Abstract
The increasing scarcity of traditional nectar sources due to climate change has led beekeepers to explore alternative floral sources. This study investigates the volatile profile, sensory characteristics, and consumer acceptability of monofloral honey derived from Capparis spinosa L., a drought-resistant Mediterranean plant. Honey samples produced by Apis mellifera ssp. sicula on Aeolian Islands (Sicily, Italy) were analyzed. Volatile organic compounds (VOCs) were extracted using headspace solid–phase microextraction (HS-SPME) and identified by gas chromatography–mass spectrometry (GC–MS), revealing 59 compounds, with dimethyl sulfide being the predominant one. Sensory evaluation using quantitative descriptive analysis (QDA) and Time Intensity (TI) analysis identified distinctive descriptors such as sweet-caramel, cabbage/cauliflower, and pungent notes. Statistical analyses confirmed correlations between specific VOCs and sensory perceptions. A consumer acceptability test involving 80 participants showed lower preference scores for caper honey in terms of aroma and overall acceptability compared to commercial multifloral honey, with differences observed across age groups. The unique aromatic profile and consumer feedback suggest that caper honey has strong potential as a niche, high-quality product, particularly within the context of climate-resilient beekeeping, offering valuable opportunities for innovation and diversification in sustainable apiculture.
1. Introduction
In recent years, the beekeeping sector has been facing increasing challenges due to the adverse effects of climate change. The rising temperatures, prolonged droughts, and shifting blooming periods are significantly affecting the availability and productivity of many floral sources traditionally used in apiculture. As a result, there is a growing interest in the identification and valorization of alternative nectar sources, particularly those derived from plants that are resilient to climate change.
Capparis spinosa L., commonly known as the caper plant, is a perennial shrub belonging to the Capparaceae family. It is a typical species of the Mediterranean ecosystem, widely distributed across arid and semiarid regions of southern Europe, North Africa, and Central Asia [1,2]. The caper plant is characterized by its remarkable drought resistance and the ability to thrive under high temperatures, making it well adapted to increasingly arid environments and a valuable nectar source during the summer months, when other floral resources are scarce. Moreover, it has been traditionally used in herbal medicine for its high amount of bioactive compounds, such as polyphenols, alkaloids, glycosides, tannins, and flavonoids [3,4,5,6]. In this regard, several studies have demonstrated its antimicrobial, antidiabetic, anti-inflammatory, and antioxidant properties [7,8,9,10].
In Italy, the caper plant is mainly found in Sicily, particularly along coastal areas and nearby small islands, with scattered populations occurring inland. Traditionally, C. spinosa is cultivated for its flower buds (capers) and fruits (caper berries), both widely used as condiments in culinary preparations. However, the reduction in pollen availability from Mediterranean plants such as citrus, whose production has declined by approximately 18% [11], has led honeybees to shift toward more drought-tolerant native species. In this context, Capparis spinosa L. has attracted growing interest as a resilient nectar source, leading to growing attention on the production of caper monofloral honey.
However, caper honey has been the subject of a limited number of studies, primarily focused on its antioxidant and antibacterial activities, as well as its potential immunomodulatory effects, suggesting that it could represent a valuable functional food [12,13,14,15].
Honey is widely recognized for its health properties due to its natural antioxidant content and associated health benefits [16]. Its chemical composition is strongly influenced by the floral source, geographical origin, environmental conditions, and post-harvest handling [17,18,19]. Among its components, volatile organic compounds (VOCs) are of particular importance, as they play a key role in determining the aroma and taste of honey and are commonly used to authenticate the botanical origin [20].
However, despite these promising findings, no studies to date have explored the chemical and sensory properties of caper honey. In particular, no data is available on its volatile composition or consumer acceptability, both of which are essential for evaluating its potential as a high-quality niche product in the honey market.
Considering the above, the present study aims to investigate the volatile profile of caper honey. Furthermore, a sensory evaluation was carried out to assess its sensory properties. This dual approach is expected to provide valuable insights into the functional and commercial potential of caper honey in the context of climate-resilient beekeeping.
2. Materials and Methods
2.1. Sampling
Caper honey samples produced by the endemic Sicilian black honeybee (Apis mellifera ssp. sicula) were provided by a local beekeeper from Aeolian Islands (Aeolian Archipelago, Sicily, Italy), who guaranteed their authenticity and botanical origin. A total of eight samples, packaged in glass containers, were collected from the 2023 and 2024 production years. The samples were transported to the laboratory, where they were stored at room temperature and protected from light until analysis. A commercial, multifloral honey was also used as a reference in the consumer’s acceptability test.
2.2. HS-SPME Conditions
The extraction of volatile compounds from honey samples was carried out by headspace solid-phase microextraction (HS-SPME). A sample of 5 g of honey was placed into a 40 mL vial, with the addition of 15 mL of NaCl saturated aqueous solution. Extraction was performed in the headspace vial kept at 40 °C using a Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) fiber of 50/30 µm film thickness (Supelco, Bellefonte, PA, USA) and housed in its manual holder (Supelco, Bellefonte, PA, USA). The extraction times consisted of an equilibration time of 30 min and an extraction time of 30 min, during which the sample was constantly stirred. Following sampling, the SPME fiber was placed into a GC injector operating in splitless mode at 260 °C and maintained for 3 min to allow thermal desorption of the analytes onto the GC capillary column.
2.3. GC-MS Analyses
The GC-MS analyses were carried out using a Shimadzu GC 2010 Plus gas chromatograph, which was interfaced with a TQMS 8040 triple quadrupole mass spectrometer (Shimadzu, Milan, Italy). For the separation of the volatile compounds, a VF-WAXms, 60 m, 0.25 mm i.d., 0.25 μm film thickness polar column (Agilent Technologies Italia S.p.A.; Milan, Italy) was used. The analytical parameters were set as follows: injector temperature, 260 °C; injection mode, splitless; oven temperature, 45 °C held for 5 min, then increased to 80 °C at a rate of 10 °C/min, and then to 240 °C at 2 °C/min; carrier gas, helium at a constant flow rate of 1.0 mL/min; transfer line temperature, 230 °C; ionization technique, electronic impact (EI) at 70 eV; acquisition range, 30–400 m/z; scan speed, 1428 (amu/s).
Each compound was identified by comparing mass spectral data with NIST’ 24 (NIST/EPA/NIH Mass Spectra Library, version 2.0, Gaithersburg, MD, USA) and FFNSC 3.0 database (Shimadzu, Kyoto, Japan), and linear retention indices (LRIs) calculated according to the Van den Dool and Kratz equation. Where available, compounds were further confirmed by the injection of analytical standards (Sigma Aldrich, Milan, Italy) [21]. Quantitative results were obtained from total ion current (TIC) peak areas (average of three replicates) and expressed as area percentage.
2.4. Sensory Analysis
Qualitative descriptive analysis (QDA) was performed on caper honey samples, following the ISO standard procedures for panel selection and judge training protocols [22]. A sensory panel consisting of 8 trained judges (4 males and 4 females) aged between 22 and 60 years, was recruited among the personnel of the University of Messina. The panelists were selected and trained in agreement with Ferreira et al. (2009) [23]. The participants have given their written consent according to the principles of the Declaration of Helsinki. The subjects did not experience any risk as a result of the sensory test. The judges signed a consent form to undergo the sensory analysis.
The panel was trained through targeted sessions using commercial honey reference samples and a list of 20 sensory attributes related to appearance, odor, taste, and texture was initially developed. Based on the frequency of citations, 16 descriptors including 2 visual descriptors (color intensity; crystallization), 7 odor descriptors (sweet-caramel; floral; fruity/citrus; woody; balsamic; pungent; cabbage/cauliflower), 6 taste descriptors (sweet; acid; bitter; salty; caper; astringent) and 1 for texture (viscosity) were selected. Subsequently, a common vocabulary was established to define and standardize the sensory descriptors, ensuring consistent interpretation. Each descriptor was thoroughly explained and discussed to eliminate ambiguity and to familiarize the panelists with the evaluation scales and procedures. During the three hours before the test sessions, participants refrained from drinking (except water), eating, and smoking.
The sensory analysis was conducted by evaluating the intensity of the descriptors on a scale from 1 to 9, where “1” indicated no perception and “9” a very intense perception. Two samples were analyzed during each test session. Between each sample, the palate was restored with water. Each sample was assessed in triplicate.
2.5. Time Intensity Analysis
Four odor descriptors (cabbage/cauliflower, pungent, floral, sweet-caramel) and one taste descriptor (sweet taste) used in QDA analysis were selected for the Time Intensity (TI) analysis. Training sessions and evaluation procedures were conducted according to the method proposed by Sokolowsky et al. (2015) [24].
The evaluation was stopped automatically after 180 s or individually by the judges when no more intensity was perceived. Data were collected at 0.5 s intervals. For each session, two honey samples were presented in a randomized order. Time Intensity was calculated according to Sokolowsky et al. (2015) [24], obtaining TI curves.
2.6. Consumer’s Acceptability Test
The consumer’s sensory acceptability of caper and multifloral honeys was evaluated by a panel of 80 untrained judges, 36 males and 44 females, ranging from 20 to 69 years old, and recruited from the Department of Veterinary Sciences (University of Messina, Messina, Italy) among habitual honey consumers. All participants involved in the study signed a written consent in alignment with the ethical standards established by the Declaration of Helsinki.
Panelists were asked to evaluate the appearance, aroma, taste, and texture of honeys.
Samples were identified with three-digit codes and served randomly on white teaspoons. After each sample was tasted, water was consumed to restore the palate. The participants expressed their judgements using a 9–point hedonic scale (1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much, and 9 = like extremely) [21]. The overall acceptability was determined using the average value of the above parameters. In addition, participants were asked to answer the questions, “Would you consume this product?” and “Would you buy this product?” with “yes” or “no”.
2.7. Statistical Analysis
The XLStat software, version 2024.1 (Addinsoft, New York, NY, USA), was used to statistically analyze the GC-MS and sensory data. Pearson’s correlation analysis was conducted to correlate GC and sensory data. One-way Analysis of Variance (ANOVA) and Duncan’s multiple range test (significance defined as p < 0.05) were applied to sensory acceptability data to determine significant differences among caper and multifloral honey samples. Bonferroni correction has been applied for multiple comparisons.
3. Results and Discussions
3.1. Volatile Aroma Profile
The volatile aroma profile of caper honey showed a complex and unique chemical composition among unifloral honeys, constituted by 59 volatile compounds including acids, alcohols, aldehydes, ketones, aromatic, furanoic, sulfur compounds, and terpenes (Table 1).

Table 1.
Volatile compounds identified in caper honey samples.
Among the detected volatiles, dimethyl sulfide, a sulfur compound, was the most abundant, accounting for 59.34 ± 0.05% of the total volatile fraction. This compound, typically associated with boiled cabbage or vegetal odors, may derive from the enzymatic degradation of sulfur-containing amino acids present in caper nectar and suggests it plays a critical role in the honey aroma profile. The dominance of sulfur compounds, including dimethyl sulfide, is uncommon in most monofloral honeys, and such high abundance could be considered a chemical marker for caper honey. However, similar sulfur-containing volatiles have been identified in honeys from certain botanical sources. For instance, in a study analyzing monofloral honeys from the Brazilian semiarid region, sulfur compounds were among the characteristic volatiles, contributing to the unique aroma profiles of those honeys. This suggests that the presence of sulfur compounds can be a distinguishing feature in honeys derived from specific floral sources [31]. Thus, the predominance of this sulfur compound in caper honey may be linked to its specific botanical origin; in fact, capers (Capparis spinosa L.) are known for producing several sulfur metabolites that characterize the flower buds and fruits’ aroma profile [32,33,34]. However, despite the high concentration in the caper honey matrix and its odor threshold (2.6 µg/kg), the sensory impact of dimethyl sulfide is relative, likely influenced by its high volatility and interaction with other aroma compounds [35].
Acids accounted for 11.59 ± 0.15% of the total volatiles, with 2-propenoic acid (5.02 ± 0.03%) and nonanoic acid (2.02 ± 0.02%) being the most abundant. Their presence is consistent with previous studies on Mediterranean honeys, where medium-chain fatty acids contribute to the honey’s pungent and slightly rancid aroma notes [36]. In contrast to typical multifloral honeys, caper honey displays a higher concentration of longer-chain acids, such as dodecanoic acid (0.51 ± 0.02%) and decanoic acid (0.45 ± 0.01%) [37].
Aldehydes (13.06 ± 0.30% of the volatile profile) with nonanal (3.09 ± 0.04%), decanal (2.11 ± 0.03%), and hexanal (2.99 ± 0.03%) compounds are commonly associated with green, fatty, and citrus-like notes and are typically derived from lipid oxidation processes. The aldehyde profile of caper honey is particularly rich compared with other monofloral honeys, which tend to be dominated by monoterpenes or phenolic derivatives [38,39].
Among alcohols (4.78 ± 0.21%), 2-ethyl-hexanol (1.84 ± 0.03%), 1-octen-3-ol (0.67 ± 0.03%), and 1-dodecanol (0.63 ± 0.03%) were notable for their relatively high abundance. These long-chain alcohols are unusual in such quantities and may arise from wax-related degradation or enzymatic activity during honey ripening. Their contribution to aroma is generally mild but may influence mouthfeel and sweetness perception [40].
Aromatic compounds, especially benzaldehyde (4.37 ± 0.04%), were present in significant amounts. Benzaldehyde contributes to almond-like notes and has been reported as a prominent volatile in several monofloral honeys. Benzaldehyde is known as a floral marker in some honeys and is biosynthetically linked to phenylalanine metabolism. Moreover, the presence of furanoic derivatives such as furfural (2.41 ± 0.04%) supports the hypothesis of Maillard-type reactions occurring during honey processing or storage [41].
Terpenes, although present in lower concentrations (1.36 ± 0.12%), include compounds like linalool (0.30 ± 0.02%) and α-terpineol (0.10 ± 0.01%), which are valuable as floral markers. These compounds contribute to the floral aroma of honey and have been used in the classification of honey botanical origins [42].
Furanoic compounds were mainly represented by furfural (2.41 ± 0.04%). This class of compounds, which also includes 2-furanmethanol, 1-(2-furanyl)-ethanone, 2,5-dimethylfuran, 2-pentylfuran, 2-acetylfuranfurfural, furfuryl alcohol, and 5-methylfurfural, is usually found at trace levels in freshly harvested honey. Chemically, they are generated via the acid-catalyzed dehydration of pentoses, and their formation can be further enhanced by nonenzymatic browning reactions during thermal processing or prolonged storage [43]. Even at low concentrations, furfural is characterized by a sweet aroma that contributes to the overall flavor complexity of honey [44]. Its concentration increases during storage and thermal processing, with a higher concentration at higher temperatures. Consequently, these compounds are commonly used to assess the quality deterioration of honey. However, because their formation is primarily influenced by post-harvest processes rather than by the botanical origin of honey, they are not suitable as floral markers [45].
3.2. Qualitative Descriptive Analysis (QDA)
Figure 1a reports the graphical representation of the quantitative descriptive analysis (QDA) of caper honey evaluated by a trained panel.
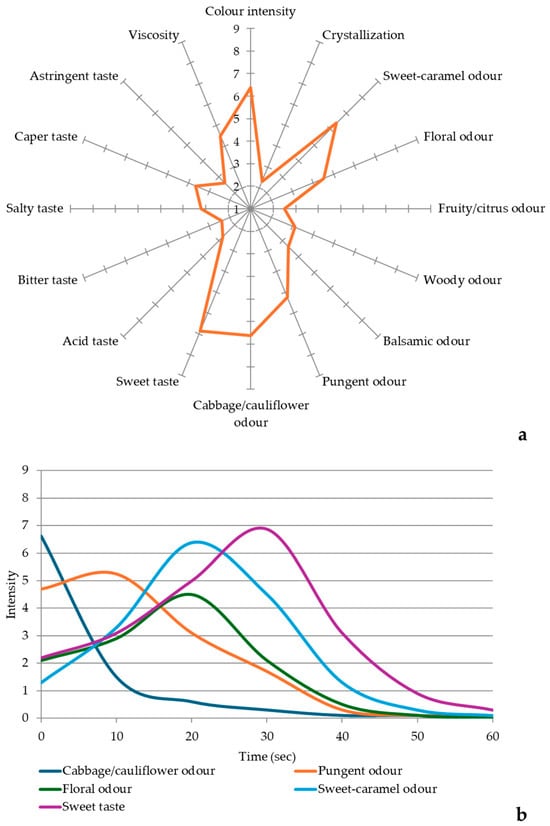
Figure 1.
(a) QDA sensory plot of caper honey sample; (b) TI curves of the main sensory attributes of caper honey.
Regarding its visual appearance, caper honey displayed a clear amber to light amber color with golden hues. It appeared clear and exhibited a low degree of crystallization. Its optical clarity indicates low levels of particulate matter and high-quality extraction practices. The low degree of crystallization is generally associated with the fructose/glucose ratio. Honey with a high fructose/glucose ratio (F/G > 1.3) typically resists crystallization, as glucose is less soluble in water and tends to crystallize easily [46].
The aroma of caper honey is distinctive and complex, dominated by moderately intense vegetal and floral notes characteristic of caper blossoms perceived as reminiscent of boiled cabbage and cooked onion. This is directly attributable to the high concentration of dimethyl sulfide (59.34 ± 0.05%), a compound known for its distinctive sulfurous aroma [33]. However, the perception of this note, although initially dominant, tends to fade over time, giving way to more pungent and sweet notes. This was assessed through the Time Intensity (TI) analysis, which allowed the identification of changes in the dominant sensory attributes over time during tasting. According to the TI curves (Figure 1b), the cabbage/cauliflower odor was the most intense during the initial 3 s, after which it rapidly declined and became almost undetectable. Its rapid decrease in perceived intensity is likely due to the high volatility and a low boiling point (37 °C) of dimethyl sulfide, which facilitates its quick dispersion at room temperature. Between approximately 3 and 14 s, the pungent odor became the dominant sensory note. This descriptor is primarily associated with the presence of organic acids, particularly 2-propenoic acid, identified in the caper honey samples.
The sweet-caramel notes identified by the panel were well supported by the presence of furfural (2.41 ± 0.04%) and other furanic compounds, such as furfuryl alcohol and 2-acetylfuran, commonly associated with Maillard reaction products [47]. Additionally, the woody and balsamic odor, as well as the citrus-like and sweet floral perceptions, were consistent with the presence of terpenes and aromatic alcohols such as linalool, cis-linalool oxide, and phenylethyl alcohol, albeit at lower concentrations (0.30 ± 0.02%, 0.50 ± 0.03%, and 0.11 ± 0.01%, respectively). These compounds have been reported to significantly contribute to the sensory complexity and floral character of monofloral honeys [48].
In TI analysis, the sweet-caramel and floral odor descriptors were initially perceived at low intensity but became predominant after approximately 14–16 s. The floral note, associated with the presence of 2-ethylhexanol, benzaldehyde, and terpenes, reached its peak intensity at around 20 s. The limited intensity of floral notes is likely due to two main factors: the high boiling points of 2-ethylhexanol and benzaldehyde, and the low concentration of terpenes in the sample. In contrast, the sweet-caramel odor became the most prominent after 14 s and persisted until the end of the analysis. This perception is primarily attributed to the aldehyde content, identified as the second-most abundant class of compound in the samples, and furfural.
When tasted by the panelists, the honey was found to be moderately sweet, with a mildly astringent, salty, and caper taste. These attributes distinguish it from more conventional monofloral honeys. A medium viscosity characterized the mouthfeel. Concerning the taste sensation of sweetness, the TI curve showed a high perceptual intensity at 30 s. This perception, which is certainly due to the sugar content of the caper honey, is also influenced by volatile compounds, especially by furan compounds [37] and alcohols [40], which can influence the perception of sweetness.
Overall, the sensory expression of caper honey reflected a distinctive aromatic identity, characterized by a sulfur-dominated base, caramel warmth, and delicate floral overlays, making it markedly different from other monofloral honeys such as citrus, which are typically dominated by monoterpenes [49,50].
The correlation heatmap between volatile compounds and sensory descriptors (Figure 2) highlighted that compounds such as benzaldehyde and furfural were strongly associated with the sweet-caramel odor of caper honey. In contrast, benzene acetaldehyde and terpenes such as α-terpineol, (E)-β-damascenone, and linalool were the major contributors to the floral aroma. The pungent odor appeared to be primarily linked to the presence of acetic acid, 2-propenoic acid, ethanol, and 2-ethyl-hexanol. Moreover, dimethyl sulfide showed a strong correlation with cabbage-like odor. These findings underscore the chemical complexity and aromatic distinctiveness of caper honey, reinforcing their potential as a valuable botanical and sensory marker for honey authentication assessment.
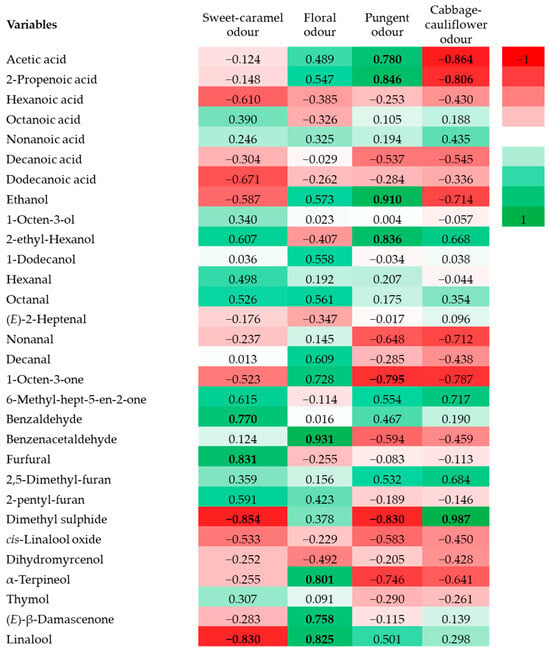
Figure 2.
Pearson’s correlation heatmap of the main volatile aroma compounds and aroma sensory descriptors of caper honey. Correlations that have reached significance are indicated in bold.
3.3. Consumer Acceptability
The consumer acceptability of caper honey was compared with a commercial multifloral honey by a panel of 80 untrained consumers. Sensory evaluation was conducted using a 9-point hedonic scale across five attributes: visual aspect, odor, taste, texture, and overall acceptability. Results (Figure 3a) indicated a statistically significant difference (p < 0.05) between the two samples for all the attributes except for texture, with multifloral honey receiving a higher mean score and an overall liking (6.72 ± 0.31) compared to caper honey (5.87 ± 0.43). The most pronounced difference was observed for taste, where multifloral honey scored significantly higher, indicating a greater consumer preference. Notably, caper honey had lower sweetness intensity, and the presence of salty and caper taste contributed to reduced acceptability among consumers preferring traditionally sweet honeys. Odor was the lowest-rated attribute for both honeys, but particularly for caper honey, suggesting a less favorable aromatic profile, probably due to its intense vegetable and pungent odor.
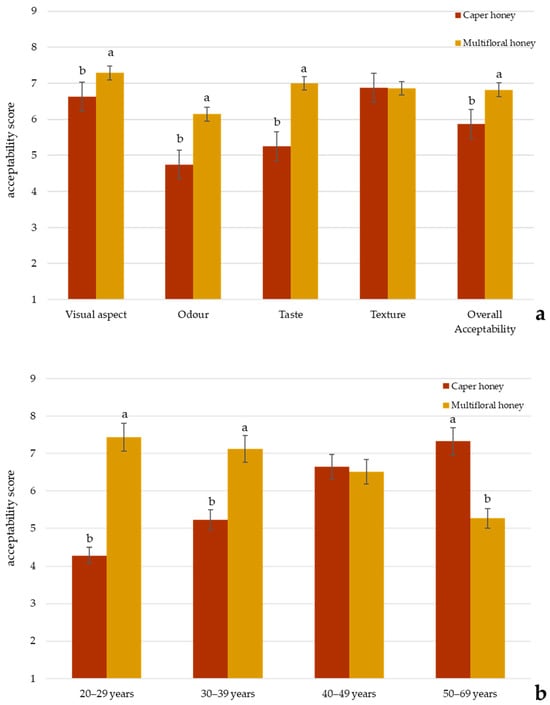
Figure 3.
(a) Consumer acceptability of different attributes for caper and multifloral honey. Different letters for a specific sensory attribute indicate statistically significant differences at p < 0.05 by Duncan’s multiple range test; (b) comparisons of overall liking of caper and multifloral honeys as a function of age group. Different letters for a specific sensory attribute indicate statistically significant differences at p < 0.05 by Duncan’s multiple range test.
However, the demographic analysis (Figure 3b) revealed age-related differences in acceptability: younger participants (20–39 years) showed a significantly higher overall liking for multifloral honey, while older participants (40–69 years) demonstrated a greater appreciation for caper honey (p < 0.05). This could be attributed to the fact that older individuals may have greater culinary experience and greater familiarity with traditional or niche food products, leading to a more developed appreciation for distinctive odors and taste, such as that of caper honey. In contrast, younger consumers may be less familiar with such sensory attributes or may tend to prefer milder, sweeter odor and taste. Therefore, the observed age-related differences in acceptability likely reflect not only physiological factors but also sociocultural influences, including product knowledge, curiosity, and appreciation for atypical or complex sensory perceptions.
Finally, Figure 4 reports the consumer responses regarding consumption and purchase intent for caper honey compared to multifloral honey. The results indicate a higher willingness to consume and purchase multifloral honey, with nearly 100% of participants expressing intent to consume it and over 90% indicating they would purchase it. In contrast, only approximately 65% of consumers reported a willingness to consume caper honey, and just under 75% would consider purchasing it.
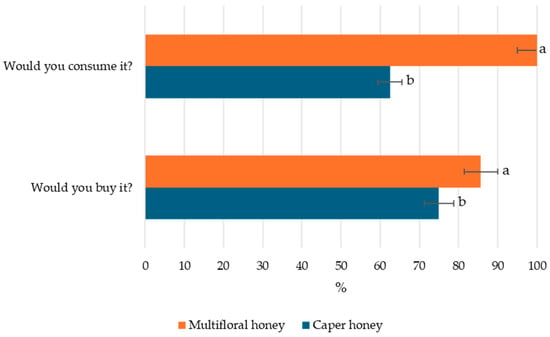
Figure 4.
Consumption and purchase intent of caper honey compared with multifloral honey. Different letters for a specific sensory attribute indicate statistically significant differences at p < 0.05 by Duncan’s multiple range test.
These findings highlight the importance of consumer segmentation in product positioning, suggesting that the lower desirability of caper honey compared to multifloral honey, particularly regarding consumption intent, may be influenced by limited consumer awareness and its less familiar sensory profile, as evidenced by the sensory evaluation results. This further suggests that, while caper honey may have limited broad-market appeal due to its pungency and lower sweetness, its complex sensory profile and intense aromatic character make it well suited as a premium, niche product for gourmet markets and high-end culinary applications.
4. Conclusions
This study provides the first integrated characterization of caper (Capparis spinosa L.) honey, focusing on its volatile composition, sensory profile, and consumer acceptability. The honey demonstrated a highly distinctive volatile fingerprint, with an unusually high concentration of sulfur compounds (particularly dimethyl sulfide) alongside aldehydes, acids, alcohols, aromatic compounds, and a moderate presence of terpenes. This unique chemical composition is strongly influenced by the caper flower phytochemistry, suggesting its potential as a botanical authenticity marker.
The sensory evaluation revealed a peculiar sensory profile, combining vegetal odor of cabbage and cauliflower, floral, and sweet-caramel odors, as well as a moderate sweetness and astringent taste. Temporal Intensity analysis indicated a shift from pungent to floral and sweet sensations over time, contributing to its perceived complexity. Consumer’s acceptability test, while indicating lower overall acceptability compared to commercial multifloral honey, also highlighted significant appreciation among older consumers with higher inclination for artisanal and niche products. These findings underscore the potential for market segmentation and the positioning of caper honey as a niche gourmet product, with relevance in climate-resilient beekeeping and biodiversity preservation. Moreover, targeted marketing strategies emphasizing provenance, biodiversity, and sensory uniqueness could enhance consumer awareness and market value.
Future research should explore broader geographical sampling, long-term storage effects on volatile stability, and functional analyses to further validate the potential of caper honey as a high-value food product.
Author Contributions
Conceptualization, A.V. and F.C.; methodology, M.M., M.T. and C.C.; software, M.T. and F.C.; validation, G.T., C.C. and F.C.; formal analysis, M.M. and M.T.; investigation, C.C.; resources, A.V. and C.C.; data curation, M.M. and M.T.; writing—original draft preparation, M.M., F.C. and G.T.; writing—review and editing, A.V. and F.C.; visualization, F.C.; supervision, A.V. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This manuscript reports on a sensory analysis conducted on honey samples with the participation of students and staff from the University of Messina, following the requirements for sensory testing as described in ISO 8586:2023 [22]. In line with current regulations on ethics in food science research, informed consent was obtained from all participants, with full assurance of data protection and privacy. The activity did not involve any novel foods, health claims, or human experimentation. Therefore, as stated in the “Ethics Review and Food-Related Research” issued by the European Commission, and according with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for research involving human subjects, our scientific project was not submitted to an ethics committee for evaluation.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VOCs | Volatile organic compounds |
| HS-SPME | Headspace solid-phase microextraction |
| DVB/CAR/PDMS | Divinylbenzene/Carboxen/Polysimethylsiloxane |
| GC-MS | Gas chromatography–mass spectrometry |
| TIC | Total ion current |
| EI | Electronic impact |
| LRIs | Linear retention indices |
| QDA | Qualitative descriptive analysis |
| TI | Time Intensity Analysis |
| ANOVA | One-way Analysis of Variance |
References
- Rahnavard, R.; Razavi, N. A review on the medical effects of Capparis spinosa L. Future Nat. Prod. 2017, 3, 44–53. [Google Scholar]
- Zarei, M.; Seyedi, N.; Maghsoudi, S.; Nejad, M.S.; Sheibani, H. Green synthesis of Ag nanoparticles on the modified graphene oxide using Capparis spinosa fruit extract for catalytic reduction of organic dyes. Inorg. Chem. Commun. 2021, 123, 108327. [Google Scholar] [CrossRef]
- Panico, A.M.; Cardile, V.; Garufi, F.; Puglia, C.; Bonina, F.; Ronsisvalle, G. Protective effect of Capparis spinosa on chondrocytes. Life Sci. 2005, 77, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, A.A.; Hernández, F. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Mazandarani, M.; Borhani, G.; Fathiazad, F. Phytochemical analysis, antioxidant activity and ecological requirements of Capparis spinosa L. in golestan and semnan provinces (north of Iran). Int. J. Med. Plants By-Prod. 2014, 1, 21–26. [Google Scholar]
- Kalantari, H.; Foruozandeh, H.; Khodayar, M.J.; Siahpoosh, A.; Saki, N.; Kheradmand, P. Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and Quercetin on tert-butyl hydroperoxide-induced acute liver damage in mice. J. Tradit. Complement. Med. 2018, 8, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.M. Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Othman, A.S. Antibacterial activity of bee and Yemeni sidr honey against some pathogenic bacterial species. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1015–1025. [Google Scholar]
- Ilyasov, R.; Gaifullina, L.; Saltykova, E.; Poskryakov, A.; Nikolenko, A. Review of the expression of antimicrobial peptide defensin in honey bees Apis mellifera L. J. Apic. Sci. 2012, 56, 115. [Google Scholar] [CrossRef]
- Mama, M.; Teshome, T.; Detamo, J. Antibacterial activity of honey against methicillin-resistant Staphylococcus aureus: A laboratory-based experimental study. Int. J. Microbiol. 2019, 2019, 7686130. [Google Scholar] [CrossRef]
- Ciriminna, R.; Angellotti, G.; Luque, R.; Pagliaro, M. The citrus economy in Sicily in the early bioeconomy era: A case study for bioeconomy practitioners. Biofuels Bioprod. Biorefining 2024, 18, 356–364. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Al-Waili, N.; Aazza, S.; Elamine, Y.; Zizi, S.; Al-Waili, T.; Al-Waili, A.; Lyoussi, B. Antioxidant and diuretic activity of co-administration of Capparis spinosa honey and propolis in comparison to furosemide. Asian Pac. J. Trop. Med. 2017, 10, 974–980. [Google Scholar] [CrossRef]
- Amer, A.M.; Abid-Alla, S.A. Antibacterial activity of Capparis spinosa honey against Staphylococcus aureus and Escherichia coli. DYSONA Life Sci. 2021, 2, 1–5. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Al Guthami, F.M.; Al Gethami, A.F.; El Fadaly, H.A. Beneficial effects of Capparis spinosa honey on the immune response of rats infected with Toxoplasma gundii. J. Pharmacopunct. 2017, 20, 112. [Google Scholar] [CrossRef]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela Lopez, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 893–920. [Google Scholar] [CrossRef]
- Guler, A.; Bakan, A.; Nisbet, C.; Yavuz, O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007, 105, 9–1125. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey volatiles as a fingerprint for botanical origin—A review on their occurrence on monofloral honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef]
- Merlino, M.; Tripodi, G.; Cincotta, F.; Prestia, O.; Miller, A.; Gattuso, A.; Verzera, A.; Condurso, C. Technological, nutritional, and sensory characteristics of gnocchi enriched with hemp seed flour. Foods 2022, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/76667.html (accessed on 15 April 2025).
- Ferreira, E.L.; Lencioni, C.; Benassi, M.T.; Barth, M.O.; Bastos, D.H.M. Descriptive sensory analysis and acceptance of stingless bee honey. Food Sci. Technol. Int. 2009, 15, 251–258. [Google Scholar] [CrossRef]
- Sokolowsky, M.; Rosenberger, A.; Fischer, U. Sensory impact of skin contact on white wines characterized by descriptive analysis, time–intensity analysis and temporal dominance of sensations analysis. Food Qual. Prefer. 2015, 39, 285–297. [Google Scholar] [CrossRef]
- Wardencki, W.; Chmiel, T.; Dymerski, T.; Biernacka, P.; Plutowska, B. Application of gas chromatography, mass spectrometry and olfactometry for quality assessment of selected food products. Ecol. Chem. Eng. 2009, 16, 287–300. [Google Scholar]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef]
- Tian, H.; Shen, Y.; Yu, H.; Chen, C. Aroma features of honey measured by sensory evaluation, gas chromatography-mass spectrometry, and electronic nose. International Int. J. Food Prop. 2018, 21, 1755–1768. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Kilic-Buyukkurt, O.; Fotouh, M.M.A.; Selli, S. Aroma active compounds of honey: Analysis with GC-MS, GC-O, and Molecular Sensory Techniques. J. Food Compost. Anal. 2024, 134, 106545. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Song, M.; Jiang, A.; Lang, Y.; Chen, L. Aromatic profiles and enantiomeric distributions of volatile compounds during the ripening of Dendropanax dentiger honey. Food Res. Int. 2024, 175, 113677. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, Y.; Yang, H.; Zhao, R.; Zhu, J.; Wang, F. Characterization of honey-like characteristic aroma compounds in Zunyi black tea and their molecular mechanisms of interaction with olfactory receptors using molecular docking. LWT 2024, 191, 115640. [Google Scholar] [CrossRef]
- da Costa, A.C.V.; Sousa, J.M.B.; da Silva, M.A.A.P.; dos Santos Garruti, D.; Madruga, M.S. Sensory and volatile profiles of monofloral honeys produced by native stingless bees of the brazilian semiarid region. Food Res. Int. 2018, 105, 110–120. [Google Scholar] [CrossRef]
- Cincotta, F.; Merlino, M.; Verzera, A.; Gugliandolo, E.; Condurso, C. Innovative process for dried caper (Capparis spinosa L.) powder production. Foods 2022, 11, 3765. [Google Scholar] [CrossRef]
- Merlino, M.; Condurso, C.; Cincotta, F.; Nalbone, L.; Ziino, G.; Verzera, A. Essential oil emulsion from caper (Capparis spinosa L.) leaves: Exploration of its antibacterial and antioxidant properties for possible application as a natural food preservative. Antioxidants 2024, 13, 718. [Google Scholar] [CrossRef]
- Condurso, C.; Mazzaglia, A.; Tripodi, G.; Cincotta, F.; Dima, G.; Maria Lanza, C.; Verzera, A. Sensory analysis and head-space aroma volatiles for the characterization of capers from different geographic origin. J. Essent. Oil Res. 2016, 28, 185–192. [Google Scholar] [CrossRef]
- Yu, P.; Yang, Y.; Sun, J.; Jia, X.; Zheng, C.; Zhou, Q.; Huang, F. Identification of volatile sulfur-containing compounds and the precursor of dimethyl sulfide in cold-pressed rapeseed oil by GC–SCD and UPLC–MS/MS. Food Chem. 2022, 367, 130741. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- Tedesco, R.; Scalabrin, E.; Malagnini, V.; Strojnik, L.; Ogrinc, N.; Capodaglio, G. Characterization of botanical origin of italian honey by carbohydrate composition and volatile organic compounds (VOCs). Foods 2022, 11, 2441. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- da Costa, A.C.V.; Sousa, J.M.B.; Bezerra, T.K.A.; da Silva, F.L.H.; Pastore, G.M.; da Silva, M.A.A.P.; Madruga, M.S. Volatile profile of monofloral honeys produced in Brazilian semiarid region by stingless bees and key volatile compounds. LWT 2018, 94, 198–207. [Google Scholar] [CrossRef]
- Machado, A.M.; Antunes, M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Volatile profile of Portuguese monofloral honeys: Significance in botanical origin determination. Molecules 2021, 26, 4970. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Sarais, G.; Congiu, F.; Marijanović, Z.; Kuś, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L∗ Cab∗ hab∘ chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Hu, Y.; Zhou, J.; Chen, L.; Lu, X. Systematic review of the characteristic markers in honey of various botanical, geographic, and entomological origins. ACS Food Sci. Technol. 2022, 2, 206–220. [Google Scholar] [CrossRef]
- Manickavasagam, G.; Saaid, M.; Lim, V. Impact of prolonged storage on quality assessment properties and constituents of honey: A systematic review. J. Food Sci. 2024, 89, 811–833. [Google Scholar] [CrossRef] [PubMed]
- Bhure, R.A.; Alam, M.; Nanda, V.; Pawar, V.M.; Saxena, S. Exploring the Impact of Thermal Processing on the Quality Attributes of Honey: A Comprehensive Review. J. Food Process Eng. 2025, 48, e70033. [Google Scholar] [CrossRef]
- Verzera, A.; Condurso, C. Sampling Techniques for the Determination of Volatile Fraction of Honey. In Comprehensive Sampling and Sample Preparation. Extraction Techniques and Applications: Food and Beverage; Elsevier: Amsterdam, The Netherlands; Academic Press: Oxford, UK, 2012; Volume 4, pp. 87–117. [Google Scholar]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Alaerjani, W.M.A.; Abu-Melha, S.; Alshareef, R.M.H.; Al-Farhan, B.S.; Ghramh, H.A.; Al-Shehri, B.M.A.; Bajaber, M.A.; Khan, K.A.; Alrooqi, M.M.; Modawe, G.A.; et al. Biochemical reactions and their biological contributions in honey. Molecules 2022, 27, 4719. [Google Scholar] [CrossRef]
- Mulheron, H.; DuBois, A.; Mayhew, E.J. Quantifying the sweetness intensity and impact of aroma in honey from four floral sources. J. Food Sci. 2024, 89, 9732–9741. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C.; Polissiou, M. Ultrasound-assisted extraction gas chromatography–mass spectrometry analysis of volatile compounds in unifloral thyme honey from Greece. Eur. Food Res. Technol. 2009, 229, 365–373. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).