Expert Perspectives on Enhancing Analytical Methods for Multi-Ingredient Dietary Supplements (MIDS): A Qualitative Study
Abstract
1. Introduction
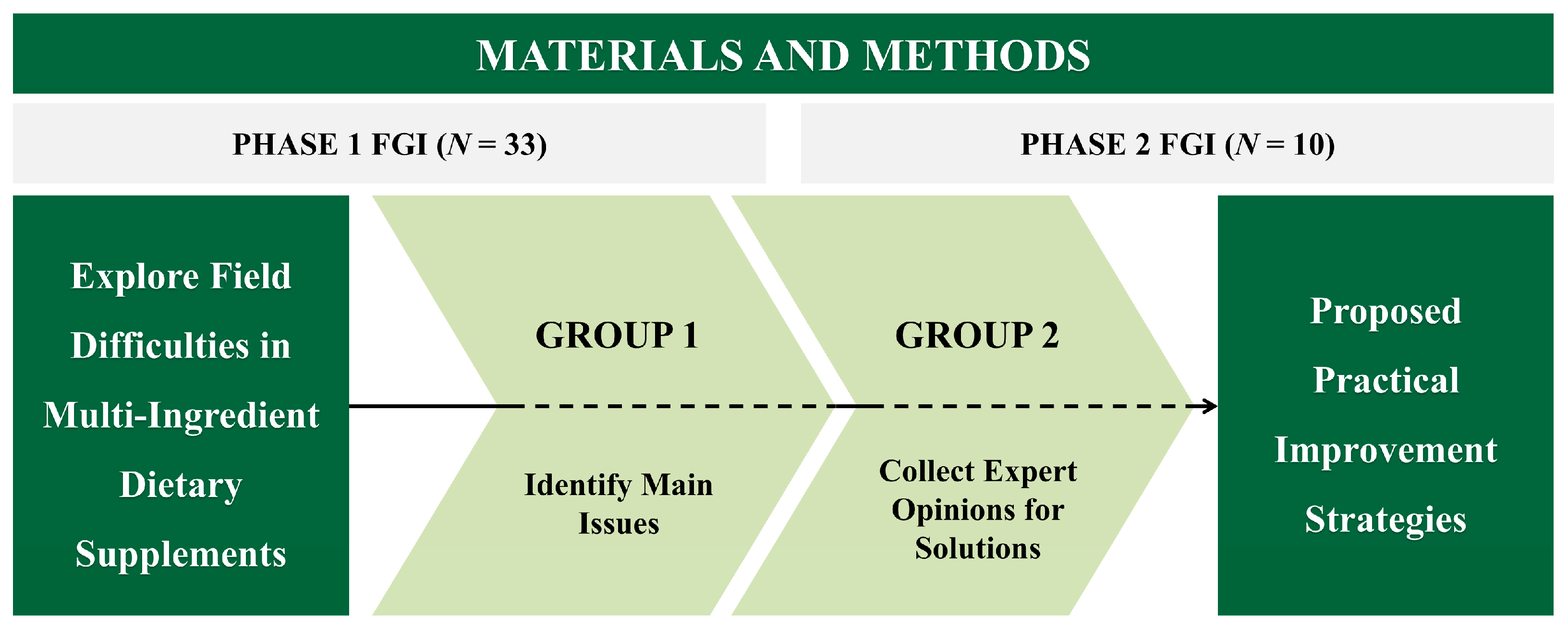
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
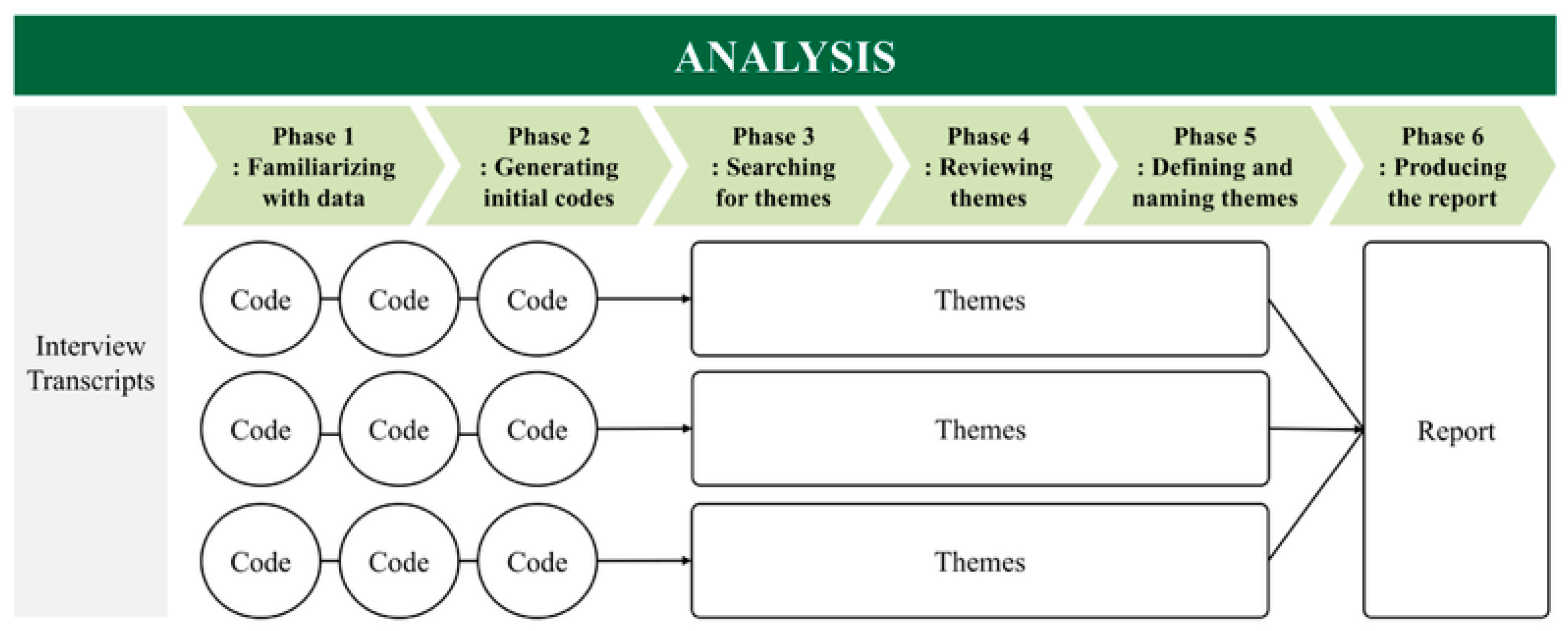
2.4. Analysis
3. Results
3.1. Participant Characteristics
3.2. Phase 1 FGI Analysis Results
3.2.1. Raw Materials and Ingredients
- Difficulties in the analysis of raw materials and ingredients
“Trace elements can get buried, and it is very difficult to actually see them.”(Professional #1)
“Trace ingredients like vitamin D and vitamin K are sometimes all detected when the content is validated, but in some situations, only half of them are detected.”(Professional #7)
“If the overdose is not applied, the content falls below the standard within the expiration date, and stability is compromised.”(Professional #1)
“Vitamin C in Acerola fruit extracts has a different peak time than synthetics, which can cause differences in analysis.”(Professional #4)
“I could see that the test for Rhodiola rosea L. extracts tended to have some impurities in it.”(Professional #16)
“Folic acid, for example, I think it is a little bit affected by pH.”(Professional #14)
“Ginsenosides themselves are affected by acid. It is affected a little bit by temperature and pH, so Rg1 becomes Rg3, and there’s damage like that.”(Professional #15)
- Analysis and interaction issues in composite ingredients
“When analyzing cranberry fruit extracts, similar anthocyanosides are sometimes detected together in the absorbance, resulting in a high content.”.(Professional #2)
“When analyzing lutein, other carotenoids such as astaxanthin, zeaxanthin, and beta-carotene can be detected together, which can skew the results.”.(Professional #10)
“It is a method that is in the Health Functional Food Code, but when catechins and amino acids were mixed, the process was done according to the process, but when the raw materials were tested alone, the result was 100, but when amino acids were mixed, it did not even come out half.”(Professional #15)
“I once used organic raw materials such as vitamin D and rice bran extract as secondary raw materials, but when the two were mixed together, the vitamin D content did not come out.”(Professional #19)
“If you mix vitamin B2 in a high magnesium product, the value of vitamin B2 is a little bit unstable.”(Professional #14)
“The flavonoids in propolis extracts are highly interfered with by excipients, and the variations are quite high.”(Professional #2)
“There was also interference between the individual recognizable forms of Saururus chinensis extract and propolis extracts.”(Professional #19)
“I put 100% inputs into the vitamin C product, but there was interference because due to vitamin C in the excipients… ”(Professional #3)
“Saw palmetto fruit extracts have a lot of assay variation and does not give the right assay.”(Professional #11)
“When you mix natural ingredients with natural ingredients, it becomes unstable.”(Professional #10)
3.2.2. Formulation
- Analytical challenges arising from formulation diversity
“There are some things that the existing formulations cannot cover because the formulations were created first and the jelly formulation was added on.”(Professional #2)
“Some of the formulations we’ve had a little trouble with are jelly.”(Professional #14)
“With jelly, you do not melt it all the way through, and then you end up cutting it up, and now you have to analyze it, and depending on how much you cut it up and what size you cut it into, you’re going to get different amounts.”(Professional #15)
“With water-soluble vitamins in soft dosage forms, there are challenges with water-based pretreatments that do not sufficiently dissolve the ingredients.”(Professional #9)
“It is not that the ingredient is not actually present, but that it has been transferred to the film. To prove this, you have to repeat experiments for each formulation, but it is difficult to continue this in reality.”.(Professional #6)
“The addition of acid in multivitamin granules or wet formulations can cause dissipation problems, depending on the activity of the water-soluble vitamins.”.(Professional #4)
“I also think now that test methods need to be formulation specific.”(Professional #13)
3.2.3. Testing Methods
- Limitations of current testing methods
“There is no separate method for glutathione, so it is difficult. Also, all phospholipids are supposed to be checked by the acetone-insoluble method, but when the phospholipids of krill oil are checked by the acetone-insoluble method, the data difference is about 30%, so it cannot be verified.”(Professional #1)
“When using the food method and the dietary supplements method, biotin was better analyzed in the food method and so on.”(Professional #14)
“The stick jelly melts at 40 to 50 degrees, but that is not in the instructions.”(Professional #29)
“I think it would be helpful to have a little more information in the manual about why they used these solvents in the instructions.”(Professional #17)
- Inter-manufacturer differences in chromatography column performance
“Technical differences between manufacturers can lead to differences in separation, sharpness, etc., and the retention time itself can vary.”(Professional #2)
“It is important to stick to the column that was used as a specific reference standard.”(Professional #7)
3.3. Phase 2 FGI Analysis Results
Expert-Recommended Analytical Solutions
- Tailored pretreatment and extraction strategies
“For vitamin B12 analysis, depending on the matrix nature of the sample, an additional enzymatic hydrolysis treatment is required to free the component from the matrix so that it can be extracted using an enzymatic treatment method such as pepsin… Vitamin K requires different pretreatment or extraction methods, such as lipase enzyme treatment to break down fats present in the sample.”(Expert #3)
“The sample pretreatment is the same as for the standard, a certain amount of sample is taken, dissolved with 0.1 N NaOH, and eluted with 10 mM phosphate buffer for analysis… The separation of rosavin isomers and rosavin in Rhodiola rosea extracts is considered possible by reducing the size of the column filler or by using buffer as the mobile phase.”(Expert #6)
“In the case of jellies, we are now experimenting with chopping them up as much as possible and controlling the sample volume. In addition, you can try freezing the jelly completely in liquid nitrogen.”(Expert #10)
“You can use dichloromethane or chloroform to separate them and then do the experiment.”(Expert #7)
- Regulatory improvements and method development
“The test method generally recommends using general-purpose and universal equipment, but it would be good to consider expensive equipment as well, because there may be expensive equipment or equipment that not everyone can afford.”(Expert #5)
“It is good to have multiple analytical methods, but as the number of analytical methods increases, the analyst needs to be responsible for whether it is appropriate to use the method.”(Expert #7)
“For the products developed so far, it is necessary to divide them into several types, select a representative matrix, and develop appropriate analytical methods.”(Expert #4)
“It will be difficult to apply all methods from the Health Functional Food Code to all matrices, but this part needs to be developed through continuous research as in other countries.”(Expert #3)
3.4. Integrated Findings from Multi-Phase FGIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIDS/MIDSs | Multi-ingredient dietary supplement(s) |
| DS/DSs | Dietary supplement(s) |
| CAGR | Compound annual growth rate |
| FGI | Focus group interviews |
| MFDS | Ministry of Food and Drug Safety |
| N (%) | Number of participants (percentage of the total participants in each category) |
| SOPs | Standard operating procedures |
| USP | United States Pharmacopeia |
| EFSA | European Food Safety Authority |
References
- Straits Research. Dietary Supplements Market Size, Growth & Trends Report by 2033. Available online: https://straitsresearch.com/report/dietary-supplements-market (accessed on 25 June 2025).
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef]
- Lam, M.; Khoshkhat, P.; Chamani, M.; Shahsavari, S.; Dorkoosh, F.A.; Rajabi, A.; Maniruzzaman, M.; Nokhodchi, A. In-Depth Multidisciplinary Review of the Usage, Manufacturing, Regulations & Market of Dietary Supplements. J. Drug Deliv. Sci. Technol. 2022, 67, 102985. [Google Scholar] [CrossRef]
- Patel, H. Comparative Analysis of Nutritional Supplement Dosage Forms. J. Glob. Econ. Bus. Financ. 2024, 6, 39–45. [Google Scholar] [CrossRef]
- Ullah, S.A.; Shahwani, G.R.; Shahwani, M.A.; Shahwani, G.M.S.; Haq, N.U.; Shahwani, N.A.S. Decomposition of Vitamins in Multivitamin Syrups of Different Brands under Various Storage Conditions. Pak-Euro J. Med. Life Sci. 2022, 5, 229–242. [Google Scholar] [CrossRef]
- Rakuša, Ž.T.; Kristl, A.; Roškar, R. Stability of Reduced and Oxidized Coenzyme Q10 in Finished Products. Antioxidants 2021, 10, 360. [Google Scholar] [CrossRef]
- N’Guessan-Gnaman, K.C.; Tuo-Kouassi, N.A.; Aka-Any-Grah, S.; Anin, A.L.; Dally, I. Stability Study of a Formulation Based on Pineapple Juice and Hibiscus Sabdariffa Concentrated Extract for the Preparation of Food Supplements. J. Drug Deliv. Ther. 2024, 14, 142–146. [Google Scholar] [CrossRef]
- Rakuša, Ž.T.; Roškar, R.; Hickey, N.; Geremia, S. Vitamin B12 in Foods, Food Supplements, and Medicines—A Review of Its Role and Properties with a Focus on Its Stability. Molecules 2023, 28, 240. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Dettlaff, K.; Jelińska, A.; Ogrodowczyk, M. Stability Studies of Parenteral Nutrition with a High Dose of Vitamin C. J. Oncol. Pharm. Pract. 2020, 26, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Kolba, N.; Tako, E. Assessing the Interactions between Zinc and Vitamin A on Intestinal Functionality, Morphology, and the Microbiome In Vivo (Gallus gallus). Nutrients 2023, 15, 2754. [Google Scholar] [CrossRef]
- Sato, A.; Takino, Y.; Yano, T.; Fukui, K.; Ishigami, A. Determination of Tissue-Specific Interaction between Vitamin C and Vitamin E in Vivo Using Senescence Marker Protein-30 Knockout Mice as a Vitamin C Synthesis Deficiency Model. Br. J. Nutr. 2022, 128, 993–1003. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, J.; Huang, Q.; Zhang, L. Editorial: Interactions of Food Components: Structural Changes and Effects on Nutritional Value. Front. Nutr. 2024, 11, 1354064. [Google Scholar] [CrossRef]
- Bawiec, P.; Sawicki, J.; Łasińska-Pracuta, P.; Czop, M.; Sowa, I.; Iłowiecka, K.; Koch, W. In Vitro Evaluation of Bioavailability of Se from Daily Food Rations and Dietary Supplements. Nutrients 2023, 15, 1511. [Google Scholar] [CrossRef]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef]
- Kurpad, A.V.; Pasanna, R.M.; Hegde, S.G.; Patil, M.; Mukhopadhyay, A.; Sachdev, H.S.; Bhat, K.G.; Sivadas, A.; Devi, S. Bioavailability and Daily Requirement of Vitamin B12 in Adult Humans: An Observational Study of Its Colonic Absorption and Daily Excretion as Measured by [13C]-Cyanocobalamin Kinetics. Am. J. Clin. Nutr. 2023, 118, 1214–1223. [Google Scholar] [CrossRef]
- Stanojević-Ristić, Z.; Mrkić, I.; Ćorac, A.; Dejanović, M.; Mitić, R.; Vitković, L.; Rašić, J.; Valjarević, D.; Valjarević, A. Healthcare Professionals’ Knowledge and Behaviors Regarding Drug–Dietary Supplement and Drug–Herbal Product Interactions. Int. J. Environ. Res. Public Health 2022, 19, 4290. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Larkin, M.; De Visser, R.; Fadden, G. Developing an Interpretative Phenomenological Approach to Focus Group Data. Qual. Res. Psychol. 2010, 7, 99–121. [Google Scholar] [CrossRef]
- Satherley, R.; Lerigo, F.; Higgs, S.; Howard, R. An Interpretative Phenomenological Analysis of the Development and Maintenance of Gluten-related Distress and Unhelpful Eating and Lifestyle Patterns in Coeliac Disease. Br. J. Health Psychol. 2022, 27, 1026–1042. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Standards and Specifications for Health Functional Foods: Official Notification (MFDS Notification No. 2025-11, 5 March 2025). 2025. Available online: https://www.mfds.go.kr/brd/m_211/view.do?seq=14880 (accessed on 12 June 2025).
- Monaggemzadeh, F.; Ebrahimi, F.; Zakeri-Milani, P.; Valizadeh, H. Effects of Formulation Variables and Storage Conditions on Light Protected Vitamin B12 Mixed Parenteral Formulations. Adv. Pharm. Bull. 2014, 4, 329–338. [Google Scholar] [CrossRef]
- Lavelli, V.; D’Incecco, P.; Pellegrino, L. Vitamin D Incorporation in Foods: Formulation Strategies, Stability, and Bioaccessibility as Affected by the Food Matrix. Foods 2021, 10, 1989. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using Thematic Analysis in Psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Naeem, M.; Ozuem, W.; Howell, K.; Ranfagni, S. A Step-by-Step Process of Thematic Analysis to Develop a Conceptual Model in Qualitative Research. Int. J. Qual. Methods 2023, 22, 16094069231205789. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R. Acerola, an Untapped Functional Superfruit: A Review on Latest Frontiers. J. Food Sci Technol. 2018, 55, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- AIRashidy, A.A.M.; Khattab, Z.; Atiyah, A. Development and Validation of Visible Spectrophotometry Method for Determination of Vitamin B9 Folic Acid in Pure and Pharmaceutical Formulations. Cent. Asian J. Theor. Appl. Sci. 2024, 5, 723–729. Available online: https://cajotas.centralasianstudies.org/index.php/CAJOTAS (accessed on 25 June 2025).
- Ložnjak, P.; García-Salinas, C.; de la Garza, R.I.D.; Bysted, A.; Jakobsen, J. The Use of a Plant Enzyme for Rapid and Sensitive Analysis of Naturally-Occurring Folates in Food by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1594, 34–44. [Google Scholar] [CrossRef]
- Ding, Y.; Choy, L.Y.; Chew, M.H.; Lin, Q.; Johns, P.W. Effects of Metal Ions on Cyanocobalamin Stability in Heated Milk Protein-based Matrices. Int. J. Food Sci. Technol. 2022, 57, 7349–7358. [Google Scholar] [CrossRef]
- Noreen, A.; Anwar, Z.; Ejaz, M.A.; Usmani, M.; Khan, T.; Sheraz, M.A.; Ahmed, S.; Mirza, T.; Khurshid, A.; Ahmad, I. Riboflavin (Vitamin B2) Sensitized Photooxidation of Ascorbic Acid (Vitamin C): A Kinetic Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 309, 123813. [Google Scholar] [CrossRef]
- Guerra, P.V.; Yaylayan, V.A. Interaction of Flavanols with Amino Acids: Postoxidative Reactivity of the B-Ring of Catechin with Glycine. J. Agric. Food Chem. 2014, 62, 3831–3836. [Google Scholar] [CrossRef]
- Rakuša, Ž.T.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]
- Portier, C.; Vigh, T.; Di Pretoro, G.; Leys, J.; Klingeleers, D.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous Twin Screw Granulation: Impact of Microcrystalline Cellulose Batch-to-Batch Variability during Granulation and Drying—A QbD Approach. Int. J. Pharm. X 2021, 3, 100077. [Google Scholar] [CrossRef]
- Sabri, L.A.; Khasraghi, A.H.; Sulaiman, H.T. Preparation and Evaluation of Oral Soft Chewable Jelly Containing Flurbiprofen. J. Adv. Pharm. Technol. Res. 2022, 13, 306–311. [Google Scholar] [CrossRef]
- Naharros-Molinero, A.; Caballo-González, M.Á.; De La Mata, F.J.; García-Gallego, S. Shell Formulation in Soft Gelatin Capsules: Design and Characterization. Adv. Healthc. Mater. 2023, 13, 2302250. [Google Scholar] [CrossRef]
- Horváth, S.; Gritti, F.; Horváth, K. Theoretical Study of the Efficiency of Liquid Chromatography Columns with Particle Size Gradient. J. Chromatogr. A 2021, 1651, 462331. [Google Scholar] [CrossRef]
- Park, J.M.; Koh, J.H.; Kim, J.M. Development of Pretreatment Method for Analysis of Vitamin B12 in Cereal Infant Formula Using Immunoaffinity Chromatography and High-Performance Liquid Chromatography. Food Sci. Anim. Resour. 2021, 41, 335–342. [Google Scholar] [CrossRef]
- Indyk, H.E.; Gill, B.D.; Wei, S.; Harvey, L.; Woollard, D.C. Quantitation of Vitamin K in Milk Products by Pre-Column Reduction HPLC–Fluorescence. Food Anal. Methods 2021, 14, 984–988. [Google Scholar] [CrossRef]
- Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.W.; Roseland, J.M.; Gusev, P.A.; Palachuvattil, J.; Dang, P.T.; Savarala, S.; Han, F.; Pehrsson, P.R.; Douglass, L.W.; Dwyer, J.T.; et al. Analytical Ingredient Content and Variability of Adult Multivitamin/Mineral Products: National Estimates for the Dietary Supplement Ingredient Database. Am. J. Clin. Nutr. 2017, 105, 526–539. [Google Scholar] [CrossRef]
- Patel, V. Performance Comparison of Rapid Petrifilm™ Test Methods vs. Conventional Test Methods for Microbiological Testing of Dietary Supplements. Curr. Dev. Nutr. 2020, 4, nzaa067_059. [Google Scholar] [CrossRef]
- Williams, M.L.; Olomukoro, A.A.; Emmons, R.V.; Godage, N.H.; Gionfriddo, E. Matrix Effects Demystified: Strategies for Resolving Challenges in Analytical Separations of Complex Samples. J. Sep. Sci. 2023, 46, 2300571. [Google Scholar] [CrossRef]
- Coskun, S.H.; Wise, S.A.; Kuszak, A.J. The Importance of Reference Materials and Method Validation for Advancing Research on the Health Effects of Dietary Supplements and Other Natural Products. Front. Nutr. 2021, 8, 786261. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Sorkin, B.C.; Lucarini, M.; Gusev, P.A.; Kuszak, A.J.; Crawford, C.; Boyd, C.; Deuster, P.A.; Saldanha, L.G.; Gurley, B.J.; et al. Analytical Challenges and Metrological Approaches to Ensuring Dietary Supplement Quality: International Perspectives. Front. Pharmacol. 2022, 12, 714434. [Google Scholar] [CrossRef]
- Mahony, L.O.; Shea, E.O.; O’Connor, E.M.; Tierney, A.; Harkin, M.; Harrington, J.; Kennelly, S.; Arendt, E.; O’Toole, P.W.; Timmons, S. A Qualitative Study of Older Adults’ and Healthcare Professionals’ Perspectives on the Potential of Functional Food Products to Support Healthy Ageing. J. Funct. Foods 2023, 107, 105689. [Google Scholar] [CrossRef]
- Ahmed, S.K. Sample Size for Saturation in Qualitative Research: Debates, Definitions, and Strategies. J. Med. Surg. Public Health 2025, 5, 100171. [Google Scholar] [CrossRef]
- Stępień, K.A.; Giebułtowicz, J. Application of Liquid Chromatography Coupled to Mass Spectrometry in Quality Assessment of Dietary Supplements-A Case Study of Tryptophan Supplements: Release Assay, Targeted and Untargeted Studies. Pharmaceuticals 2022, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Bošnir, J.; Bevardi, M.; Hećimović, I.; Budeč, M.; Cindrić, I.J.; Kober, R.; Jurak, G.; Lasić, D.; Brkić, D.; Racz, A. Optimization of Sample Preparation Procedure for Determination of Fat-Soluble Vitamins in Milk and Infant Food by HPLC Technique. Processes 2024, 12, 1530. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, T.; Ho, C.-T.; Huang, Q.; Wu, Q.; Zhang, M. Improving the Stability and Bioavailability of Tea Polyphenols by Encapsulations: A Review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Abe-Matsumoto, L.T.; Sampaio, G.R.; Bastos, D.H.M. Do the Labels of Vitamin A, C, and E Supplements Reflect Actual Vitamin Content in Commercial Supplements? J. Food Compos. Anal. 2018, 72, 141–149. [Google Scholar] [CrossRef]
- Bailey, R.L. Current Regulatory Guidelines and Resources to Support Research of Dietary Supplements in the United States. Crit. Rev. Food Sci. Nutr. 2020, 60, 298–309. [Google Scholar] [CrossRef]
- United States Pharmacopeia. About U.S. Pharmacopeia. Available online: https://www.usp.org/about (accessed on 16 September 2025).
- European Food Safety Authority. About Us|EFSA. Available online: https://www.efsa.europa.eu/en/about/about-efsa (accessed on 16 September 2025).
| Topic | Questions |
|---|---|
| Participant demographics |
|
| Raw material and ingredient |
|
| Formulation |
|
| Testing methods |
|
| Other |
|
| Topic | Questions |
|---|---|
| Participant demographics |
|
| Expert-recommended analytical solutions |
|
| Other |
|
| Target Group | Division | Category | N (%) 1 |
|---|---|---|---|
| Phase 1: MIDS 2 industry workers entry 2 | Main task | Analytical | 7 (21.2%) |
| Quality control | 13 (39.4%) | ||
| Research and development | 10 (30.3%) | ||
| Materials research | 3 (9.1%) | ||
| Experience | <10 years | 12 (36.4%) | |
| 10–20 years | 9 (27.3%) | ||
| 20–30 years | 10 (30.3%) | ||
| ≥30 years | 2 (6.1%) | ||
| Phase 2: Academic and industry experts | Main task | Analytical | 7 (70%) |
| Design and development | 3 (30%) | ||
| Experience | <10 years | 1 (10%) | |
| 10–20 years | 5 (50%) | ||
| 20–30 years | 3 (30%) | ||
| ≥30 years | 1 (10%) |
| Phase | Theme | Sub-Theme | Code |
|---|---|---|---|
| Phase 1: MIDS 1 industry workers | Raw material and ingredient | Difficulties in the analysis of raw materials and ingredients | Difficulties in measuring ingredient levels |
| Ingredient loss during distribution | |||
| Analytical difficulties due to the presence of isomers | |||
| Analysis and interaction issues in composite ingredients | Content changes due to raw material interactions | ||
| Analytical difficulties due to peak time differences between raw materials | |||
| Formulation | Analytical challenges arising from formulation diversity | Analytical difficulties due to specific formulations | |
| Ingredient-level changes due to formulation interference | |||
| Testing methods | Limitations of current testing methods | Absence of established test methods | |
| Lack of raw material-specific guidelines | |||
| Inter-manufacturer differences in chromatography column performance | Column-to-column variation between manufacturers | ||
| Inconsistent column selection criteria | |||
| Phase 2: Academic and industry experts | Expert-recommended analytical solutions | Tailored pretreatment and extraction strategies | Solutions to raw material analysis issues |
| Solutions to formulation-related challenges | |||
| Regulatory improvements and method development | Improvements in testing methods | ||
| Procedural and institutional improvements |
| Division | Standard Material | Identified Challenges | Proposed Solutions |
|---|---|---|---|
| Vitamin B12 | Vitamin B12 | Significant reduction or undetectable levels due to accompanying components (Vitamin C, Rhodiola rosea L. extracts) |
|
| Vitamin K2 | Vitamin K2 | Decreased values associated with magnesium as a co-formulant |
|
| Vitamin B9 | Vitamin B9 | Variations due to differences in standard materials and pretreatment solvents specified in the Standards and Specifications for Health Functional Foods |
|
| Rhodiola rosea L. | Rosavin | Analytical complexity due to the presence of isomers (rosavin and its isomers) |
|
| Marigold flower extracts | Lutein, zeaxanthin | Inflated values due to structurally similar carotenoids |
|
| Haematococcus pluvialis extracts | Astaxanthin | Inflated values due to structurally similar carotenoids |
|
| Bilberry extracts | Anthocyanosides | Inflated values due to the presence of similar series of accompanying ingredients |
|
| Cranberry fruit extracts | Proanthocyanidins | Inflated values due to the presence of similar carotenoids |
|
| Vitamin D | Vitamin D | Interference from multi-ingredient formulations and component non-detection |
|
| Biotin | Biotin | Interference from multi-ingredient formulations and component non-detection |
|
| Vitamin C | Vitamin C | Numerical fluctuations caused by the accompanying ingredients |
|
| Propolis extracts | Total flavonoids | Numerical variations due to interfering excipients |
|
| Saw palmetto fruit extracts | Fatty acid | Numerical fluctuations caused by the accompanying ingredients (e.g., Ginkgo biloba leaf extracts) |
|
| Pantothenic acid | Pantothenic acid | Decreased values near the end of shelf life |
|
| Glutathione | Glutathione | Absence of an official testing method |
|
| Natural ingredients | Natural lemon extract powder | Difficulty in peak alignment between natural samples and synthetic standards |
|
| Jelly formulation | Complexity of the extraction process |
| |
| Soft capsule | Ingredient migration into capsule shell complicates analysis |
| |
| Solid formulation | Variation in results due to pretreatment methods |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, I.; Park, H.J.; Ko, K.S.; Kim, H.; Oh, J. Expert Perspectives on Enhancing Analytical Methods for Multi-Ingredient Dietary Supplements (MIDS): A Qualitative Study. Foods 2025, 14, 3598. https://doi.org/10.3390/foods14213598
Ko I, Park HJ, Ko KS, Kim H, Oh J. Expert Perspectives on Enhancing Analytical Methods for Multi-Ingredient Dietary Supplements (MIDS): A Qualitative Study. Foods. 2025; 14(21):3598. https://doi.org/10.3390/foods14213598
Chicago/Turabian StyleKo, Ingyeong, Hae Jin Park, Kwang Suk Ko, Hyunsoo Kim, and Jieun Oh. 2025. "Expert Perspectives on Enhancing Analytical Methods for Multi-Ingredient Dietary Supplements (MIDS): A Qualitative Study" Foods 14, no. 21: 3598. https://doi.org/10.3390/foods14213598
APA StyleKo, I., Park, H. J., Ko, K. S., Kim, H., & Oh, J. (2025). Expert Perspectives on Enhancing Analytical Methods for Multi-Ingredient Dietary Supplements (MIDS): A Qualitative Study. Foods, 14(21), 3598. https://doi.org/10.3390/foods14213598








