zamir, D.; Fernie, A.R.; Fait, A. Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations. PLoS Genet. 2012, 8, e1002612. [Google Scholar] [CrossRef] [PubMed]
- Causse, M.; Duffe, P.; Gomez, M.C.; Buret, M.; Damidaux, R.; Zamir, D.; Gur, A.; Chevalier, C.; Lemaire-Chamley, M.; Rothan, C. A Genetic Map of Candidate Genes and QTLs Involved in Tomato Fruit Size and Composition. J. Exp. Bot. 2004, 55, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive Metabolic Profiling and Phenotyping of Interspecific Introgression Lines for Tomato Improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar] [CrossRef]
- Paterson, A.H.; Damon, S.; Hewitt, J.D.; Zamir, D.; Rabinowitch, H.D.; Lincoln, S.E.; Lander, E.S.; Tanksley, S.D. Mendelian Factors Underlying Quantitative Traits in Tomato: Comparison across Species, Generations, and Environments. Genetics 1991, 127, 181–197. [Google Scholar] [CrossRef]
- Tanksley, S.D.; Grandillo, S.; Fulton, T.M.; Zamir, D.; Eshed, Y.; Petiard, V.; Lopez, J.; Beck-Bunn, T. Advanced Backcross QTL Analysis in a Cross Between an Elite Processing Line of Tomato and Its Wild Relative, L. pimpinellifolium. Theor. Appl. Genet. 1996, 92, 213–224. [Google Scholar] [CrossRef]
- Fulton, T.M.; Nelson, J.C.; Tanksley, S.D. Introgression and DNA Marker Analysis of Lycopersicon peruvianum, a Wild Relative of the Cultivated Tomato, into Lycopersicon esculentum, Followed Through Three Successive Backcross Generations. Theor. Appl. Genet. 1997, 95, 895–902. [Google Scholar] [CrossRef]
- Goldman, I.L.; Paran, I.; Zamir, D. Quantitative Trait Locus Analysis of a Recombinant Inbred Line Population Derived from a Lycopersicon esculentum × Lycopersicon cheesmanii Cross. Theor. Appl. Genet. 1995, 90, 925–932. [Google Scholar] [CrossRef]
- Bernacchi, D.; Beck-Bunn, T.; Emmatty, D.; Eshed, Y.; Inai, S.; Lopez, J.; Petiard, V.; Sayama, H.; Uhlig, J.; Zamir, D.; et al. Advanced Backcross QTL Analysis of Tomato. II. Evaluation of near-Isogenic Lines Carrying Single-Donor Introgressions for Desirable Wild QTL-Alleles Derived from Lycopersicon hirsutum and L. pimpinellifolium. Theor. Appl. Genet. 1998, 97, 170–180. [Google Scholar] [CrossRef]
- Bernacchi, D.; Beck-Bunn, T.; Eshed, Y.; Lopez, J.; Petiard, V.; Uhlig, J.; Zamir, D.; Tanksley, S. Advanced Backcross QTL Analysis in Tomato. I. Identification of QTLs for Traits of Agronomic Importance from Lycopersicon hirsutum. Theor. Appl. Genet. 1998, 97, 381–397. [Google Scholar] [CrossRef]
- Yelle, S.; Hewitt, J.D.; Robinson, N.L.; Damon, S.; Bennett, A.B. Sink Metabolism in Tomato Fruit: III. Analysis of Carbohydrate Assimilation in a Wild Species. Plant Physiol. 1988, 87, 737–740. [Google Scholar] [CrossRef]
- Yelle, S.; Chetelat, R.T.; Dorais, M.; Deverna, J.W.; Bennett, A.B. Sink Metabolism in Tomato Fruit: IV. Genetic and Biochemical Analysis of Sucrose Accumulation. Plant Physiol. 1991, 95, 1026–1035. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Deverna, J.W.; Bennett, A.B. Introgression into Tomato (Lycopersicon esculentum) of the L. chmielewskii Sucrose Accumulator Gene (Sucr) Controlling Fruit Sugar Composition. Theor. Appl. Genet. 1995, 91, 327–333. [Google Scholar] [CrossRef]
- Levin, I.; Gilboa, N.; Yeselson, E.; Shen, S.; Schaffer, A.A. Fgr, a Major Locus That Modulates the Fructose to Glucose Ratio in Mature Tomato Fruits. Theor. Appl. Genet. 2000, 100, 256–262. [Google Scholar] [CrossRef]
- Eshed, Y.; Zamir, D. An Introgression Line Population of Lycopersicon Pennellii in the Cultivated Tomato Enables the Identification and Fine Mapping of Yield-Associated QTL. Genetics 1995, 141, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Fridman, E.; Carrari, F.; Liu, Y.-S.; Fernie, A.R.; Zamir, D. Zooming in on a Quantitative Trait for Tomato Yield Using Interspecific Introgressions. Science 2004, 305, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.J.; Carrari, F.; Bauke, A.; Overy, S.; Hill, S.A.; Quick, P.W.; Fernie, A.R.; Sweetlove, L.J. Fruit Carbohydrate Metabolism in an Introgression Line of Tomato with Increased Fruit Soluble Solids. Plant Cell Physiol. 2005, 46, 425–437. [Google Scholar] [CrossRef]
- Zanor, M.I.; Osorio, S.; Nunes-Nesi, A.; Carrari, F.; Lohse, M.; Usadel, B.; Kühn, C.; Bleiss, W.; Giavalisco, P.; Willmitzer, L.; et al. RNA Interference of LIN5 in Tomato Confirms Its Role in Controlling Brix Content, Uncovers the Influence of Sugars on the Levels of Fruit Hormones, and Demonstrates the Importance of Sucrose Cleavage for Normal Fruit Development and Fertility. Plant Physiol. 2009, 150, 1204–1218. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Huang, S.; Hu, J.; Wang, Q.; Tang, Y.; Yang, T.; Asmutola, P.; Wang, J.; Yu, Q. Enhanced Soluble Sugar Content in Tomato Fruit Using CRISPR/Cas9-Mediated SlINVINH1 and SlVPE5 Gene Editing. PeerJ 2021, 9, e12478. [Google Scholar] [CrossRef]
- Çolak, N.G.; Eken, N.T.; Ülger, M.; Frary, A.; Doğanlar, S. Exploring Wild Alleles from Solanum pimpinellifolium with the Potential to Improve Tomato Flavor Compounds. Plant Sci. 2020, 298, 110567. [Google Scholar] [CrossRef]
- Miao, S.; Wei, X.; Zhu, L.; Ma, B.; Li, M. The Art of Tartness: The Genetics of Organic Acid Content in Fresh Fruits. Hortic. Res. 2024, 11, uhae225. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, Z.; Wang, H.; Cui, X.; Sun, S. Mapping of Gene Regulating Citric Acid Content in Tomato Fruit. Acta Hortic. Sin. 2024, 51, 67–76. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A Chemical Genetic Roadmap to Improved Tomato Flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, J.; Pandey, M.K.; Varshney, R.K.; Huang, L.; Luo, H.; Liu, N.; Chen, W.; Lei, Y.; Liao, B.; et al. Dissection of the Genetic Basis of Yield-Related Traits in the Chinese Peanut Mini-Core Collection Through Genome-Wide Association Studies. Front. Plant Sci. 2021, 12, 637284. [Google Scholar] [CrossRef] [PubMed]
- Achnine, L.; Huhman, D.V.; Farag, M.A.; Sumner, L.W.; Blount, J.W.; Dixon, R.A. Genomics-Based Selection and Functional Characterization of Triterpene Glycosyltransferases from the Model Legume Medicago Truncatula. Plant J. 2005, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Shibata, M.; Nishiyama, Y.; Springob, K.; Kitayama, M.; Shimada, N.; Aoki, T.; Ayabe, S.-I.; Saito, K. Differential Gene Expression Profiles of Red and Green Forms of Perilla Frutescens Leading to Comprehensive Identification of Anthocyanin Biosynthetic Genes. FEBS J. 2008, 275, 3494–3502. [Google Scholar] [CrossRef]
- Widodo; Patterson, J.H.; Newbigin, E.D.; Tester, M.; Bacic, A.; Roessner, U. Metabolic Responses to Salt Stress of Barley (Hordeum vulgare L.) Cultivars, Sahara and Clipper, Which Differ in Salinity Tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite Biodiversity in Pepper (Capsicum) Fruits of Thirty-Two Diverse Accessions: Variation in Health-Related Compounds and Implications for Breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Carreno-Quintero, N.; Bouwmeester, H.J.; Keurentjes, J.J.B. Genetic Analysis of Metabolome-Phenotype Interactions: From Model to Crop Species. Trends Genet. 2013, 29, 41–50. [Google Scholar] [CrossRef]
- Luo, J. Metabolite-Based Genome-Wide Association Studies in Plants. Curr. Opin. Plant Biol. 2015, 24, 31–38. [Google Scholar] [CrossRef]
- Sauvage, C.; Segura, V.; Bauchet, G.; Stevens, R.; Do, P.T.; Nikoloski, Z.; Fernie, A.R.; Causse, M. Genome-Wide Association in Tomato Reveals 44 Candidate Loci for Fruit Metabolic Traits. Plant Physiol. 2014, 165, 1120–1132. [Google Scholar] [CrossRef]
- Ye, J.; Li, W.; Ai, G.; Li, C.; Liu, G.; Chen, W.; Wang, B.; Wang, W.; Lu, Y.; Zhang, J.; et al. Genome-Wide Association Analysis Identifies a Natural Variation in Basic Helix-Loop-Helix Transcription Factor Regulating Ascorbate Biosynthesis via D-Mannose/L-Galactose Pathway in Tomato. PLoS Genet. 2019, 15, e1008149. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Ge, P.; Li, F.; Zhu, L.; Wang, Y.; Tao, J.; Zhang, X.; Dong, H.; Gai, W.; et al. A 21-Bp InDel in the Promoter of STP1 Selected During Tomato Improvement Accounts for Soluble Solid Content in Fruits. Hortic. Res. 2023, 10, uhad009. [Google Scholar] [CrossRef]
- Davies, J.N.; Hobson, G.E. The Constituents of Tomato Fruit—The Influence of Environment, Nutrition, and Genotype. Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Bastías, A.; López-Climent, M.; Valcárcel, M.; Rosello, S.; Gómez-Cadenas, A.; Casaretto, J.A. Modulation of Organic Acids and Sugar Content in Tomato Fruits by an Abscisic Acid-Regulated Transcription Factor. Physiol. Plant 2011, 141, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Khefifi, H.; Dumont, D.; Costantino, G.; Doligez, A.; Brito, A.C.; Bérard, A.; Morillon, R.; Ollitrault, P.; Luro, F. Mapping of QTLs for Citrus Quality Traits Throughout the Fruit Maturation Process on Clementine (Citrus reticulata × C. sinensis) and Mandarin (C. Reticulata blanco) Genetic Maps. Tree Genet. Genomes 2022, 18, 40. [Google Scholar] [CrossRef]
- Husain, S.E.; James, C.; Shields, R.; Foyer, C.H. Manipulation of Fruit Sugar Composition but Not Content in Lycopersicon esculentum Fruit by Introgression of an Acid Invertase Gene from Lycopersicon pimpinellifolium. New Phytol. 2001, 150, 65–72. [Google Scholar] [CrossRef][Green Version]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, S.; Zhang, J.; He, H.; Sun, H.; Tian, S.; Gong, G.; Zhang, H.; Levi, A.; Tadmor, Y.; et al. A Tonoplast Sugar Transporter Underlies a Sugar Accumulation QTL in Watermelon. Plant Physiol. 2018, 176, 836–850. [Google Scholar] [CrossRef]
- Jinggui, F.; Xudong, Z.; Haifeng, J.; Chen, W. Research Advances on Physiological Function of Plant Sucrose Synthase. Nanjing Nongye Daxue Xuebao 2017, 40, 759–768. [Google Scholar]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhao, T.; Luo, J.; Liu, X.; Jiang, J. CYTOSOLIC INVERTASE2 regulates flowering and reactive oxygen species-triggered programmed cell death in tomato. Plant Physiol. 2024, 196, 1110–1125. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Lian, L.; Song, J.; Du, X.; Liu, W.; Zhao, W.; Yang, L.; Li, C.; Qin, Y.; et al. Systematic Analysis of the Sugar Accumulation Mechanism in Sucrose- and Hexose- Accumulating Cherry Tomato Fruits. BMC Plant Biol. 2022, 22, 303. [Google Scholar] [CrossRef]
- Klann, E.M.; Hall, B.; Bennett, A.B. Antisense Acid Invertase (TIV1) Gene Alters Soluble Sugar Composition and Size in Transgenic Tomato Fruit. Plant Physiol. 1996, 112, 1321–1330. [Google Scholar] [CrossRef]
- Hackel, A.; Schauer, N.; Carrari, F.; Fernie, A.R.; Grimm, B.; Kühn, C. Sucrose Transporter LeSUT1 and LeSUT2 Inhibition Affects Tomato Fruit Development in Different Ways. Plant J. 2006, 45, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, C.; Wang, M.; Li, T.; Liu, X.; Jiang, J. Plasma Membrane-Localized SlSWEET7a and SlSWEET14 Regulate Sugar Transport and Storage in Tomato Fruits. Hortic. Res. 2021, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Shammai, A.; Petreikov, M.; Yeselson, Y.; Faigenboim, A.; Moy-Komemi, M.; Cohen, S.; Cohen, D.; Besaulov, E.; Efrati, A.; Houminer, N.; et al. Natural Genetic Variation for Expression of a SWEET Transporter among Wild Species of Solanum lycopersicum (Tomato) Determines the Hexose Composition of Ripening Tomato Fruit. Plant J. 2018, 96, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, X.; Yang, J.; Li, H.; Ma, B.; Zhang, K.; Zhang, Y.; Cheng, L.; Ma, F.; Li, M. Heterologous Expression of the Apple Hexose Transporter MdHT2.2 Altered Sugar Concentration with Increasing Cell Wall Invertase Activity in Tomato Fruit. Plant Biotechnol. J. 2020, 18, 540–552. [Google Scholar] [CrossRef]
- Jung, B.; Ludewig, F.; Schulz, A.; Meißner, G.; Wöstefeld, N.; Flügge, U.-I.; Pommerrenig, B.; Wirsching, P.; Sauer, N.; Koch, W.; et al. Identification of the Transporter Responsible for Sucrose Accumulation in Sugar Beet Taproots. Nat. Plants 2015, 1, 14001. [Google Scholar] [CrossRef]
- Zhu, L.; Li, B.; Wu, L.; Li, H.; Wang, Z.; Wei, X.; Ma, B.; Zhang, Y.; Ma, F.; Ruan, Y.-L.; et al. MdERDL6-Mediated Glucose Efflux to the Cytosol Promotes Sugar Accumulation in the Vacuole Through up-Regulating TSTs in Apple and Tomato. Proc. Natl. Acad. Sci. USA 2021, 118, e2022788118. [Google Scholar] [CrossRef]
- Petreikov, M.; Shen, S.; Yeselson, Y.; Levin, I.; Bar, M.; Schaffer, A.A. Temporally Extended Gene Expression of the ADP-Glc Pyrophosphorylase Large Subunit (AgpL1) Leads to Increased Enzyme Activity in Developing Tomato Fruit. Planta 2006, 224, 1465–1479. [Google Scholar] [CrossRef]
- Petreikov, M.; Yeselson, L.; Shen, S.; Levin, I.; Schaffer, A.A.; Efrati, A.; Bar, M. Carbohydrate Balance and Accumulation during Development of Near-Isogenic Tomato Lines Differing in the AGPase-L1 Allele. J. Am. Soc. Hortic. Sci. 2009, 134, 134–140. [Google Scholar] [CrossRef]
- Qin, G.; Zhu, Z.; Wang, W.; Cai, J.; Chen, Y.; Li, L.; Tian, S. A Tomato Vacuolar Invertase Inhibitor Mediates Sucrose Metabolism and Influences Fruit Ripening. Plant Physiol. 2016, 172, 1596–1611. [Google Scholar] [CrossRef]
- Ariizumi, T.; Higuchi, K.; Arakaki, S.; Sano, T.; Asamizu, E.; Ezura, H. Genetic Suppression Analysis in Novel Vacuolar Processing Enzymes Reveals Their Roles in Controlling Sugar Accumulation in Tomato Fruits. J. Exp. Bot. 2011, 62, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Takei-Hoshi, R.; Yoshikawa, I.; Nishida, K.; Kobayashi, M.; Kusano, M.; Lu, Y.; Ariizumi, T.; Ezura, H.; Otagaki, S.; et al. Functional Disruption of Cell Wall Invertase Inhibitor by Genome Editing Increases Sugar Content of Tomato Fruit without Decrease Fruit Weight. Sci. Rep. 2021, 11, 21534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, H.; Wu, M.; Zhou, Y.; Yu, C.; Yang, Q.; Rolland, F.; Van de Poel, B.; Bouzayen, M.; Hu, N.; et al. A Vacuolar Invertase Gene SlVI Modulates Sugar Metabolism and Postharvest Fruit Quality and Stress Resistance in Tomato. Hortic. Res. 2025, 12, uhae283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lyu, H.; Chen, J.; Cao, X.; Du, R.; Ma, L.; Wang, N.; Zhu, Z.; Rao, J.; Wang, J.; et al. Releasing a Sugar Brake Generates Sweeter Tomato Without Yield Penalty. Nature 2024, 635, 647–656. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Turečková, V.; Xue, G.-P.; Fernie, A.R.; Mueller-Roeber, B.; Balazadeh, S. The NAC Transcription Factor SlNAP2 Regulates Leaf Senescence and Fruit Yield in Tomato. Plant Physiol. 2018, 177, 1286–1302. [Google Scholar] [CrossRef]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an Auxin Response Factor, Is Involved in Chlorophyll and Sugar Accumulation during Tomato Fruit Development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Bui, T.P.; Le, N.T.; Nguyen, C.X.; Le, M.T.T.; Dao, N.T.; Phan, Q.; Van Le, T.; To, H.M.T.; Pham, N.B.; et al. Disrupting Sc-uORFs of a Transcription Factor bZIP1 Using CRISPR/Cas9 Enhances Sugar and Amino Acid Contents in Tomato (Solanum lycopersicum). Planta 2023, 257, 57. [Google Scholar] [CrossRef]
- Wu, X.; Huo, R.; Yuan, D.; Zhao, L.; Kang, X.; Gong, B.; Lü, G.; Gao, H. Exogenous GABA Improves Tomato Fruit Quality by Contributing to Regulation of the Metabolism of Amino Acids, Organic Acids and Sugars. Sci. Hortic. 2024, 338, 113750. [Google Scholar] [CrossRef]
- Li, F.; Lin, J.; Ahiakpa, J.K.; Gai, W.; Tao, J.; Ge, P.; Zhang, X.; Mu, Y.; Ye, J.; Zhang, Y. ZF Protein C2H2-71 Regulates the Soluble Solids Content in Tomato by Inhibiting LIN5. J. Integr. Agric. 2025, 24, 2190–2202. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Li, J.; Dong, H.; Hu, Z.; Xia, X.; Yu, J.; Zhou, Y. Manipulating the Light Systemic Signal HY5 Greatly Improve Fruit Quality in Tomato. Adv. Sci. 2025, 12, e2500110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.; Luo, Z.; Lyu, L.; Wang, C.; Zhu, L.; Ma, F.; Li, M.; Han, D. Overexpression of a Transcription Factor MdWRKY126 Altered Soluble Sugar Accumulation in Apple and Tomato Fruit. Hortic. Plant J. 2025, 11, 989–998. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Hu, T.; Zhang, F.; Wang, B.; Li, C.; Yang, T.; Li, H.; Lu, Y.; Giovannoni, J.J.; et al. An InDel in the Promoter of Al-Activated Malate Transporter9 Selected During Tomato Domestication Determines Fruit Malate Contents and Aluminum Tolerance. Plant Cell 2017, 29, 2249–2268. [Google Scholar] [CrossRef]
- Osorio, S.; Vallarino, J.G.; Szecowka, M.; Ufaz, S.; Tzin, V.; Angelovici, R.; Galili, G.; Fernie, A.R. Alteration of the Interconversion of Pyruvate and Malate in the Plastid or Cytosol of Ripening Tomato Fruit Invokes Diverse Consequences on Sugar but Similar Effects on Cellular Organic Acid, Metabolism, and Transitory Starch Accumulation. Plant Physiol. 2013, 161, 628–643. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Berberich, T.; Tanaka, S.; Nishiyama, M.; Kanayama, Y.; Kojima, S.; Muramoto, K.; Kusano, T. A Novel Strategy to Produce Sweeter Tomato Fruits with High Sugar Contents by Fruit-Specific Expression of a Single bZIP Transcription Factor Gene. Plant Biotechnol. J. 2016, 14, 1116–1126. [Google Scholar] [CrossRef]
- Jenner, H.L.; Winning, B.M.; Millar, A.H.; Tomlinson, K.L.; Leaver, C.J.; Hill, S.A. NAD Malic Enzyme and the Control of Carbohydrate Metabolism in Potato Tubers. Plant Physiol. 2001, 126, 1139–1149. [Google Scholar] [CrossRef]
- Laval-Martin, D.; Farineau, J.; Diamond, J. Light versus Dark Carbon Metabolism in Cherry Tomato Fruits: I. Occurrence of Photosynthesis. Study of the Intermediates. Plant Physiol. 1977, 60, 872–876. [Google Scholar] [CrossRef]
- Leegood, R.C.; Walker, R.P. Regulation and Roles of Phosphoenolpyruvate Carboxykinase in Plants. Arch. Biochem. Biophys. 2003, 414, 204–210. [Google Scholar] [CrossRef]
- Famiani, F.; Cultrera, N.G.M.; Battistelli, A.; Casulli, V.; Proietti, P.; Standardi, A.; Chen, Z.-H.; Leegood, R.C.; Walker, R.P. Phosphoenolpyruvate Carboxykinase and Its Potential Role in the Catabolism of Organic Acids in the Flesh of Soft Fruit During Ripening. J. Exp. Bot. 2005, 56, 2959–2969. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Li, M.; Liu, Z.; You, C.-X.; Wang, D.-M.; Zhai, H.; Hao, Y.-J. Molecular Cloning of Three Malic Acid Related Genes MdPEPC, MdVHA-A, MdcyME and Their Expression Analysis in Apple Fruits. Sci. Hortic. 2009, 122, 404–408. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, C.; Dong, W.; Jiang, Q.; Wang, D.; Li, S.; Chen, M.; Liu, C.; Sun, C.; Chen, K. Transcriptome and Metabolome Analyses of Sugar and Organic Acid Metabolism in Ponkan (Citrus reticulata) Fruit During Fruit Maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bauchet, G.; Grenier, S.; Samson, N.; Segura, V.; Kende, A.; Beekwilder, J.; Cankar, K.; Gallois, J.-L.; Gricourt, J.; Bonnet, J.; et al. Identification of Major Loci and Genomic Regions Controlling Acid and Volatile Content in Tomato Fruit: Implications for Flavor Improvement. New Phytol. 2017, 215, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic Evidence for Complex Domestication History of the Cultivated Tomato in Latin America. Mol. Biol. Evol. 2020, 37, 1118–1132. [Google Scholar] [CrossRef]
- Gai, W.; Yang, F.; Yuan, L.; Ul Haq, S.; Wang, Y.; Wang, Y.; Shang, L.; Li, F.; Ge, P.; Dong, H.; et al. Multiple-Model GWAS Identifies Optimal Allelic Combinations of Quantitative Trait Loci for Malic Acid in Tomato. Hortic. Res. 2023, 10, uhad021. [Google Scholar] [CrossRef]
- Ruggieri, V.; Francese, G.; Sacco, A.; D’Alessandro, A.; Rigano, M.M.; Parisi, M.; Milone, M.; Cardi, T.; Mennella, G.; Barone, A. An Association Mapping Approach to Identify Favourable Alleles for Tomato Fruit Quality Breeding. BMC Plant Biol. 2014, 14, 337. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Ding, Q.; Huang, X.; Zhang, Y.; Zou, Z.; Li, M.; Cui, L.; Zhang, J. Association Mapping of Main Tomato Fruit Sugars and Organic Acids. Front. Plant Sci. 2016, 7, 1286. [Google Scholar] [CrossRef]
- Gai, W.; Yuan, L.; Yang, F.; Ahiakpa, J.K.; Li, F.; Ge, P.; Zhang, X.; Tao, J.; Wang, F.; Yang, Y.; et al. Genome-Wide Variants and Optimal Allelic Combinations for Citric Acid in Tomato. Hortic. Res. 2024, 11, uhae070. [Google Scholar] [CrossRef]
- Xiaoa, F.; Yang, Z.; Zhua, L. Low Temperature and Weak Light Affect Greenhouse Tomato Growth and Fruit Quality. J. Plant Sci. 2018, 6, 16–24. [Google Scholar]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime Supplemental LED Inter-Lighting Improves Growth and Yield of Single-Truss Tomatoes by Enhancing Photosynthesis in Both Winter and Summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Yang, S.; Jiang, C.; Liu, Y.; Zhang, N.; Sun, X.; Zhang, Y.; Zhu, K.; Peng, Y.; et al. Light Quality Regulates Plant Biomass and Fruit Quality Through a Photoreceptor-Dependent HY5-LHC/CYCB Module in Tomato. Hortic. Res. 2023, 10, uhad219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, K.; Wang, X.; Yan, J.; Zhu, H.; Zhang, N.; Wang, Y.; Zhao, Q.; Liu, Y.; Bu, X.; et al. Manipulation of Artificial Light Environment Improves Plant Biomass and Fruit Nutritional Quality in Tomato. J. Adv. Res. 2025, 75, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Zuo, J.; Watkins, C.B.; Wang, Q.; Liang, H.; Zheng, Y.; Liu, M.; Ji, Y. Sugar Accumulation and Fruit Quality of Tomatoes Under Water Deficit Irrigation. Postharvest Biol. Technol. 2023, 195, 112112. [Google Scholar] [CrossRef]
- Obadi, A.; Alharbi, A.; Alomran, A.; Alghamdi, A.G.; Louki, I.; Alkhasha, A.; Alqardaeai, T. Enhancement in Tomato Yield and Quality Using Biochar Amendments in Greenhouse under Salinity and Drought Stress. Plants 2024, 13, 1634. [Google Scholar] [CrossRef]
- Wu, K.; Hu, C.; Wang, J.; Guo, J.; Sun, X.; Tan, Q.; Zhao, X.; Wu, S. Comparative Effects of Different Potassium Sources on Soluble Sugars and Organic Acids in Tomato. Sci. Hortic. 2023, 308, 111601. [Google Scholar] [CrossRef]
- Wan, X.; Zhu, C.; Li, Q.; Su, L.; Li, Y.; Wu, H.; Jiang, W.; Lu, T.; Yu, H. Chloride Modulates Carbohydrate Metabolism and Ethylene Synthesis in Tomato Fruits. Plant J. 2025, 122, e70132. [Google Scholar] [CrossRef]
- Boufeldja, L.; Brandt, D.; Guzman, C.; Vitou, M.; Boudard, F.; Morel, S.; Servent, A.; Dhuique-Mayer, C.; Ollier, L.; Duchamp, O.; et al. Effect of Elevated Carbon Dioxide Exposure on Nutrition-Health Properties of Micro-Tom Tomatoes. Molecules 2022, 27, 3592. [Google Scholar] [CrossRef]
- Karim, M.F.; Hao, P.; Nordin, N.H.B.; Qiu, C.; Zeeshan, M.; Khan, A.A.; Shamsi, I.H. Effects of CO2 Enrichment by Fermentation of CRAM on Growth, Yield and Physiological Traits of Cherry Tomato. Saudi J. Biol. Sci. 2020, 27, 1041–1048. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding Crops to Feed 10 Billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Bai, Y.; Dougherty, L.; Li, M.; Fazio, G.; Cheng, L.; Xu, K. A Natural Mutation-Led Truncation in One of the Two Aluminum-Activated Malate Transporter-like Genes at the Ma Locus Is Associated with Low Fruit Acidity in Apple. Mol. Genet. Genom. 2012, 287, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.; Le, N.T.; Heo, J.; Sim, S.-C. Low-Density SNP Markers with High Prediction Accuracy of Genomic Selection for Bacterial Wilt Resistance in Tomato. Front. Plant Sci. 2024, 15, 1402693. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, Y.; Li, M.; Xiong, S.; Huang, L.; Zhang, S.; Zhang, W.; Liang, X.; Wang, W.; Tang, K.; et al. Biofortification of tomatoes with beta-carotene through targeted gene editing. Int. J. Biol. Macromol. 2025, 327 Pt 2, 147396. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Li, S.; Shi, Z.; Zou, Y.; Zhang, Y.; Huang, X.; Yang, D.; Yang, Y.; Li, Z.; Xu, C. Engineering Source-Sink Relations by Prime Editing Confers Heat-Stress Resilience in Tomato and Rice. Cell 2025, 188, 530–549.e20. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient Increase of Ɣ-Aminobutyric Acid (GABA) Content in Tomato Fruits by Targeted Mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef]
- Fishman, S.; Génard, M. A Biophysical Model of Fruit Growth: Simulation of Seasonal and Diurnal Dynamics of Mass. Plant Cell Environ. 1998, 21, 739–752. [Google Scholar] [CrossRef]
- Zhou, H.; Kang, S.; Génard, M.; Vercambre, G.; Chen, J. Integrated Model Simulates Bigger, Sweeter Tomatoes under Changing Climate Under Reduced Nitrogen and Water Input. Hortic. Res. 2023, 10, uhad045. [Google Scholar] [CrossRef]
- Beauvoit, B.P.; Colombié, S.; Monier, A.; Andrieu, M.-H.; Biais, B.; Bénard, C.; Chéniclet, C.; Dieuaide-Noubhani, M.; Nazaret, C.; Mazat, J.-P.; et al. Model-Assisted Analysis of Sugar Metabolism Throughout Tomato Fruit Development Reveals Enzyme and Carrier Properties in Relation to Vacuole Expansion. Plant Cell 2014, 26, 3224–3242. [Google Scholar] [CrossRef]
- Chen, J.; Beauvoit, B.; Génard, M.; Colombié, S.; Moing, A.; Vercambre, G.; Gomès, E.; Gibon, Y.; Dai, Z. Modelling Predicts Tomatoes Can Be Bigger and Sweeter If Biophysical Factors and Transmembrane Transports Are Fine-Tuned During Fruit Development. New Phytol. 2021, 230, 1489–1502. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Holme, I.B.; Gregersen, P.L.; Brinch-Pedersen, H. Induced Genetic Variation in Crop Plants by Random or Targeted Mutagenesis: Convergence and Differences. Front. Plant Sci. 2019, 10, 1468. [Google Scholar] [CrossRef]
- Gao, C. Genome Engineering for Crop Improvement and Future Agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Zhang, S.; Yao, J.; Chai, S.; Yadav, V.; Athar, H.-R.; Rahman, M.U.; Wang, L.; Wang, X. Ectopic Expression of VvFUS3, B3-Domain Transcription Factor, in Tomato Influences Seed Development via Affecting Endoreduplication and Hormones. Hortic. Plant J. 2022, 8, 351–360. [Google Scholar] [CrossRef]
- Zsögön, A.; Cermak, T.; Voytas, D.; Peres, L.E.P. Genome Editing as a Tool to Achieve the Crop Ideotype and De Novo Domestication of Wild Relatives: Case Study in Tomato. Plant Sci. 2017, 256, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of Wild Tomato Is Accelerated by Genome Editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Du, M.; Sun, C.; Deng, L.; Zhou, M.; Li, J.; Du, Y.; Ye, Z.; Huang, S.; Li, T.; Yu, J.; et al. Molecular Breeding of Tomato: Advances and Challenges. J. Integr. Plant Biol. 2025, 67, 669–721. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).