Optimization of Anthocyanin Extraction from Purple Sweet Potato Peel (Ipomea batata) Using Sonotrode Ultrasound-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Extraction of Anthocyanins
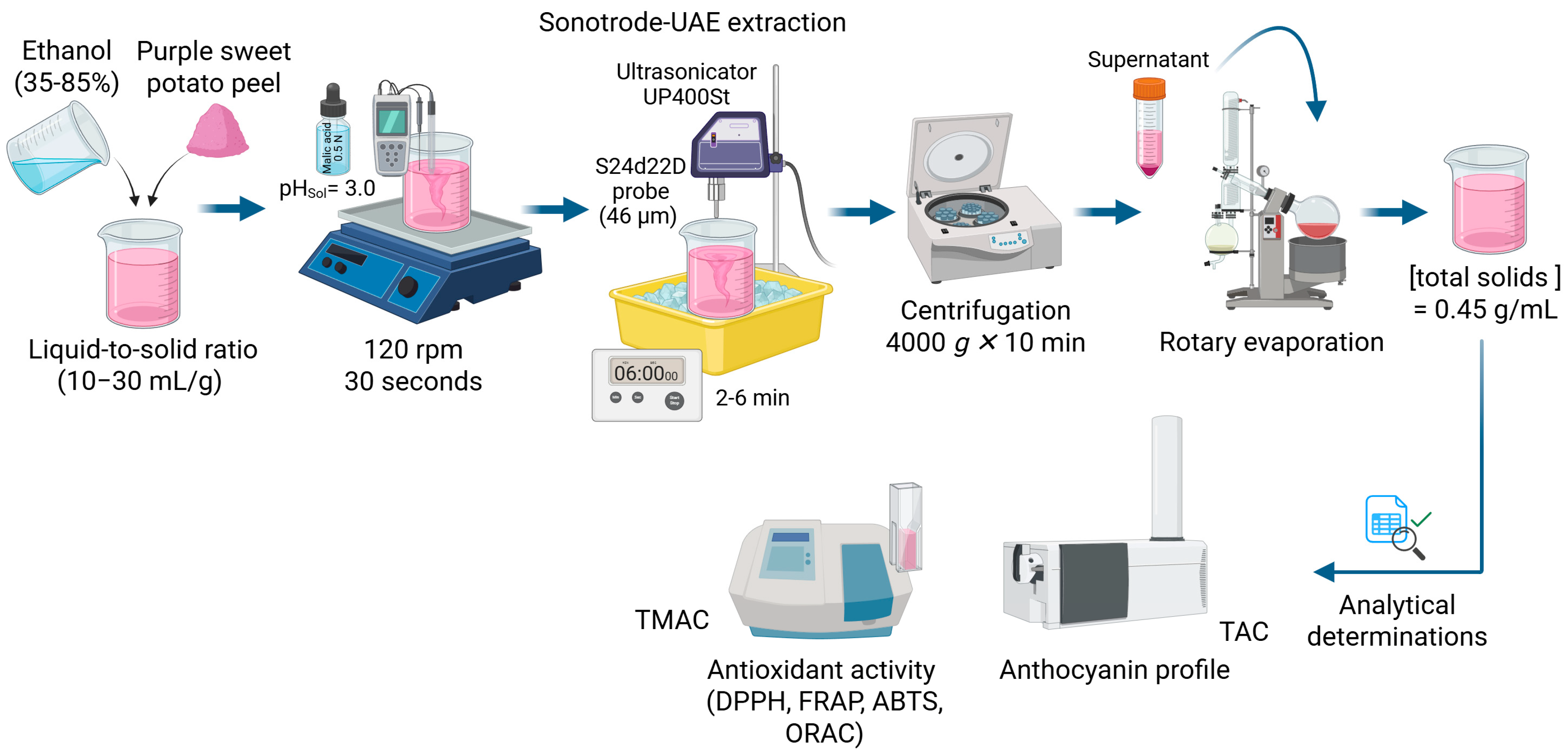
2.3.1. Sonotrode-UAE
2.3.2. Agitation
2.4. Anthocyanin Content and Profile
2.4.1. Total Monomeric Anthocyanin Content (TMAC)
2.4.2. Detection and Identification of Anthocyanin Profile
2.5. Determination of In Vitro Antioxidant Activity
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Cation Decolourization Assay
2.5.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.5.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Sonotrode-UAE on Anthocyanin Content
3.1.1. Effect of Sonotrode-UAE on TMAC
3.1.2. Effect of Sonotrode-UAE on Anthocyanin Profile
3.2. Effect of Sonotrode-UAE on In Vitro Antioxidant Activity
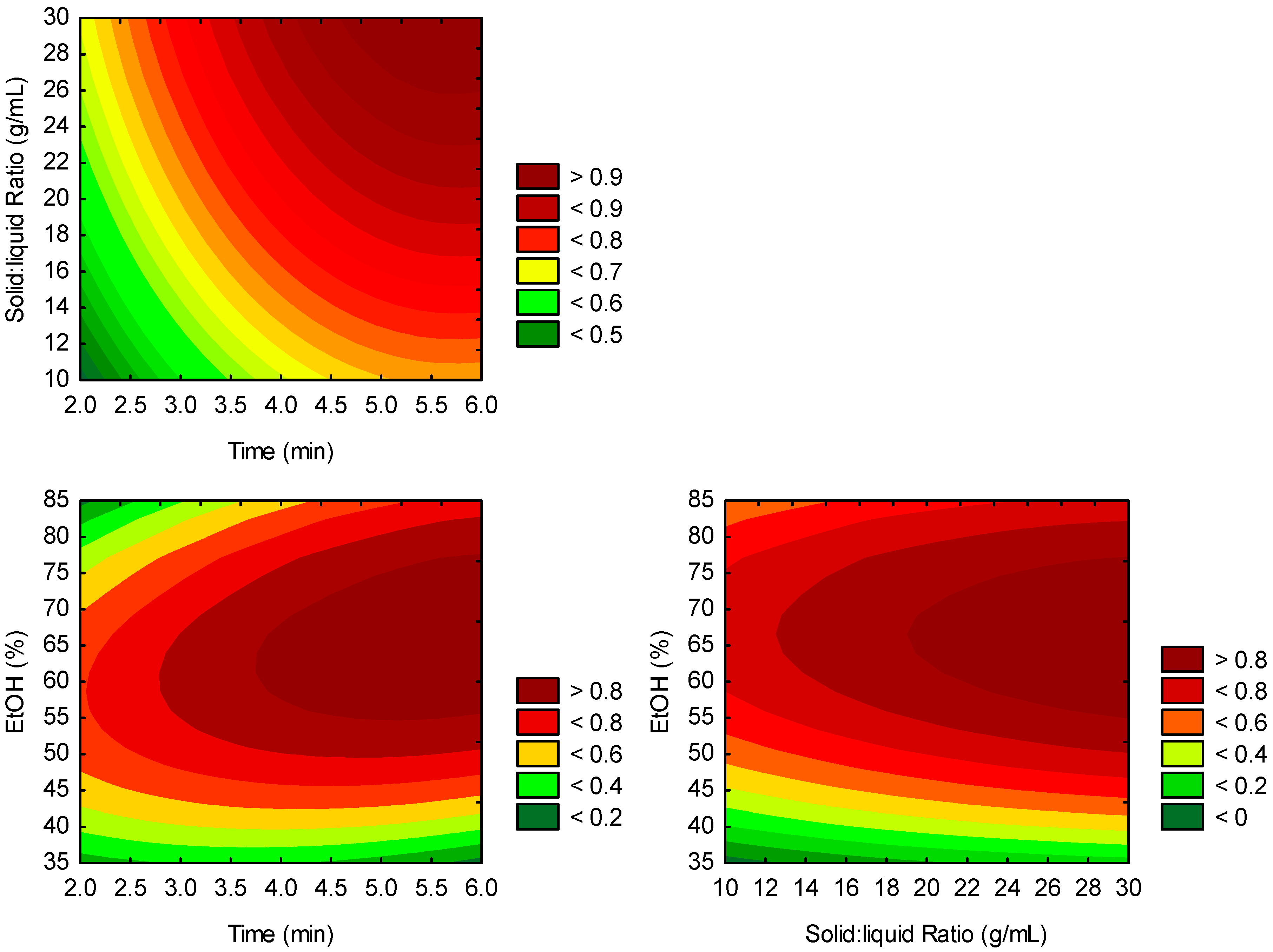
3.3. Process Optimization and Validation of the Model
3.4. Comparison with Magnetic Agitation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Peak nº | RT (min) | Formula | Tof MS (m/z) | MS/MS (Fragment Ions (m/z)) | Identification |
|---|---|---|---|---|---|
| 1 | 13.29 | C33H41O21 | 773.2141 | 612.1654/450.1107/287.0550 | Cy-3-soph-glc |
| 2 | 17.07 | C34H43O21 | 787.2296 | 625.1765/463.1239/301.0707 | Peo-3-soph-5-glc |
| 2 | 26.03 | C40H45O23 | 893.2342 | 731.1807/449.1077/287.0550 | Cy-3-p-hydroxybenzoylsoph-5-glc |
| 3 | 27.55 | C42H47O24 | 935.2443 | 773.1904/449.1086/287.0544 | Cy-3-(6‴-caffeoyl soph)-5-glc |
| 4 | 29.72 | C41H47O23 | 907.2512 | 745.1976/463.1240/301.0707 | Peo-3-hydroxybenzoylsoph-5-glc |
| 5 | 32.36 | C43H49O24 | 949.2608 | 787.2059/449.1073/287.0542 | Cy-3-feruloyl soph-5-glc |
| 6 | 35.48 | C42H47O24 | 935.2457 | 773.1926/449.1074/287.0552 | Cy-3-caffeoyl soph-5-glc |
| 7 | 35.90 | C44H51O24 | 963.2778 | 801.2241/463.1238/301.0707 | Peo-3-feruloyl soph-5-glc |
| 8 | 38.09 | C43H49O24 | 949.2616 | 787.2078/463.1233/301.0703 | Peo-3-caffeoyl soph-5-glc |
| 9 | 40.21 | C51H53O27 | 1097.2761 | 935.2227/449.1055/287.0549 | Cy-3-dicaffeoyl soph-5-glc |
| 10 | 40.31 | C49H51O26 | 1055.2672 | 893.2138/449.1079/287.0555 | Cy-3-caffeoyl-p-hydroxybenzoyl-soph-glc |
| 11 | 44.11 | C52H55O27 | 1111.2934 | 949.2386/449.1077/287.0547 | Cy-3-caffeoyl-feruloyl soph-5-glc |
| 12 | 45.21 | C52H55O27 | 1111.2928 | 949.2389/463.1229/301.0698 | Peo-3-dicaffeoyl-soph-5-glc |
| 13 | 45.23 | C50H53O26 | 1069.2793 | 907.2286/463.1233/301.0698 | Peo-3-caffeoyl-p-hydroxybenzoyl soph-5-glc |
| 14 | 46.35 | C49H51O25 | 1039.2695 | 877.2158/433.1117/271.0597 | Pg-3-caffeoyl-p-hydroxybenzoyl soph-5-glc |
| 15 | 49.31 | C53H57O27 | 1125.3080 | 963.2550/463.1235/301.0698 | Peo-3-caffeoyl-feruloyl soph-5-glc |
| 16 | 52.57 | C51H55O26 | 1083.2977 | 921.2438/463.123/301.0706 | Peo-3-(6′-p′-hydroxybenzoyl-6″-feruloyl soph)-5-glc |
| 17 | 13.29 | C33H41O21 | 773.2141 | 612.1654/450.1107/287.0550 | Cy-3-soph-glc |
References
- Grant, K.R.; Gallardo, R.K.; McCluskey, J.J. Consumer Preferences for Foods with Clean Labels and New Food Technologies. Agribusiness 2021, 37, 764–781. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Paola Floridas-Galán, J.; Paredes-Concepción, P. Bioactive Compounds from Agroindustrial Byproducts and Their Applications: From Traditional Methods to Green Technologies. Sci. Agropecu. 2024, 15, 461–476. [Google Scholar] [CrossRef]
- Frosi, I.; Balduzzi, A.; Moretto, G.; Colombo, R.; Papetti, A. Towards Valorization of Food-Waste-Derived Pectin: Recent Advances on Their Characterization and Application. Molecules 2023, 28, 6390. [Google Scholar] [CrossRef]
- Di Maro, M.; Gargiulo, L.; Gomez d’Ayala, G.; Duraccio, D. Exploring Antimicrobial Compounds from Agri-Food Wastes for Sustainable Applications. Int. J. Mol. Sci. 2024, 25, 13171. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Sanches Silva, A. Natural Pigments from Food Wastes: New Approaches for the Extraction and Encapsulation. Curr. Opin. Green Sustain. Chem. 2024, 47, 100929. [Google Scholar] [CrossRef]
- Dhiman, S.; Thakur, B.; Kaur, S.; Ahuja, M.; Gantayat, S.; Sarkar, S.; Singh, R.; Tripathi, M. Closing the Loop: Technological Innovations in Food Waste Valorisation for Global Sustainability. Discov. Sustain. 2025, 6, 258. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Screening Factors to Affect Ultrasound-Assisted Extraction of (Poly)Phenols from Date Palm Seeds. Front. Chem. 2024, 12, 1409393. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Olivo, A.O.; Durán-Castañeda, A.C.; López-Cárdenas, F.G.; de Rodríguez-Romero, J.J.; Sánchez-Burgos, J.A.; Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M. Advances in Ultrasound-Assisted Extraction of Bioactive Compounds (Antioxidant Compounds) from Agrofood Waste. In Advanced Technologies in Wastewater Treatment: Food Processing Industry; Elsevier: Amsterdam, Netherlands, 2023; pp. 217–246. ISBN 9780323885102. [Google Scholar]
- Razola-Díaz, M.d.C.; Verardo, V.; Guerra-Hernández, E.J.; García-Villanova Ruiz, B.; Gómez-Caravaca, A.M. Response Surface Methodology for the Optimization of Flavan-3-Ols Extraction from Avocado By-Products via Sonotrode Ultrasound-Assisted Extraction. Antioxidants 2023, 12, 1409. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization Model for Ultrasonic-Assisted and Scale-up Extraction of Anthocyanins from Pyrus Communis ‘Starkrimson’ Fruit Peel. Food. Chem. 2019, 297, 124993. [Google Scholar] [CrossRef]
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-De-cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of Sonotrode Ultrasonic-Assisted Extraction of Proanthocyanidins from Brewers’ Spent Grains. Antioxidants 2019, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Aurora-Vigo, E.F.; Villagrán, Z.; Rodríguez-Lafitte, E.; Ruvalcaba-Gómez, J.M.; Solano-Cornejo, M.Á.; Zamora-Gasga, V.M.; Montalvo-González, E.; Gómez-Rodríguez, H.; Aceves-Aldrete, C.E.; et al. Design of Experiments for Optimizing Ultrasound-Assisted Extraction of Bioactive Compounds from Plant-Based Sources. Molecules 2023, 28, 7752. [Google Scholar] [CrossRef]
- Szpisják-Gulyás, N.; Al-Tayawi, A.N.; Horváth, Z.H.; László, Z.; Kertész, S.; Hodúr, C. Methods for Experimental Design, Central Composite Design and the Box–Behnken Design, to Optimise Operational Parameters: A Review. Acta Aliment. 2023, 52, 521–537. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 June 2025).
- Zhao, M.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.; Sun, F.; Li, Y.; Xia, X. Monitoring Dynamic Changes in Chicken Freshness at 4 °C and 25 °C Using PH-Sensitive Intelligent Films Based on Sodium Alginate and Purple Sweet Potato Peel Extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B.; Vega-Maturino, S. Coloring Potential of Anthocyanins from Purple Sweet Potato Paste: Ultrasound-Assisted Extraction, Enzymatic Activity, Color and Its Application in Ice Pops. Food Chem. Adv. 2023, 3, 100358. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent PH Indicator Film Composed of Agar/Potato Starch and Anthocyanin Extracts from Purple Sweet Potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Liu, T.; Qin, R.; Xue, J.; Li, H.; Liu, Y. Fabrication of PH-Responsive Chitosan-Based Hydrogel Beads via Electrostatic Layer-by-Layer Assembly for Visual Monitoring of Pork Freshness. Food Hydrocoll. 2025, 166, 111356. [Google Scholar] [CrossRef]
- Pang, G.; Zhou, C.; Zhu, X.; Chen, L.; Guo, X.; Kang, T. Colorimetric Indicator Films Developed by Incorporating Anthocyanins into Chitosan-based Matrices. J. Food Saf. 2023, 43, e13045. [Google Scholar] [CrossRef]
- Yun, D.; Wu, Y.; Yong, H.; Tang, C.; Chen, D.; Kan, J.; Liu, J. Recent Advances in Purple Sweet Potato Anthocyanins: Extraction, Isolation, Functional Properties and Applications in Biopolymer-Based Smart Packaging. Foods 2024, 13, 3485. [Google Scholar] [CrossRef]
- Matsufuji, H.; Kido, H.; Misawa, H.; Yaguchi, J.; Otsuki, T.; Chino, M.; Takeda, M.; Yamagata, K. Stability to Light, Heat, and Hydrogen Peroxide at Different PH Values and DPPH Radical Scavenging Activity of Acylated Anthocyanins from Red Radish Extract. J. Agric. Food Chem. 2007, 55, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Safinta Nurindra Rahmadhia, S.N.R.; Afist Azkiya Sidqi, A.A.S.; Yanas Anggana Saputra, Y.A.S. Physical Properties of Tapioca Starch-Based Film Indicators with Anthocyanin Extract from Purple Sweet Potato (Ipomea batatas L.) and Response to PH Changes. Sains Malays 2023, 52, 1685–1697. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.; Yun, D.; Wang, Z.; Tang, C.; Liu, J. A New Method to Prepare Color-Changeable Smart Packaging Films Based on the Cooked Purple Sweet Potato. Food Hydrocoll. 2023, 137, 108397. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Z.; Zhang, J.; Li, N.; Wei, Z.; Zhang, Y.; Wu, X.; Liu, J.; Gao, Q.; Sun, X.; et al. Exopolysaccharide Riclin and Anthocyanin-Based Composite Colorimetric Indicator Film for Food Freshness Monitoring. Carbohydr. Polym. 2023, 314, 120882. [Google Scholar] [CrossRef]
- Huang, S.; Wang, G.; Lin, H.; Xiong, Y.; Liu, X.; Li, H. Preparation and Dynamic Response Properties of Colorimetric Indicator Films Containing PH-Sensitive Anthocyanins. Sens. Actuators Rep. 2021, 3, 100049. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Mu, T.; Li, Q.; Richel, A. Preparation of Cellulose Nanocrystals from Purple Sweet Potato Peels by Ultrasound-Assisted Maleic Acid Hydrolysis. Food Chem. 2023, 403, 134496. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.-S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus Microwave as Green Processes for Extraction of Rosmarinic, Carnosic and Ursolic Acids from Rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Guo, Y.; He, J.; Liu, G.; Li, S.; Barba, F.J.; Jaffrin, M.Y. Green Ultrasound-Assisted Extraction of Anthocyanin and Phenolic Compounds from Purple Sweet Potato Using Response Surface Methodology. Int. Agrophys. 2016, 30, 113–122. [Google Scholar] [CrossRef]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J. Optimisation of Aqueous Two-Phase Extraction of Anthocyanins from Purple Sweet Potatoes by Response Surface Methodology. Food Chem. 2013, 141, 3034–3041. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Lucas-González, R.; Carrillo, C.; Purriños, L.; Pateiro, M.; Bermúdez, R.; Lorenzo, J.M. Effect of Different Pre-Treatment on Acrylamide Content, Nutrition Value, Starch Digestibility and Anthocyanin Bioaccessibility of Purple Sweet Potato (Ipomoea batata) Deep-Fried Chips. Food Chem. 2024, 460, 140535. [Google Scholar] [CrossRef]
- Zoubiri, L.; Bakir, S.; Barkat, M.; Carrillo, C.; Capanoglu, E. Changes in the Phenolic Profile, Antioxidant Capacity and in Vitro Bioaccessibility of Two Algerian Grape Varieties, Cardinal and Dabouki (Sabel), during the Production of Traditional Sun-Dried Raisins and Homemade Jam. J. Berry Res. 2019, 9, 709–724. [Google Scholar] [CrossRef]
- Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of Pulsed Electric Fields for Obtaining Antioxidant Extracts from Fish Residues. Antioxidants 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay Iris. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Huan, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Han, Y.; Gu, Z.; Chen, D. Optimizing Conditions for Anthocyanins Extraction from Purple Sweet Potato Using Response Surface Methodology (RSM). LWT Food Sci. Technol. 2008, 41, 155–160. [Google Scholar] [CrossRef]
- Ke, F.; Liu, D.; Qin, J.; Yang, M. Functional PH-Sensitive Film Containing Purple Sweet Potato Anthocyanins for Pork Freshness Monitoring and Cherry Preservation. Foods 2024, 13, 736. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, J.S.; Choi, D.S.; Jung, M.Y. Characterization and Quantitation of Anthocyanins in Purple-Fleshed Sweet Potatoes Cultivated in Korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS. J. Agric. Food Chem. 2013, 61, 3148–3158. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin Changes in the Korean Purple-Fleshed Sweet Potato, Shinzami, as Affected by Steaming and Baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Liu, Y. Optimization of Extraction of Natural Pigment from Purple Sweet Potato by Response Surface Methodology and Its Stability. J. Chem. 2013, 2013, 590512. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 Analytical Profile of Extracts Obtained from Purple Sweet Potato after Green Ultrasound-Assisted Extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; Wang, C.; Xiao, X.L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Im, Y.R.; Kim, I.; Lee, J. Phenolic Composition and Antioxidant Activity of Purple Sweet Potato (Ipomoea batatas (L.) Lam.): Varietal Comparisons and Physical Distribution. Antioxidants 2021, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as Potential Source of Antioxidants. Characterization, Optimization of Extraction Parameters and Bioactive Properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Guclu, G.; Dagli, M.M.; Aksay, O.; Keskin, M.; Kelebek, H.; Selli, S. Comparative Elucidation on the Phenolic Fingerprint, Sugars and Antioxidant Activity of White, Orange and Purple-Fleshed Sweet Potatoes (Ipomoea batatas L.) as Affected by Different Cooking Methods. Heliyon 2023, 9, e18684. [Google Scholar] [CrossRef] [PubMed]
- Prakash Maran, J.; Manikandan, S. Response Surface Modeling and Optimization of Process Parameters for Aqueous Extraction of Pigments from Prickly Pear (Opuntia ficus-indica) Fruit. Dye. Pigment. 2012, 95, 465–472. [Google Scholar] [CrossRef]
- Moraga-Babiano, L.; Lucas-González, R.; Domínguez-Valencia, R.; Gaona-Ruiz, M.; Carrillo, C.; Echegaray, N.; Pateiro, M.; Lorenzo, J.M. Encapsulated Purple Sweet Potato Peel Extract as Antioxidant and Sustainable Colourant to Preserve the Quality of Beef Burgers during the Shelf Life. Food Chem. 2025, 487, 144657. [Google Scholar] [CrossRef]
- Collings, D.A. Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]

| Variable | Definition and Units | Nomenclature | Value/Range |
|---|---|---|---|
| Fixed | pH | 3.0 | |
| Power | 180 W | ||
| Probe | S24d22D | ||
| Amplitude | 46 µm; 100% | ||
| Upper temperature limit | 50 °C | ||
| Independent | Extraction Time (min) | t (x1) | 2–6 |
| Liquid-to-solid ratio v/w | v/w (x2) | 10–30 mL/g | |
| Ethanol concentration (v/v %) | EtOH (x3) | 35–85% | |
| Dependent | Total monomeric anthocyanin content (mg/g PSPP) | TMAC (y1) | |
| Total anthocyanin content (mg/g PSPP) | TAC (y2) | ||
| Peonidin-3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside (mg/g) | Peo-3-ch (y3) | ||
| Cyanidin-3-caffeoyl-p-hydroxybenzoyl-sophoroside-glucoside (mg/g) | Cy-3-ch (y4) | ||
| Peonidin-3-hydroxybenzoylsophoroside-5-glucoside (mg/g) | Peo-3-h (y5) | ||
| ABTS (mg ascorbic acid equivalents/g PSPP) | ABTS (y6) | ||
| DPPH (mg Trolox equivalents/g PSPP) | DPPH (y7) | ||
| FRAP (µmol Fe2+/g PSPP) | FRAP (y8) | ||
| ORAC (mg Trolox equivalents/g PSPP) | ORAC (y9) |
| Independent Variables | Dependent Variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMAC, TAC, and Main Anthocyanins (mg/g PSPP) | Antioxidant Activity (mg/g PSPP) | |||||||||||
| t | v/w | EtOH | TMAC | TAC | Peo-3-ch | Cy-3-ch | Peo-3-h | ABTS | DPPH | FRAP | ORAC | |
| No | x1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | Y8 | Y9 |
| 1 | 2 (−1) | 10 (−1) | 60 (0) | 1.54 ± 0.00 | 1.65 ± 0.00 | 0.80 ± 0.01 | 0.18 ± 0.00 | 0.19 ± 0.00 | 11.40 ± 0.94 | 11.20 ± 2.01 | 132.31 ± 3.78 | 31.71 ± 0.96 |
| 2 | 6 (+1) | 10 (−1) | 60 (0) | 1.67 ± 0.06 | 2.12 ± 0.02 | 1.04 ± 0.01 | 0.27 ± 0.00 | 0.22 ± 0.00 | 11.86 ± 0.09 | 13.75 ± 0.04 | 150.53 ± 4.45 | 37.41 ± 0.47 |
| 3 | 2 (−1) | 30 (+1) | 60 (0) | 2.44 ± 0.08 | 2.68 ± 0.01 | 1.29 ± 0.01 | 0.36 ± 0.00 | 0.27 ± 0.00 | 15.74 ± 0.56 | 15.13 ± 1.80 | 190.35 ± 2.63 | 45.38 ± 3.80 |
| 4 | 6 (+1) | 30 (+1) | 60 (0) | 2.40 ± 0.11 | 3.07 ± 0.07 | 1.47 ± 0.03 | 0.41 ± 0.01 | 0.31 ± 0.00 | 15.91 ± 0.00 | 18.16 ± 0.36 | 203.61 ± 4.50 | 52.68 ± 1.32 |
| 5 | 2 (−1) | 20 (0) | 35 (−1) | 0.32 ± 0.00 | 0.27 ± 0.00 | 0.06 ± 0.00 | 0.00 ± 0.00 | 0.12 ± 0.00 | 9.86 ± 1.61 | 8.53 ± 0.43 | 58.01 ± 2.24 | 31.72 ± 0.00 |
| 6 | 6 (+1) | 20 (0) | 35 (−1) | 0.63 ± 0.01 | 0.57 ± 0.01 | 0.23 ± 0.01 | 0.01 ± 0.00 | 0.15 ± 0.00 | 11.98 ± 0.81 | 13.60 ± 0.00 | 108.88 ± 12.49 | 33.73 ± 0.00 |
| 7 | 2 (−1) | 20 (0) | 85 (+1) | 0.16 ± 0.01 | 0.04 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.00 | 8.96 ± 1.20 | 5.97 ± 0.39 | 53.60 ± 7.20 | 25.28 ± 0.63 |
| 8 | 6 (+1) | 20 (0) | 85 (+1) | 2.20 ± 0.07 | 2.71 ± 0.02 | 1.28 ± 0.01 | 0.44 ± 0.00 | 0.25 ± 0.00 | 13.29 ± 0.50 | 13.40 ± 1.12 | 173.26 ± 7.45 | 35.51 ± 2.24 |
| 9 | 4 (0) | 10 (−1) | 35 (−1) | 0.38 ± 0.01 | 0.41 ± 0.00 | 0.15 ± 0.00 | 0.01 ± 0.00 | 0.11 ± 0.00 | 7.30 ± 0.76 | 6.83 ± 0.57 | 61.67 ± 11.42 | 23.58 ± 2.79 |
| 10 | 4 (0) | 30 (+1) | 35 (−1) | 0.45 ± 0.01 | 0.30 ± 0.02 | 0.06 ± 0.00 | 0.00 ± 0.00 | 0.13 ± 0.00 | 11.67 ± 0.79 | 12.57 ± 0.93 | 122.56 ± 8.91 | 39.85 ± 3.12 |
| 11 | 4 (0) | 10 (−1) | 85 (+1) | 1.57 ± 0.00 | 1.42 ± 0.04 | 0.60 ± 0.01 | 0.22 ± 0.00 | 0.19 ± 0.00 | 11.35 ± 0.06 | 12.96 ± 2.04 | 163.09 ± 0.00 | 31.60 ± 3.69 |
| 12 | 4 (0) | 30 (+1) | 85 (+1) | 1.68 ± 0.01 | 2.03 ± 0.03 | 0.90 ± 0.02 | 0.31 ± 0.00 | 0.22 ± 0.00 | 12.65 ± 0.50 | 10.79 ± 1.99 | 143.68 ± 0.23 | 35.86 ± 1.85 |
| 13 | 4 (0) | 20 (0) | 60 (0) | 2.66 ± 0.34 | 2.70 ± 0.00 | 1.29 ± 0.00 | 0.36 ± 0.00 | 0.28 ± 0.00 | 17.12 ± 0.00 | 17.41 ± 0.00 | 194.12 ± 6.75 | 40.40 ± 1.93 |
| 14 | 4 (0) | 20 (0) | 60 (0) | 2.09 ± 0.06 | 2.52 ± 0.00 | 1.23 ± 0.00 | 0.32 ± 0.00 | 0.26 ± 0.00 | 16.31 ± 0.00 | 18.19 ± 0.00 | 192.26 ± 9.80 | 50.57 ± 2.47 |
| 15 | 4 (0) | 20 (0) | 60 (0) | 2.01 ± 0.02 | 2.19 ± 0.03 | 1.06 ± 0.02 | 0.27 ± 0.00 | 0.24 ± 0.00 | 16.52 ± 0.00 | 18.67 ± 0.00 | 204.31 ± 2.69 | 57.36 ± 0.82 |
| TMAC | TAC | Peo−3−ch | Cy−3−ch | Peo−3−h | ABTS | DPPH | FRAP | ORAC | |
|---|---|---|---|---|---|---|---|---|---|
| βo | 2.25 (0.001) | 2.47 (0.000) | 1.19 (0.000) | 0.32 (0.000) | 0.26 (0.000) | 17.71 (0.000) | 16.5 (0.000) | 196.90 (0.000) | 47.7 (0.000) |
| β1 | 0.31 (0.148) | 0.48 (0.032) | 0.23 (0.028) | 0.07 (0.029) | 0.04 (0.060) | 1.52 (0.034) | 1.96 (0.015) | 25.25 (0.045) | 2.91 (0.227) |
| β2 | 0.23 (0.263) | 0.31 (0.115) | 0.14 (0.12) | 0.05 (0.086) | 0.03 (0.144) | 2.39 (0.006) | 1.49 (0.040) | 19.08 (0.100) | 6.18 (0.033) |
| β3 | 0.48 (0.045) | 0.58 (0.016) | 0.28 (0.014) | 0.12 (0.005) | 0.02 (0.239) | 0.68 (0.248) | 0.50 (0.398) | 22.82 (0.061) | 0.16 (0.942) |
| β12 | −0.04 (0.877) | −0.02 (0.929) | −0.02 (0.895) | −0.01 (0.759) | 0.00 (0.959) | 1.19 (0.167) | 0.12 (0.880) | −1.24 (0.930) | 0.40 (0.899) |
| β13 | 0.43 (0.149) | 0.59 (0.050) | 0.28 (0.051) | 0.11 (0.027) | 0.05 (0.087) | 0.55 (0.490) | 1.19 (0.179) | 17.20(0.256) | 2.54 (0.435) |
| β23 | 0.01 (0.965) | 0.18 (0.468) | 0.10 (0.400) | 0.03 (0.478) | 0.00 (0.952) | −0.77 (0.345) | −1.98 (0.049) | −20.08 (0.195) | −3.00 (0.361) |
| β11 | −0.22 (0.450) | −0.11 (0.658) | −0.04 (0.748) | −0.02 (0.660) | −0.02 (0.462) | −1.22 (0.173) | −1.47 (0.122) | −26.00 (0.121) | −3.77 (0.279) |
| β22 | −0.02 (0.932) | 0.02 (0.928) | 0.00 (0.984) | 0.000 (0.897) | 0.01 (0.804) | −1.50 (0.108) | −0.46 (0.585) | −1.69 (0.908) | −2.13 (0.523) |
| β33 | −1.21 (0.006) | −1.46 (0.002) | −0.76 (0.001) | −0.19 (0.003) | −0.100 (0.009) | −5.47 (0.001) | −5.25 (0.001) | −72.46 (0.003) | −12.85 (0.009) |
| R2 | 0.877 | 0.933 | 0.941 | 0.938 | 0.87 | 0.947 | 0.939 | 0.909 | 0.858 |
| CV | 13.02 | 10.73 | 10.47 | 12.52 | 10.23 | 4.81 | 5.33 | 7.86 | 7.24 |
| (eq 1) | |
| (eq 2) | |
| (eq 3) | Peo-3-ch |
| (eq 4) | Cy-3-ch |
| (eq 5) | Peo-3-h |
| (eq 6) | |
| (eq 7) | |
| (eq 8) |
| Predictive | Experimental | Mean | SD | CV | |
|---|---|---|---|---|---|
| TMAC (mg/g) | 2.50 | 2.38 | 2.44 | 0.1 | 2.44 |
| TAC (mg/g) | 3.15 | 2.63 | 2.89 | 0.26 | 8.92 |
| Peonidin-3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside (mg/g) | 1.51 | 1.25 | 1.38 | 0.13 | 9.40 |
| Cyanidin-3-caffeoyl-p-hydroxybenzoyl-sophoroside-glucoside (mg/g) | 0.42 | 0.35 | 0.39 | 0.04 | 9.59 |
| Peonidin-3-hydroxybenzoylsophoroside-5-glucoside (mg/g) | 0.32 | 0.28 | 0.30 | 0.02 | 6.92 |
| ABTS (mg TE/g) | 20.08 | 18.78 | 19.43 | 0.7 | 3.36 |
| DPPH (mg AAE/g) | 18.13 | 18.17 | 18.15 | 0.0 | 0.12 |
| FRAP (µmol Fe2+/g) | 212.29 | 254.26 | 233.27 | 21.0 | 9.00 |
| ORAC (mg TE/g) | 51.29 | 60.26 | 55.77 | 4.5 | 8.04 |
| Sonotrode-UAE | Magnetic Agitation | |
|---|---|---|
| Cyanidin-3-sophoroside-glucoside | 28.00 ± 1.46 a | 27.69 ± 1.69 a |
| Peonidin-3-sophoroside-5-glucoside | 33.88 ± 1.13 a | 31.34 ± 1.91 a |
| Cyanidin-3p-hydroxybenzoylsophoroside-5-glucoside | 133.95 ± 2.34 a | 133.95 ± 2.34 a |
| Cyanidin-3-(6‴-caffeoyl sophoroside)-5-glucoside | 2.52 ± 0.16 a | 2.63 ± 0.14 a |
| Peonidin-3-hydroxybenzoylsophoroside-5-glucoside | 275.11 ± 9.8 a | 251.07 ± 15.73 a |
| Cyanidin-3-feruloyl sophoroside-5-glucoside | 43.09 ± 1.67 a | 39.81 ± 3.1 b |
| Peonidin-3-feruloyl sophoroside-5-glucoside | 56.9 ± 4.44 a | 49.48 ± 2.13 b |
| Peonidin-3-caffeoyl sophoroside-5-glucoside | 118.15 ± 7.87 a | 97.64 ± 5.64 b |
| Peonidin derivative 5 | 27.48 ± 2.92 a | 23.29 ± 0.57 b |
| Cyanidin-3-caffeoyl-p-hydroxybenzoyl-sophoroside-glucoside | 348.12 ± 9.82 a | 312.59 ± 6.8 b |
| Cyanidin-3-caffeoyl-feruloyl sophoroside-5-glucoside | 91.86 ± 1.44 a | 80.13 ± 1.58 b |
| Peonidin-3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | 1253.63 ± 30.46 a | 1009.88 ± 21.31 b |
| Peonidin-3-caffeoyl-feruloyl sophoroside-5-glucoside | 202.59 ± 3.41 a | 156.7 ± 2.03 b |
| Peonidin-3-(6′-p′-hydroxybenzoyl-6″-feruloyl sophoroside)-5-glucoside | 39.91 ± 1.15 a | 31.31 ± 1.26 b |
| Glycosilated | 61.88 ± 2.34 a | 59.03 ± 3.57 a |
| Monoacylated | 629.73 ± 16.03 a | 568.24 ± 32.49 b |
| Diacylated | 1936.11 ± 44.69 a | 1590.61 ± 30.35 b |
| TAC | 2627.72 ± 61.69 a | 2217.88 ± 65.06 b |
| DPPH (mg TE/g) | 18.17 ± 0.31 a | 10.41 ± 0.4 b |
| ABTS (mg AAE/g) | 18.78 ± 0.48 a | 14.92 ± 0.61 b |
| FRAP (µmol Fe2+/g) | 254.26 ± 7.93 a | 172.98 ± 14.43 b |
| ORAC (mg TE/g) | 60.26 ± 4.53 a | 48.31 ± 1.86 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas-González, R.; Pateiro, M.; Domínguez-Valencia, R.; Carrillo, C.; Lorenzo, J.M. Optimization of Anthocyanin Extraction from Purple Sweet Potato Peel (Ipomea batata) Using Sonotrode Ultrasound-Assisted Extraction. Foods 2025, 14, 2686. https://doi.org/10.3390/foods14152686
Lucas-González R, Pateiro M, Domínguez-Valencia R, Carrillo C, Lorenzo JM. Optimization of Anthocyanin Extraction from Purple Sweet Potato Peel (Ipomea batata) Using Sonotrode Ultrasound-Assisted Extraction. Foods. 2025; 14(15):2686. https://doi.org/10.3390/foods14152686
Chicago/Turabian StyleLucas-González, Raquel, Mirian Pateiro, Rubén Domínguez-Valencia, Celia Carrillo, and José M. Lorenzo. 2025. "Optimization of Anthocyanin Extraction from Purple Sweet Potato Peel (Ipomea batata) Using Sonotrode Ultrasound-Assisted Extraction" Foods 14, no. 15: 2686. https://doi.org/10.3390/foods14152686
APA StyleLucas-González, R., Pateiro, M., Domínguez-Valencia, R., Carrillo, C., & Lorenzo, J. M. (2025). Optimization of Anthocyanin Extraction from Purple Sweet Potato Peel (Ipomea batata) Using Sonotrode Ultrasound-Assisted Extraction. Foods, 14(15), 2686. https://doi.org/10.3390/foods14152686











