Functional Quality and Microbiological Stability of Grape Juice During Processing by UV-C Light Compared to Mild Heat Treatment and Evolution of the Parameters During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Culture Media
2.2. Extraction of Grape Juice
2.3. Characterization of Grape Juice
2.4. Microbial Inactivation Kinetics
2.4.1. Inoculum Preparation
2.4.2. Application of UV-C Light Treatments
2.4.3. Determination of the Delivered UV-C Dose
2.4.4. Application of Mild Thermal Treatment (MTT)
2.4.5. Enumeration of Microorganisms
2.5. Storage Stability
Microbiological Stability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Grape Juice
3.2. Selection of UV-C Processing Time
3.3. Selection of the Processing Time for the Mild Thermal Treatment at 75 °C
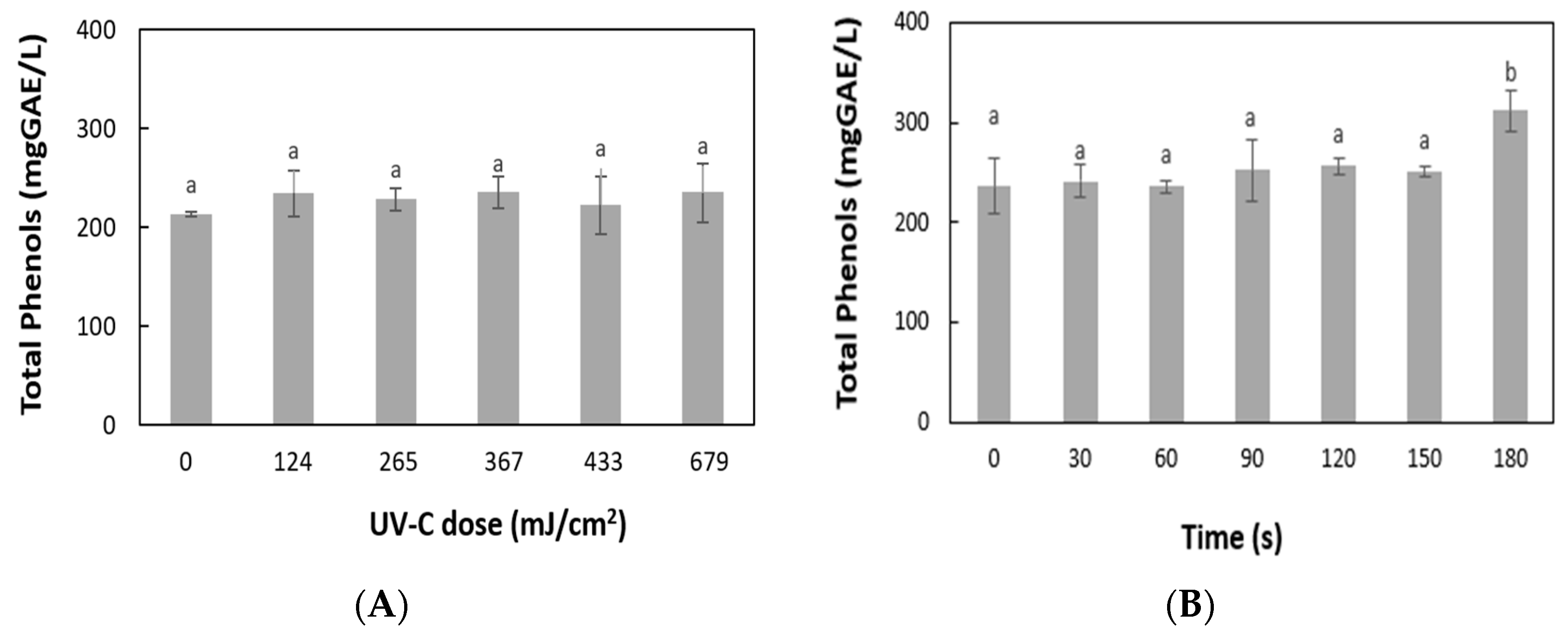
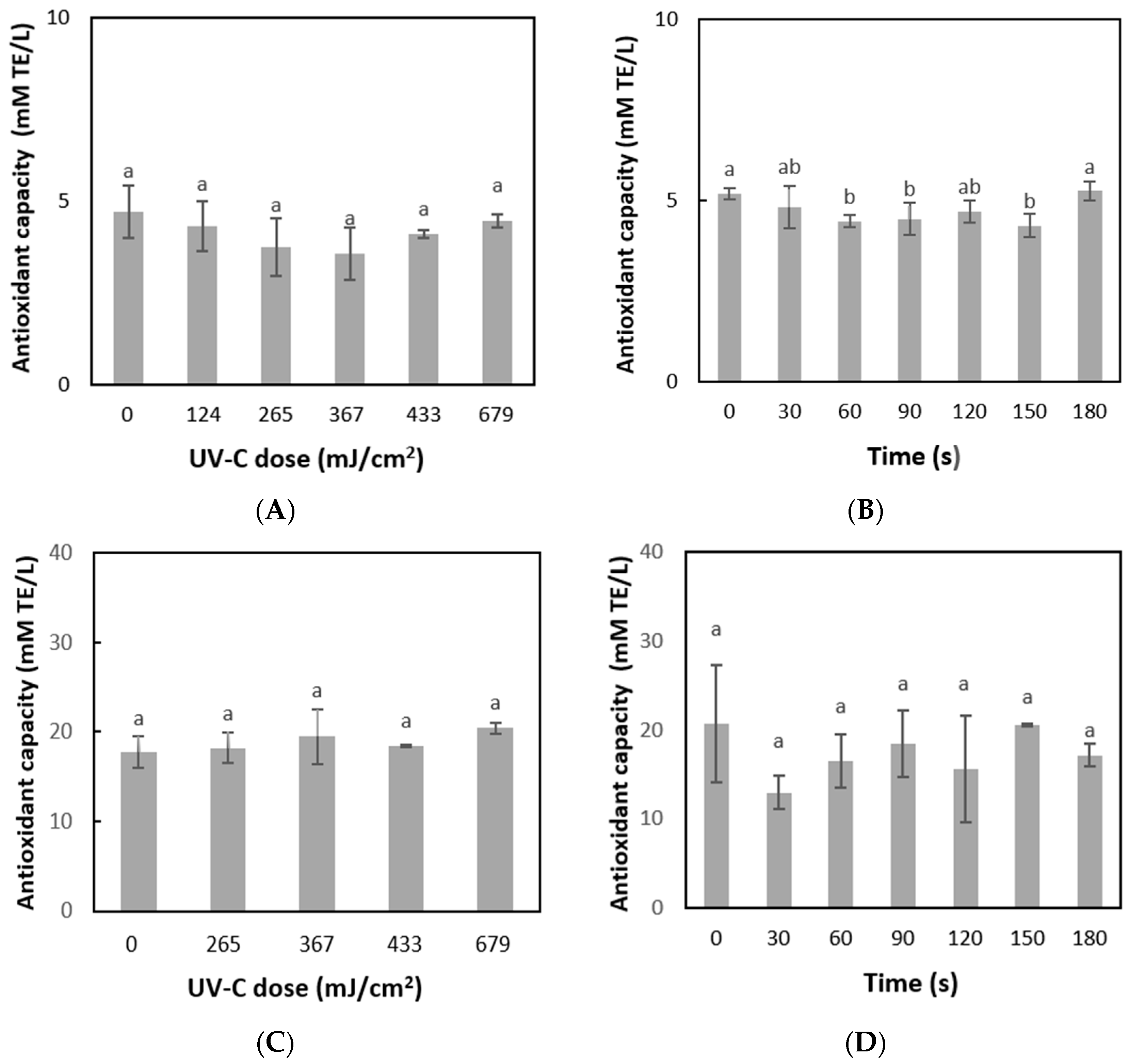
3.4. Effect of UV-C Light and Mild Heat Treatment on Total Phenol Content and Antioxidant Activity
3.5. Juice Stability in Refrigerated Storage
3.5.1. Evolution of pH, Soluble Solids, and Color
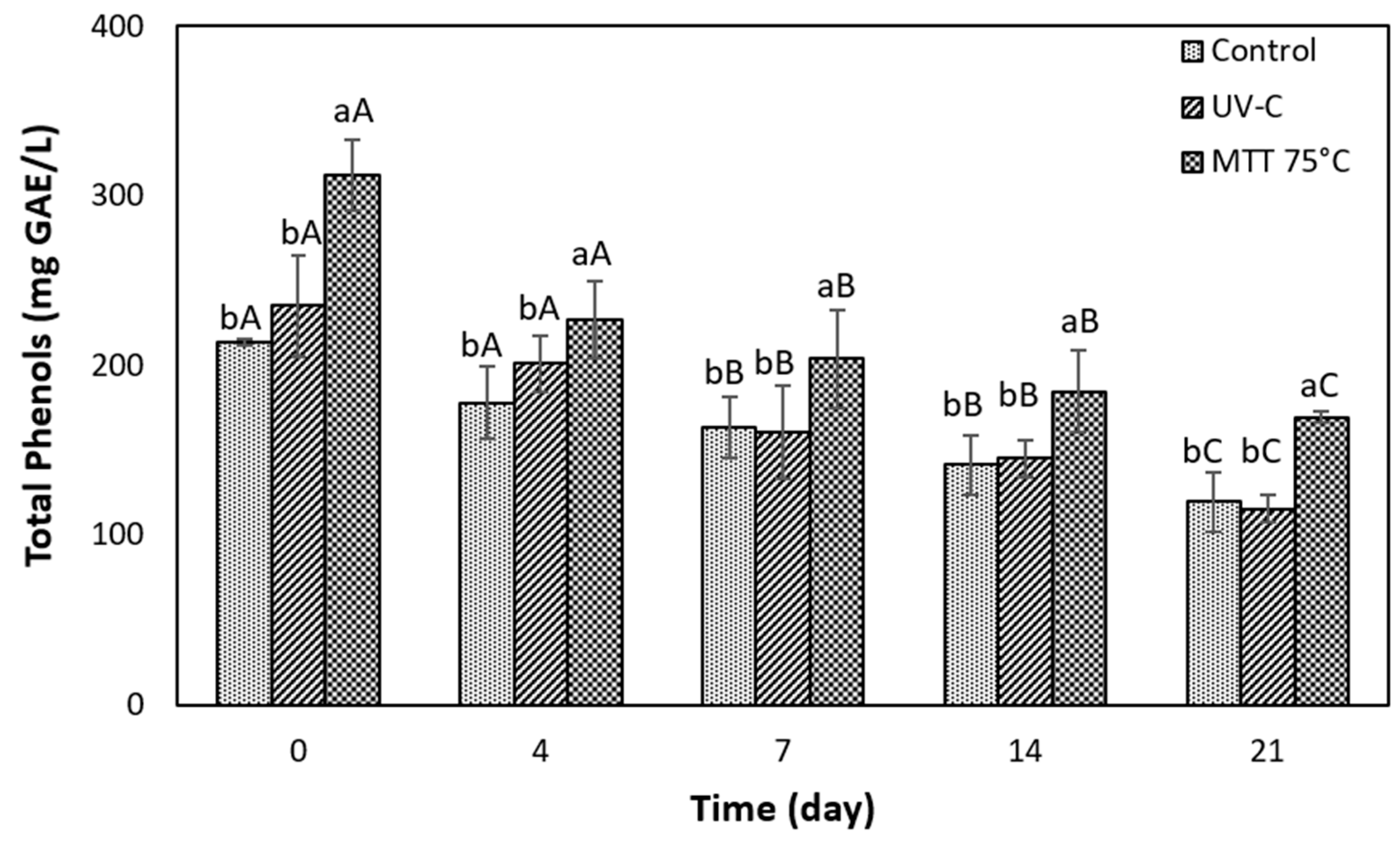
3.5.2. Evolution of Total Phenols During Storage
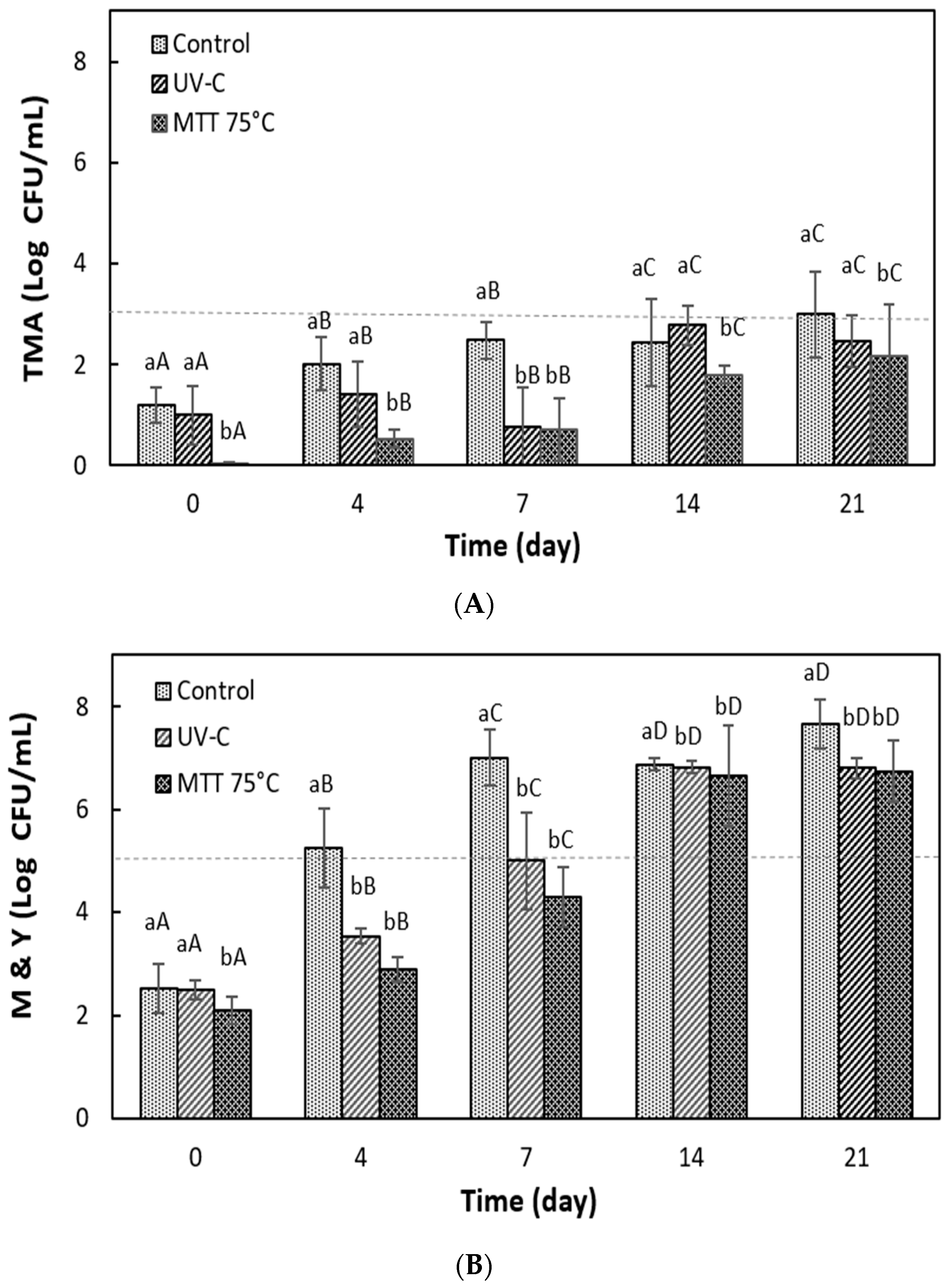
3.5.3. Microbiological Stability of Grape Juice During Refrigerated Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV-C | Short-wave ultraviolet light |
| MTT | Mild thermal treatment |
| TP | Total phenols |
| AC | Antioxidant capacity |
| GAE | Galic acid equivalent |
| TE | Trolox equivalent |
| α | Absorption coefficient |
| AOAC | Association of the Official Analytical Chemists |
| DL | Detection limit |
| LAB | Lactic acid bacteria |
References
- Mansur, A.R.; Lee, H.S.; Lee, C.J. A Review of the Efficacy of Ultraviolet C Irradiation for Decontamination of Pathogenic and Spoilage Microorganisms in Fruit Juices. J. Microbiol. Biotechnol. 2023, 33, 419–429. [Google Scholar] [CrossRef]
- Scharf, S.; Sandmann, M. Effects of Pulsed Electric Fields on the Physicochemical and Sensory Properties of Thompson Seedless Grapes. Beverages 2023, 9, 82. [Google Scholar] [CrossRef]
- Tipton, S.; Morris, J.; Main, G.; Sharp, C.; MCNEW, R. GRAPE JUICE AS AN EXTENDER AND SWEETENER FOR BLUEBERRY DRINKS. J. Food Qual. 1999, 22, 275–285. [Google Scholar] [CrossRef]
- Parihar, S.; Sharma, D.; Bypass, N.D. A Breif Overview on Vitis vinifera. Sch. Acad. J. Pharm. 2021, 9531, 231–239. [Google Scholar] [CrossRef]
- dos Santos Lima, M.; Silva Dantas, B.; Araújo Carvalho, A.J.d.B.; Pereira, G.E.; Colombo Pimentel, T.; Magnani, M. A novel method for ultra-fast determination of phenolics with performance comparable to UPLC/DAD: Method development and validation on analysis of seedless table grapes. J. Food Compos. Anal. 2024, 134, 106511. [Google Scholar] [CrossRef]
- Nasser, M.; Cheikh-Ali, H.; Hijazi, A.; Merah, O.; Al-Rekaby, A.E.A.N.; Awada, R. Phytochemical profile, antioxidant and antitumor activities of green grape juice. Processes 2020, 8, 507. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Rojo, M.C.; Arroyo López, F.N.; Lerena, M.C.; Mercado, L.; Torres, A.; Combina, M. Evaluation of different chemical preservatives to control Zygosaccharomyces rouxii growth in high sugar culture media. Food Control 2015, 50, 349–355. [Google Scholar] [CrossRef]
- Sourri, P.; Tassou, C.C.; Nychas, G.J.E.; Panagou, E.Z. Fruit Juice Spoilage by Alicyclobacillus: Detection and Control Methods—A Comprehensive Review. Foods 2022, 11, 747. [Google Scholar] [CrossRef]
- Orizano-ponce, E.; Char, C.; Sepúlveda, F.; Ortiz-viedma, J. Heat sensitization of Escherichia coli by the natural antimicrobials vanillin and emulsified citral in blended carrot-orange juice. Food Microbiol. 2022, 107, 104058. [Google Scholar] [CrossRef]
- Krug, M.; Chapin, T.; Danyluk, M.; Goodrich-Schneider, R.; Schneider, K.; Harris, L.; Worobo, R. Outbreaks of Foodborne Disease Associated with Fruit and Vegetable Juices, 1922–2019. Edis 2020, 1–7. [Google Scholar] [CrossRef]
- Rangel, J.M.; Sparling, P.H.; Crowe, C.; Griffin, P.M.; Swerdlow, D.L. Epidemiology of Escherichia coli. Emerg. Infect. Dis. 2005, 11, 603–609. [Google Scholar] [CrossRef]
- Pierscianowski, J.; Popović, V.; Biancaniello, M.; Bissonnette, S.; Zhu, Y.; Koutchma, T. Continuous-flow UV-C processing of kale juice for the inactivation of E. coli and assessment of quality parameters. Food Res. Int. 2021, 140, 110085. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Wu, S.J.; Chen, B.Y.; Huang, H.W.; Wang, C.Y. Effect of high-pressure processing and thermal pasteurization on overall quality parameters of white grape juice. J. Sci. Food Agric. 2017, 97, 3166–3172. [Google Scholar] [CrossRef]
- Souza, V.R.; Koutchma, T. Ultraviolet Light Microbial Inactivation in Liquid Foods. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 146–170. [Google Scholar]
- Sauceda-Gálvez, J.N.; Tió-Coma, M.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Effect of single and combined UV-C and ultra-high pressure homogenisation treatments on inactivation of Alicyclobacillus acidoterrestris spores in apple juice. Innov. Food Sci. Emerg. Technol. 2020, 60, 102299. [Google Scholar] [CrossRef]
- Rajan, A.; Radhakrishnan, M. Green Technologies for Sustainable Food Production and Preservation: An Overview of Ohmic Heating, Infrared Heating and UV Light Technology. In Sustainable Food Science- A comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2023; Volume 4, pp. 184–197. ISBN 9780128241660. [Google Scholar]
- Santhirasegaram, V.; Razali, Z.; George, D.S.; Somasundram, C. Comparison of UV-C treatment and thermal pasteurization on quality of Chokanan mango (Mangifera indica L.) juice. Food Bioprod. Process. 2015, 94, 313–321. [Google Scholar] [CrossRef]
- Falguera, V.; Garvín, A.; Garza, S. Effect of UV—Vis Photochemical Processing on Pear Juices from Six Different Varieties. Food Bioprocess Technol. 2014, 7, 84–92. [Google Scholar] [CrossRef]
- Pala, Ç.; Kirca Toklucu, A. Microbial, physicochemical and sensory properties of UV-C processed orange juice and its microbial stability during refrigerated storage. LWT—Food Sci. Technol. 2013, 50, 426–431. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ruiz-López, I.I.; Pacheco-Aguirre, F.M.; Guerrero-Beltrán, J.Á.; Ávila-Sosa, R.; Ochoa-Velasco, C.E. Ultraviolet-C light effect on physicochemical, bioactive, microbiological, and sensorial characteristics of carrot (Daucus carota) beverages. Food Sci. Technol. Int. 2016, 22, 536–546. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Palgan, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. The Effect of Ultraviolet Light on Microbial Inactivation and Quality Attributes of Apple Juice. Food Bioprocess Technol. 2012, 5, 680–686. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Guerrero Beltrán, J.Á. Short-wave ultraviolet-C light effect on pitaya (Stenocereus griseus) juice inoculated with Zygosaccharomyces bailii. J. Food Eng. 2013, 117, 34–41. [Google Scholar] [CrossRef]
- Association of the Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Gaithersbg, MD, USA, 2016. [Google Scholar]
- Koutchma, T. Fundamentals of UV Light Emission, Transmission, and Absorption. In Preservation and Shelf Life Extension; Academic Press: Cambridge, MA, USA, 2014; pp. 1–3. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ortiz-viedma, J.; Bastias-montes, J.M.; Char, C.; Vega, C.; Quintriqueo, A.; Gall, M.; Flores, M.; Aguilera, J.M.; Miranda, J.M.; Barros-vel, J. Sequential Biorefining of Bioactive Compounds of High Functional Value from Calafate Pomace (Berberis microphylla) Using Supercritical CO2 and Pressurized Liquids. Antioxidants 2023, 12, 323. [Google Scholar] [CrossRef]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Jimenez, P.; Romero, N. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rahn, R.O. Potassium Iodide as a Chemical Actinometer for 254 nm Radiation: Use of lodate as an Electron Scavenger. Photochem. Photobiol. 1997, 66, 450–455. [Google Scholar] [CrossRef]
- MINSAL. Reglamento Sanitario de los Alimentos—Ministerio de Salud; MINSAL: Santiago, Chile, 2015. [Google Scholar]
- Unluturk, S.; Atilgan, M. UV-C Irradiation of Freshly Squeezed Grape Juice and Modeling Inactivation Kinetics. J. Food Process Eng. 2014, 37, 438–449. [Google Scholar] [CrossRef]
- Tarifa, M.C.; Agustín, M.d.R.; Brugnoni, L.I. Biological control of foodborne pathogens by lactic acid bacteria: A focus on juice processing industries. Rev. Argent. Microbiol. 2023, 55, 378–386. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Ortíz, R.; Benavides, J.; Mura, E.; Lissi, E. Use of the ORAC-pyrogallol red/ORAC-fluorescein ratio to assess the quality of antioxidants in Chilean wines. J. Chil. Chem. Soc. 2011, 56, 764–767. [Google Scholar] [CrossRef]
- Müller, A.; Noack, L.; Greiner, R.; Stahl, M.R.; Posten, C. Effect of UV-C and UV-B treatment on polyphenol oxidase activity and shelf life of apple and grape juices. Innov. Food Sci. Emerg. Technol. 2014, 26, 498–504. [Google Scholar] [CrossRef]
- Hinds, L.M.; O’Donnell, C.P.; Akhter, M.; Tiwari, B.K. Principles and mechanisms of ultraviolet light emitting diode technology for food industry applications. Innov. Food Sci. Emerg. Technol. 2019, 56, 102153. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, J.; Pan, Z.; Cheng, Y.; Zhang, Y.; Li, N. Effect of heat treatment, pH, sugar concentration, and metal ion addition on green color retention in homogenized puree of Thompson seedless grape. LWT 2014, 55, 595–603. [Google Scholar] [CrossRef]
- Usaga, J.; Worobo, R.W.; Moraru, C.I.; Padilla-Zakour, O.I. Time after apple pressing and insoluble solids influence the efficiency of the UV treatment of cloudy apple juice. LWT—Food Sci. Technol. 2015, 62, 218–224. [Google Scholar] [CrossRef]
- Unluturk, S.; Atilgan, M.R. Microbial Safety and Shelf Life of UV-C Treated Freshly Squeezed White Grape Juice. J. Food Sci. 2015, 80, M1831–M1841. [Google Scholar] [CrossRef]
- Ramesh, T.; Yaparatne, S.; Tripp, C.P.; Nayak, B.; Amirbahman, A. Ultraviolet Light-Assisted Photocatalytic Disinfection of Escherichia coli and Its Effects on the Quality Attributes of White Grape Juice. Food Bioprocess Technol. 2018, 11, 2242–2252. [Google Scholar] [CrossRef]
- Kaya, Z.; Unluturk, S. Processing of clear and turbid grape juice by a continuous flow UV system. Innov. Food Sci. Emerg. Technol. 2016, 33, 282–288. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. UV-C light inactivation of single and composite microbial populations in tangerine-orange juice blend. Evaluation of some physicochemical parameters. Food Bioprod. Process. 2019, 117, 149–159. [Google Scholar] [CrossRef]
- Gouma, M.; Gayán, E.; Raso, J.; Condón, S.; Álvarez, I. Inactivation of spoilage yeasts in apple juice by UV–C light and in combination with mild heat. Innov. Food Sci. Emerg. Technol. 2015, 32, 146–155. [Google Scholar] [CrossRef]
- Casco, M.d.l.A.; Schelegueda, L.; Andreone, A.; Schenk, M.; Campos, C.A.; Guerrero, S.N. A novel approach using UV-C light assisted by mild heat and natamycin as a hurdle strategy to preserve juice from non-commercial-grade apples. Food Control 2025, 171, 111147. [Google Scholar] [CrossRef]
- Donsingha, S.; Assatarakul, K. Kinetics model of microbial degradation by UV radiation and shelf life of coconut water. Food Control 2018, 92, 162–168. [Google Scholar] [CrossRef]
- Antonio-Gutiérrez, O.; López-Díaz, A.; Palou, E.; López-Malo, A.; Ramírez-Corona, N. Characterization and effectiveness of short-wave ultraviolet irradiation reactors operating in continuous recirculation mode to inactivate Saccharomyces cerevisiae in grape juice. J. Food Eng. 2019, 241, 88–96. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the inactivation of vegetative bacteria by thermal treatments: Mode of action, influence of environmental factors and inactivation kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal Treatments for Fruit and Vegetable Juices and Beverages: A Literature Overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Genova, G.; Tosetti, R.; Tonutti, P. Berry ripening, pre-processing and thermal treatments affect the phenolic composition and antioxidant capacity of grape (Vitis vinifera L.) juice. J. Sci. Food Agric. 2016, 96, 664–671. [Google Scholar] [CrossRef]
- Ferrario, M.; Guerrero, S.; Char, C. Optimisation of minimal processing variables to preserve the functional quality and colour of carrot juice by means of the response surface methodology. Int. J. Food Sci. Technol. 2017, 52, 864–871. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. A comparative study on the effect of conventional thermal pasteurisation, microwave and ultrasound treatments on the antioxidant activity of five fruit juices. Food Sci. Technol. Int. 2015, 22, 288–301. [Google Scholar] [CrossRef]
- Biancaniello, M.; Popović, V.; Avila, C.; Ros-Polski, V.; Koutchma, T. Feasibility of a Novel Industrial-Scale Treatment of Green Cold-Pressed Juices by UV-C Light Exposure. Beverages 2018, 4, 29. [Google Scholar] [CrossRef]
- de Souza, V.R.; Popović, V.; Bissonnette, S.; Ros, I.; Mats, L.; Duizer, L.; Warriner, K.; Koutchma, T. Quality changes in cold pressed juices after processing by high hydrostatic pressure, ultraviolet-c light and thermal treatment at commercial regimes. Innov. Food Sci. Emerg. Technol. 2020, 64, 102398. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhu, Y.; Wang, S.; Hu, X.; Ling, M.; Li, D.; Duan, C.; Mu, H.; Zhu, B.; et al. Exploring the aroma profiles and color characteristics of chardonnay wines from the eastern foothills of Ningxia Helan Mountain in China: A Flavoromics approach. Food Chem. X 2024, 24, 102038. [Google Scholar] [CrossRef]
- Wietrzynski, W.; Engel, B.D. Chlorophyll biogenesis sees the light. Nat. Plants 2021, 7, 380–381. [Google Scholar] [CrossRef]
- Gök, S.B. UV-C Treatment of Apple and Grape Juices by Modified UV-C Reactor Based on Dean Vortex Technology: Microbial, Physicochemical and Sensorial Parameters Evaluation. Food Bioprocess Technol. 2021, 14, 1055–1066. [Google Scholar] [CrossRef]
- Muche, B.M.; Speers, R.A.; Rupasinghe, H.P.V. Storage Temperature Impacts on Anthocyanins Degradation, Color Changes and Haze Development in Juice of “Merlot” and “Ruby” Grapes (Vitis vinifera). Front. Nutr. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; Taha, N.A.; EL-Khateeb, Y.A. Storability of ‘Thompson Seedless’ grapes: Using biopolymer coating chitosan and polyvinyl alcohol blending with salicylic acid and antioxidant enzymes activities during cold storage. Sci. Hortic. 2019, 249, 314–321. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| pH | 3.7 ± 0.1 |
| Soluble solids (°Brix) | 25.0 ± 0.7 |
| % Acidity (g tartaric acid/100 mL) | 0.60 ± 0.01 |
| α254 nm (cm−1) | 44.2 ± 1.2 |
| Color | |
| L* | 53.20 ± 0.07 |
| a* | −5.00 ± 0.05 |
| b* | 28.6 ± 0.2 |
| Total phenols [mg GAE/L] | 213.7 ± 2.1 |
| Antioxidant capacity | |
| ORAC [mM TE/L] | 4.7 ± 0.7 |
| DPPH [mM TE/L] | 17.8 ± 1.7 |
| Treatment | Time (Day) | pH | Soluble Solids (°Brix) | Color Parameters | |||
|---|---|---|---|---|---|---|---|
| Lab* | a* | b* | ∆E | ||||
| Control | 0 | 3.7 ± 0.1 aA | 25.0 ± 0.7 aA | 53.1 ± 0.4 aA | −4.2 ± 0.9 cA | 28.4 ± 0.6 aA | 0.00 ± 0.00 bA |
| 4 | 3.78 ± 0.08 aA | 25.0 ± 0.7 aA | 46 ± 1 bB | 3 ± 1 bB | 26.0 ± 0.5 aA | 10 ± 1 aB | |
| 7 | 3.8 ± 0.1 aA | 24.6 ± 0.6 2 aA | 48 ± 1 bB | 4.0 ± 0.4 bB | 26 ± 1 aA | 10.11 ± 0.03 aB | |
| 14 | 3.83 ± 0.08 aA | 22 ± 1 aB | 50.1 ± 0.3 bAB | 2.4 ± 0.3 bB | 26.9 ± 0.9 aA | 7.4 ± 0.7 aB | |
| 21 | 3.9 ± 0.1 aA | 20 ± 2 aC | 48.2 ± 0.2 bB | 4 ± 1 bB | 26 ± 1 aA | 9.35 ± 0.09 aB | |
| UV-C 569 mJ/cm2 | 0 | 3.76 ± 0.09 aA | 24.6 ± 0.9 aA | 50.0 ± 0.6 cA | −2 ± 1 bA | 26.6 ± 0.2 bA | 4 ± 1 aA |
| 4 | 3.77 ± 0.08 aA | 24.6 ± 0.7 aA | 44.2 ± 0.2 cB | 5 ± 1 aB | 25.0 ± 0.4 bA | 8.9 ± 0.4 aB | |
| 7 | 3.79 ± 0.09 aA | 24.4 ± 0.8 aA | 43 ± 1 cB | 6.1 ± 0.3 aB | 25.0 ± 0.9 bA | 11 ± 1 aB | |
| 14 | 3.79 ± 0.05 aA | 23.0 ± 0.4 aB | 46 ± 1 cAB | 6.1 ± 0.2 aB | 24 ± 1 bA | 10 ± 2 aB | |
| 21 | 3.80 ± 0.06 aA | 21.6 ± 0.5 aC | 45 ± 1 cB | 6.1 ± 0.7 aB | 24.9 ± 0.7 bA | 9 ± 2 aB | |
| Thermal treatment 75 °C, 180 s | 0 | 3.7 ± 0.1 aA | 25.6 ± 0.7 aA | 52.1 ± 0.1 bA | −0.3 ± 0.2 cA | 27 ± 1 aA | 4.3 ± 0.7 aA |
| 4 | 3.77 ± 0.07 aA | 25.4 ± 0.6 aA | 53.0 ± 0.6 aB | 0.2 ± 0.2 cB | 26 ± 1 aA | 1.4 ± 0.5 bB | |
| 7 | 3.79 ± 0.06 aA | 25.0 ± 0.1 aA | 53.2 ± 0.1 aB | −0.1 ± 0.2 cB | 26 ± 2 aA | 1.5 ± 0.6 bB | |
| 14 | 3.81 ± 0.06 aA | 23 ± 2 aB | 55 ± 1 aAB | −0.2 ± 0.3 cB | 27 ± 1 aA | 2 ± 1 bB | |
| 21 | 3.84 ± 0.08 aA | 20 ± 2 aC | 54.8 ± 0.9 aB | −0.3 ± 0.4 cB | 26 ± 1 aA | 3 ± 1 bB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Char, C.; Vegas, C.; Romero, N.; Puente-Diaz, L.; Ortiz-Viedma, J.; Flores, M. Functional Quality and Microbiological Stability of Grape Juice During Processing by UV-C Light Compared to Mild Heat Treatment and Evolution of the Parameters During Cold Storage. Foods 2025, 14, 2056. https://doi.org/10.3390/foods14122056
Char C, Vegas C, Romero N, Puente-Diaz L, Ortiz-Viedma J, Flores M. Functional Quality and Microbiological Stability of Grape Juice During Processing by UV-C Light Compared to Mild Heat Treatment and Evolution of the Parameters During Cold Storage. Foods. 2025; 14(12):2056. https://doi.org/10.3390/foods14122056
Chicago/Turabian StyleChar, Cielo, Carla Vegas, Nalda Romero, Luis Puente-Diaz, Jaime Ortiz-Viedma, and Marcos Flores. 2025. "Functional Quality and Microbiological Stability of Grape Juice During Processing by UV-C Light Compared to Mild Heat Treatment and Evolution of the Parameters During Cold Storage" Foods 14, no. 12: 2056. https://doi.org/10.3390/foods14122056
APA StyleChar, C., Vegas, C., Romero, N., Puente-Diaz, L., Ortiz-Viedma, J., & Flores, M. (2025). Functional Quality and Microbiological Stability of Grape Juice During Processing by UV-C Light Compared to Mild Heat Treatment and Evolution of the Parameters During Cold Storage. Foods, 14(12), 2056. https://doi.org/10.3390/foods14122056







