Abstract
The Maypole apple is a new, promising species of small apples with a prominent flavor and deep red flesh and peel. This study divided Maypole apples into outer flesh, inner flesh, and peel, and used subcritical water at 100–175 °C for 10–30 min to extract various phytochemicals (procyanidin B2 (PB2), 5-caffeoylquinic acid (5CQA), and epicatechin). The obtained Maypole apple extracts and extraction residues in this work were analyzed using a SEM, HPLC, FT-IR, and UV-Vis spectrophotometer. Under different subcritical water extraction conditions, this work found the highest extraction rate: to be PB2 from the peel (4.167 mg/mL), 5CQA (2.296 mg/mL) and epicatechin (1.044 mg/mL) from the inner flesh. In addition, this work regressed the quadratic equations of the specific yield through ANOVA and found that temperature is a more significant affecting factor than extraction time. This aspect of the study suggests that phytochemicals could be obtained from the Maypole apple using the new extraction method of subcritical water.
1. Introduction
The Maypole apple is a type of crabapple that has been evaluated as a potential pollinizer for major apple cultivars [1]. It has been reported that phytochemical composition and contents significantly vary between the apple peel and flesh [2,3]. Because of the dark red color of the flesh and peel of Maypole apples, they are expected to contain a considerable amount of phytochemicals. Phytochemicals have been reported to have different benefits for human health, such as controlling immune and inflammatory responses, inhibiting cancer cell growth, and preventing lipid oxidation [3,4,5]. This work focuses on the extraction of phytochemicals such as procyanidin B2 (PB2), 5-caffeoylquinic acid (5CQA), and epicatechin from Maypole apples.
Procyanidins are polymers or oligomers of flavonoids, which are commonly found in various fruits and plants [6]. PB2 is an antioxidant that is primarily found in procyanidin extracts from grape seeds [7,8]. Some apple varieties also accumulate PB2 in their flesh and peel [9]. PB2 has been reported to have various beneficial effects, including antitumor [10], anti-DNA damage [11], anti-inflammatory [12], and hair growth-activating effects [13].
5CQA is a caffeic acid ester that is also known as chlorogenic acid. 5CQA is commonly found in plants, including fruits, vegetables, and coffee [14,15]. In addition, a high amount of 5CQA has been found in apples [16]. 5CQA is a dietary polyphenolic compound with various significant therapeutic effects, including antioxidant, antibacterial, hepatoprotective, cardioprotective, anti-inflammatory, antipyretic, neuroprotective, anti-obesity, antiviral, antimicrobial, antihypertensive, and central nervous system stimulation effects [16,17,18].
Epicatechin is a natural flavonoid found in green tea [19]. It has been reported to have a substantial antioxidant effect that contributes to its therapeutic effect. Epicatechin has been reported to exhibit beneficial effects in controlling sugar levels in diabetic patients [20], show antioxidant effects [21], prevent cardiovascular disease [22], improve exercise performance [23], and inhibit cancer [24].
Traditionally, organic solvents such as methanol and ethanol have been used to extract phytochemical compounds from various natural products [25,26,27,28]. Methanol is toxic to human health, and some regions and cultures prohibit the use of ethanol in food processing, necessitating the use of alternative solvents. Moreover, the latest research shows that even small amounts of alcohol can affect health [29]. Water, in contrast, is a green solvent that is non-toxic to human health and the environment, and has no cultural problems. In addition, replacing organic solvents with water in phytochemical compound extraction is expected to lead to significant cost reduction. In the present study, water was used to extract PB2, 5CQA, and epicatechin from Maypole apples.
If water is heated above 374 °C and pressurized above 22 MPa, it becomes supercritical. Supercritical water has low viscosity, low surface tension, and a high diffusion coefficient [30]. In addition, supercritical water could behave as a nonpolar solvent, meaning that ionic compounds are poorly soluble in it [31]. Thus, supercritical water can serve as a good solvent for nonpolar compounds [32,33,34].
Subcritical water is milder than supercritical water. This study defines subcritical water as water under high pressure at 100–374 °C. Although some bioactive compounds could be degraded at a high temperature [35], it has been reported that tannic acid, as a typical polyphenolic compound, has major degradation at temperatures above 230 °C [36]. Therefore, the use of subcritical water up to 175 °C is mild for this work. It is reported that subcritical water contains a range of hydrogen and hydroxide ions [37]. In addition, previous studies have indicated that the dielectric constant of subcritical water at 250 °C in the liquid state (ε = 27) is similar to that of methanol (ε = 33) and ethanol (ε = 24) at 25 °C [38,39]. This indicates that subcritical water may have good solubility for natural products, comparable to that of methanol or ethanol. In addition, with the increase in water temperature to 200 °C, the surface tension of water decreases by nearly 50%, making it easier for the subcritical water to wet the raw materials and thus increasing the extraction efficiency [40,41]. Furthermore, the pressurized hot water is expected to effectively break the cell wall, especially for the peel of the Maypole apple, and extract the inner biological components. Owing to its various advantages, subcritical water has been reported to be a new and promising extraction medium for the extraction of various phytochemicals from various natural products [42,43,44].
The present study aimed to investigate the optimal temperature and time for the subcritical water extraction of PB2, 5CQA, and epicatechin from various parts of Maypole apples. This study also provides more experimental data for future use of subcritical water extraction to extract different phytochemicals from natural resources.
2. Materials and Methods
2.1. Materials
Maypole apples, used as the raw material, were picked from the Nakahira farm in Matsukawa-Cho, Nagano Prefecture, Japan, in early September. The pure water used in this study was distilled using a distillation apparatus (Auto Still WS 200; Yamato Scientific Co. Ltd., Tokyo, Japan). The PB2 standard was purchased from Fuji Film Wako Junyaku Co. Ltd., Osaka, Japan. (-)-Epicatechin was purchased from Hayashi Kasei Co., Ltd. Nagoya, Japan. 5CQA (98% content) was purchased from Kanto Kagaku Co., Ltd., Tokyo, Japan. Acetic acid (99.5% purity) and acetonitrile (99.8% purity) were purchased from Fujifilm Wako Pure Chemical Industries Ltd., Osaka, Japan.
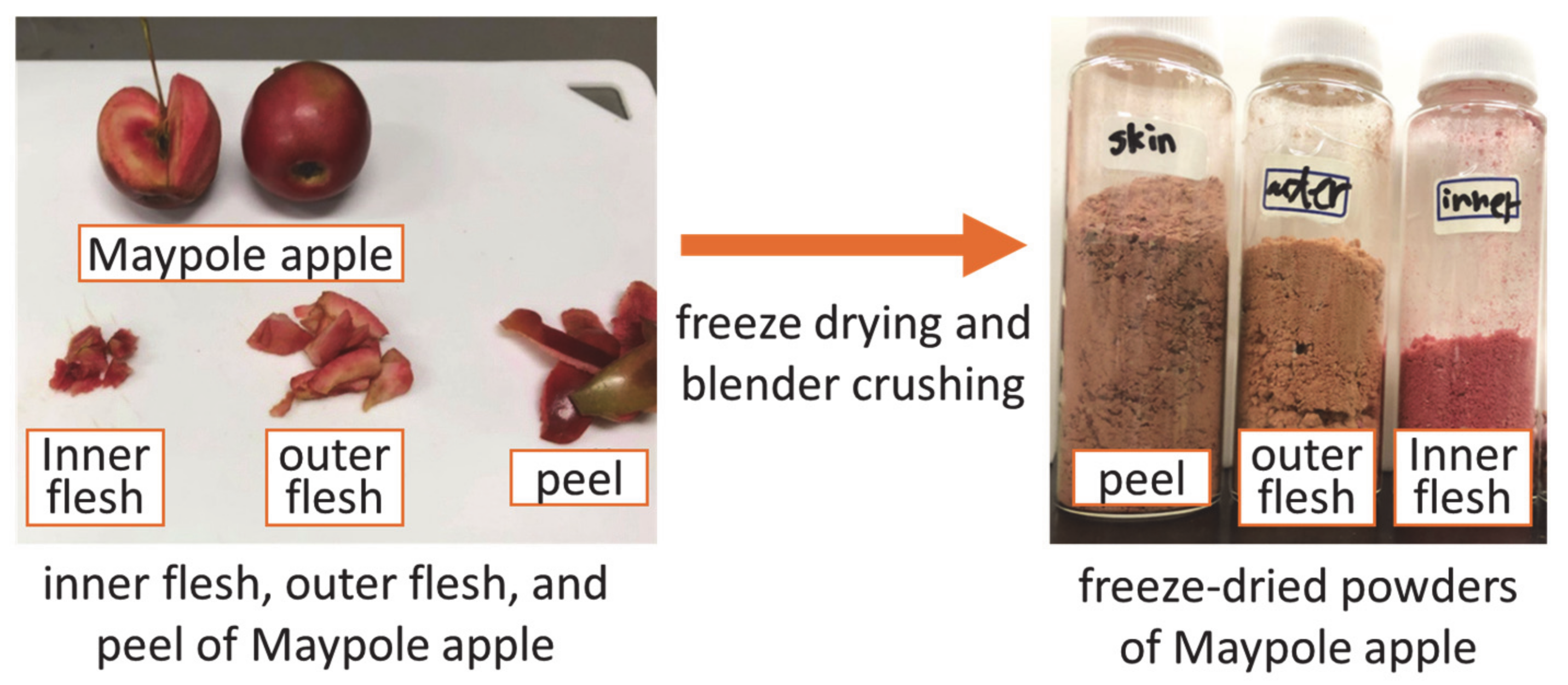
Figure 1 shows a flow chart illustrating the preprocessing of Maypole apples. Apples were washed with tap water and peeled using a peeler. Subsequently, the apples were divided into three parts: the peel, outer flesh, and inner flesh, which were freeze-dried (Eyela FDU-1200; Rikakikai Co., Ltd., Tokyo, Japan) for 24 h and crushed into powder. After freeze-drying, the moisture contents of the peel, outer flesh, and inner flesh were 6.53 wt.%, 9.43 wt.%, and 10.94 wt.%, respectively, which was tested using a halogen moisture meter (65g0001g; AS ONE Corp., Osaka, Japan).
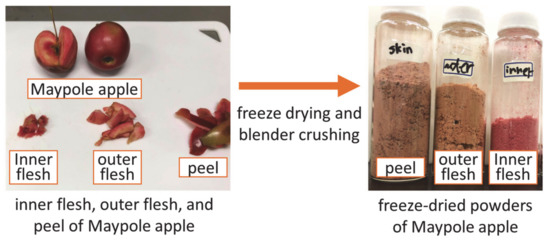
Figure 1.
Pre-processing of different parts of Maypole apples.
2.2. Methods
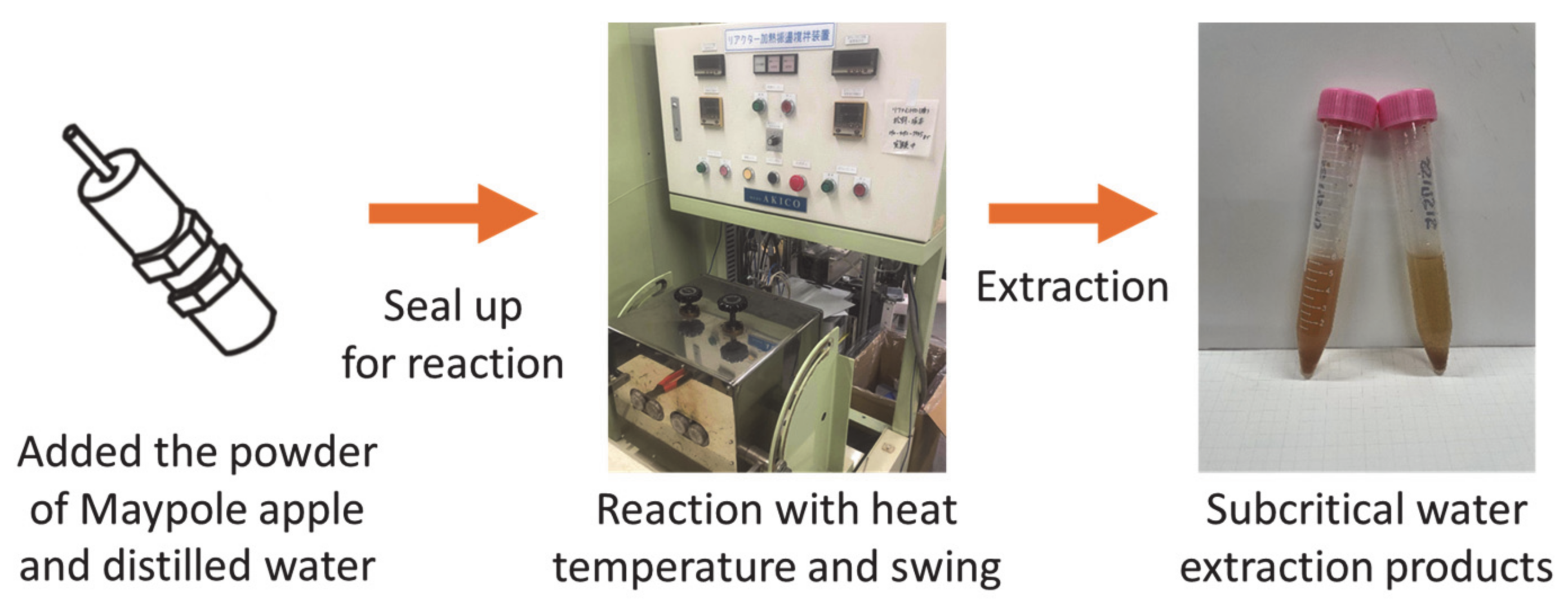
Figure 2 shows the batch high-temperature swing device (AKICO Co., Ltd., Tokyo, Japan). A sample of 0.2 g freeze-dried Maypole apple powder and 7 mL distilled water were added to an 8 mL SUS-316 reactor and hermetically sealed. The reactor was then loaded into an electric furnace (NMF-13AD, Isuzu Corporation, Tokyo, Japan) and heated to the desired temperature (100–175 °C). The swing device was set to 60 times/min. After 10–30 min of reaction, the reactor was removed from the electric furnace and quenched in a cold water bath with water flow under ambient conditions. Cooling took 10–20 min, depending on the reaction temperature. After the reactor was cooled, it was removed from the water bath, and its lid was removed so as to collect the extract and residue. In order to prevent bioactive compounds from reacting with light, the extracts were wrapped in aluminum foil and stored at <6 °C for further characterization.
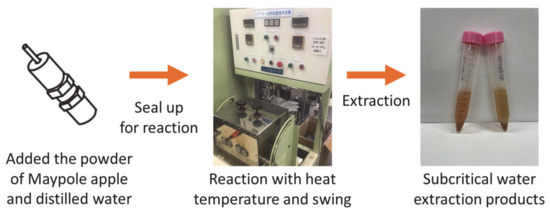
Figure 2.
Extraction of different parts of the Maypole apple by subcritical water.
2.3. Analytical Methods
2.3.1. Scanning Electron Microscopy (SEM) Analysis and Imaging of Residues
The morphologies of the residues were observed using SEM (S-4300, Hitachi, Tokyo, Japan). The samples were sputter-coated with gold (RMC-Eiko RE vacuum coater, Eiko Engineering Co., Ltd., Tokyo, Japan) at 100 millitorrs under 7 mA for 300 s prior to SEM in order to capture a zoomed-in image of the samples.
2.3.2. High-Performance Liquid Chromatography (HPLC)
Subcritical extracts were centrifuged at 2500 rpm for 10 min prior to HPLC. The centrifuged extracts were added with 5 μL of 10 g/L o-cresol (99% purity, Fujifilm Wako Pure Chemical Industries Co., Ltd., Osaka, Japan) as an intermediate marker to 1 mL of supernatant. Ten microliters of the obtained solution was sampled using an HPLC autosampler (SIL-10 AF, Shimadzu Corporation, Kyoto, Japan) and injected into the HPLC apparatus. The flow rate was 1.0 mL/min, and the wavelength was set to 280 nm for PB2 [45,46], 5CQA [47,48], and epicatechin [49,50].
Because it is difficult to resolve all individual peaks, a gradient method with two mobile phases [51] was used. Mobile phase A consisted of 2.5 vol.% aqueous acetic acid, and mobile phase B was acetonitrile. The gradient conditions were set as follows: 0 min A–B (97:3), 5 min A–B (91:9), 15 min A–B (84:16), 33 min A–B (64:36), 38 min A–B (0:100), 48 min A–B (97:3), and 60 min A–B (97:3).
HPLC pumps A (LC10-AD, Shimadzu Corporation, Kyoto, Japan) and B (LC20-AD, Shimadzu Corporation, Kyoto, Japan) were used. The separation column STR ODS-II (4.6 mm × 250 mm; Shinwa Chemical Industry, Kyoto, Japan) was used at a temperature of 40 °C. The HPLC detector was an SDP-M10A diode array detector (Shimadzu Corp., Kyoto, Japan).
2.3.3. Fourier Transform Infrared (FT-IR) Spectroscopy
FT-IR spectroscopy (Spectrum Two, PerkinElmer Ltd., Buckinghamshire, UK), in the wavenumber range of 400–4000 cm−1, was used to analyze Maypole apple solids and subcritical water extraction residues.
2.3.4. Total Phenolic Content (TPC) Analysis
The TPC was determined using the Folin-Ciocalteu reagent. Specifically, 1 mL of the extract was added to 1 mL of the Folin-Ciocalteu reagent in a dark room. After 3–5 min, 7 mL of distilled water and 1 mL of 7.5 wt.% sodium carbonate were added to the mixture. The solution was placed in a dark room for 3 h until all reactions were completed. The absorbance of the solution was measured at 765 nm on a single-beam UV-Vis spectrophotometer (V-550, JASCO, Tokyo, Japan) [52,53]. This procedure was performed three times for each sample, and the average absorbance was calculated.
3. Results and Discussions
3.1. Appearance of the Extracts
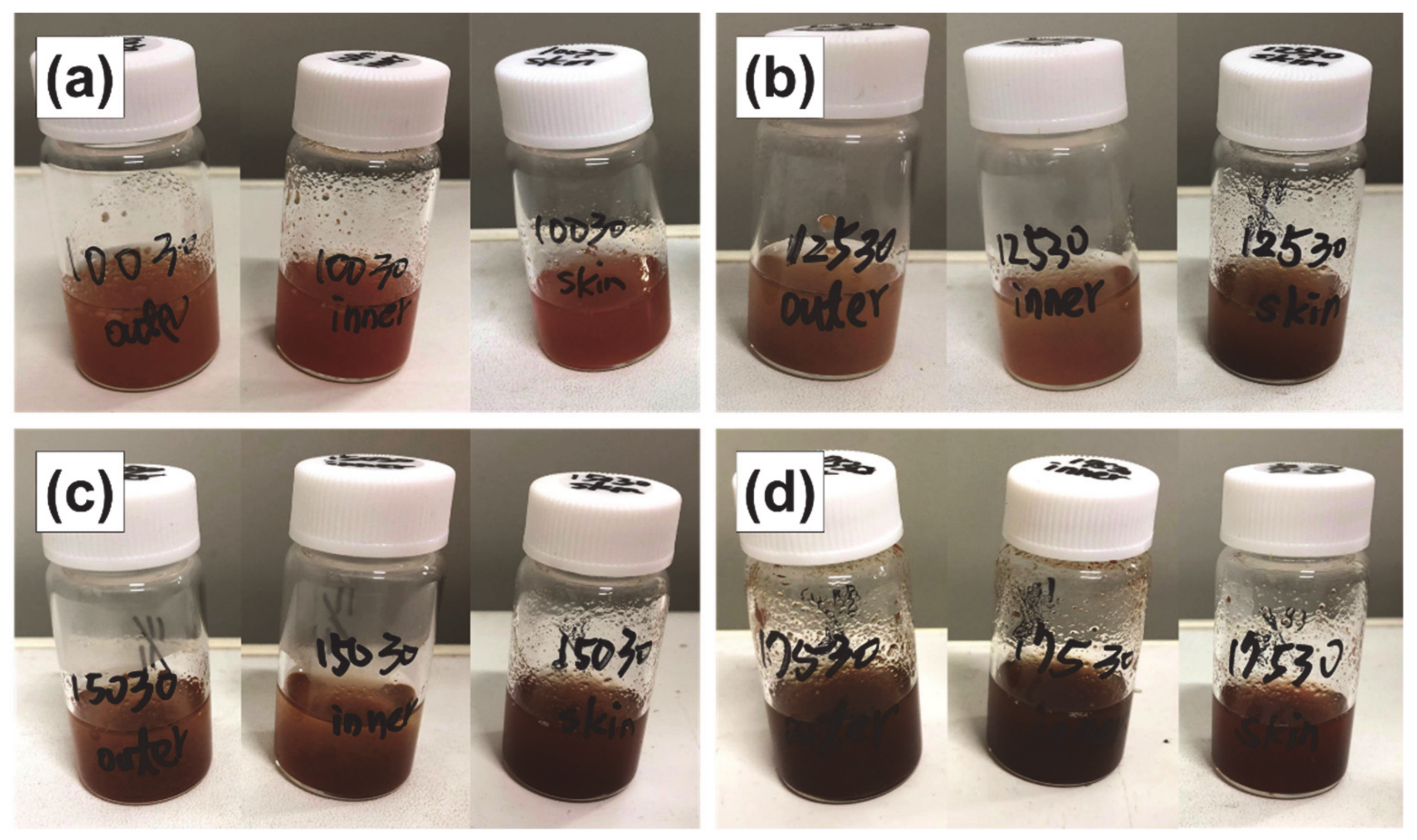
As discussed below, an extraction time of 30 min is optimal; therefore, the extract obtained under these conditions is shown. As shown in Figure 3, with the increase in extraction temperature from 100 to 175 °C, the color of the subcritical water extracts became darker; thus, the total extracted content is expected to increase with extraction temperature.
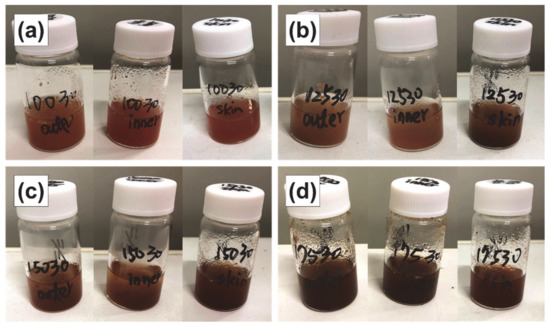
Figure 3.
Subcritical water extracts of the outer flesh, inner flesh, and peel of Maypole apples at (a) 100 °C, (b) 125 °C, (c) 150 °C, and (d) 175 °C for 30 min.
3.2. SEM and Imaging of the Residues
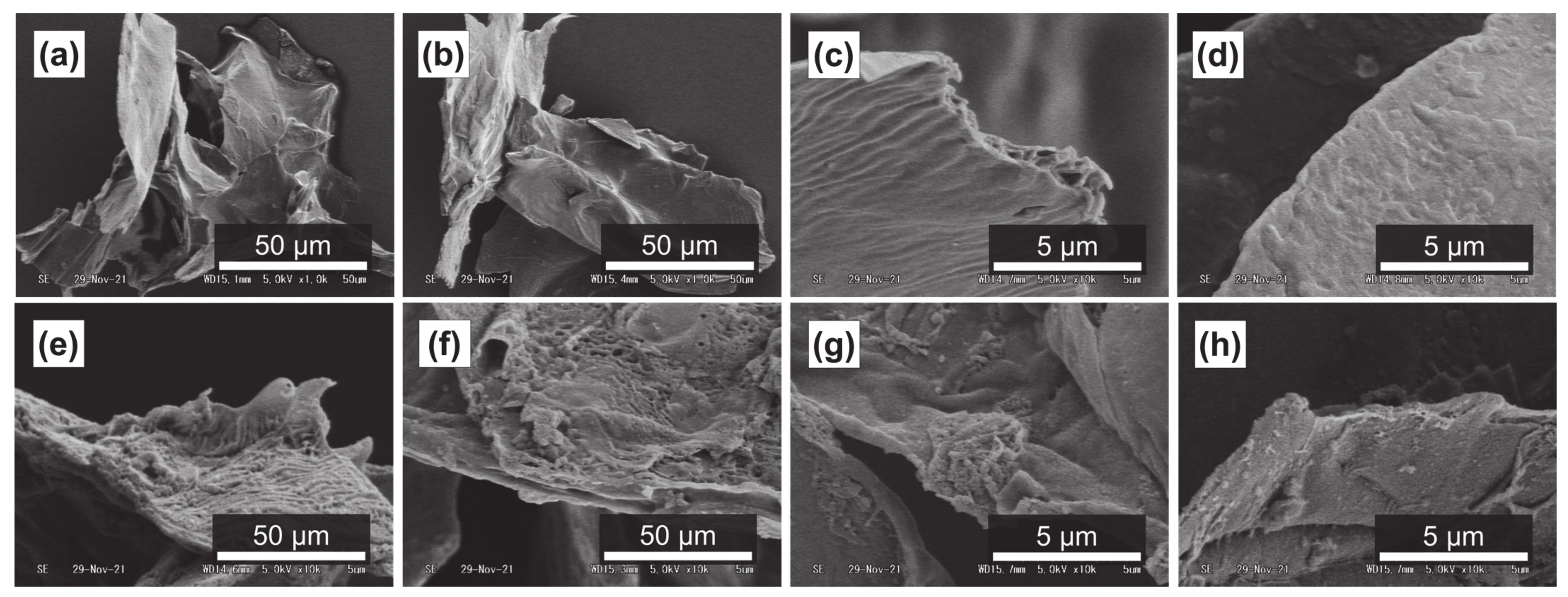
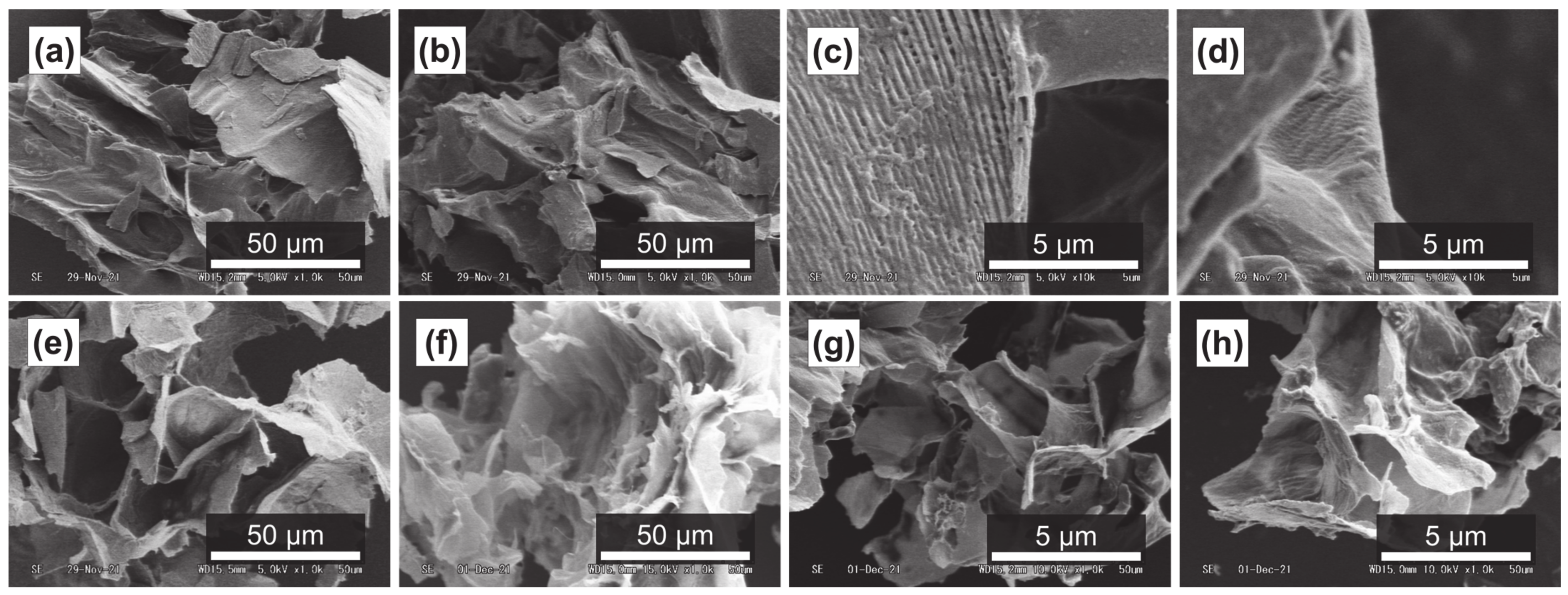
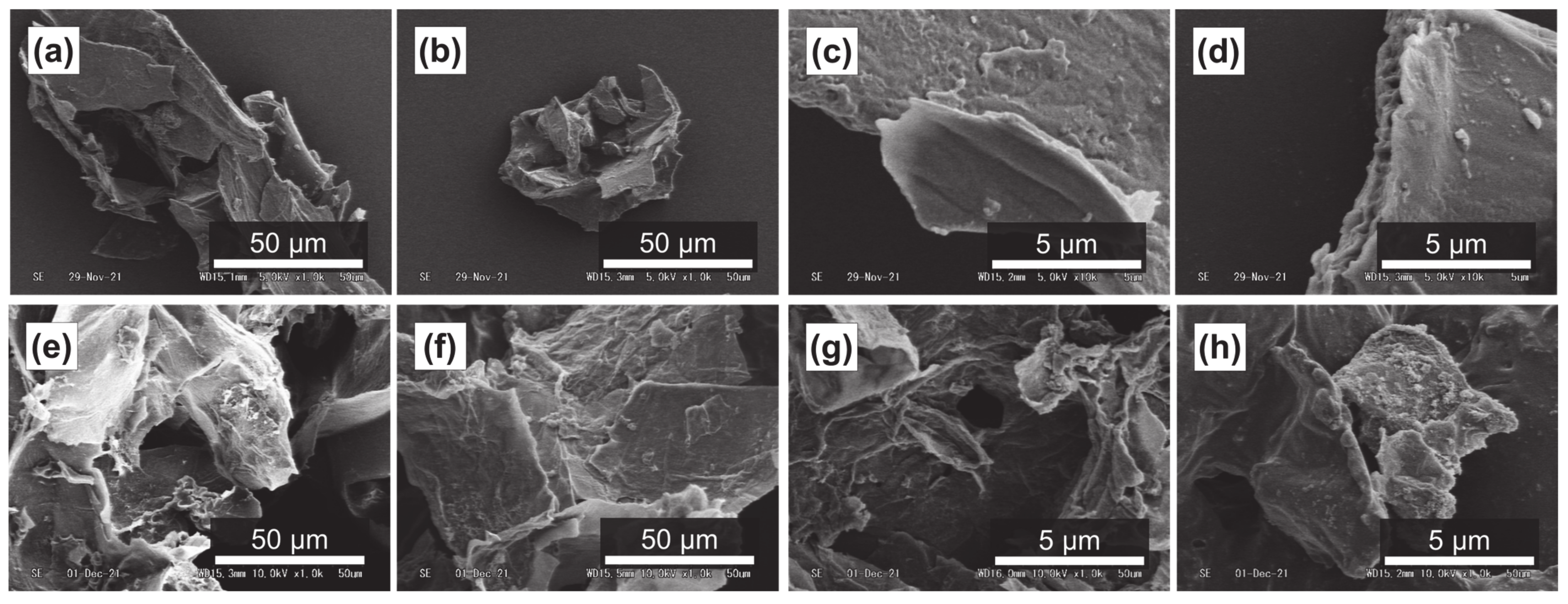
It has been reported that subcritical water can effectively decompose polysaccharides in natural products [54,55]. Figure 4a–d, Figure 5a–d and Figure 6a–d show SEM images of the outer flesh, inner flesh, and peel of Maypole apples, respectively, before subcritical water treatment. These images can be used to observe the morphology of the cell wall, which is primarily composed of multiple polysaccharides [56]. As shown in Figure 4e–h, Figure 5e–h and Figure 6e–h, the SEM images indicate that under high temperatures and pressures, subcritical water can effectively break the structure of cells and penetrate them. Subcritical water is expected to directly dissolve active ingredients inside the cells of Maypole apples.
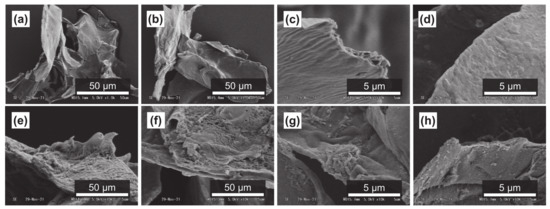
Figure 4.
SEM images of the outer flesh of Maypole apples: (a,b) raw under ×1.0k magnification; (c,d) raw under ×10.0k magnification; (e) treated with 100 °C subcritical water for 10 min; (f) treated with 150 °C subcritical water for 10 min; (g) treated with 175 °C subcritical water for 10 min; and (h) treated with 175 °C subcritical water for 20 min.
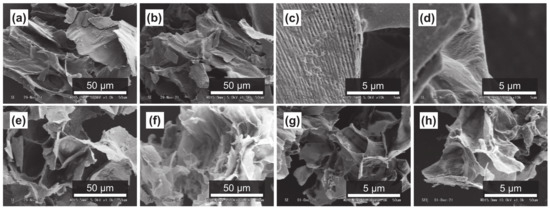
Figure 5.
SEM images of the inner flesh of Maypole apples: (a,b) raw under ×1.0k magnification; (c,d) raw under ×10.0k magnification; (e) treated with 100 °C subcritical water for 10 min; (f) treated with 100 °C subcritical water for 30 min; (g) treated with 175 °C subcritical water for 10 min; and (h) treated with 175 °C subcritical water for 30 min.
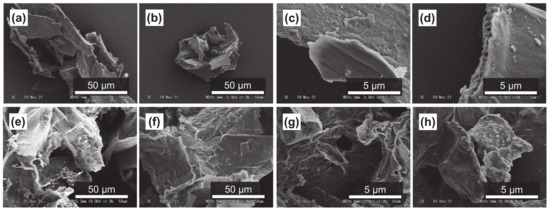
Figure 6.
SME images of the peel of Maypole apples: (a,b) raw under ×1.0k magnification; (c,d) raw under ×10.0k magnification; (e) treated with 100 °C subcritical water for 10 min; (f) treated with 100 °C subcritical water for 30 min; (g) treated with 175 °C subcritical water for 10 min; and (h) treated with 175 °C subcritical water for 30 min.
3.3. HPLC Results
HPLC showed the characteristic peaks of PB2, 5CQA, and epicatechin. We obtained PB2, 5CQA, and epicatechin yields by calculating the peak areas under the calibration curves of standard samples. After calculating the weight of the powder used, the specific yield was calculated using Equation (1).
3.3.1. Yield of PB2
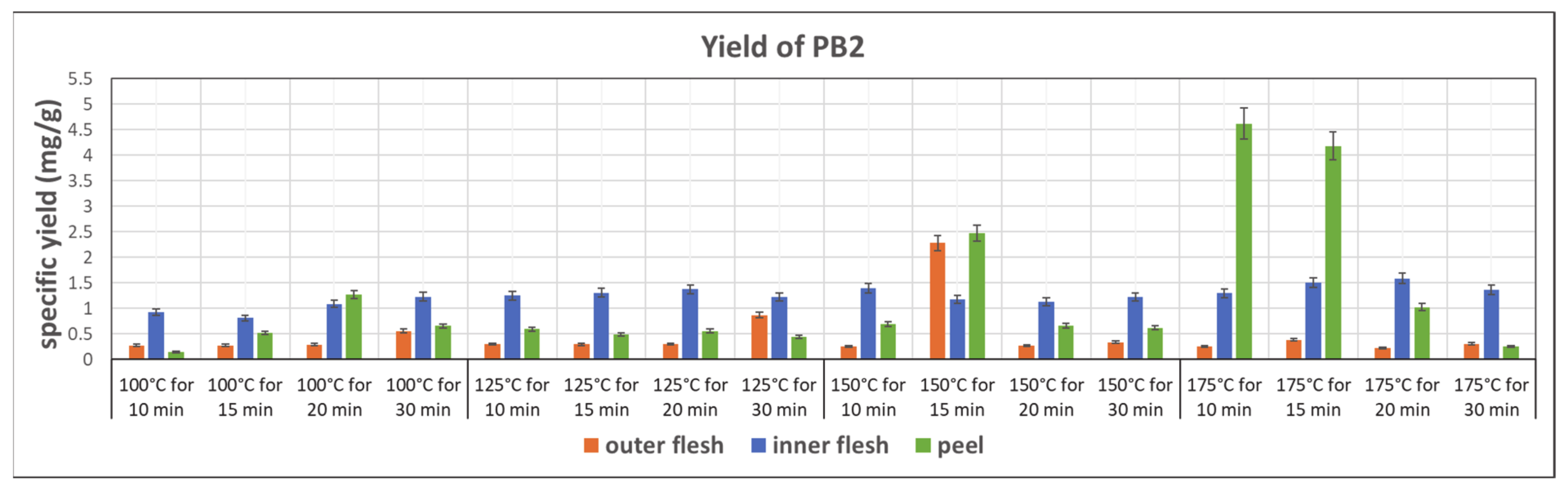
Figure 7 shows the specific yield of PB2 in subcritical water extracts from the outer flesh, inner flesh, and peel, under different conditions. The peel of Maypole apples yielded the maximum amount of PB2. In addition, the yield of PB2 is strongly related to the extraction temperature. Generally, higher temperatures result in higher PB2 contents. The highest PB2 content (4.167 mg/g) can be observed in the extract obtained at 175 °C and 10 min from the peel. Because of the thick epidermal cell walls, subcritical water at the highest tested temperature (175 °C) and high pressure broke down tissue fibers and extracted PB2 the most effectively. However, the results also show that bioactive PB2 easily decomposes at 175 °C. Therefore, subcritical water extraction of PB2 should be accomplished within 10–15 min.
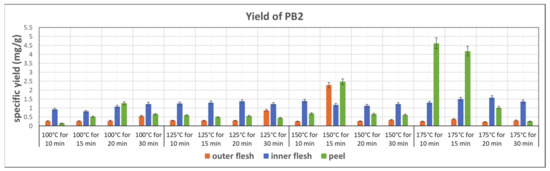
Figure 7.
Specific yield of the subcritical extraction of PB2 from the outer flesh, inner flesh, and peel of Maypole apples at different temperatures and extraction times.
3.3.2. Yield of 5CQA
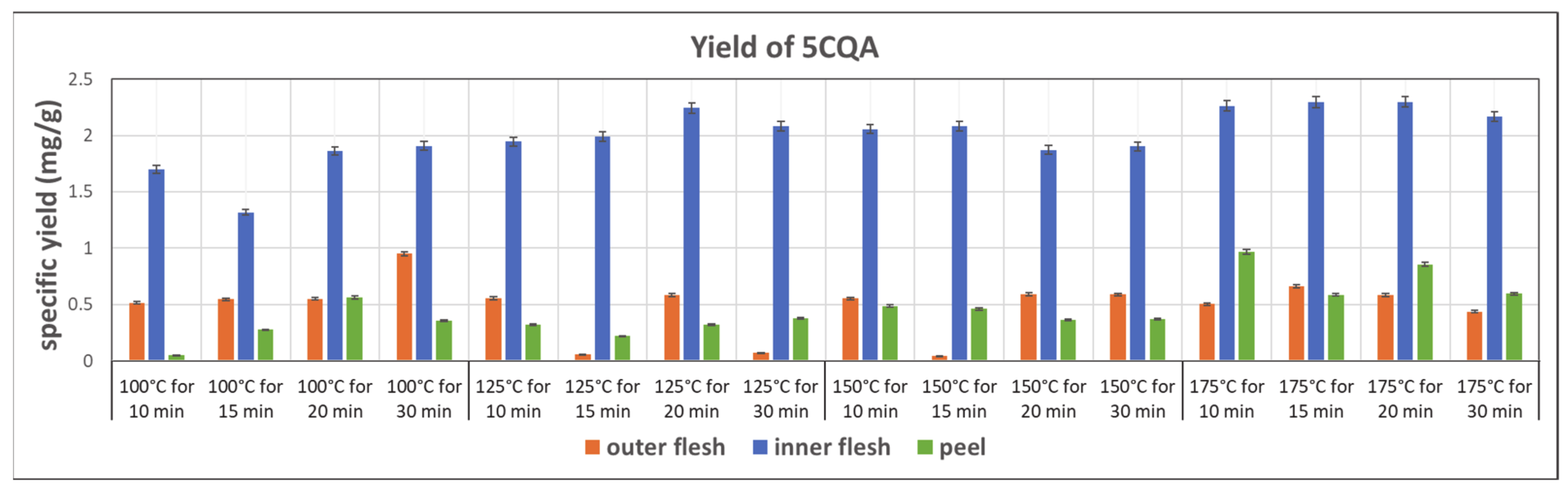
The yields of 5CQA in the subcritical water extraction from the outer flesh, inner flesh, and peel are shown in Figure 8. In general, compared to other parts of Maypole apples, the inner flesh yields more than three times more 5CQA in the extraction with subcritical water. In addition, 5CQA did not significantly decompose at 175 °C within 30 min. The highest 5CQA content (2.296 mg/g) can be observed in the extract from the inner flesh obtained at 175 °C and 20 min.
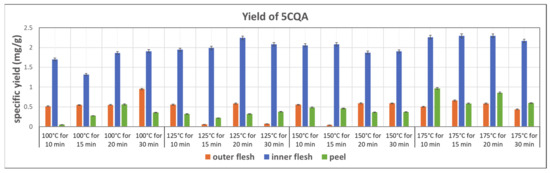
Figure 8.
Specific yield of the subcritical extraction of 5CQA from the outer flesh, inner flesh, and peel of Maypole apples at different temperatures and extraction times.
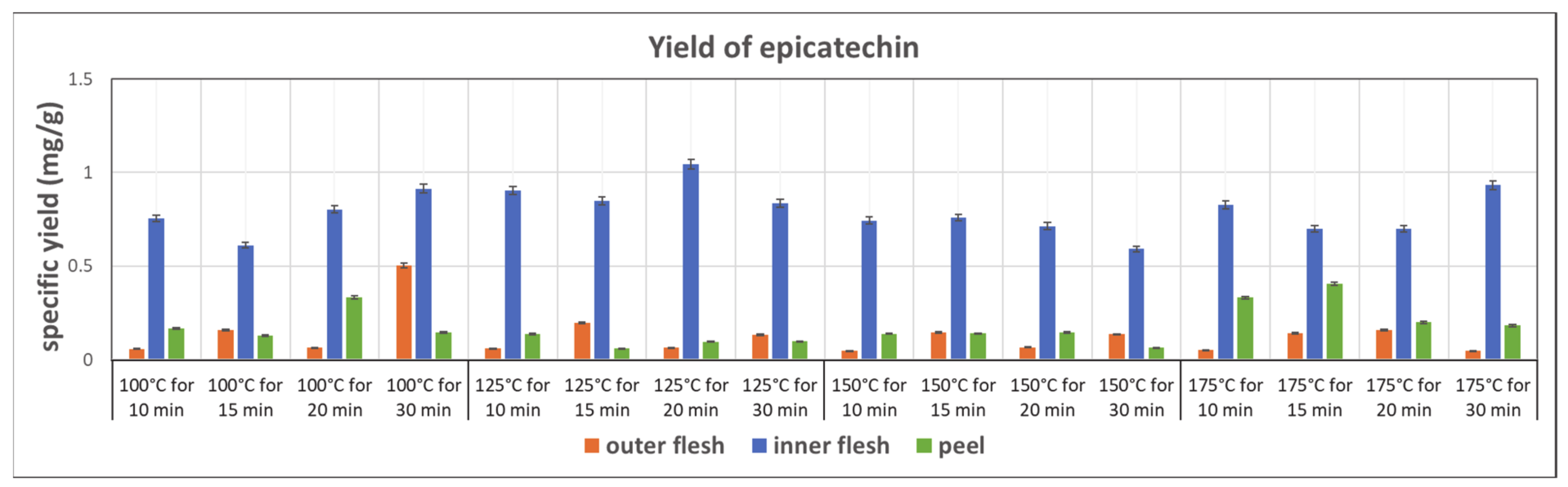
3.3.3. Yield of Epicatechin
Figure 9 shows the yields of epicatechin obtained in the subcritical water extraction of the outer flesh, inner flesh, and peel, under different conditions. The inner flesh of Maypole apples yielded remarkably high epicatechin contents, with the highest extraction yield of 1.044 mg/g obtained at 125 °C and 20 min. The yield of epicatechin in the inner flesh was double that in the outer flesh and peel. Therefore, different parts of the Maypole apple should be used for extracting different phytochemical compounds.
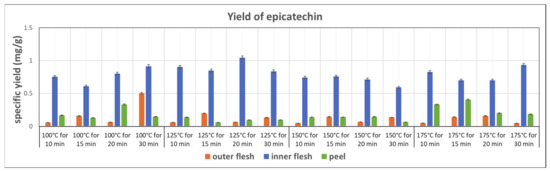
Figure 9.
Specific yield of the subcritical extraction of epicatechin from the outer flesh, inner flesh, and peel of Maypole apples at different temperatures and extraction times.
3.3.4. Analysis of Variance (ANOVA)
This work utilizes ANOVA to evaluate the correlation between the extraction rate of phytochemicals in Maypole apples and subcritical water extraction conditions. Temperature (100–175 °C) and extraction time (10–30 min) were selected as two independent factors of subcritical water extraction. From the data shown in Figure 7, Figure 8 and Figure 9, the highest extract rate of the three types of phytochemicals from Maypole apples were observed, which were PB2 from peel extracts and 5CQA and epicatechin from the inner flesh extracts, respectively.
Table 1 presents the ANOVA of these three sets of data. The F-test result indicates that the subcritical water extraction affecting factors of PB2, 5CQA, and epicatechin are temperature (°C) > Time (min). In addition, the lower p-value indicates that temperature has a more significant predictability in extraction rate than extraction time.

Table 1.
ANOVA for experimental parameters.
Additionally, this work found the predictive quadratic models for predicting yields of PB2 in peel ((Equation (2), p-value = 0.0074, <0.05) and 5CQA in inner flesh ((Equation (3), p-value = 0.0456, <0.05) were statistically significant. In these models, f(x, y) is the specific yield of the extracted liquid (mg/mL), x is the subcritical water temperature (°C), and y is the reaction time (min).
In addition, this work also demonstrates other significant quadratic regression equations, including the specific yield of 5CQA from the peel ((Equation (4), p-value = 0.0038, <0.05), and the specific yield of epicatechin from the peel ((Equation (5), p-value = 0.0456, <0.05). In these equations, f(x, y) is the specific yield of the extracted liquid (mg/mL), x is the subcritical water temperature (°C), and y is the reaction time (min).
Therefore, it is considered that different phytochemicals can be obtained from different parts of Maypole apples. In addition, the temperature (°C) of the subcritical water is expected to be a more important factor than the extraction time (min).
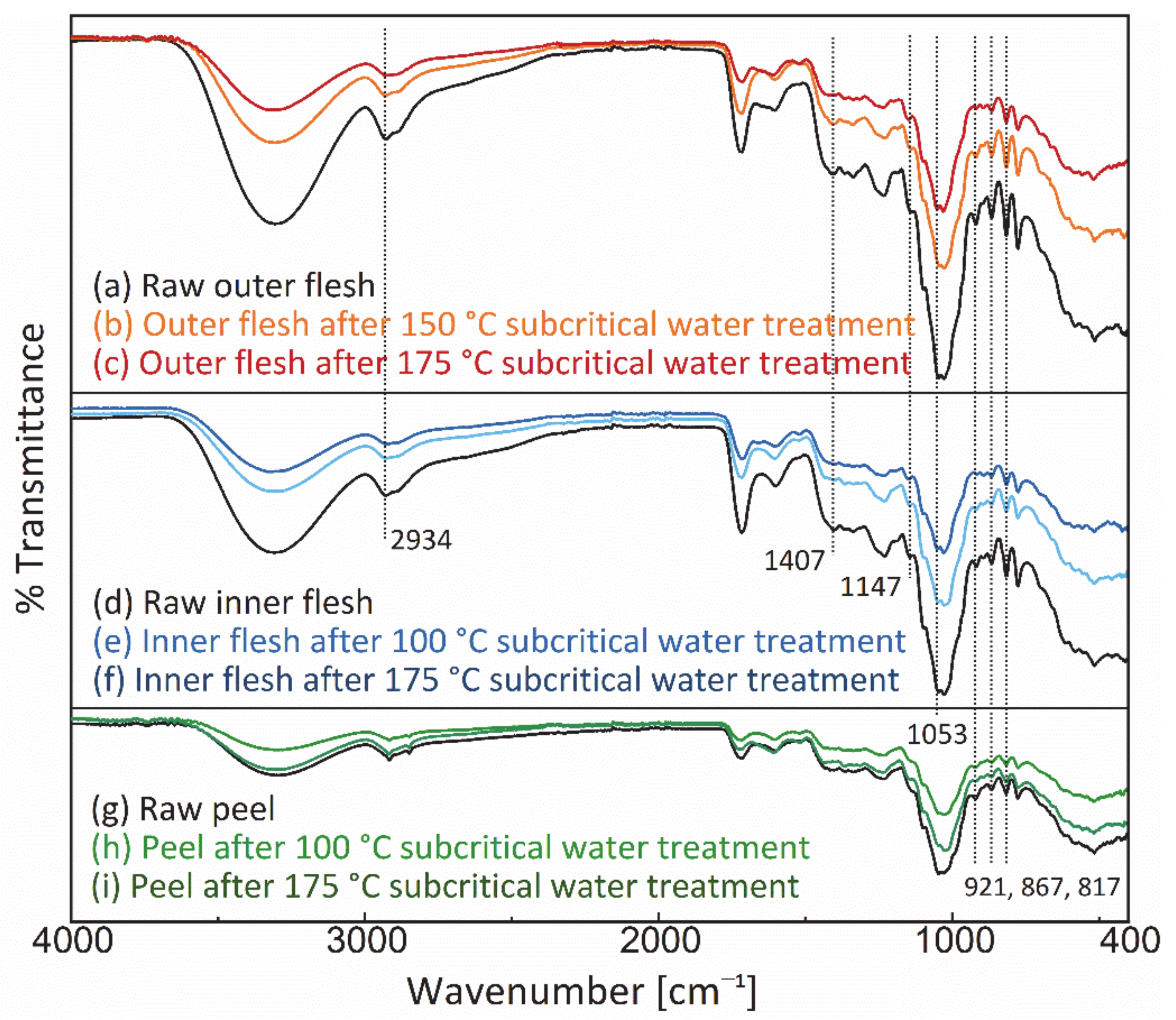
3.4. Analysis of FT-IR Spectra
Figure 10 shows the FT-IR spectra of the Maypole apple outer flesh, inner flesh, and peel before and after subcritical water treatment at 175 °C. In the FT-IR spectra of the outer and inner flesh, the moderate intensity band at 2934 cm−1, the strong band at 1147 cm−1, and the highest intensity band at 1053 cm−1 are characteristic bands of glucose; they correspond to C–H asymmetric stretching, C–O stretching, and C–O, C–C–C asymmetric tensile vibration [57]. In addition, the moderately intense band at 1407 cm−1 was attributed to the deformation vibrations of O-C–H, C–O–H, and C–C–H in fructose [58]. In addition, the moderately intense bands at 921, 867, and 817 cm−1 were assigned to the C–H deformation vibrations of fructose and the C–H stretching and CH3 rocking vibrations of pectin (a polysaccharide), respectively [59].
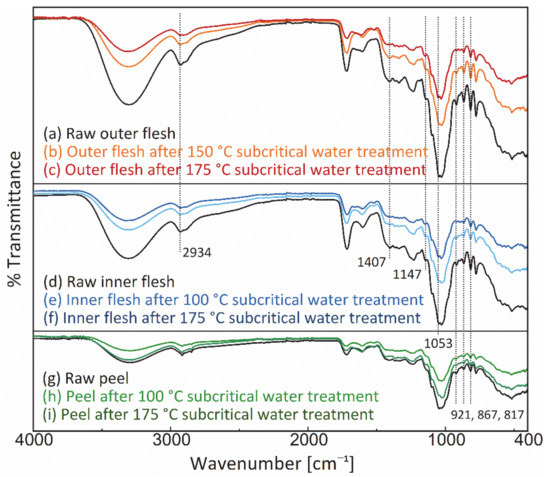
Figure 10.
FT-IR spectrum of the outer flesh, inner flesh, and peel of Maypole apples.
In the present study, no new characteristic peaks were observed in the FT-IR spectra of different parts of Maypole apples, thus suggesting that subcritical water extraction at 175 °C did not generate new significantly observable or potentially toxic chemicals. Additionally, the peak intensities of the extraction residue were lower than those of the respective raw materials, indicating that subcritical water can extract the characteristic phytochemical components of Maypole apples.
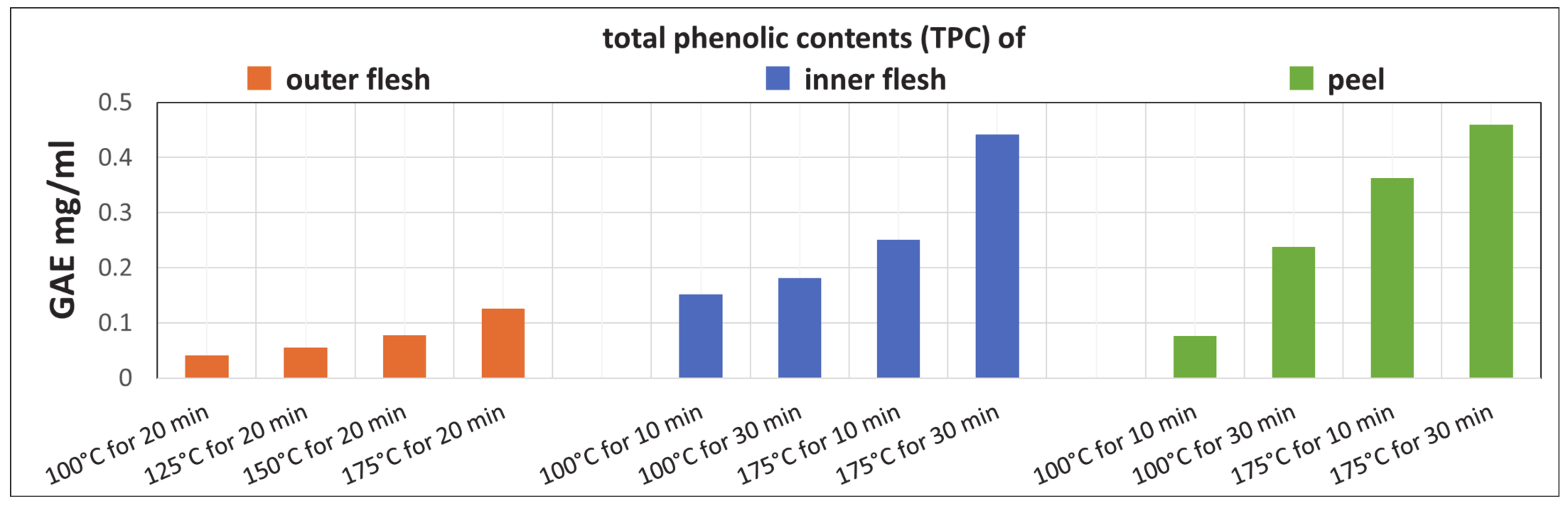
3.5. TPC Analysis
Figure 11 shows that the TPCs of the Maypole apple outer flesh, inner flesh, and peel extracts increase with the extraction temperature and time. In addition, the TPCs of the raw inner flesh and peel (which have a dark red color) are significantly higher than that of the outer flesh (which is lighter in color).
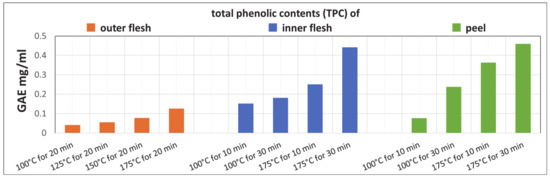
Figure 11.
TPC after subcritical extraction of the outer flesh, inner flesh, and peel of Maypole apples at different temperatures and extraction times.
The results indicate that extraction from Maypole apples with subcritical water at 175 °C for 30 min yields high TPC. In addition, in subcritical water environments up to 175 °C, high-molecular-weight polyphenols are expected to decompose, forming other compounds (phenolic, etc.) that become extractable.
4. Conclusions
In this study, subcritical water was successfully used to extract phytochemicals, including PB2, 5CQA, and epicatechin, from various parts of Maypole apples. SEM images showed that under high temperatures and pressures, subcritical water could effectively break the structure of cells and penetrate into the Maypole apple cells. The results of HPLC showed that the phytochemical components of various parts of the apple could be extracted from subcritical water under different conditions. Particularly, the content of PB2 was the highest (4.167 mg/g) in the extract from the peel obtained at 175 °C and 10 min extraction time. The 5CQA content was the highest (2.296 mg/g) in the extract from the inner flesh obtained at 175 °C and 20 min. The epicatechin content was the highest (1.044 mg/g) in the extract from the inner flesh obtained at 125 °C and 20 min. The concentration of phenolic compounds in the extract was the highest at 175 °C and 20–30 min. Moreover, the ANOVA results showed that the temperature of the subcritical water extraction is expected to be a more critical factor than the extraction time.
According to the results of this study, subcritical water can effectively extract phytochemicals from Maypole apples. Subcritical water extraction does not require the use of organic solvents, and is environmentally friendly. This work further provides more experimental data of the extraction of phytochemicals using subcritical water.
Author Contributions
Conceptualization, M.T., L.Z. and W.D.; data curation, M.T., L.Z., S.M., T.W. and W.D.; investigation, M.T., L.Z., S.M., W.D. and H.K.; methodology, M.T., L.Z. and W.D.; project administration, M.G. and H.K.; resources, M.G. and H.K.; supervision, M.G. and H.K.; visualization, M.T., L.Z., S.M. and W.D.; writing—original draft, M.T., L.Z., W.D. and H.K.; writing—review and editing, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kwon, S.-I.; Yoo, J.; Lee, J.; Moon, Y.-S.; Choi, C.; Jung, H.Y.; Lee, D.H.; Kim, C.K.; Kang, I.-K. Evaluation of crab apples for apple production in high-density apple orchards. J. Plant Biotechnol. 2015, 42, 271–276. [Google Scholar] [CrossRef]
- He, X.; Liu, R.H. Phytochemicals of Apple Peels: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2008, 56, 9905–9910. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Hollman, P.; Katan, M. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517–520. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, P.; Yu, F.; Zhang, Z.; Cai, Q.; Lu, W.; Li, B.; Qin, W.; Cheng, M.; Wang, H. Grape seed procyanidin B2 ameliorates hepatic lipid metabolism disorders in db/db mice. Mol. Med. Rep. 2017, 16, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. Cell. Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, T.; Yang, S.; Li, X.; Song, B.; Wang, Y.; Lin, Q.; Cao, J. Analysis of phenolic components and related biological activities of 35 apple (Malus pumila Mill.) cultivars. Molecules 2020, 25, 4153. [Google Scholar] [CrossRef]
- Ito, H.; Kobayashi, E.; Li, S.-H.; Hatano, T.; Sugita, D.; Kubo, N.; Shimura, S.; Itoh, Y.; Tokuda, H.; Nishino, H. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002, 50, 2400–2403. [Google Scholar] [CrossRef]
- Sakano, K.; Mizutani, M.; Murata, M.; Oikawa, S.; Hiraku, Y.; Kawanishi, S. Procyanidin B2 has anti- and pro-oxidant effects on metal-mediated DNA damage. Free. Radic. Biol. Med. 2005, 39, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, B.; Li, X.; Yu, F.; Cai, Q.; Zhang, Z.; Cheng, M.; Gao, H. Anti-inflammatory effects of grape seed procyanidin B2 on a diabetic pancreas. Food Funct. 2015, 6, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Takahashi, T.; Watanabe, Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine 2000, 7, 529–536. [Google Scholar] [CrossRef]
- Park, J.B. 5-Caffeoylquinic acid and caffeic acid orally administered suppress P-selectin expression on mouse platelets. J. Nutr. Biochem. 2009, 20, 800–805. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J. Agric. Food Chem. 2006, 54, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-O-Caffeoylquinic acid: A spectroscopic study and biological screening for antimicrobial activity. LWT Food Sci. Technol. 2016, 65, 471–479. [Google Scholar] [CrossRef]
- Bonita, J.S.; Mandarano, M.; Shuta, D.; Vinson, J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol. Res. 2007, 55, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Makino, H. Production of decaffeinated green tea leaves using liquefied dimethyl ether. Food Bioprod. Process. 2013, 91, 376–380. [Google Scholar] [CrossRef]
- Taylor, R. Insulin resistance and type 2 diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.; Alañón, M. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Okiyama, D.C.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E. Pressurized liquid extraction of flavanols and alkaloids from cocoa bean shell using ethanol as solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef]
- Trugo, L.C.; Macrae, R. Chlorogenic acid composition of instant coffees. Analyst 1984, 109, 263–266. [Google Scholar] [CrossRef]
- Palma, M.; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [Google Scholar] [CrossRef]
- GBD 2016 Alcohol Collaborators, Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [CrossRef]
- Ding, X.; Sheehan, J.D.; Zhang, T.; Shen, Z.; Wang, Y.; Jiang, Z.; Fang, T. Experimental-computational approach for elucidating the dissolution behavior of potassium phosphates in near- and supercritical water. J. Supercrit. Fluids 2022, 181, 105488. [Google Scholar] [CrossRef]
- Kritzer, P. Corrosion in high-temperature and supercritical water and aqueous solutions: A review. J. Supercrit. Fluids 2004, 29, 1–29. [Google Scholar] [CrossRef]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of protein and amino acids from deoiled rice bran by subcritical water hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Weingärtner, H.; Franck, E.U. Supercritical water as a solvent. Angew. Chem. Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lei, Y.; Shen, Z.; Yu, Y.; Zhou, Q.; Wei, J.; Fang, T. Experimental Determination and Modeling of the Solubility of Sodium Chloride in Subcritical Water from (568 to 598) K and (10 to 25) MPa. J. Chem. Eng. Data 2017, 62, 3374–3390. [Google Scholar] [CrossRef]
- Machmudah, S.; Lestari, S.D.; Widiyastuti; Wahyudiono; Kanda, H.; Winardi, S.; Goto, M. Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. J. Supercrit. Fluids 2018, 133, 615–624. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Ruenngam, D.; Quitain, A.T.; Tanaka, M.; Sasaki, M.; Goto, M. Reaction kinetics of hydrothermal hydrolysis of hesperidin into more valuable compounds under supercritical carbon dioxide conditions. J. Supercrit. Fluids 2012, 66, 215–220. [Google Scholar] [CrossRef]
- Chhouk, K.; Wahyudiono; Kanda, H.; Goto, M. Efficacy of supercritical carbon dioxide integrated hydrothermal extraction of Khmer medicinal plants with potential pharmaceutical activity. J. Environ. Chem. Eng. 2018, 6, 2944–2956. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Hydrolysis of biopolymers in near-critical and subcritical water. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–107. [Google Scholar]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono; Kanda, H.; Sasaki, M.; Goto, M. Hot compressed water extraction of lignin by using a flow-through reactor. Eng. J. 2015, 19, 25–44. [Google Scholar] [CrossRef]
- Kodama, S.; Shoda, T.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Extraction of β–glucan by hydrothermal liquidization of barley grain in a semi-batch reactor. Sep. Sci. Technol. 2016, 51, 278–289. [Google Scholar] [CrossRef]
- Kodama, S.; Shoda, T.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Enhancing pressurized water extraction of β-glucan from barley grain by adding CO2 under hydrothermal conditions. Chem. Eng. Process. Process Intensif. 2015, 97, 45–54. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Armenta-Vázquez, M.E.; Castro-Arredondo, S.I.; Espinosa-Plascencia, A.; del Refugio Robles-Burgueño, M.; González-Ríos, H.; González-León, A.; Balandrán-Quintana, R.R. Potential of polyphenols from an aqueous extract of apple peel as inhibitors of free radicals: An experimental and computational study. J. Mol. Struct. 2013, 1035, 61–68. [Google Scholar] [CrossRef]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Products Formed in Model Wine Solutions Involving Anthocyanins, Procyanidin B2, and Acetaldehyde. J. Agric. Food Chem. 1996, 44, 2402–2407. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Wong, S.K.; Lim, Y.Y.; Ling, S.K.; Chan, E.W.C. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacogn. Res. 2014, 6, 67. [Google Scholar]
- Chafer, A.; Pascual-Martí, M.C.; Salvador, A.; Berna, A. Supercritical fluid extraction and HPLC determination of relevant polyphenolic compounds in grape skin. J. Sep. Sci. 2005, 28, 2050–2056. [Google Scholar] [CrossRef]
- Gottumukkala, R.V.S.S.; Nadimpalli, N.; Sukala, K.; Subbaraju, G.V. Determination of Catechin and Epicatechin Content in Chocolates by High-Performance Liquid Chromatography. Int. Sch. Res. Not. 2014, 2014, 628196. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Wang, C.; Sun, W.; Zhang, Z.; Cheng, W. A gradient HPLC method for the quality control of chlorogenic acid, linarin and luteolin in Flos Chrysanthemi Indici suppository. J. Pharm. Biomed. Anal. 2007, 43, 753–757. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Möller , M.; Nilges , P.; Harnisch, F.; Schröder, U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Machmudah, S.; Wahyudiono, K.H.; Sasaki, M.; Goto, M. Subcritical water extraction and direct formation of microparticulate polysaccharide powders from Ganoderma lucidum. Int. J. Technol. 2014, 1, 40–50. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring Polysaccharide Dynamics in the Plant Cell Wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; Leon, A.d. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Euimica 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Mohamed, G.F.; Shaheen, M.S.; Khalil, S.K.; Hussein, A.; Kamil, M.M. Application of FT-IR spectroscopy for rapid and simultaneous quality determination of some fruit products. Nat. Sci. 2011, 9, 21–31. [Google Scholar]
- Hazarika, S.; Hebb, R.L.; Rizvi, S.S.H. Prediction of Ripening Stage of Cameo Apple Using Fourier-Transform Infrared Spectroscopy. Int. J. Fruit Sci. 2018, 18, 188–198. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).