New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends
Abstract
1. Introduction
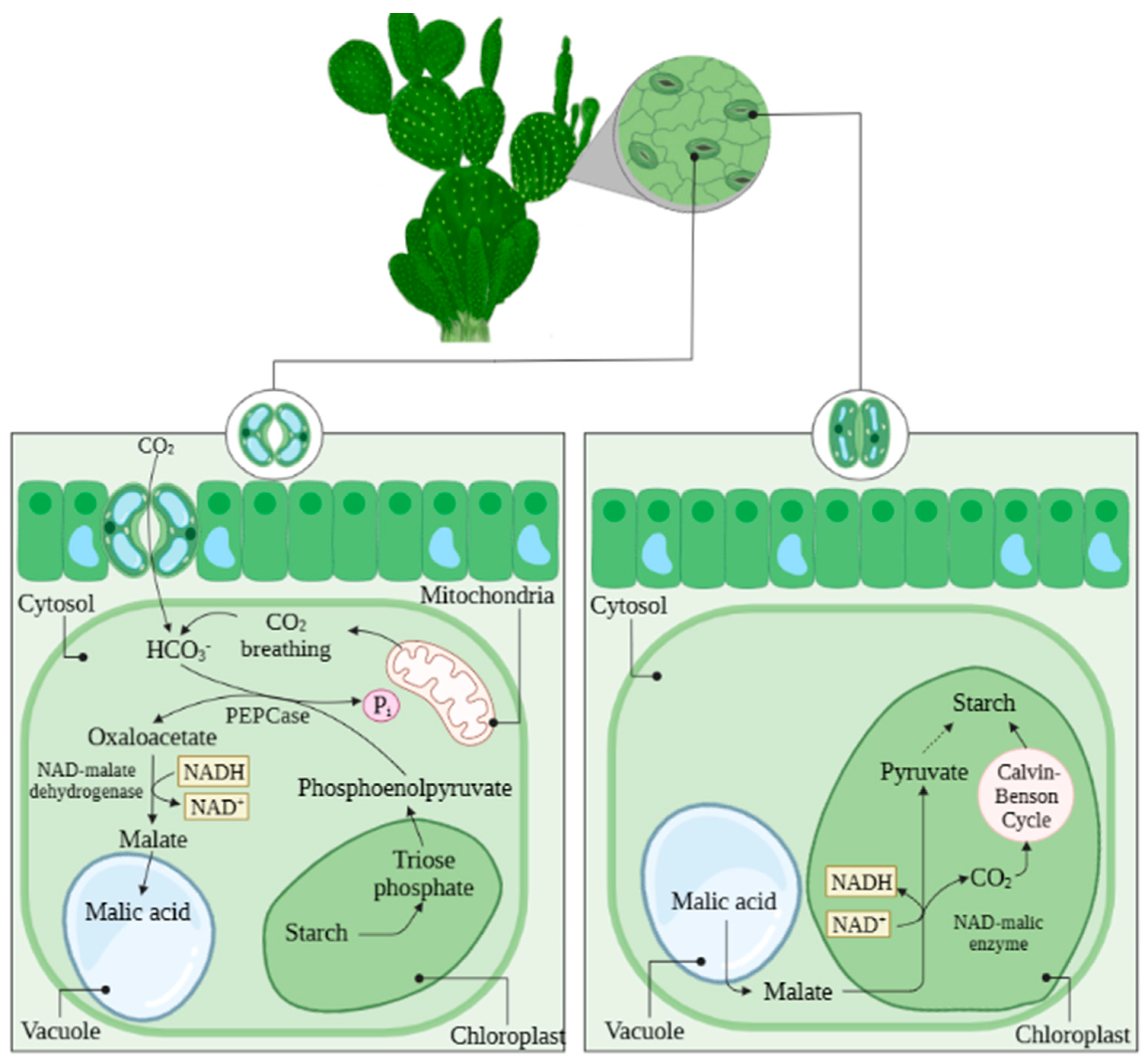
2. Importance of Cacti in the Semi-Arid Region
3. Contemplating an Integrative and Circular Production of Cactus Species for Human Consumption Considering the United Nations SDGs
4. Relevant Cacti for Food Industry
4.1. Opuntia ficus-indica
4.2. Cereus peruvianus
4.3. Hylocereus undatus
4.4. Potential Toxins and Allergens from Cacti
5. Functional Ingredients Derived from Cactus Species for the Food Industry
5.1. Dietary Fiber
5.1.1. Mucilage
5.1.2. Pectin
5.2. Phytochemicals
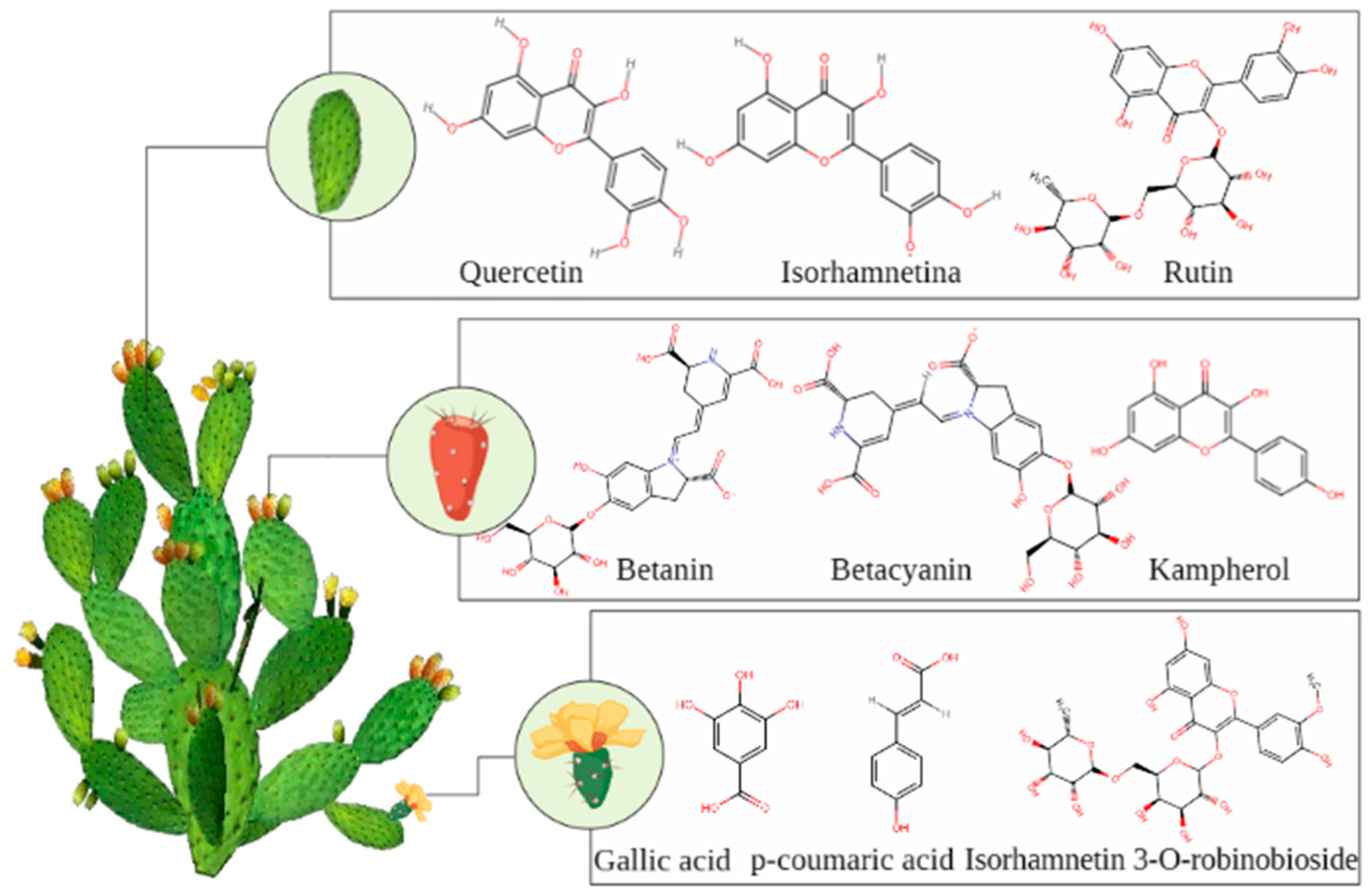
5.2.1. Polyphenols
5.2.2. Flavonoids
5.3. Oil from Cactus Sources
| Parameter | Typical Value |
|---|---|
| Fatty Acid Profile [106] | |
| Myristic acid 14:0 | 0.10 |
| Palmitic acid 16:0 | 12.29 |
| Palmitoleic acid 16:1 (9Z) | 0.75 |
| 7Z, 10Z-Hexadecadienoic acid, 16:2 (6Z, 9Z) | 0.05 |
| Stearic acid 18:0 | 3.92 |
| Oleic acid 18:1 (9Z) | 17.61 |
| Vaccenic acid 18:1 (11E) | 6.29 |
| 13-Octadecanoic acid 18:1 (13E) | 0.17 |
| Linoleic acid 18:2 (9Z, 12Z) | 57.98 |
| Linolenic acid 18:3 (9Z, 12Z, 15Z) | 0.21 |
| Arachidic acid 20:0 | 0.33 |
| Gondoic acid 20:1 (11Z) | 0.10 |
| Methyl-9-eicosenoate | 0.22 |
| Physical Properties [107] | |
| Density (g/cm3 at 20 °C) | ~0.92 |
| Refractive index (at 20 °C) | ~1.47 |
| Smoke point (°C) | ~200 |
| Chemical Properties | |
| Iodine value (g I2/100g) | ~90–100 |
| Acid value (mg KOH/g) | ~1 |
| Peroxide value (meq O2/kg) | ~10 |
| Sensory Properties | |
| Color | Yellow to greenish |
| Aroma/flavor | Light, nutty |
6. Future Trends for Technological Application of Cacti-Derived Ingredients
6.1. Bakery
6.2. Food Dyes
6.3. Probiotic
6.4. Functional Beverages
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Albuquerque, J.G.; Escalona-Buendía, H.B.; de Aquino, J.S.; da Vasconcelos, M.A.S. Nopal Beverage (Opuntia ficus-indica) as a Non-Traditional Food: Sensory Properties, Expectations, Experiences, and Emotions of Low-Income and Food-Insecure Brazilian Potential Consumers. Food Res. Int. 2022, 152, 110910. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Becerra, E.; de los Angeles Aguilera-Barreiro, M.; Contreras-Padilla, M.; Pérez-Torrero, E.; Rodriguez-Garcia, M.E. Nopal Cladodes (Opuntia ficus indica): Nutritional Properties and Functional Potential. J. Funct. Foods 2022, 95, 105183. [Google Scholar] [CrossRef]
- Daniloski, D.; D’Cunha, N.M.; Speer, H.; McKune, A.J.; Alexopoulos, N.; Panagiotakos, D.B.; Petkoska, A.T.; Naumovski, N. Recent Developments on Opuntia Spp., Their Bioactive Composition, Nutritional Values, and Health Effects. Food Biosci. 2022, 47, 101665. [Google Scholar] [CrossRef]
- Cardoso, D.B.; de Carvalho, F.F.R.; de Medeiros, G.R.; Guim, A.; Cabral, A.M.D.; Véras, R.M.L.; dos Santos, K.C.; Dantas, L.C.N.; de Nascimento, A.G.O. Levels of Inclusion of Spineless Cactus (Nopalea cochenillifera Salm Dyck) in the Diet of Lambs. Anim. Feed Sci. Technol. 2019, 247, 23–31. [Google Scholar] [CrossRef]
- Gusha, J.; Halimani, T.E.; Katsande, S.; Zvinorova, P.I. The Effect of Opuntia ficus indica and Forage Legumes Based Diets on Goat Productivity in Smallholder Sector in Zimbabwe. Small Rumin. Res. 2015, 125, 21–25. [Google Scholar] [CrossRef]
- Mayer, J.A.; Cushman, J.C. Nutritional and Mineral Content of Prickly Pear Cactus: A Highly Water-use Efficient Forage, Fodder and Food Species. J. Agron. Crop Sci. 2019, 205, 625–634. [Google Scholar] [CrossRef]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An Overview and Update on the Chemical Composition and Potential Health Benefits of Opuntia ficus-indica (L.) Miller. J. Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; del Gómez-García, M.R.; Valverde, M.E.; Paredes-López, O. Nopal (Opuntia Spp.) and Its Effects on Metabolic Syndrome: New Insights for the Use of a Millenary Plant. Curr. Pharm. Des. 2019, 25, 3457–3477. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef]
- Cuevas-Gonzalez, P.F.; Liceaga, A.M.; Aguilar-Toala, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Koutroulis, A.G. Dryland Changes under Different Levels of Global Warming. Sci. Total Environ. 2019, 655, 482–511. [Google Scholar] [CrossRef]
- de Cavalcante, A.M.B.; Sampaio, A.C.P. Modeling the Potential Distribution of Cacti under Climate Change Scenarios in the Largest Tropical Dry Forest Region in South America. J. Arid Environ. 2022, 200, 104725. [Google Scholar] [CrossRef]
- da Jardim, A.M.R.F.; do Araújo Júnior, G.N.; da Silva, M.V.; dos Santos, A.; da Silva, J.L.B.; Pandorfi, H.; de Oliveira-Júnior, J.F.; de Teixeira, A.H.C.; Teodoro, P.E.; de Lima, J.L.M.P.; et al. Using Remote Sensing to Quantify the Joint Effects of Climate and Land Use/Land Cover Changes on the Caatinga Biome of Northeast Brazilian. Remote Sens. 2022, 14, 1911. [Google Scholar] [CrossRef]
- Dubeux, J.C.B.; dos Santos, M.V.F.; da Cunha, M.V.; dos Santos, D.C.; de Souza, R.T.A.; de Mello, A.C.L.; de Souza, T.C. Cactus (Opuntia and Nopalea) Nutritive Value: A Review. Anim. Feed Sci. Technol. 2021, 275, 114890. [Google Scholar] [CrossRef]
- Abidi, S.; Ben Salem, H.; Vasta, V.; Priolo, A. Supplementation with Barley or Spineless Cactus (Opuntia ficus indica f. Inermis) Cladodes on Digestion, Growth and Intramuscular Fatty Acid Composition in Sheep and Goats Receiving Oaten Hay. Small Rumin. Res. 2009, 87, 9–16. [Google Scholar] [CrossRef]
- De Araújo, F.F.; de Farias, D.P.; Neri-Numa, I.A.; Pastore, G.M. Underutilized Plants of the Cactaceae Family: Nutritional Aspects and Technological Applications. Food Chem. 2021, 362, 130196. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, Y.; Martínez-Huélamo, M.; Pedraza-Chaverri, J.; Ramírez, V.; Martínez-Tagüeña, N.; Trujillo, J. Ethnobotanical, Nutritional and Medicinal Properties of Mexican Drylands Cactaceae Fruits: Recent Findings and Research Opportunities. Food Chem. 2020, 312, 126073. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed Editora: Porto Alegre, Brazil, 2017. [Google Scholar]
- Messerschmid, T.F.E.; Wehling, J.; Bobon, N.; Kahmen, A.; Klak, C.; Los, J.A.; Nelson, D.B.; dos Santos, P.; de Vos, J.M.; Kadereit, G. Carbon Isotope Composition of Plant Photosynthetic Tissues Reflects a Crassulacean Acid Metabolism (CAM) Continuum in the Majority of CAM Lineages. Perspect. Plant Ecol. Evol. Syst. 2021, 51, 125619. [Google Scholar] [CrossRef]
- Nosek, M.; Gawrońska, K.; Rozpądek, P.; Szechyńska-Hebda, M.; Kornaś, A.; Miszalski, Z. Withdrawal from Functional Crassulacean Acid Metabolism (CAM) Is Accompanied by Changes in Both Gene Expression and Activity of Antioxidative Enzymes. J. Plant Physiol. 2018, 229, 151–157. [Google Scholar] [CrossRef]
- Mabotja, M.B.; Gerrano, A.S.; Venter, S.L.; du Plooy, C.P.; Kudanga, T.; Amoo, S.O. Nutritional Variability in 42 Cultivars of Spineless Cactus Pear Cladodes for Crop Improvement. South Afr. J. Bot. 2021, 142, 140–148. [Google Scholar] [CrossRef]
- Du Toit, A.; de Wit, M.; Osthoff, G.; Hugo, A. Antioxidant Properties of Fresh and Processed Cactus Pear Cladodes from Selected Opuntia ficus-indica and O. Robusta Cultivars. South Afr. J. Bot. 2018, 118, 44–51. [Google Scholar] [CrossRef]
- Vieira, É.d.A.; Alcântara, M.A.; Albuquerque dos Santos, N.; Gondim, A.D.; Iacomini, M.; Mellinger, C.; Cordeiro, A.M.T.d.M. Mucilages of Cacti from Brazilian Biodiversity: Extraction, Physicochemical and Technological Properties. Food Chem. 2021, 346, 128892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Runting, R.K.; Webb, E.L.; Edwards, D.P.; Carrasco, L.R. Coordinated Intensification to Reconcile the ‘Zero Hunger’ and ‘Life on Land’ Sustainable Development Goals. J. Environ. Manag. 2021, 284, 112032. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, K.A.; Fatah, F.A.; Kruseman, G.; Erenstein, O. Projecting Food Demand in 2030: Can Uganda Attain the Zero Hunger Goal? Sustain. Prod. Consum. 2021, 28, 1140–1163. [Google Scholar] [CrossRef]
- Kent, K.; Murray, S.; Penrose, B.; Auckland, S.; Godrich, S.; Lester, E.; Visentin, D. Food Insecure Households Faced Greater Challenges Putting Healthy Food on the Table during the COVID-19 Pandemic in Australia. Appetite 2022, 169, 105815. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Rocamora-Montiel, B.; Legua, P.; Hernández, F.; López-Lluch, D. Economic Estimation of Cactus Pear Production and Its Feasibility in Spain. Trends Food Sci. Technol. 2020, 103, 379–385. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun Kim, S.; Wong, J.W.C. Sustainable Processing of Food Waste for Production of Bio-Based Products for Circular Bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-Wealth: Biowaste Valorization into Valuable Bio(Nano)Materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Da Costa, G.M.; Aona, L.Y.; Marinho, L.C. Is That a Cactus on the Roof? Cactus Succul. J. 2021, 93, 149–152. [Google Scholar] [CrossRef]
- Dick, M.; Dal Magro, L.; Rodrigues, R.C.; de Oliveira Rios, A.; Flôres, S.H. Valorization of Opuntia Monacantha (Willd.) Haw. Cladodes to Obtain a Mucilage with Hydrocolloid Features: Physicochemical and Functional Performance. Int. J. Biol. Macromol. 2019, 123, 900–909. [Google Scholar] [CrossRef]
- Le Houérou, H.N. The Role of Cacti (Opuntia spp.) in Erosion Control, Land Reclamation, Rehabilitation and Agricultural Development in the Mediterranean Basin. J. Arid Environ. 1996, 33, 135–159. [Google Scholar] [CrossRef]
- Badii, M.H.; Flores, A.E. Prickly Pear Cacti Pests and Their Control in Mexico. Fla. Entomol. 2001, 84, 503. [Google Scholar] [CrossRef]
- Ortega-Baes, P.; Sühring, S.; Sajama, J.; Sotola, E.; Alonso-Pedano, M.; Bravo, S.; Godínez-Alvarez, H. Diversity and Conservation in the Cactus Family. In Desert Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 157–173. [Google Scholar]
- Mufas, A.H.M.; Perera, O.D.A.N. Study on Development of Pitaya Fruit (Hylocereus undatus) Incorporated Ice Cream; an Alternative Solution to the Pitaya Cultivators in Sri Lanka. In Proceedings of the Third International Symposium, Oluvil, Sri Lanka, 6–7 July 2013; pp. 6–7. [Google Scholar]
- Ruiz-Torralba, A.; Guerra-Hernández, E.J.; García-Villanova, B. Antioxidant Capacity, Polyphenol Content and Contribution to Dietary Intake of 52 Fruits Sold in Spain. CyTA J. Food 2018, 16, 1131–1138. [Google Scholar] [CrossRef]
- Zhen, T.; JingRui, L.; Xiang, W.; XiaoLei, W.; BinBin, G.; HongBo, G. Cloning of Nitrate Reductase Gene of Lettuce and Effect of Exogenous γ-Aminobutyric Acid on Gene Expression and Nitrate Content in Leaves under High Nitrogen Level. Acta Bot. Boreali-Occident. Sin. 2015, 35, 1098–1105. [Google Scholar]
- Hossain, F.M.d.; Numan, S.M.d.; Akhtar, S. Cultivation, Nutritional Value, and Health Benefits of Dragon Fruit (Hylocereus Spp.): A Review. Int. J. Hortic. Sci. Technol. 2021, 8, 239–249. [Google Scholar]
- Liu, B.; Zhang, G.; Murphy, A.; De Koeyer, D.; Tai, H.; Bizimungu, B.; Si, H.; Li, X.-Q. Differences between the Bud End and Stem End of Potatoes in Dry Matter Content, Starch Granule Size, and Carbohydrate Metabolic Gene Expression at the Growing and Sprouting Stages. J. Agric. Food Chem. 2016, 64, 1176–1184. [Google Scholar] [CrossRef]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef]
- Mazari, A.; Yahiaoui, K.; Fedjer, Z.; Mahdeb, A. Physical Characteristics, Phytochemical Content and Antioxidant Activity of Cactus Pear Fruits Growing in Northeast Algeria. J. Prof. Assoc. Cactus Dev. 2018, 20, 177–195. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, A.R.; Mohamed, H.I.; Al-Otaibi, H.H.; Ramadan, K.M.A.; Elkatry, H.O. Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production. Agronomy 2023, 13, 439. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Campos-Mendiola, R.; Hurtado-Salgado, M.A.; Muthuswamy Ponniah, J. Composición Química del Mucílago de Nopal en Diferentes Etapas de Madurez. Agrociencia 2022, 56i2, 2726. [Google Scholar] [CrossRef]
- Allen, J.C.; Issa, J.Y.; Cai, W. Calcium Content, in Vitro Digestibility, and Bioaccessibility in Leaves of Spinach (Spinacia oleracea), Sweet Potato (Ipomea batatas), and Drumstick Tree (Moringa oleifera). F1000 Res. 2014, 3, 65. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, V.; Mohamed, M.B.N.; Shukla, A.K.; Mangalassery, S.; Dayal, D. Establishment and Performance of Cactus (Opuntia ficus-indica) Accessions at Initial Stages under Shed Net in Semi-Arid Region of Rajasthan. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1983–1988. [Google Scholar] [CrossRef]
- Stambouli-Essassi, S.; Harrabi, R.; Bouzid, S.; Harzallah-Skhiri, F. Evaluation of the Efficiency of Opuntia ficus-indica Cladode Cuttings for Vegetative Multiplication. Not. Bot. Horti. Agrobot. Cluj Napoca 2015, 43, 521–527. [Google Scholar] [CrossRef]
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical Analysis of Nutritional Content of Prickly Pads (Opuntia ficus indica) at Varied Ages in an Organic Harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef]
- del Díaz, M.S.S.; de la Rosa, A.-P.B.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia Spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med Cell Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Izquierdo-Vega, J.; Delgado-Olivares, L.; Vargas-Mendoza, N.; Álvarez-González, I.; Morales-González, Á.; et al. Opuntia Spp. in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 2. Plants 2022, 11, 2333. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Chougui, N.; Sahi, Y.; Belkacemi, M. Comparative Study between the Different Compartments of Opuntia ficus-indica L. In Proceedings of the Inside Food Symposium, Leuven, Belgium, 9–12 April 2013; pp. 9–12. [Google Scholar]
- Machado, F.A.P.S.A.; Oliveira, A.J.B.; Mangolin, C.A.; Gobbi Filho, L.; Machado, M.F.P.S. Polysaccharide Production from Callus Cultures of Cereus peruvianus Mill. (Cactaceae). Crop. Breed. Appl. Biotechnol. 2004, 4, 313–316. [Google Scholar] [CrossRef]
- Saag, L.M.K.; Sanderson, G.R.; Moyna, P.; Ramos, G. Cactaceae Mucilage Composition. J. Sci. Food Agric. 1975, 26, 993–1000. [Google Scholar] [CrossRef]
- Martin, P.; Faria-Tavares, J.; Mangolin, C.; Machado, M.d.F. Somaclones of Cereus Peruvianus Mill. (Cactaceae) with High Morphological Divergence May Generate New Varieties of Ornamental Cacti and Provide Relevant Chemical Compounds. Preprints.org 2018, 1, 2018010128. [Google Scholar]
- Mangolin, C.A.; Ottoboni, L.M.; Machado, M.F. Two-Dimensional Electrophoresis of Cereus peruvianus (Cactaceae) Callus Tissue Proteins. Electrophoresis 1999, 20, 626–629. [Google Scholar] [CrossRef]
- Som, A.M.; Ahmat, N.; Abdul Hamid, H.A.; Azizuddin, N. A Comparative Study on Foliage and Peels of Hylocereus undatus (White Dragon Fruit) Regarding Their Antioxidant Activity and Phenolic Content. Heliyon 2019, 5, e01244. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the Nature-Inspired Pigments, in Health and Diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- García-Menaya, J.M.; Cordobés-Durán, C.; Bobadilla, P.; Ledesma, A.; Pérez-Rangel, I. Hypersensitivity Systemic Reaction to Cactus Fruit (Opuntia ficus-indica). Allergy 2009, 64, 1689–1690. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Greco, G.; Gentile, C.; Settanni, L.; Inglese, P. Effect of Opuntia ficus-indica Mucilage Edible Coating on Quality, Nutraceutical, and Sensorial Parameters of Minimally Processed Cactus Pear Fruits. Agronomy 2021, 11, 1963. [Google Scholar] [CrossRef]
- Saparbekova, A.; Latif, A.; Altekey, A. Risks of Microbiological Contamination of Fruits and Vegetables Used for Food. Bull. Innov. Univ. Eurasia 2021, 82, 97–102. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Paula, C.D.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia Spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Viernstein, H.; Loeppert, R.; Praznik, W. Prebiotic Potential and Chemical Characterization of the Poly and Oligosaccharides Present in the Mucilage of Opuntia ficus-indica and Opuntia joconostle. Food Chem. 2021, 362, 130167. [Google Scholar] [CrossRef]
- Guevara-Arauza, J.C.; de Jesús Ornelas-Paz, J.; Pimentel-González, D.J.; Rosales Mendoza, S.; Soria Guerra, R.E.; Paz Maldonado, L.M.T. Prebiotic Effect of Mucilage and Pectic-Derived Oligosaccharides from Nopal (Opuntia ficus-indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Loeppert, R.; Viernstein, H.; Praznik, W. The Effect of Cladode Drying Techniques on the Prebiotic Potential and Molecular Characteristics of the Mucilage Extracted from Opuntia ficus-indica and Opuntia joconostle. Sci. Pharm. 2020, 88, 43. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Sampaio, K.B.; Menezes, F.N.D.D.; de Assis, P.O.A.; dos Santos Lima, M.; de Oliveira, M.E.G.; de Souza, E.L.; de Cássia Ramos do Egypto Queiroga, R. In Vitro Evaluation of Potential Prebiotic Effects of a Freeze-Dried Juice from Pilosocereus Gounellei (A. Weber Ex K. Schum. Bly. Ex Rowl) Cladodes, an Unconventional Edible Plant from Caatinga Biome. 3 Biotech 2020, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, M.; Salazar-Montoya, J.A.; Rodríguez-Páez, L.I.; Ramos-Ramírez, E.G. In Vitro Fermentation of Oligosaccharides Obtained from Enzymatic Hydrolysis of Opuntia streptacantha Mucilage. J. Sci. Food Agric. 2019, 99, 2883–2891. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; Trejo, C.; Arroyo-Peña, V.B.; Sánchez Urdaneta, A.B.; Balois Morales, R. Diversity of Unavailable Polysaccharides and Dietary Fiber in Domesticated Nopalito and Cactus Pear Fruit (Opuntia Spp.). Chem. Biodivers. 2012, 9, 1599–1610. [Google Scholar] [CrossRef]
- Hussain, S.; Alamri, M.S.; Mohamed, A.A.; Ibraheem, M.A.; Qasem, A.A.A.; Shamlan, G.; Ababtain, I.A. Exploring the Role of Acacia (Acacia seyal) and Cactus (Opuntia ficus-indica) Gums on the Dough Performance and Quality Attributes of Breads and Cakes. Foods 2022, 11, 1208. [Google Scholar] [CrossRef]
- Uebelhack, R.; Busch, R.; Alt, F.; Beah, Z.-M.; Chong, P.-W. Effects of Cactus Fiber on the Excretion of Dietary Fat in Healthy Subjects: A Double Blind, Randomized, Placebo-Controlled, Crossover Clinical Investigation. Curr. Ther. Res. 2014, 76, 39–44. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Can Nopal Cactus (Opuntia ficus-indica L. Miller) Treat Obesity? Obes. Med 2022, 30, 100390. [Google Scholar] [CrossRef]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.P.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Fig “Ficus Carica L.” and Its by-Products: A Decade Evidence of Their Health-Promoting Benefits towards the Development of Novel Food Formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Feugang, J.M. Nutritional and Medicinal Use of Cactus Pear (Opuntia Spp.) Cladodes and Fruits. Front. Biosci. 2006, 11, 2574. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Cárdenas, A. Pectins from Opuntia Spp.: A Short Review. J. Prof. Assoc. Cactus Dev. 2003, 5, 17–29. [Google Scholar]
- Costa, K.P.B.; Reichembach, L.H.; de Oliveira Petkowicz, C.L. Pectins with Commercial Features and Gelling Ability from Peels of Hylocereus Spp. Food Hydrocoll. 2022, 128, 107583. [Google Scholar] [CrossRef]
- Cárdenas, A.; Goycoolea, F.M.; Rinaudo, M. On the Gelling Behaviour of ‘Nopal’ (Opuntia ficus indica) Low Methoxyl Pectin. Carbohydr. Polym. 2008, 73, 212–222. [Google Scholar] [CrossRef]
- Mohamed, S.K.; Alazhary, A.M.; Al-Zaqri, N.; Alsalme, A.; Alharthi, F.A.; Hamdy, M.S. Cost-Effective Adsorbent from Arabinogalactan and Pectin of Cactus Pear Peels: Kinetics and Thermodynamics Studies. Int. J. Biol. Macromol. 2020, 150, 941–947. [Google Scholar] [CrossRef]
- Blanco-Pérez, F.; Steigerwald, H.; Schülke, S.; Vieths, S.; Toda, M.; Scheurer, S. The Dietary Fiber Pectin: Health Benefits and Potential for the Treatment of Allergies by Modulation of Gut Microbiota. Curr. Allergy Asthma Rep. 2021, 21, 43. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Zhao, Q.; Wen, A.; Li, L.; Zhang, Y. The Regulating Effect of Tibet Opuntia ficus-indica (Linn.) Mill. Polysaccharides on the Intestinal Flora of Cyclophosphamide-Induced Immunocompromised Mice. Int. J. Biol. Macromol. 2022, 207, 570–579. [Google Scholar] [CrossRef]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.M.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Aguilar-López, M.; Pérez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) Protects from Metabolic Endotoxemia by Modifying Gut Microbiota in Obese Rats Fed High Fat/Sucrose Diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red Pitaya Betacyanins Protects from Diet-Induced Obesity, Liver Steatosis and Insulin Resistance in Association with Modulation of Gut Microbiota in Mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Ali, S.K.; Mahmoud, S.M.; El-Masry, S.S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Aboel-Ainin, M.A. Phytochemical Screening and Characterization of the Antioxidant, Anti-Proliferative and Antibacterial Effects of Different Extracts of Opuntia ficus-indica Peel. J. King Saud. Univ. Sci. 2022, 34, 102216. [Google Scholar] [CrossRef]
- Rockett, F.C.; de Oliveira Schmidt, H.O.; Schmidt, L.; Rodrigues, E.; Tischer, B.; Ruffo de Oliveira, V.; Lima da Silva, V.; Rossini Augusti, P.; Flôres, S.H.; Rios, A. Phenolic Compounds and Antioxidant Activity in Vitro and in Vivo of Butia and Opuntia Fruits. Food Res. Int. 2020, 137, 109740. [Google Scholar] [CrossRef] [PubMed]
- González-Ponce, H.A.; Martínez-Saldaña, M.C.; Tepper, P.G.; Quax, W.J.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Betacyanins, Major Components in Opuntia Red-Purple Fruits, Protect against Acetaminophen-Induced Acute Liver Failure. Food Res. Int. 2020, 137, 109461. [Google Scholar] [CrossRef] [PubMed]
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R.A. Oligosaccharides of Pitaya (Dragon Fruit) Flesh and Their Prebiotic Properties. Food Chem. 2010, 120, 850–857. [Google Scholar] [CrossRef]
- Malfa, G.A.; Di Giacomo, C.; Cardia, L.; Sorbara, E.E.; Mannucci, C.; Calapai, G. A Standardized Extract of Opuntia ficus-indica (L.) Mill and Olea europaea L. Improves Gastrointestinal Discomfort: A double-blinded Randomized-controlled Study. Phytother. Res. 2021, 35, 3756–3768. [Google Scholar] [CrossRef]
- Remes-Troche, J.M.; Taboada-Liceaga, H.; Gill, S.; Amieva-Balmori, M.; Rossi, M.; Hernández-Ramírez, G.; García-Mazcorro, J.F.; Whelan, K. Nopal Fiber (Opuntia ficus-indica) Improves Symptoms in Irritable Bowel Syndrome in the Short Term: A Randomized Controlled Trial. Neurogastroenterol. Motil. 2021, 33, e13986. [Google Scholar] [CrossRef]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.S.; Cano, M.P. In Vitro Gastrointestinal Stability, Bioaccessibility and Potential Biological Activities of Betalains and Phenolic Compounds in Cactus Berry Fruits (Myrtillocactus geometrizans). Food Chem. 2021, 342, 128087. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory Potential of Prickly Pears and Their Isolated Bioactives against Digestive Enzymes Linked to Type 2 Diabetes and Inflammatory Response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef]
- del Laguna, B.C.C.; Flores Gallegos, A.C.; Ascacio Valdés, J.A.; Iliná, A.; Galindo, A.S.; Castañeda Facio, A.O.; Esparza González, S.C.; Herrera, R.R. Physicochemical and Functional Properties of the Undervalued Fruits of Cactus Cylindropuntia Imbricate (“Xoconostle”) and Antioxidant Potential. Biocatal. Agric. Biotechnol. 2022, 39, 102245. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS Profiling of Secondary Metabolites from Opuntia ficus-indica Cladode, Peel and Fruit Pulp Extracts and Their Antioxidant, Neuroprotective Effect in Rats with Aluminum Chloride Induced Neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- El Otmani, S.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Chemical Composition and in Vitro Digestibility of Alternative Feed Resources for Ruminants in Mediterranean Climates: Olive Cake and Cactus Cladodes. J. Agric. Sci. 2019, 157, 260–271. [Google Scholar] [CrossRef]
- Dick, M.; Limberger, C.; Cruz Silveira Thys, R.; de Oliveira Rios, A.; Hickmann Flôres, S. Mucilage and Cladode Flour from Cactus (Opuntia monacantha) as Alternative Ingredients in Gluten-Free Crackers. Food Chem. 2020, 314, 126178. [Google Scholar] [CrossRef]
- De Santiago, E.; Juániz, I.; Cid, C.; De Peña, M.-P. Extraction of (Poly)Phenolic Compounds of Cactus (Opuntia ficus-indica (L.) Mill.) Cladodes. Food Anal. Methods 2021, 14, 1167–1175. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Matboli, M.; Saad, M.; Hasanin, A.H.; Saleh, L.A.; Baher, W.; Bekhet, M.M.; Eissa, S. New Insight into the Role of Isorhamnetin as a Regulator of Insulin Signaling Pathway in Type 2 Diabetes Mellitus Rat Model: Molecular and Computational Approach. Biomed. Pharmacother. 2021, 135, 111176. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Fiori, L.; Ciolli, M.; Aprea, E. Prickly Pear Seed Oil Extraction, Chemical Characterization and Potential Health Benefits. Molecules 2021, 26, 5018. [Google Scholar] [CrossRef]
- De Wit, M.; Motsamai, V.K.; Hugo, A. Cold-Pressed Cactus Pear Seed Oil: Quality and Stability. Grasas Aceites 2021, 72, e415. [Google Scholar] [CrossRef]
- Nounah, I.; Chbani, M.; Matthäus, B.; Charrouf, Z.; Hajib, A.; Willenberg, I. Profile of Volatile Aroma-Active Compounds of Cactus Seed Oil (Opuntia ficus-indica) from Different Locations in Morocco and Their Fate during Seed Roasting. Foods 2020, 9, 1280. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Cafarella, C.; Aprea, E.; Ferrentino, G.; Gasparini, A.; Buzzanca, C.; Micalizzi, G.; Dugo, P.; Mondello, L.; Rigano, F. Supercritical Fluid Extraction of Oils from Cactus Opuntia ficus-indica L. and Opuntia Dillenii Seeds. Foods 2023, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Dantas, D.L.; Viera, V.B.; Soares, J.K.B.; dos Santos, K.M.O.; do Egito, A.S.; de Figueirêdo, R.M.F.; Lima, M.d.S.; Machado, N.A.F.; de Souza, M.d.F.V.; da Conceição, M.L.; et al. Pilosocereus gounellei (Xique-Xique) Flour: Improving the Nutritional, Bioactive, and Technological Properties of Probiotic Goat-Milk Yogurt. LWT 2022, 158, 113165. [Google Scholar] [CrossRef]
- Machado, T.A.D.G.; Pacheco, M.T.B.; Queiroga, R.d.C.R.d.E.; Cavalcante, L.M.; Bezerril, F.F.; Ormenese, R.d.C.S.C.; Garcia, A.d.O.; Nabeshima, E.H.; Pintado, M.M.E.; Oliveira, H.M.L. Nutritional, Physicochemical and Sensorial Acceptance of Functional Cookies Enriched with Xiquexique (Pilosocereus gounellei) Flour. PLoS ONE 2021, 16, e0255287. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of Prickly Pear (Opuntia ficus-indica L.) Peel Flour as an Innovative Ingredient in Biscuits Formulation. LWT 2020, 124, 109155. [Google Scholar] [CrossRef]
- Msaddak, L.; Abdelhedi, O.; Kridene, A.; Rateb, M.; Belbahri, L.; Ammar, E.; Nasri, M.; Zouari, N. Opuntia ficus-indica Cladodes as a Functional Ingredient: Bioactive Compounds Profile and Their Effect on Antioxidant Quality of Bread. Lipids Health Dis. 2017, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.F.M.; El-Anany, A.M.; Mousa, H.M.; Hamad, E.M. Nutritional and Sensory Characteristics of Bread Enriched with Roasted Prickly Pear (Opuntia ficus-indica) Seed Flour. Food Funct. 2020, 11, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, Z.; Li, G.; Hu, L.; Chen, J.; Hu, Y. Make Your Packaging Colorful and Multifunctional: The Molecular Interaction and Properties Characterization of Natural Colorant-Based Films and Their Applications in Food Industry. Trends Food Sci. Technol. 2022, 124, 259–277. [Google Scholar] [CrossRef]
- Utpott, M.; Assis, R.Q.; Pagno, C.H.; Pereira Krigger, S.; Rodrigues, E.; de Oliveira Rios, A.; Hickmann Flôres, S. Evaluation of the Use of Industrial Wastes on the Encapsulation of Betalains Extracted from Red Pitaya Pulp (Hylocereus polyrhizus) by Spray Drying: Powder Stability and Application. Food Bioproc. Tech. 2020, 13, 1940–1953. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Sáenz, C. Microparticles of Yellow-Orange Cactus Pear Pulp (Opuntia ficus-indica) with Cladode Mucilage and Maltodextrin as a Food Coloring in Yogurt. LWT 2021, 138, 110672. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, M.G.; Amaya-Guerra, C.A.; Quintero-Ramos, A.; Pérez-Carrillo, E.; Meléndez-Pizarro, C.O. Use of Red Cactus Pear (Opuntia ficus-indica) Encapsulated Powder to Pigment Extruded Cereal. J. Food Qual. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Lugo-Zarate, L.; del Cruz-Cansino, N.S.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Arias-Rico, J.; Cervantes-Elizarrarás, A. Evaluation of Physicochemical, Microbiological, and Antioxidant Properties of a Drinkable Yogurt Added with Ultrasonicated Purple Cactus Pear (Opuntia ficus-indica) Juice Powder. J. Food Process. Preserv. 2021, 45, e15720. [Google Scholar] [CrossRef]
- Da Nunes, A.R.C.; Mangolin, C.A.; Braz de Oliveira, A.J.; Gonçalves, R.A.C.; da Avincola, A.S.; Ribeiro de Almeida, R.T.; Pilau, E.J.; de Fatima Pires da Silva Machado, M. Cereus peruvianus Mill. (Cactaceae) as a Source of Natural Antioxidants: Phenolic Compounds and Antioxidant Activity of Cladode Extracts in Two Collection Periods. Curr. Res. Food Sci. 2022, 5, 984–991. [Google Scholar] [CrossRef]
- Oliveira, J.M.C.; de Souza, E.L.; de Lima, K.Y.G.; dos Lima, M.S.; Viera, V.B.; Queiroga, R.d.C.R.d.E.; de Oliveira, M.E.G. Physicochemical Parameters, Phytochemical Profile and Antioxidant Properties of a New Beverage Formulated with Xique-Xique (Pilosocereus gounellei) Cladode Juice. Foods 2021, 10, 1970. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Jäger, H.; Knorr, D.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Impact of Pulsed Electric Fields, High Hydrostatic Pressure, and Thermal Pasteurization on Selected Characteristics of Opuntia Dillenii Cactus Juice. LWT Food Sci. Technol. 2017, 79, 534–542. [Google Scholar] [CrossRef]
- Otálora, M.C.; de Jesús Barbosa, H.; Perilla, J.E.; Osorio, C.; Nazareno, M.A. Encapsulated Betalains (Opuntia ficus-indica) as Natural Colorants. Case Study: Gummy Candies. LWT 2019, 103, 222–227. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Youssef, K.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Flavonol Profile of Cactus Fruits (Opuntia ficus-indica) Enriched Cereal-Based Extrudates: Authenticity and Impact of Extrusion. Food Res. Int. 2015, 78, 442–447. [Google Scholar] [CrossRef]
- Tabarestani, P.S.; Kashiri, M.; Maghsoudlou, Y.; Shahiri Tabarestani, H.; Ghorbani, M. Effect of Opuntia Pulp as a Clean Label Ingredient on Techno-functional Properties of Meat-free Burger. Int. J. Food Sci. Technol. 2022, 57, 3982–3989. [Google Scholar] [CrossRef]
- Panda, S.K.; Behera, S.K.; Witness Qaku, X.; Sekar, S.; Ndinteh, D.T.; Nanjundaswamy, H.M.; Ray, R.C.; Kayitesi, E. Quality Enhancement of Prickly Pears (Opuntia Sp.) Juice through Probiotic Fermentation Using Lactobacillus Fermentum—ATCC 9338. LWT 2017, 75, 453–459. [Google Scholar] [CrossRef]
- Verón, H.E.; Gauffin Cano, P.; Fabersani, E.; Sanz, Y.; Isla, M.I.; Fernández Espinar, M.T.; Gil Ponce, J.V.; Torres, S. Cactus Pear (Opuntia ficus-indica) Juice Fermented with Autochthonous Lactobacillus plantarum S-811. Food Funct. 2019, 10, 1085–1097. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Phytochemical and Nutritional Significance of Cactus Pear. Eur. Food Res. Technol. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- Alcántara-Zavala, A.E.; de Dios Figueroa-Cárdenas, J. Shelf Life, Physicochemical and Antioxidant Properties of Red Cactus Pear Pulque Processed by Ohmic Heating and by Conventional Pasteurization. Int. J. Gastron. Food Sci. 2022, 28, 100497. [Google Scholar] [CrossRef]
- El-Sayed, H.; Ramadan, M. Production of Probiotic-Fermented Rice Milk Beverage Fortified with Cactus Pear and Physalis Pulp. Zagazig J. Agric. Res. 2020, 47, 165–177. [Google Scholar] [CrossRef]
- Hou, L.; Kou, X.; Wang, H.; Liu, Y.; Xie, B.; Yang, X. Study on the Processing Technology of Compound Fruit and Vegetable Beverage of Cactus. Storage Process 2013, 13, 42–46. [Google Scholar]
- Tsegay, Z.T.; Gebremedhin, K.M. Physicochemical and Sensory Properties of Wine Produced from Blended Cactus Pear (Opuntia ficus-indica) and Lantana camara (L. camara ) Fruits. J. Food Qual. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Ayed, L.; Hamdi, M. Manufacture of a Beverage from Cactus Pear Juice Using “Tea Fungus” Fermentation. Ann. Microbiol. 2015, 65, 2293–2299. [Google Scholar] [CrossRef]
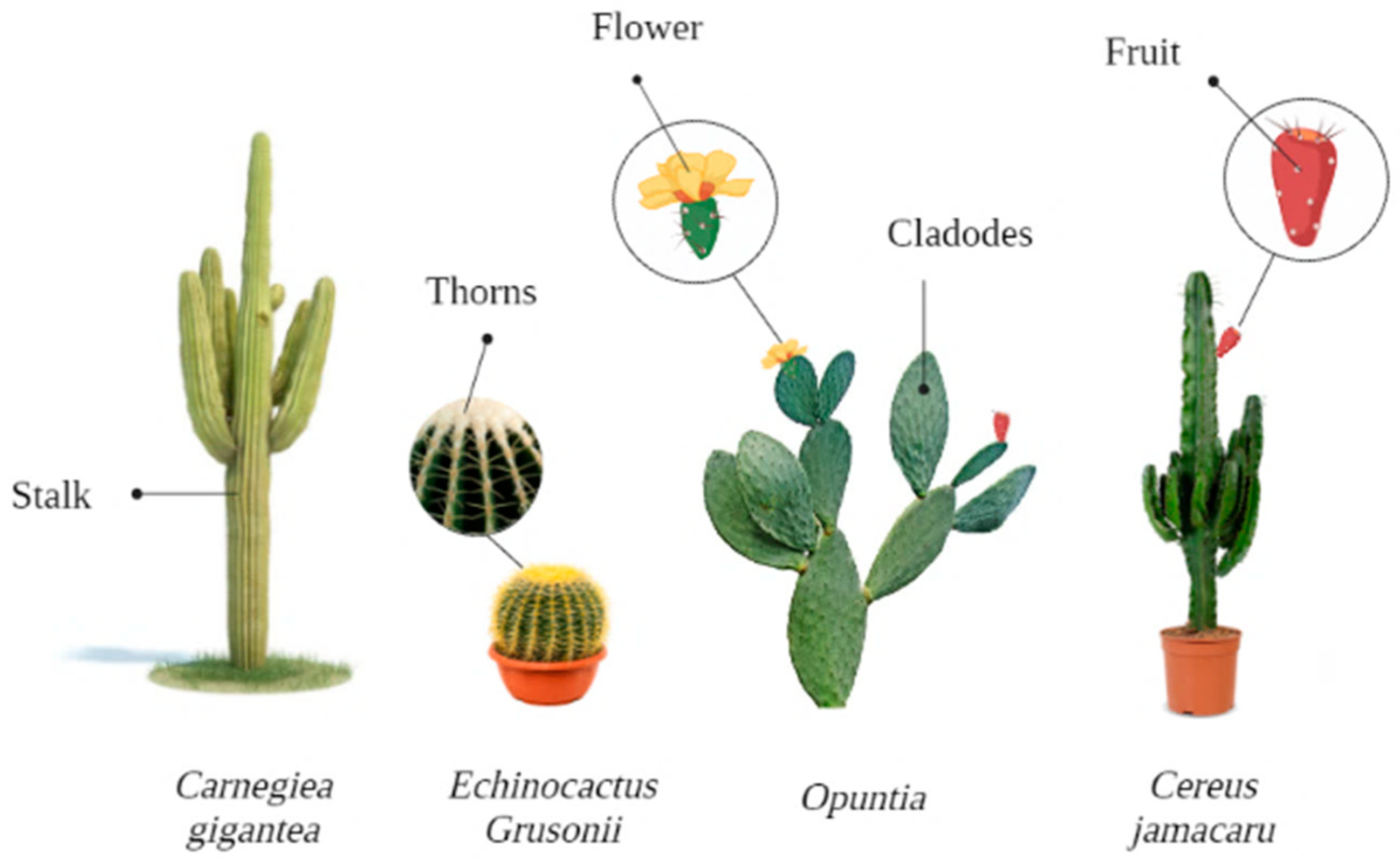
| Species | Edible Part | Traditional Use |
|---|---|---|
| Opuntia fícus-indica Opuntia cochenillifera | Cladodes | As a vegetable in salads, jelly, flour, or extracts |
| Fruits | Consumption of fresh pulp, jelly, and beverage | |
| Flowers | Extracts used in folk medicine | |
| Cereus peruvianus Cereus jamacaru Cereus hilmanianus | Cladodes | Extracts used in folk medicine |
| Fruits and seeds | Consumed fresh or in culinary preparations | |
| Pilosocereus gounellei Pilosocereus pachycladus | Cladodes | As a vegetable in salads, jelly, flour, or extracts |
| Fruits | Consumed fresh or in culinary preparations | |
| Hylocereus undatus | Fruits | Consumed fresh or in culinary preparations |
| Flowers | Extracts used in folk medicine | |
| Selenicereus grandifloras | Fruits | Consumed fresh or in culinary preparations |
| Stenocereus thurberi | ||
| Tacinga inamoena |
| Cacti Specie | Opuntia ficus-indica | Cereus peruvianus | Hylocereus spp. | |
|---|---|---|---|---|
| Nutrient | Nopal Cladodes | Prickly Pear Fruit | Peruvian Apple Cactus Fruit | Dragon Fruit |
| Moisture (g/100 g) | 90–94 | 88–92 | 88–92 | 80–90 |
| Protein (g/100 g) | 1.2–2.0 | 0.5–1.1 | 0.6–0.9 | 0.2–1.2 |
| Fat (g/100 g) | 0.1–0.5 | 0.3–0.6 | 0.1–0.3 | 0.1–0.7 |
| Carbohydrates (g/100 g) | 3.0–4.5 | 7.0–12.0 | 6.0–10.0 | 8.0–18.0 |
| Dietary Fiber (g/100 g) | 2.2–4.0 | 3.0–5.0 | 1.0–2.0 | 1.0–3.0 |
| Ash (g/100 g) | 1.1–1.6 | 0.8–1.2 | 0.4–0.7 | 0.4–0.8 |
| Minerals | ||||
| Calcium (mg/100 mg) | 220–320 | 30–55 | 20–30 | 6–15 |
| Magnesium (mg/100 mg) | 52–85 | 15–30 | 10–20 | 10–20 |
| Potassium (mg/100 mg) | 300–430 | 220–330 | 200–300 | 200–400 |
| Sodium (mg/100 mg) | 21–45 | 3–15 | 1–10 | 0–50 |
| Iron (mg/100 mg) | 0.5–2.0 | 0.3–1.5 | 0.2–0.5 | 0.3–0.7 |
| Vitamins | ||||
| Vitamin A µg/100 g | 50–100 | 20–50 | Not found in the literature | 10–40 |
| Vitamin C mg/100 g | 9–25 | 10–40 | 5–20 | 2–25 |
| Vitamin E mg/100 g | 0.5–1.5 | 0.3–1.0 | Not found in the literature | Not found in the literature |
| Phytochemicals | ||||
| Polyphenols mg/100 g | 50–200 | 30–100 | Not found in the literature | 10–30 |
| Flavonoids mg/100 g | 10–50 | 10–30 | Not found in the literature | Not found in the literature |
| Betalains mg/100 g | Not significant | 10–50 (peel) | Not found in the literature | 10–50 (peel) |
| Cacti Component | Health Benefits | Applications in the Food Industry | References |
|---|---|---|---|
| Dietary Fiber | Promotes gut health, supports weight management, and maintains healthy blood sugar levels. | Used as a natural thickening agent in sauces, soups, and bakery items. Enhances the nutritional profile of food products. Reduces calorie intake by inducing satiety. | [63,64,65,66,67] |
| Mucilage | Potential prebiotic benefits due to the presence of monosaccharides such as xylose. | Used as a thickening agent and stabilizer. Applications include the development of edible coatings, bioplastics, encapsulating agents, and gluten-free foods. | [23,68] |
| Pectin | Promotes gut health, aids in controlling blood sugar levels, and helps lower cholesterol levels. Potential prebiotic properties. | Used for its gelling, thickening, and stabilizing properties in the production of jellies, jams, marmalades, and certain types of candy. Acts as a fat substitute in baked goods to stabilize acidic protein drinks such as yogurt. | [69,70,71,72,73,74,75] |
| Polyphenols | Potent antioxidant, anti-inflammatory, anticancer properties, antihyperglycemic activity, and potential benefits in obesity, cardiovascular and inflammatory diseases, diabetes, and gastric ulcers. | Used as food resources and in folk medicine, potential use in nutraceutical and cosmetic industries. Serves as a source of food coloring agents and bioethanol and biogas production. Contains beneficial compounds such as lignans, sterols, esters, saponins, and alkaloids. | [3,22,27,50,76,77,78,79,80,81,82,83] |
| Flavonoids | Anti-inflammatory, antiaging, antioxidant, antimicrobial, anticancer, hepatoprotective, reproductive system protective, antiobesity, and antidiabetic effects. | Significant component in the formulation of functional food and drugs. Known to regulate signaling pathways (e.g., NF-kB, PI3K/AKT, MAPK). | [84,85,86] |
| Opuntia ficus-indica (Prickly pear seed oil) | Rich in linoleic acid and tocopherols, including vitamin E. High sterol content, particularly β-sitosterol. Strong antioxidant properties. Relatively high smoke point. Pleasant taste and aroma. | Used as cooking oil; a functional ingredient in food formulations such as salad dressings, dips, and spreads; and a carrier oil for other flavors and bioactive ingredients. Additionally, valuable in the cosmetic industry. | [87,88,89,90] |
| Species | Probiotic Strain | Results | Reference |
|---|---|---|---|
| Opuntia fícus-indica and Opuntia joconostle | Lacticaseibacillus rhamnosus GG (ATCC 53103), Lactobacillus acidophilus (DSM 13241), and Bifidobacterium longum subsp. Infantis (ATCC 15697) | All strains were able to use mucilage as substrate, although with lower maximum growth than glucose. | [64] |
| Opuntia ficus-indica | Colon microbiota obtained from a fecal sample from a healthy adult volunteer | The mucilage increased Lactobacillus growth by up to 23.8% and produced a slight decrease in enterococci, enterobacteria, staphylococci, and clostridia. | [65] |
| Opuntia fícus-indica and Opuntia joconostle | Lacticaseibacillus rhamnosus GG (ATCC 53103), Lactobacillus acidophilus (DSM 13241), Bifidobacterium longum ssp. Infantis (ATCC 15697), and Bifidobacterium animalis ssp. Lactis Bb-12 (DSM 15954) | Mucilage fermentability by selected probiotics was relatively low, 11–27% compared to glucose, and decreased with increasing levels of galacturonic acids in the molecules. Therefore, its fermentability by probiotic species can be attributed more to its structural characteristics and monosaccharide composition than to its dimensions. | [66] |
| Pilosocereus gounellei | Lactobacillus acidophilus LA-05, Lacticaseibacillus casei L-26, and Lacticaseibacillus paracasei L-10 | Juice from Pilosocereus gounellei cladodes showed positive scores for prebiotic activity in all probiotics examined, indicating a selective stimulatory effect on these microorganisms to the detriment of enteric pathogens. | [67] |
| Opuntia streptacantha | Lactobacillus acidphilus, Lacticaseibacillus casei, and Bifidobacterium animalis subsp. lactis | The mucilage fractions stimulated the in vitro growth of commercial probiotics, with behavior similar to that obtained with commercial inulin. | [68] |
| Material | Study | Results | References |
|---|---|---|---|
| Polysaccharides extracted from Tibet Opuntia ficus-indica (Linn.) Mill. | Cyclophosphamate-induced immunocompromised mice | Ingestion of polysaccharides significantly regulated the relative abundance of Lactobacillus, Bacterioides, and Akkermansia, and the new dominant intestinal bacterial species were Deferribacteres, Actinomycetes, Firmicutes, Tenericutes, Actinomycetes, and Pasteurella. Polysaccharides can effectively increase the metabolic level of lysine synthesis and decomposition, regulate the level of gene expression after immune disorders, and improve the overall health of immunodeficient mice. | [81] |
| Cooked Opuntia ficus-indica | Sample of 36 female volunteers, with an obesity group consisting of 25 women and another group consisting of 11 women of normal weight. | It was reported that intervention with Opuntia Ficus-indica in the diet of women induced changes in the intestinal microbiota in both groups of women, associated with biochemical and anthropometric parameters. | [82] |
| Dried cladodes of Opuntia ficus-indica | Obese rats fed a high-fat, high-sucrose diet | The addition of Opuntia ficus-indica cladodes to the diet of rats can ameliorate specific biochemical parameters of obesity, such as total cholesterol, GIP, APP, leptin, and peptides, modifying the intestinal microbiota. Therefore, dried cladodes of Opuntia ficus-indica have the ability to modify the intestinal microbiota and reduce metabolic endotoxemia and other biochemical abnormalities related to obesity, supporting their use as a functional food and prebiotic. | [83] |
| Red pitaya betacyanins | Male mice fed low-fat diet, high-fat diet, and high-fat diet plus Hylocereus polyrhizus | The addition of red pitaya betacyanins to the diet protects against diet-induced obesity and related metabolic diseases, is associated with improving the inflammatory state, modulating the intestinal microbiota, decreasing the proportion of Firmicutes and Bacteroidetes, and increasing the relative abundance of Akkermansia at the genus level. | [84] |
| Opuntia ficus-indica extracts | Antioxidant activity, in vitro antitumor activity, and antimicrobial activity | Antimicrobial activity against pathogenic viruses and bacteria was observed in extracts of Opuntia ficus-indica. Selective cytotoxicity towards cancer cells. | [85] |
| Butia and Opuntia fruit hydroethanolic extracts | Antioxidant capacity in vitro and in vivo | The hydroethanolic extracts of Butia and Opuntia fruits showed antioxidant effects in vitro and in vivo, as well as no toxic effect in vivo. | [86] |
| Opuntia robusta and Opuntia streptacantha fruit extracts | In vitro and in vivo study with mice | Therapeutic treatments with extracts of Opuntia reduced biochemical, molecular, and histological markers of liver injury (in vivo) and hepatocytes (in vitro). Opuntia extracts reduced the increased APAP expression of the stress-related gene Gadd45b, and exerted diverse effects on the antioxidant-related genes Sod2, Gclc, and Hmox1, independent of their ability to clear ROS. | [87] |
| White and red pitaya extracts | Study of the effect of hydrolysis of artificial human gastric juice, hydrolysis of human α-amylase, and the growth of probiotics (Lactobacillus delbrueckii and Bifidobacterium bifidum) | Prebiotic properties were observed in the extracts, which included resistance to the acidic conditions of the human stomach, partial resistance to human salivary α-amylase, and the ability to stimulate the growth of lactobacilli and bifidobacteria. | [88] |
| Standardized cladode extracts of Opuntia ficus-indica and Olea europaea | Study conducted with one hundred healthy participants with gastrointestinal discomfort | The group treated with the extract showed a significant improvement in gastrointestinal quality of life (GIQLI) and in the Gastroesophageal Reflux Disease Symptom Rating Scale. | [89] |
| Fiber of Opuntia ficus-indica | Study carried out with 60 patients recruited with a diagnosis of irritable bowel syndrome | Dietary supplementation with fiber from Opuntia ficus-indica was associated with short-term improvement in symptoms of irritable bowel syndrome. | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.d.B. New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods 2023, 12, 2494. https://doi.org/10.3390/foods12132494
Monteiro SS, Almeida RL, Santos NC, Pereira EM, Silva AP, Oliveira HML, Pasquali MAdB. New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods. 2023; 12(13):2494. https://doi.org/10.3390/foods12132494
Chicago/Turabian StyleMonteiro, Shênia Santos, Raphael Lucas Almeida, Newton Carlos Santos, Emmanuel Moreira Pereira, Amanda Priscila Silva, Hugo Miguel Lisboa Oliveira, and Matheus Augusto de Bittencourt Pasquali. 2023. "New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends" Foods 12, no. 13: 2494. https://doi.org/10.3390/foods12132494
APA StyleMonteiro, S. S., Almeida, R. L., Santos, N. C., Pereira, E. M., Silva, A. P., Oliveira, H. M. L., & Pasquali, M. A. d. B. (2023). New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods, 12(13), 2494. https://doi.org/10.3390/foods12132494








