Abstract
The application of stilbenes in the food industry is being considered because of their biological activities. Piceatannol, pterostilbene and ε-viniferin have awakened the industry’s interest. However, before they can be commercialized, we must first guarantee their safety for consumers. The present work reviews the toxicological studies performed with these stilbenes. A wide variety of studies has demonstrated their cytotoxic effects in both cancer and non-cancerous cell lines. In contrast, although DNA damage was detected by some authors, in vitro genotoxic studies on the effects of piceatannol, pterostilbene, and ε-viniferin remain scarce. None of the three reviewed substances have been evaluated using the in vitro tests required by the European Food Safety Authority (EFSA) as the first step in genotoxicity testing. We did not find any study on the toxic effects of these stilbenes in vivo. Thus, more studies are needed to confirm their safe use before they can be authorized as additive in the food industry.
1. Introduction
During the last decades, the interest in polyphenolic phytochemicals has increased markedly due to their beneficial properties [1]. Natural polyphenols are abundant in fruits, vegetables, whole grains, and foods and beverages derived from them such as chocolate, wine, olive oil, or tea; thus making it the most important phytochemical present in the human diet [2]. These compounds are highly diversified and comprise several subgroups of phenolic compounds ranging from simple substances, including phenolic acids and stilbenes, to complex polymerized molecules, such as tannins [3].
Natural stilbenes are secondary metabolites produced by plants to protect themselves against stressful conditions such as ultraviolet irradiation, excessive heat and fungal or bacterial infections [2]. Structurally, stilbenes are characterized by the presence of a 1, 2-diphenylethylene nucleus [4] and they can be found in E, or trans, and Z, or cis configurations, the trans form being the one that exhibits more potent pharmacological activities [5,6]. Moreover, these compounds exist as monomers, such as resveratrol, piceatannol, or pterostilbene, and oligomers, like ε-viniferin [1] (Figure 1).
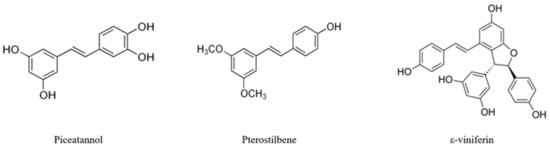
Figure 1.
Chemical structures of piceatannol, pterostilbene, and ε-viniferin.
There are more than 400 natural stilbenes reported, however, they are only distributed in a small and heterogeneous group of plants such as wine grape (Vitis vinifera), peanut (Arachis hypogaea), and some tree species (Pinus and Picea genera) because stilbene synthase, the key enzyme involved in stilbene biosynthesis, is not ubiquitously expressed [4]. In general, the highest amount of stilbenes is found in grapes and wine derivatives. However, data related with the available concentrations of these compounds from different sources is very scarce because it depends on the variety of grapes, agricultural and environmental factors (soil, temperature, pathogen attack) and the complexity of the qualitative and quantitative analysis of different stilbenes [7,8]. Moreover, residues produced during wine making such as grape pomaces and other grape juice solids contain high polyphenol concentrations and are important sources of many stilbene compounds, which is interesting because sustainability in food production has become an area of utmost importance [9].
These compounds have been widely used in the manufacture of industrial dyes, laser dyes, optical brighteners, phosphors, and scintillators [5]. However, in recent years, stilbenes and their analogues have awakened the interest of the scientific community due to their diverse spectrum of biological applications such as anticarcinogenic, antiproliferative, antiangiogenic, antimicrobial, antileukemic, anti-inflammatory, antioxidant, antimutagenic, and antigenotoxic agents, and as a vasodilator [2,10,11], among others [6]. Furthermore, numerous studies have indicated a positive effect of these compounds against diseases related to oxidative stress including cancer, cardiovascular, and autoimmune diseases [12], aging [13] and neurodegenerative pathologies [1]. These preventive effects of stilbenes are mainly due to their antioxidant activity by scavenging free radicals, but recent lines of evidence suggest that they can also interact directly with multiple intracellular signaling cascades involved in the development of numerous pathologies [2]. Moreover, the use of stilbenes as natural preservatives has recently become an area of growing interest because synthetic additives are increasingly rejected by consumers, who now give preference to ingredients from natural sources [14].
These new applications of stilbenes in the food industry have caused some concern regarding their safety for consumers since the intake of these stilbenes may increase. In this sense, the estimate human consumption of stilbenes depends on many factors such as the type of diet and food processing, leading to a large variability of the exposure scenario [7,8]. Then, a toxicological evaluation is required by the European Food Safety Authority (EFSA) prior to their commercial use. The first approach to determining the toxicity effects of any compound should be the use of in vitro cytotoxicity tests to define basal cytotoxicity, which is directly related to cell death induction. Following the EFSA’s Panel on Food Additives and Nutrient Sources added to Food (2012) guidelines, a step-wise approach is recommended for the evaluation of data on the genotoxic potential of these compounds, starting with a basic battery of two in vitro tests, comprised of the bacterial reverse-mutation assay (Ames test, OECD 471) and the micronucleus test (OECD 487). In the case of inconclusive, contradictory, or equivocal results, it may be appropriate to conduct further in vitro testing [15]. Additional in vivo studies are also needed before its commercialization. These studies include genotoxicity, toxicity (subchronic, chronic, and carcinogenicity), reproductive, and developmental toxicity testing, etc. [15,16]. Therefore, besides their well-known beneficial effects, stilbenes may also exhibit toxic effects. The toxicity of trans-resveratrol, the most extensively studied stilbene, has been evaluated by other authors [17,18]. This stilbene has been categorized as GRAS (Generally Recognized as Safe) by the US Food and Drug Administration (FDA) [19]. In addition, trans-resveratrol with ≥99% (w/w) purity has obtained EFSA approval as a novel food [16]. In this sense, because of its safe status, properties, and consumer acceptance, some resveratrol derivatives such as piceatannol, pterostilbene, and ε-viniferin have recently piqued the interest of industries [20]. However, very few reports have analyzed the toxicity of these derivatives. In this regard, the aim of the present work was to review and provide a compilation of the scientific publications focused on in vitro and in vivo toxicological studies of piceatannol, pterostilbene, and ε-viniferin carried out to date.
2. Cytotoxicity in In Vitro Studies Performed with Stilbenes
Cytotoxicity studies are the first approach in defining the toxic effects of any compound since they are simple, fast, and have a high sensitivity. These assays define the basal toxicity related to cell induction and are a first step in evaluating the safety of the tested molecules [21]. In this regard, the results of the cytotoxic and morphological studies carried out thus far in piceatannol, pterostilbene, and ε-viniferin are shown in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6. It is interesting to point out that, although stilbenes have been used in traditional medicine since ancient times [22], most of the studies concerning the cytotoxicity of these stilbenes have been published recently, between the years 2001 and 2020.

Table 1.
In vitro cytotoxicity studies performed with piceatannol.

Table 2.
In vitro morphological studies performed with piceatannol.

Table 3.
In vitro cytotoxicity studies performed with pterostilbene.

Table 4.
In vitro morphological studies performed with pterostilbene.

Table 5.
In vitro cytotoxicity studies performed with trans-ε- viniferin.

Table 6.
In vitro morphological studies performed with ε-viniferin.
The most frequently used biomarker to assess the cytotoxic effects of these stilbenes is the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. It measures the mitochondrial metabolic rate and indirectly reflects the viable cell number [23]. This is one of the most popular techniques for screening the effects of compounds on cultured cells. However, some stilbenes exhibit MTT-reducing activity which can lead to inaccurate readings [23]. In this sense, several authors have used alternative biomarkers of cell viability such as the trypan blue dye exclusion test (TBET), cell counting kits (CCK), water soluble tetrazolium salt-1 (WST-1), Sulforhodamine B (SRB) assay, neutral red uptake (NRU), lactate dehydrogenase (LDH) activity, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8) assay, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay or automated cell counting (CC108).
Additionally, it seems that some polyphenols induce cytotoxicity in a cell type-selective manner [24]. In relation to the experimental models selected, it should be emphasized that most experiments have been performed in human cancer cell lines. This is because their main purpose was to assess the potential of these compounds as anticancer agents, since these stilbenes can modulate cellular oxidative stress levels and induce DNA damage. Moreover, these compounds, in combination with chemotherapeutics, can have chemoprotective and synergistic effects [25,26], which is of great interest for potential therapeutic uses. However, although stilbenes are not known to exhibit toxicity toward normal cell lines [27], cytotoxic effects have also been recorded after piceatannol, pterostilbene, and ε-viniferin exposure in non-cancer cell lines. The studies performed on these cells are far fewer and the results differ depending on the stilbene tested, cell lines used, assay performed, and exposure conditions. In this sense, it is also important to evaluate the effects of these stilbenes in normal cell lines to assert their safety before they can be used for industrial use. In general, although high concentrations were required to obtain an IC50 value up to 400 µM, a decrease in cell proliferation was recorded in a time- and dose-dependent manner. This effect was even observed at lower concentrations such as 30 µM for piceatannol [28], 40 µM for pterostilbene [29], and 20 µM for ε-viniferin [30]. These findings are relevant since non-cancerous cells are usually more sensitive, and the results could be easily extrapolated to human systems [31].
The cytotoxic effects of piceatannol are described in (Table 1). Lymphoma cells such as HL-60 cells [24,32,33,34,35,36], L1210 cells [35,37], or K562 cells [35,36,38] have been the most extensively used to study this stilbene, followed by melanoma cells [39,40,41,42], and colon [37,43,44], prostate [45], or liver [24] cancer cell lines. Contradictory cytotoxic results have been obtained since different methods and cell lines have been used. In general, most of the authors stated that piceatannol has cytotoxic effects in a dose- and time-dependent manner in cancer cells lines at concentrations between 20–100 µM after 24 and 48 h of exposure. Moreover, cytotoxic effects have also been reported in non-cancerous cells treated with piceatannol [28,32,46]. Similar to that observed in cancer cells, concentrations from 30 µM affected the cell viability of normal HUVEC cells after 48 h [28]. In contrast, higher concentrations were necessary to observe toxic effects in the two non-tumor oral human cells, HGF (gingival fibroblast) and HPC (pulp cells), reaching CC50 values at 364 µM and 414 µM after 24 h of exposure [32]. The results show high variability as a function of the non-cancerous cell line model selected for the test. The toxicity of this stilbene seems to be related to the ortho-dihydroxyl groups on the phenyl ring, also known as catechol. This is in agreement with other authors who stated that the hydroxylation of resveratrol in positions 3’ and 4’ resulted in increased cytotoxicity [47]. Thus, some authors have reported that the toxic effects of piceatannol are even more potent than those exhibited by trans-resveratrol, pterostilbene, or trans-stilbene-oxide [23,47,48,49,50,51].
In contrast, very few authors reported tan absence of cytotoxic effects after exposure to piceatannol in different leukemic cell lines at concentrations up to 50 µM after 24 h and 48 h, and up to 100 µM after 48 h of exposure [37,38,48]. Moreover, high concentrations of piceatannol (400 µM) showed a non-cytotoxic effect in murine melanoma cell lines [42].
In order to complete these results, morphological assays were performed by these authors (Table 2). The results showed that this compound induced apoptosis in a dose-dependent manner causing cell shrinkage, chromatin and nuclear condensation, and apoptotic bodies. Low concentrations (1 µM) of the compound can induce spherical apoptotic beads after 48 h of exposure in SK-Mel-28 cancer cells [40]. In contrast, it is interesting to point out that no study has been performed to evaluate the effects of piceatannol in the morphology of non-cancerous cells.
The results of the in vitro cytotoxicity studies carried out with pterostilbene are shown in (Table 3). A comparison between all cytotoxic studies is difficult since the exposure conditions, cell lines, and endpoints differed. In general, most of the authors indicated that this stilbene shows cytotoxic effects in several cell models at different conditions in a range of 25–100 µM. The lowest IC50 value reported was 1.81 µM in SOSP-9607 cells after 24 h of exposure measured by the MTT assay [49].
On the other hand, although the IC50 values for non-cancerous cell lines could not always been calculated, a reduction in cell viability was observed after exposure to pterostilbene. The percentage of cell viability of Chang human liver cells was reduced to 75% after exposure to 100 µM of this stilbene after 24 h [50]. Moreover, a very important decrease in cell proliferation was observed in CRL-158 human placenta cells exposed to pterostilbene at concentrations of 40 and 80 µM resulting in reductions of 61.8% and 72.2% as compared to the control [29].
Pterostilbene is expected to be a potent cytotoxic agent since the introduction of one or more methoxy groups into the stilbene structure was previously observed to increase the cytotoxicity of stilbene derivatives [43]. This agrees with the results obtained by several authors comparing the effect of this stilbene with other structurally modified stilbenes, observing that pterostilbene exhibits more potent effects than resveratrol, piceatannol, trans-3,5,4’-trimethoxystilbene, and 3,5,4’-triacetylstilbene [45,64,68,84,87].
Moreover, the cytotoxicity study of pterostilbene has been completed with several morphological assays (Table 4). The methods used for this purpose were fluorescence microscopy using acridine orange (AO) and ethidium bromide (EB), staining with 4, 6-diamidino-2-phenylindole (DAPI) or Hoechst 33342, and electron microscopy. Low concentrations of pterostilbene caused morphological changes indicating the induction of apoptosis in different cells. The SOSP-9607 cell line treated for 24 h with 1 µM of pterostilbene showed loss of confluence [49]. Moreover, MCF-7 cells exposed to 5 µM for 24 h suffered shrinkage, membrane and cytoplasmic blebbings and chromatin condensation [72]. Moreover, in the case of pterostilbene, no morphological assays were performed on non-cancer cell lines.
The cytotoxic studies performed with ε-viniferin are reported in Table 5. In general, concentrations ranging from 10–200 µM of ε-viniferin caused a significant decrease in the cell viability of cancer cells in a time- and concentration-dependent manner. Low IC50 values for trans-ε-viniferin were found in HL-60, HepG2, and AGS carcinoma cell lines with values of 5.6 µM ± 1.4, 7.7 µM ± 0.2, and 9.3 µM ± 0.3, respectively [93]. Moreover, ε-viniferin cytotoxicity in non-cancerous cells has also been demonstrated [30,32,46]. Chowdhury et al. (2005) [32] stated that the 50% cytotoxic concentrations of (-)-ε-viniferin in human oral cell lines HGF, HPC, and HPLF were 111 µM, 146 µM, and 94 µM, respectively, which is of interest since ε-viniferin concentrations of 100–200 µM were used in most of the studies performed. Moreover, only 49.9 µM of this compound was required to inhibit the growth by half in MRC-5 normal human lung cells [46]. Higher concentrations were needed to induce toxicity in the non-transformed human hepatocyte cell line HH4, and the IC50 values obtained after 24 and 48 h of exposure were 192.7 µM and 177.9 µM, respectively.
This compound’s lack of cytotoxicity has also been demonstrated in various cancer and non-cancer cell lines (SW480, L1210, K562, HCT116, PC12, HepG2, and Chang cells) [37,99,100,101]. It is interesting to indicate that, although different exposure times have been evaluated (24–96 h), the absence of toxic effects in some cases may be due the low concentrations studied (10, 30, and 50 µM) [37,99,100].
Furthermore, since ε-viniferin is a resveratrol dimer, it can possess a trans or cis configuration. Moreover, this stilbene is a chiral molecule that can cause dextrorotation (−) and levorotation (+). Most of the authors did not specify which ε-viniferin configuration was evaluated. Among those who reported the configuration, trans-ε-viniferin was the most studied was because it is more stable than the cis configuration. The effects of both isomers have been evaluated by Kim et al., (2002) [94]. Concentrations up to 100 µM of both cis and trans isomers induced similar cytotoxic effects in C6, HepG2, HeLa, MCF-7, and HT-29 cancer cell lines after 70 h of exposure. Moreover, the IC50 values obtained in all cell lines were comparable for both configurations [94]. Furthermore, (−)-ε-viniferin was also selected by several authors, but only Chang et al. (2017) [102] evaluated the cytotoxic effects of (+)-ε-viniferin, hindering the comparison between both configurations.
The morphological changes produced by ε-viniferin have been reported by four authors, as far as we know. The main results of these studies are described in Table 6. After exposure to 100 µM for 24 h, and 95 µM and 130 µM for 48 h different cancer cells (HL-60 and C6) suffered chromatin condensation, nuclear fragmentation and contraction [96,104]. Thus, it seems that a prolonged exposure to this compound does not result in more damage. Moreover, low concentrations of ε-viniferin (30 µM and 60 µM) for 48 and 72 h did not produce apoptotic changes in SW480 and HT144 cancer cell lines [99,103], evidencing that concentrations higher than 60 µM are needed to induce ultrastructural damage. Finally, it should be emphasized that nuclear staining with Hoechst was the only technique performed in these assays and there were no studies evaluating the effect of ε-viniferin in non-cancerous cells.
3. Genotoxicity in In Vitro Studies Performed with Stilbenes
In general, very few in vitro studies have been performed to investigate the potential genotoxic effects and the DNA damage produced by piceatannol, pterostilbene, or ɛ-viniferin. In fact, there is no research whose main objective has focused on this aspect. Specifically, only 11, 10, and 3 studies of piceatannol, pterostilbene and ε-viniferin, respectively, are related to this topic (Table 7).

Table 7.
In vitro genotoxicity and DNA damage studies performed with piceatannol, pterostilbene and ε-viniferin.
The Guidance for submission for food additive evaluations of the EFSA Panel on Food Additives and Nutrient Sources added to Food [15,116] reported that the mutagenic and genotoxic potential of new additives must be assessed in view of the adverse consequences of genetic damage to human health. To address genotoxicity studies, EFSA guidelines indicate two mandatory tests for all food additives, the Ames test and the in vitro mammalian cell micronucleus test. These tests meet the basic requirements to cover the three genetic endpoints with the minimum number of tests.
Among all the studies conducted with piceatannol, only Makena and Chung (2007) [110] performed one of the two tests required by the EFSA for the evaluation of its genotoxic potential. These authors carried out the Ames test using only one Salmonella typhimurium strain (TA102), out of the 5 strains recommended by the EFSA. They showed a non-mutagenic effect at 50 µg/plate of piceatannol in the presence and absence of metabolic activation (rat liver S9 mix). However, the main objective of their work was not to evaluate the potential genotoxicity of piceatannol, but to demonstrate the antimutagenic effect of this compound against the mutations induced by benzidine at 50, 100, and 200 µg/plate in the TA102 strain. In addition to this work, there are also two reports that use the comet assay to evaluate the DNA damage produced by piceatannol in different cell lines. The comet assay is an efficient tool to measure single and double-strand DNA breaks at the cellular level [85]. Thus, Azmi et al. (2005) [109] stated that piceatannol produced more damage than resveratrol in the DNA of human peripheral lymphocytes at 10, 20, and 50 µM of piceatannol in the presence of Cu (II); however, no data for piceatannol without Cu (II) was reported. On the other hand, the other study only focused on demonstrating the protective effect of this compound. Ovesná et al. (2006) [35] showed a decrease in the DNA damage produced by H2O2 in L1210, K562, and HL-60 cell lines at 1, 2.5, and 5 µmol/L. Moreover, other techniques such as flow cytometry, western blot analysis and electrophoresis have indicated that piceatannol produces DNA damage, electrophoresis being the most widely used assay [32,34,53,60,112,113]. In general, different studies have demonstrated that piceatannol produces fragmentation in a dose-dependent manner in some cell lines such as HL-60, HSC-2 [32], U937 [53], A549, and HepG2 [112] by electrophoresis. To date, no in vitro micronucleus assays have been performed with piceatannol as required by the EFSA to ensure its safety as far as we know.
In relation to genotoxicity and DNA damage studies performed with pterostilbene, different techniques such as the micronucleus test, comet assay, electrophoresis, western blot analysis, and the TUNEL assay have been performed. Rossi et al. (2013) [114] stated that pterostilbene does not produce micronuclei at concentrations of 20, 40, and 80 µM in CHO-K1 cells after 3 h of exposure. Furthermore, they confirmed that this stilbene reduced basal DNA damage present in untreated cells under these same conditions by the comet assay. Moreover, the latter authors observed that 80 µM of pterostilbene can reduce the oxidative damage produced by H2O2 as measured by the comet assay but it did not show a protective effect against the induction of micronuclei produced by H2O2. Furthermore, antimutagenic effects of pterostilbene against 4-nitroquinoline-N-oxide have been detected by the comet assay at 50 µM [67]. Similar to piceatannol reports, most of the studies performed with pterostilbene used electrophoresis. Different authors have evidenced that pterostilbene can produce DNA fragmentation in different cell lines such as HeLa [74,82], MCF-7 [62,72], PC3 [62], and MOLT4 [69] at different concentrations (from 10 to 200 µM) and exposure periods (from 12 to 48 h). Despite being required by the EFSA, no Ames test studies have been performed with this substance thus far.
Among the three stilbenes studied in this review, ɛ-viniferin has been the least studied in regard to its genotoxic and DNA-damaging potential. Kim et al. (2002) [94] performed the Ames test in order to evaluate the antimutagenic potential of ɛ-viniferin. They used the TA100 strain of Salmonella typhimurium, exhibiting its antimutagenic potential at a concentration of 35.2 g/plate. However, no information about the mutagenic potential of the substance was reported for this assay. In addition, more recent studies have demonstrated that ε-viniferin produces DNA damage in the A431 cell line by the comet assay [115] and the C6 cell line by the TUNEL assay [104].
As the results showed, none of the three reviewed substances have been assessed by both in vitro tests (Ames test and micronucleus assay) required by the EFSA as the first step in genotoxicity testing. Moreover, most of these studies have been carried out on cancer cell lines and their main objective was not to study the genotoxic potential of these stilbenes as required by the EFSA for all food additives to ensure consumer safety. In this sense, the DNA damage has been investigated as a possible mechanism of cytotoxicity against cancer cells.
Taking into account these results, we consider it necessary and scientifically relevant to evaluate the performance of the in vitro genotoxicity assays and the DNA damage caused by these stilbenes prior to their use in the food industry.
4. Toxicological In Vivo Studies Performed with Stilbenes
Studies focused on assessing the toxicity of substances using in vivo models are necessary to guarantee the safety of their use. In this sense, in vivo toxicity studies of piceatannol, pterostilbene, and ε-viniferin in rodents with potential application in the food industry (novel foods, food additives, etc.) are very scarce, and none have fulfilled the assessment required by the EFSA [15,116]. These studies compromise genotoxicity and other toxicity studies such as subchronic and carcinogenicity studies, etc. [103]. It has only been in recent years that studies have been performed to assess the protective effect of these substances against stress and disease in rodents [117,118,119,120].
With respect to piceatannol, as far as we know, only two authors have evaluated its potential toxic effect. Kiliç (2019) [118] showed that albino mice administered a dose of 4 mg/kg/day IP for 7 days did not show significant differences in biochemical parameters such as superoxide dismutase, catalase, and malonyldialdehyde as compared to the control group. There was no observable nuclear signal of rabbit monoclonal antibody against proliferating cell nuclear antigen or hepatic DNA damage in the treated group. With respect to the results of the histological analysis, apoptotic hepatocytes were rarely observed in animals exposed to piceatannol. Moreover, Shi and Fu (2019) [120] showed that 10 mg/kg/day of piceatannol administered orally via gastric gavage did not induce testicular toxicity. Additionally, beneficial effects such as a marked improvement in mRNA- and protein-expression levels of Nrf2 and its regulated genes and proteins were observed in rats.
The first study that investigated the safety profile of pterostilbene was conducted by Ruiz et al. (2009) [121]. They demonstrated that mice exposed to pterostilbene during 28 days at a dose up to 3000 mg/kg/day caused no mortality during the experimental period. Histopathologic examination and evaluation of biochemical parameters also revealed no alterations regarding organ weight or clinical signs. However, the red blood cell number and hematocrit increased after polyphenol administration as compared to the control group (Ruiz et al., 2009). Later, Riche et al. (2013) [122] assessed the toxicity of pterostilbene in mice after IV administration of 30 mg/kg/day for 23 days. Even at this high dose, pterostilbene was found to be pharmacologically safe as its administration was accompanied by no systemic or organ related toxicity. Moreover, these authors evaluated the long-term safety of pterostilbene administration in a randomized double-blind placebo-controlled trial in humans [122]. They reported that daily doses from 100 mg to 250 mg in adults with hyperlipidemia did not produce a significant adverse drug reaction on hepatic, renal, or glucose markers, with pterostilbene being well-tolerated twice daily. The data available in animal and human models suggests that this compound does not have significant toxic effects. However, the existing information is not adequate to justify the positive effects of this compound in humans after prolonged administration beyond the recommended dietary dose [119]. To our knowledge, no in vivo studies about the safety profile of ε-viniferin were described in the scientific literature. In this sense, it is imperative to perform clinical animal research and human trials to address the safety of ε-viniferin after acute and chronic administration prior to its industrial use.
Taking into account all these facts, further research should include study designs aimed to investigate the safety of these stilbenes in in vivo models. More studies are needed which focus on genotoxicity, subchronic, and chronic toxic effects, etc. to portray the comprehensive safety aspects and to reinforce its human relevancy and market prospects.
5. Conclusions
Considering the increasing interest in stilbenes as additives in the food industry, toxicological assays are needed to assure their safety. The present review describes the available data on the cytotoxic, mutagenic, and genotoxic aspects of piceatannol, pterostilbene, and ε-viniferin. Their cytotoxic effects depend on the cell lines used, assays performed, and exposure conditions. In general, most of the authors stated that these compounds exhibit toxic effects not only in cancer cells but in non-cancer cell lines. Moreover, the DNA damage induced by these compounds has been demonstrated by several methods as a possible mechanism of cytotoxicity. However, the in vitro genotoxic potential of piceatannol, pterostilbene, and ε-viniferin has been poorly studied and no studies following EFSA guidelines were performed. The largest gap in the toxicity assessment of these compounds is the lack of in vivo studies, since most of the authors have evaluated their beneficial properties but have not evaluated their in vivo toxicity. Thus, in order to guarantee the safe use of piceatannol, pterostilbene, and ε-viniferin, more studies are needed such as toxicokinetic, genotoxicity, subchronic, chronic, and carcinogenicity assays, etc. to fulfill the EFSA’s recommendations.
Author Contributions
C.M.-P.: Writing—Review & Editing, Supervision; A.I.P.: Writing—Review & Editing, Supervision; M.P.: Writing—Review & Editing; S.P.: Writing—Review & Editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FEDER/Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación and INIA provided the financial support for this project (RTA2015-00005-C02-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Freyssin, A.; Page, G.; Fauconneau, B.; Rioux Bilan, A. Natural Stilbenes Effects in Animal Models of Alzheimer’s Disease. Neural Regen. Res. 2020, 15, 843–849. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Cutrim, C.S.; Cortez, M.A.S. A Review on Polyphenols: Classification, Beneficial Effects and Their Application in Dairy Products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Likhtenshtein, G.I. Stilbenes Synthesis and Applications. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Khan, Z.A.; Iqbal, A.; Shahzad, S.A. Synthetic Approaches toward Stilbenes and Their Related Structures. Mol. Divers. 2017, 21, 483–509. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A Review of Dietary Stilbenes: Sources and Bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Cicatiello, C.; Franco, S.; Pancino, B.; Blasi, E. The Value of Food Waste: An Exploratory Study on Retailing. J. Retail. Consum. Serv. 2016, 30, 96–104. [Google Scholar] [CrossRef]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial Activity of Resveratrol Structural Analogues: A Mechanistic Evaluation of the Structure-Activity Relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Loh, Y.C.; Tew, W.Y.; Yam, M.F. Vasorelaxant Effect of 3, 5, 4′-Trihydroxy-Trans-Stilbene (Resveratrol) and Its Underlying Mechanism. Inflammopharmacology 2020, 28, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the Treatment of Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Silva, P.; Sureda, A.; Tur, J.A.; Andreoletti, P.; Cherkaoui-Malki, M.; Latruffe, N. How Efficient Is Resveratrol as an Antioxidant of the Mediterranean Diet, towards Alterations during the Aging Process? Free Radic. Res. 2019, 53, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A. Side Streams of Plant Food Processing As a Source of Valuable Compounds: Selected Examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Opinion. Guidance for Submission for Food Additive Evaluations. EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Safety of Synthetic Trans-resveratrol as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4368. [Google Scholar] [CrossRef]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher Dose of Resveratrol Elevated Cardiovascular Disease Risk Biomarker Levels in Overweight Older Adults—A Pilot Study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Abdel-rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J.; et al. The Safety and Regulation of Natural Products Used as Foods and Food Ingredients. Toxicol. Sci. 2011, 123, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12. [Google Scholar] [CrossRef]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New Advances in Active Packaging Incorporated with Essential Oils or Their Main Components for Food Preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Billack, B.; Radkar, V.; Adiabouah, C. In Vitro Evaluation of the Cytotoxic and Antiproliferative Properties of Resveratrol and Several of Its Analogs. Cell. Mol. Biol. Lett. 2008, 13, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Haza, A.I. Selective Apoptotic Effects of Piceatannol and Myricetin in Human Cancer Cells. J. Appl. Toxicol. 2012, 32, 986–993. [Google Scholar] [CrossRef]
- Farrand, L.; Byun, S.; Kim, J.Y.; Im-Aram, A.; Lee, J.; Lim, S.; Lee, K.W.; Suh, J.Y.; Lee, H.J.; Tsang, B.K. Piceatannol Enhances Cisplatin Sensitivity in Ovarian Cancer via Modulation of P53, X-Linked Inhibitor of Apoptosis Protein (XIAP), and Mitochondrial Fission. J. Biol. Chem. 2013, 288, 23740–23750. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lin, C.W.; Yang, S.F.; Sheu, G.T.; Yu, Y.Y.; Chen, M.K.; Chiou, H.L. A Combination of Pterostilbene with Autophagy Inhibitors Exerts Efficient Apoptotic Characteristics in Both Chemosensitive and Chemoresistant Lung Cancer Cells. Toxicol. Sci. 2014, 137, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial Activity of Resveratrol Analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef]
- Hu, W.H.; Dai, D.K.; Zheng, B.Z.Y.; Duan, R.; Dong, T.T.X.; Qin, Q.W.; Tsim, K.W.K. Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-Angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor. Molecules 2020, 25, 3769. [Google Scholar] [CrossRef]
- Tolba, M.F.; Abdel-Rahman, S.Z. Pterostilbine, an Active Component of Blueberries, Sensitizes Colon Cancer Cells to 5-Fluorouracil Cytotoxicity. Sci. Rep. 2015, 5, 15239. [Google Scholar] [CrossRef]
- Zghonda, N.; Yoshida, S.; Araki, M.; Kusunoki, M.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Greater Effectiveness of ε-Viniferin in Red Wine than Its Monomer Resveratrol for Inhibiting Vascular Smooth Muscle Cell Proliferation and Migration. Biosci. Biotechnol. Biochem. 2011, 75, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer, A. Cancer Cell Lines Involving Cancer Stem Cell Populations Respond to Oxidative Stress. Biotechnol. Rep. 2018, 17, 24–30. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-Specificity and Apoptosis-Inducing Activity of Stilbenes and Flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar]
- Fritzer-Szekeres, M.; Savinc, I.; Horvath, Z.; Saiko, P.; Pemberger, M.; Graser, G.; Bernhaus, A.; Ozsvar-Kozma, M.; Grusch, M.; Jaeger, W.; et al. Biochemical effects of piceatannol in human HL-60 promyelocytic leukemia cells—Synergism with Ara-C. Int. J. Oncol. 2008, 33, 887–892. [Google Scholar] [CrossRef]
- Kang, C.H.; Moon, D.O.; Choi, Y.H.; Choi, I.W.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Piceatannol Enhances TRAIL-Induced Apoptosis in Human Leukemia THP-1 Cells through Sp1- and ERK-Dependent DR5 up-Regulation. Toxicol. Vitr. 2011, 25, 605–612. [Google Scholar] [CrossRef]
- Ovesná, Z.; Kozics, K.; Bader, Y.; Saiko, P.; Handler, N.; Erker, T.; Szekeres, T. Antioxidant Activity of Resveratrol, Piceatannol and 3,3′,4,4′,5,5′-Hexahydroxy-Trans-Stilbenein Three Leukemia Cell Lines. Oncol. Rep. 2006, 16, 617–624. [Google Scholar] [CrossRef]
- Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Roberti, M.; Pizzirani, D.; Meli, M.; Dusonchet, L.; Gebbia, N.; Abbadessa, V.; Crosta, L.; et al. Pterostilbene and 3′-Hydroxypterostilbene Are Effective Apoptosis-Inducing Agents in MDR and BCR-ABL-Expressing Leukemia Cells. Int. J. Biochem. Cell Biol. 2005, 37, 1709–1726. [Google Scholar] [CrossRef]
- Ha, D.T.; Chen, Q.C.; Hung, T.M.; Youn, U.J.; Ngoc, T.M.; Thuong, P.T.; Kim, H.J.; Seong, Y.H.; Min, B.S.; Bae, K. Stilbenes and Oligostilbenes from Leaf and Stem of Vitis Amurensis and Their Cytotoxic Activity. Arch. Pharm. Res. 2009, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Roslie, H.; Chan, K.M.; Rajab, N.F.; Velu, S.S.; Kadir, S.A.I.A.S.A.; Bunyamin, I.; Weber, J.F.F.; Thomas, N.F.; Majeed, A.B.A.; Myatt, G.; et al. 3,5-Dibenzyloxy-4′-Hydroxystilbene Induces Early Caspase-9 Activation during Apoptosis in Human K562 Chronic Myelogenous Leukemia Cells. J. Toxicol. Sci. 2012, 37, 13–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, M.; Zhang, Z.; Gao, T. Piceatannol Induced Apoptosis through Up-Regulation of MicroRNA-181a in Melanoma Cells. Biol. Res. 2017, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. Grape Polyphenol Resveratrol and the Related Molecule 4-Hydroxystilbene Induce Growth Inhibition, Apoptosis, S-Phase Arrest, and Upregulation of Cyclins A, E, and B1 in Human SK-Mel-28 Melanoma Cells. J. Agric. Food Chem. 2003, 51, 4576–4584. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The Grape and Wine Polyphenol Piceatannol Is a Potent Inducer of Apoptosis in Human SK-Mel-28 Melanoma Cells. Eur. J. Nutr. 2004, 43, 275–284. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, Y.J. Piceatannol Inhibits Melanogenesis by Its Antioxidative Actions. Biol. Pharm. Bull. 2007, 30, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, O.; Wiśniewski, J.; Bielawska-Pohl, A.; Paprocka, M.; Duarte, N.; Ferreira, M.J.U.; Duś, D.; Michalak, K. Stilbenes as Multidrug Resistance Modulators and Apoptosis Inducers in Human Adenocarcinoma Cells. Anticancer Res. 2010, 30, 4587–4593. [Google Scholar] [PubMed]
- Wolter, F.; Clausnitzer, A.; Akoglu, B.; Stein, J. Piceatannol, a Natural Analog of Resveratrol, Inhibits Progression through the s Phase of the Cell Cycle in Colorectal Cancer Cell Lines. J. Nutr. 2002, 132, 298–302. [Google Scholar] [CrossRef]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-Resveratrol and Piceatannol Administered Orally Suppress and Inhibit Tumor Formation and Growth in Prostate Cancer Xenografts. Prostate 2013, 73, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis Vinifera L. Pinot Noir Grape Cane Extract: Isolation, Characterization, in Vitro Antioxidant Capacity and Anti-Proliferative Effect on Cancer Cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, Prooxidant and Cytotoxic Activity of Hydroxylated Resveratrol Analogues: Structure-Activity Relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Alas, S.; Bonavida, B. Inhibition of Constitutive STAT3 Activity Sensitizes Resistant Non-Hodgkin’s Lymphoma and Multiple Myeloma to Chemotherapeutic Drug-Mediated Apoptosis. Clin. Cancer Res. 2003, 9, 316–326. [Google Scholar]
- Liu, Y.; Wang, L.; Wu, Y.; Lv, C.; Li, X.; Cao, X.; Yang, M.; Feng, D.; Luo, Z. Pterostilbene Exerts Antitumor Activity against Human Osteosarcoma Cells by Inhibiting the JAK2/STAT3 Signaling Pathway. Toxicology 2013, 304, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Hasiah, A.H.; Ghazali, A.R.; Weber, J.F.F.; Velu, S.; Thomas, N.F.; Inayat Hussain, S.H. Cytotoxic and Antioxidant Effects of Methoxylated Stilbene Analogues on HepG2 Hepatoma and Chang Liver Cells: Implications for Structure Activity Relationship. Hum. Exp. Toxicol. 2011, 30, 138–144. [Google Scholar] [CrossRef]
- Wieder, T.; Prokop, A.; Bagci, B.; Essmann, F.; Bernicke, D.; Schulze-Osthoff, K.; Dörken, B.; Schmalz, H.G.; Daniel, P.T.; Henze, G. Piceatannol, a Hydroxylated Analog of the Chemopreventive Agent Resveratrol, Is a Potent Inducer of Apoptosis in the Lymphoma Cell Line BJAB and in Primary, Leukemic Lymphoblasts. Leukemia 2001, 15, 1735–1742. [Google Scholar] [CrossRef]
- Radkar, V.; Hardej, D.; Lau-Cam, C.; Billack, B. Evaluation of Resveratrol and Piceatannol Cytotoxicity in Macrophages, T Cells, and Skin Cells. Arh. Hig. Rada Toksikol. 2007, 58, 293–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.H.; Park, C.; Lee, J.O.; Kim, G.Y.; Lee, W.H.; Choi, Y.H.; Ryu, C.H. Induction of Apoptosis by Piceatannol in Human Leukemic U937 Cells through Down-Regulation of Bcl-2 and Activation of Caspases. Oncol. Rep. 2008, 19, 961–967. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L. The Grape and Wine Constituent Piceatannol Inhibits Proliferation of Human Bladder Cancer Cells via Blocking Cell Cycle Progression and Inducing Fas/Membrane Bound Fas Ligand-Mediated Apoptotic Pathway. Mol. Nutr. Food Res. 2008, 52, 408–418. [Google Scholar] [CrossRef]
- Rüweler, M.; Gülden, M.; Maser, E.; Murias, M.; Seibert, H. Cytotoxic, Cytoprotective and Antioxidant Activities of Resveratrol and Analogues in C6 Astroglioma Cells in Vitro. Chem. Biol. Interact. 2009, 182, 128–135. [Google Scholar] [CrossRef]
- Liu, W.H.; Chang, L. Sen. Piceatannol Induces Fas and FasL Up-Regulation in Human Leukemia U937 Cells via Ca2+/P38α MAPK-Mediated Activation of c-Jun and ATF-2 Pathways. Int. J. Biochem. Cell Biol. 2010, 42, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Arai, D.; Kataoka, R.; Otsuka, S.; Kawamura, M.; Maruki-Uchida, H.; Sai, M.; Ito, T.; Nakao, Y. Piceatannol Is Superior to Resveratrol in Promoting Neural Stem Cell Differentiation into Astrocytes. Food Funct. 2016, 7, 4432–4441. [Google Scholar] [CrossRef]
- Takasawa, R.; Akahane, H.; Tanaka, H.; Shimada, N.; Yamamoto, T.; Uchida-Maruki, H.; Sai, M.; Yoshimori, A.; Tanuma, S.-i. Piceatannol, a Natural Trans-Stilbene Compound, Inhibits Human Glyoxalase I. Bioorg. Med. Chem. Lett. 2017, 27, 1169–1174. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Xie, J.; Hu, Y.; Zhang, Y. Anti-Tumor Effect of Piceatannol through Induction of Cell Apoptosis via up-Regulation of MicroRNA-125b Expression on Pancreatic Cancer. Int. J. Clin. Exp. Med. 2017, 10, 14495–14502. [Google Scholar]
- Siedlecka-Kroplewska, K.; Ślebioda, T.; Kmieć, Z. Induction of Autophagy, Apoptosis and Aquisition of Resistance in Response to Piceatannol Toxicity in MOLT-4 Human Leukemia Cells. Toxicol. Vitr. 2019, 59, 12–25. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Guo, Q.; Liu, Y.; Zhao, Y.; Wu, Y.; Sun, B.; Wang, Q.; Liu, J.; Han, J. Investigation of Binary and Ternary Systems of Human Serum Albumin with Oxyresveratrol/Piceatannol and/or Mitoxantrone by Multipectroscopy, Molecular Docking and Cytotoxicity Evaluation. J. Mol. Liq. 2020, 311, 113364. [Google Scholar] [CrossRef]
- Chakraborty, A.; Gupta, N.; Ghosh, K.; Roy, P. In Vitro Evaluation of the Cytotoxic, Anti-Proliferative and Anti-Oxidant Properties of Pterostilbene Isolated from Pterocarpus Marsupium. Toxicol. Vitr. 2010, 24, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Ho, C.T.; Wang, Y.J. Pterostilbene Induces Autophagy and Apoptosis in Sensitive and Chemoresistant Human Bladder Cancer Cells. Mol. Nutr. Food Res. 2010, 54, 1819–1832. [Google Scholar] [CrossRef]
- Nutakul, W.; Sobers, H.S.; Qiu, P.; Dong, P.; Decker, E.A.; McClements, D.J.; Xiao, H. Inhibitory Effects of Resveratrol and Pterostilbene on Human Colon Cancer Cells: A Side-by-Side Comparison. J. Agric. Food Chem. 2011, 59, 10964–10970. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Khan, S.I.; Tabanca, N.; Wedge, D.E.; Manly, S.P.; Cutler, S.J.; Coy, M.R.; Becnel, J.J.; Neff, S.A.; Gloer, J.B. Biological Activity of Peanut (Arachis hypogaea) Phytoalexins and Selected Natural and Synthetic Stilbenoids. J. Agric. Food Chem. 2011, 59, 1673–1682. [Google Scholar] [CrossRef]
- Harun, Z.; Ghazali, A.R. Potential Chemoprevention Activity of Pterostilbene by Enhancing the Detoxifying Enzymes in the HT-29 Cell Line. Asian Pac. J. Cancer Prev. 2012, 13, 6403–6407. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Prosperini, A.; Font, G.; Ruiz, M.J. Effect of Polyphenols on Enniatins-Induced Cytotoxic Effects in Mammalian Cells. Toxicol. Mech. Methods 2012, 22, 687–695. [Google Scholar] [CrossRef]
- Mena, S.; Rodríguez, M.L.; Ponsoda, X.; Estrela, J.M.; Jäättela, M.; Ortega, A.L. Pterostilbene-Induced Tumor Cytotoxicity: A Lysosomal Membrane Permeabilization-Dependent Mechanism. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Siedlecka-Kroplewska, K.; Jozwik, A.; Kaszubowska, L.; Kowalczyk, A.; Boguslawski, W. Pterostilbene Induces Cell Cycle Arrest and Apoptosis in MOLT4 Human Leukemia Cells. Folia Histochem. Cytobiol. 2012, 50, 574–580. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Wang, X.; Zhang, J.; Han, W.; Feng, L.; Sun, J.; Jin, H.; Wang, X.J. Pterostilbene Simultaneously Induces Apoptosis, Cell Cycle Arrest and Cyto-Protective Autophagy in Breast Cancer Cells. Am. J. Transl. Res. 2012, 4, 44–51. [Google Scholar] [PubMed]
- Pino, M.A.; Pietka-Ottlik, M.; Billack, B. Ebselen Analogues Reduce 2-Chloroethyl Ethyl Sulphide Toxicity in A-431 Cells. Arh. Hig. Rada Toksikol. 2013, 64, 77–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Bodipati, N.; Krishna Peddinti, R.; Roy, P. Role of Isothiocyanate Conjugate of Pterostilbene on the Inhibition of MCF-7 Cell Proliferation and Tumor Growth in Ehrlich Ascitic Cell Induced Tumor Bearing Mice. Exp. Cell Res. 2014, 320, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Wawszczyk, J.; Kapral, M.; Hollek, A.; Węglarz, L. In Vitro Evaluation of Antiproliferative and Cytotoxic Properties of Pterostilbene against Human Colon Cancer Cells. Acta Pol. Pharm. Drug Res. 2014, 71, 1051–1055. [Google Scholar]
- Zhang, B.; Wang, X.Q.; Chen, H.Y.; Liu, B.H. Involvement of the Nrf2 Pathway in the Regulation of Pterostilbene-Induced Apoptosis in HeLa Cells via ER Stress. J. Pharmacol. Sci. 2014, 126, 216–229. [Google Scholar] [CrossRef]
- Ko, C.P.; Lin, C.W.; Chen, M.K.; Yang, S.F.; Chiou, H.L.; Hsieh, M.J. Pterostilbene Induce Autophagy on Human Oral Cancer Cells through Modulation of Akt and Mitogen-Activated Protein Kinase Pathway. Oral Oncol. 2015, 51, 593–601. [Google Scholar] [CrossRef]
- Wu, C.H.; Hong, B.H.; Ho, C.T.; Yen, G.C. Targeting Cancer Stem Cells in Breast Cancer: Potential Anticancer Properties of 6-Shogaol and Pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene Induces Apoptosis and Cell Cycle Arrest in Diffuse Large B-Cell Lymphoma Cells. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Xie, B.; Xu, Z.; Hu, L.; Chen, G.; Wei, R.; Yang, G.; Li, B.; Chang, G.; Sun, X.; Wu, H.; et al. Pterostilbene Inhibits Human Multiple Myeloma Cells via ERK1/2 and JNK Pathway in Vitro and in Vivo. Int. J. Mol. Sci. 2016, 17, 1927. [Google Scholar] [CrossRef] [PubMed]
- Adiabouah Achy-Brou, C.A.; Billack, B. A Comparative Assessment of the Cytotoxicity and Nitric Oxide Reducing Ability of Resveratrol, Pterostilbene and Piceatannol in Transformed and Normal Mouse Macrophages. Drug Chem. Toxicol. 2017, 40, 36–46. [Google Scholar] [CrossRef]
- Hung, C.M.; Liu, L.C.; Ho, C.T.; Lin, Y.C.; Way, T. Der. Pterostilbene Enhances TRAIL-Induced Apoptosis through the Induction of Death Receptors and Downregulation of Cell Survival Proteins in TRAIL-Resistance Triple Negative Breast Cancer Cells. J. Agric. Food Chem. 2017, 65, 11179–11191. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Di, S.; Feng, X.; Liu, D.; Jiang, S.; Hu, W.; Qin, Z.; Li, Y.; Lv, J.; et al. Pterostilbene Exerts Anticancer Activity on Non-Small-Cell Lung Cancer via Activating Endoplasmic Reticulum Stress. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Bin, W.H.; Da, L.H.; Xue, Y.; Jing, B.W. Pterostilbene (3′,5′-Dimethoxy-Resveratrol) Exerts Potent Antitumor Effects in HeLa Human Cervical Cancer Cells via Disruption of Mitochondrial Membrane Potential, Apoptosis Induction and Targeting m-TOR/PI3K/Akt Signalling Pathway. JBUON 2018, 23, 1384–1389. [Google Scholar]
- Chang, H.P.; Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Juan, Y.N.; Tsao, J.W.; Chiu, H.Y.; Yang, J.S. Pterostilbene Modulates the Suppression of Multidrug Resistance Protein 1 and Triggers Autophagic and Apoptotic Mechanisms in Cisplatin-Resistant Human Oral Cancer CAR Cells via AKT Signaling. Int. J. Oncol. 2018, 52, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; AlSharif, D.; Mazza, C.; Syar, P.; Al Sharif, M.; Fata, J.E. Resveratrol and Pterostilbene Exhibit Anticancer Properties Involving the Downregulation of HPV Oncoprotein E6 in Cervical Cancer Cells. Nutrients 2018, 10, 243. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Liu, X.; Li, X.; Cao, Y.; Bai, Y.; Qi, F. Pterostilbene Inhibits Amyloid-β-Induced Neuroinflammation in a Microglia Cell Line by Inactivating the NLRP3/Caspase-1 Inflammasome Pathway. J. Cell. Biochem. 2018, 119, 7053–7062. [Google Scholar] [CrossRef]
- Liu, K.F.; Liu, Y.X.; Dai, L.; Li, C.X.; Wang, L.; Liu, J.; Lei, J.D. A Novel Self-Assembled PH-Sensitive Targeted Nanoparticle Platform Based on Antibody-4arm-Polyethylene Glycol-Pterostilbene Conjugates for Co-Delivery of Anticancer Drugs. J. Mater. Chem. B 2018, 6, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in Vitro and in Vivo Analysis. Front. Oncol. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Tan, K.T.; Chen, P.W.; Li, S.; Ke, T.M.; Lin, S.H.; Yang, C.C. Pterostilbene Inhibits Lung Squamous Cell Carcinoma Growth in Vitro and in Vivo by Inducing S Phase Arrest and Apoptosis. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef]
- Jung, J.H.; Shin, E.A.; Kim, J.H.; Sim, D.Y.; Lee, H.; Park, J.E.; Lee, H.J.; Kim, S.H. NEDD9 Inhibition by MiR-25-5p Activation Is Critically Involved in Co-Treatment of Melatonin-and Pterostilbene-Induced Apoptosis in Colorectal Cancer Cells. Cancers 2019, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene Activates the Nrf2-Dependent Antioxidant Response to Ameliorate Arsenic-Induced Intracellular Damage and Apoptosis in Human Keratinocytes. Front. Pharmacol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, S.Y.; Wang, S.Y.; Lin, J.A.; Yen, G.C. Pterostilbene Enhances Cytotoxicity and Chemosensitivity in Human Pancreatic Cancer Cells. Biomolecules 2020, 10, 709. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, D.; Luo, Q.; Li, J.; Liu, J. Pterostilbene Inhibits Human Renal Cell Carcinoma Cells Growth and Induces DNA Damage. Biol. Pharm. Bull. 2020, 43, 258–265. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; Zhang, S.; Li, P.; Wang, T.; Ho, C.T.; Pan, M.H.; Bai, N. Chemical Characterization of Main Bioactive Constituents in Paeonia Ostii Seed Meal and GC-MS Analysis of Seed Oil. J. Food Biochem. 2020, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, E.J.; Sung, H.C.; Chung, S.K.; Park, H.D.; Choi, W.C. Cytotoxic and Antimutagenic Stilbenes from Seeds of Paeonia lactiflora. Arch. Pharm. Res. 2002, 25, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Billard, C.; Izard, J.C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.P. Comparative Antiproliferative and Apoptotic Effects of Resveratrol, ε-Viniferin and Vine-Shots Derived Polyphenols (Vineatrols) on Chronic B Lymphocytic Leukemia Cells and Normal Human Lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Park, Y.H.; Choi, S.W.; Yang, E.K.; Lee, W.J. Resveratrol Derivatives Potently Induce Apoptosis in Human Promyelocytic Leukemia Cells. Exp. Mol. Med. 2003, 35, 467–474. [Google Scholar] [CrossRef]
- Muhtadi Hakim, E.H.; Juliawaty, L.D.; Syah, Y.M.; Achmad, S.A.; Latip, J.; Ghisalberti, E.L. Cytotoxic Resveratrol Oligomers from the Tree Bark of Dipterocarpus hasseltii. Fitoterapia 2006, 77, 550–555. [Google Scholar] [CrossRef]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative Activities of Resveratrol and Related Compounds in Human Hepatocyte Derived HepG2 Cells Are Associated with Biochemical Cell Disturbance Revealed by Fluorescence Analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef]
- Marel, A.K.; Lizard, G.; Izard, J.C.; Latruffe, N.; Delmas, D. Inhibitory Effects of Trans-Resveratrol Analogs Molecules on the Proliferation and the Cell Cycle Progression of Human Colon Tumoral Cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.M.; Monti, J.P. Protective Effect of ε-Viniferin on β-Amyloid Peptide Aggregation Investigated by Electrospray Ionization Mass Spectrometry. Bioorg. Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Rohaiza, S.; Yaacob, W.A.; Din, L.B.; Nazlina, I. Cytotoxic Oligostilbenes from Shorea Hopeifolia. Afr. J. Pharm. Pharmacol. 2011, 5, 1272–1277. [Google Scholar] [CrossRef]
- Chang, C.I.; Chien, W.C.; Huang, K.X.; Hsu, J.L. Anti-Inflammatory Effects of Vitisinol A and Four Other Oligostilbenes from Ampelopsis Brevipedunculata Var. Hancei. Molecules 2017, 22, 1195. [Google Scholar] [CrossRef]
- Nivelle, L.; Aires, V.; Rioult, D.; Martiny, L.; Tarpin, M.; Delmas, D. Molecular Analysis of Differential Antiproliferative Activity of Resveratrol, Epsilon Viniferin and Labruscol on Melanoma Cells and Normal Dermal Cells. Food Chem. Toxicol. 2018, 116, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.; Apaydın, E.; Önder, N.İ.; Şen, M.; Ayrım, A.; Öğünç, Y.; İncesu, Z. Apoptotic Effects of ε-Viniferin in Combination with Cis-Platin in C6 Cells. Cytotechnology 2018, 70, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Vion, E.; Page, G.; Bourdeaud, E.; Paccalin, M.; Guillard, J.; Rioux Bilan, A. Trans ε-Viniferin Is an Amyloid-β Disaggregating and Anti-Inflammatory Drug in a Mouse Primary Cellular Model of Alzheimer’s Disease. Mol. Cell. Neurosci. 2018, 88, 1–6. [Google Scholar] [CrossRef]
- Courtois, A.; Garcia, M.; Krisa, S.; Atgié, C.; Sauvant, P.; Richard, T.; Faure, C. Encapsulation of ϵ-Viniferin in Onion-Type Multi-Lamellar Liposomes Increases Its Solubility and Its Photo-Stability and Decreases Its Cytotoxicity on Caco-2 Intestinal Cells. Food Funct. 2019, 10, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Aja, I.; Begoña Ruiz-Larrea, M.; Courtois, A.; Krisa, S.; Richard, T.; Ruiz-Sanz, J.I. Screening of Natural Stilbene Oligomers from Vitis Vinifera for Anticancer Activity on Human Hepatocellular Carcinoma Cells. Antioxidants 2020, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Padial, C.; Puerto, M.; del Mar Merchán-Gragero, M.; Moreno, F.J.; Richard, T.; Cantos-Villar, E.; Pichardo, S. Cytotoxicity Studies of a Stilbene Extract and Its Main Components Intended to Be Used as Preservative in the Wine Industry. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Resveratrol-Cu(II) Induced DNA Breakage in Human Peripheral Lymphocytes: Implications for Anticancer Properties. FEBS Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef]
- Makena, P.S.; Chung, K.T. Effects of Various Plant Polyphenols on Bladder Carcinogen Benzidine-Induced Mutagenicity. Food Chem. Toxicol. 2007, 45, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C.; Lin, C.-Y.; Lin, H.-Y.; Wu, J.M. AKT/MTOR as Novel Targets of Polyphenol Piceatannol Possibly Contributing to Inhibition of Proliferation of Cultured Prostate Cancer Cells. ISRN Urol. 2012, 2012, 272697. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, J.; Balaraman Ravindrran, M. Chitosan/Poly (Lactic Acid)-Coated Piceatannol Nanoparticles Exert an in Vitro Apoptosis Activity on Liver, Lung and Breast Cancer Cell Lines. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Molagoda, I.M.N.; Park, C.; Kwon, T.K.; Yun, S.J.; Kim, W.J.; Kim, G.Y.; Choi, Y.H. Piceatannol-Induced Apoptosis Is Reversed by N-Acetyl-L-Cysteine through Restoration of XIAP Expression. Biol. Pharm. Bull. 2018, 41, 1372–1378. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Antonioletti, R.; Viglianti, A.; Traversi, G.; Leone, S.; Basso, E.; Cozzi, R. Scavenging of Hydroxyl Radical by Resveratrol and Related Natural Stilbenes after Hydrogen Peroxide Attack on DNA. Chem. Biol. Interact. 2013, 206, 175–185. [Google Scholar] [CrossRef]
- Baechler, S.A.; Schroeter, A.; Dicker, M.; Steinberg, P.; Marko, D. Topoisomerase II-Targeting Properties of a Grapevine-Shoot Extract and Resveratrol Oligomers. J. Agric. Food Chem. 2014, 62, 780–788. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Opinion. Scientific Opinion on Genotoxicity Testing Strategies Applicable to Food and Feed Safety Assessment. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Caillaud, M.; Guillard, J.; Richard, D.; Milin, S.; Chassaing, D.; Paccalin, M.; Page, G.; Bilan, A.R. Trans ε Viniferin Decreases Amyloid Deposits and Inflammation in a Mouse Transgenic Alzheimer Model. PLoS ONE 2019, 14, e212663. [Google Scholar] [CrossRef]
- Kiliç, V. Piceatannol Mediated Modulation of Oxidative Stress and Regeneration in the Liver of Endotoxemic Mice. J. Med. Food 2019, 22, 594–601. [Google Scholar] [CrossRef]
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising Therapeutic Potential of Pterostilbene and Its Mechanistic Insight Based on Preclinical Evidence. Eur. J. Pharmacol. 2016, 789, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fu, L. Piceatannol Inhibits Oxidative Stress through Modification of Nrf2-Signaling Pathway in Testes and Attenuates Spermatogenesis and Steroidogenesis in Rats Exposed to Cadmium during Adulthood. Drug Des. Devel. Ther. 2019, 13, 2811–2824. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Fernández, M.; Picó, Y.; Mañes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary Administration of High Doses of Pterostilbene and Quercetin to Mice Is Not Toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of Safety from a Human Clinical Trial with Pterostilbene. J. Toxicol. 2013, 2013. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).