Comparative Study of Oxidative Structural Modifications of Unadsorbed and Adsorbed Proteins in Whey Protein Isolate-Stabilized Oil-in-Water Emulsions under the Stress of Primary and Secondary Lipid Oxidation Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
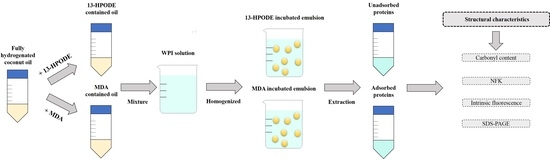
2.2. Preparation of Primary LPO Products (13-HPODE)
2.3. Preparation of Secondary LPO Products (MDA)
2.4. Emulsion Preparation
2.5. Recovery of Unadsorbed and Adsorbed Proteins
2.6. Determination of Protein Concentrations
2.7. Measurement of the Protein Carbonyl Content
2.8. NFK
2.9. Intrinsic Tryptophan Fluorescence
2.10. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Concentrations of Unadsorbed and Adsorbed Proteins
3.2. Measurement of the Protein Carbonyl Content
3.3. Measurement of NFK
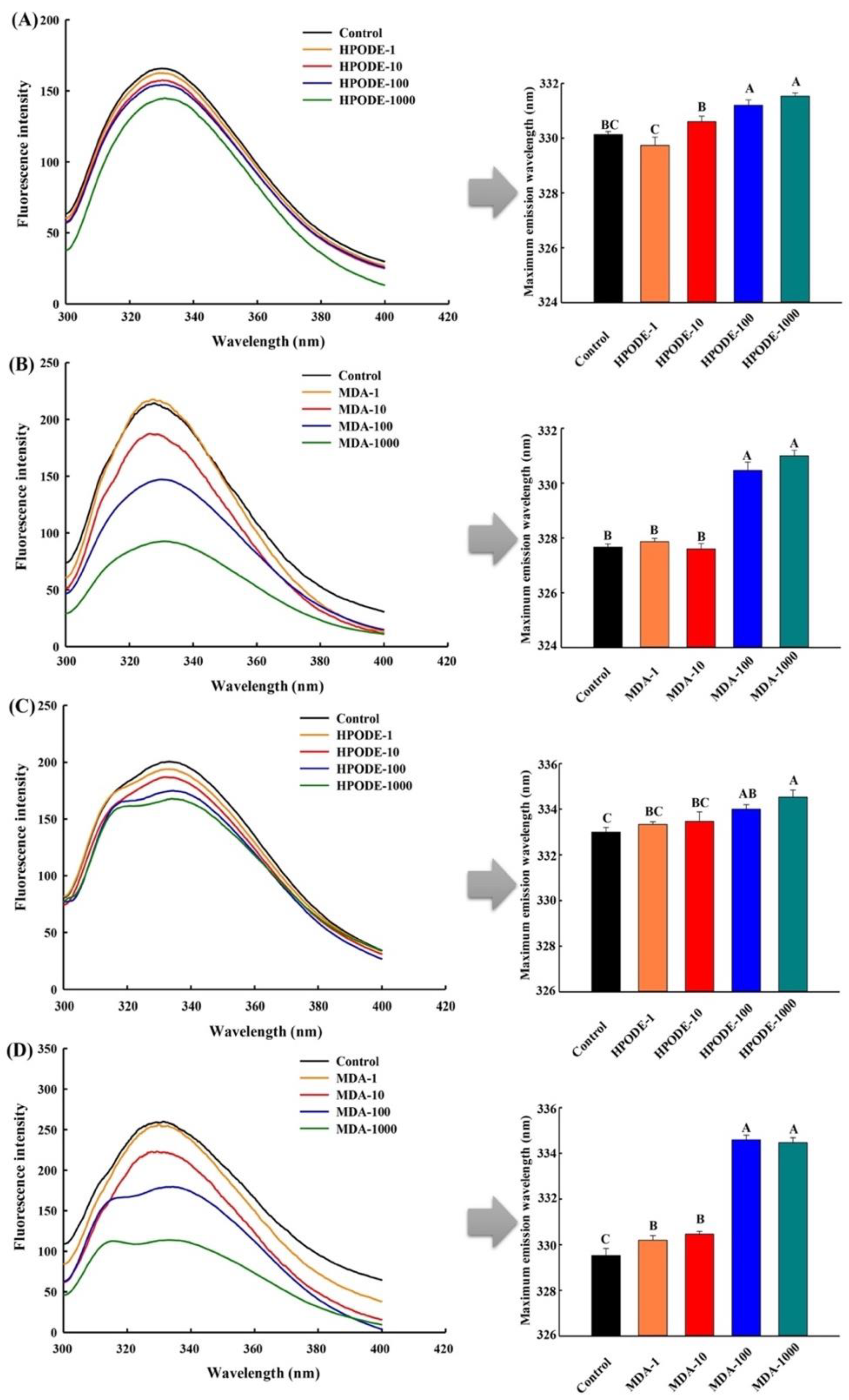
3.4. Measurement of Intrinsic Tryptophan Fluorescence
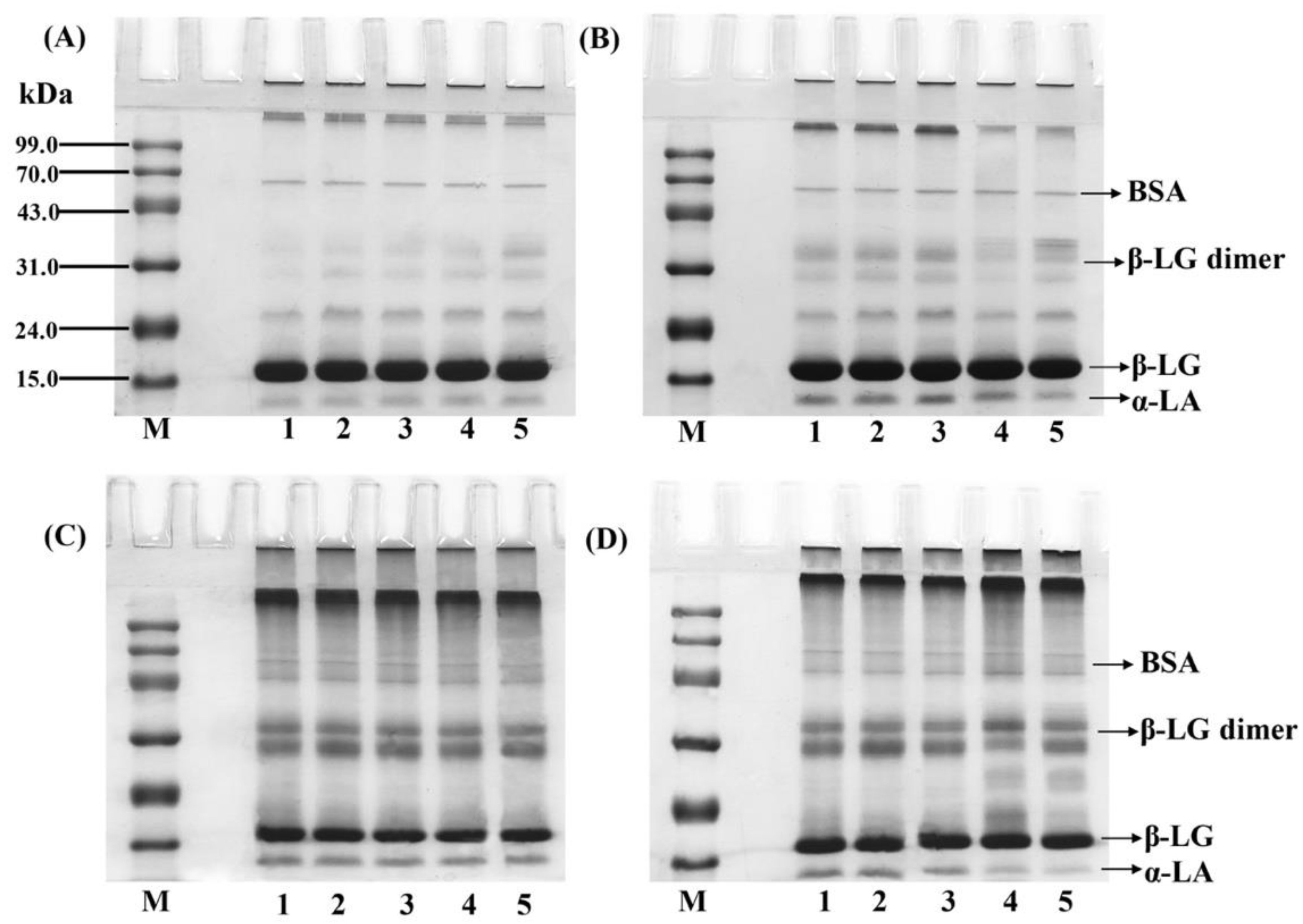
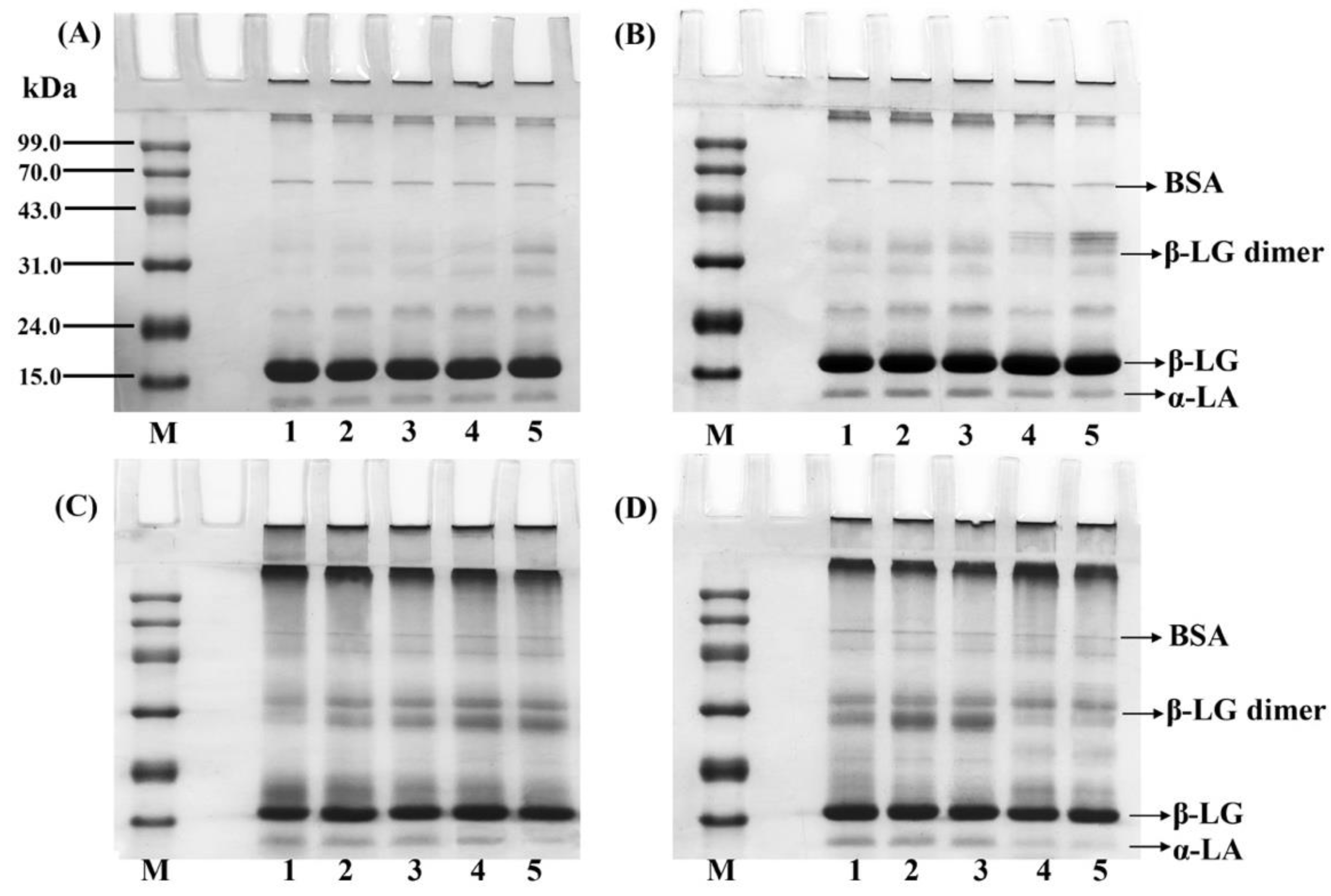
3.5. SDS-PAGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.L.; Liu, C.; Zhang, S.Y.; Li, J.S.; Zheng, H.Y.; Jin, H.; Xu, J. Comparison of different protein emulsifiers on physicochemical properties of β-carotene-loaded nanoemulsion: Effect on formation, stability, and in vitro digestion. Nanomaterial 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Zheng, H.Y.; Zhang, S.Y.; Li, J.S.; Zhu, X.Q.; Jin, H.; Xu, J. Improvement of protein emulsion stability through glycosylated black bean protein covalent interaction with (−)-epigallocatechin-3-gallate. RSC Adv. 2021, 11, 2546–2555. [Google Scholar] [CrossRef]
- Cucu, T.; Devreese, B.; Mestdagh, F.; Kerkaert, B.; De Meulenaer, B. Protein-lipid interactions during the incubation of whey proteins with autoxidizing lipids. Int. Dairy J. 2011, 21, 427–433. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Zhao, C.; Yi, J.H.; Liu, N.; Cao, Y.G.; Decker, E.A.; McClements, D.J. Impact of interfacial composition on lipid and protein co-oxidation in oil-in-water emulsions containing mixed emulisifers. J. Agric. Food Chem. 2018, 66, 4458–4468. [Google Scholar] [CrossRef]
- Obando, M.; Papastergiadis, A.; Li, S.S.; De Meulenaer, B. Impact of lipid and protein co-oxidation on digestibility of dairy proteins in oil-in-water (o/w) emulsions. J. Agric. Food Chem. 2015, 63, 9820–9830. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde measurement in oxidized foods: Evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef]
- Papastergiadis, A.; Fatouh, A.; Jacxsens, L.; Lachat, C.; Shrestha, K.; Daelman, J.; Kolsteren, P.; Van Langenhove, H.; De Meulenaer, B. Exposure assessment of Malondialdehyde, 4-Hydroxy-2-(E)-Nonenal and 4-Hydroxy-2-(E)-Hexenal through specific foods available in Belgium. Food Chem. Toxicol. 2014, 73, 51–58. [Google Scholar] [CrossRef]
- Wu, X.J.; Li, F.; Wu, W. Effects of oxidative modification by 13-hydroperoxyoctadecadienoic acid on the structure and functional properties of rice protein. Food Res. Int. 2020, 132, 109096. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.M.; Hua, Y.F. Structural modification of soy protein by the lipid peroxidation product malondialdehyde. J. Sci. Food Agric. 2009, 89, 1416–1423. [Google Scholar] [CrossRef]
- Zhu, Z.S.; Fang, R.; Zhao, D.; Huang, M. Effect of malondialdehyde on oil-in-water emulsifying behavior and Maillard reaction of chicken sarcoplasmic protein in emulsion. Colloid Surf. B Biointerfaces 2020, 191, 111016. [Google Scholar] [CrossRef]
- Mestdagh, F.; Kerkaert, B.; Cucu, T.; De Meulenaer, B. Interaction between whey proteins and lipids during light-induced oxidation. Food Chem. 2011, 126, 1190–1197. [Google Scholar] [CrossRef]
- Li, Y.; Niu, H.L.; Liu, H.T.; Liu, Q.; Kong, B.H. Effect of porcine plasma protein with limited hydrolyzation coupled with Tween 20 on the physical and oxidative stability of oil-in-water emulsions. Food Biophys. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: Lentil, pea, and faba bean proteins. Food Res. Int. 2017, 100, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Berton, C.; Ropers, M.H.; Guibert, D.; Solé, V.; Genot, C. Modifications of interfacial proteins in oil-in-water emulsions prior to and during lipid oxidation. J. Agric. Food Chem. 2012, 60, 8659–8671. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008, 80, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, Y.L. Inhibition of lipid oxidation in oil-in-water emulsions by interface adsorbed myofibrillar protein. J. Agric. Food Chem. 2015, 63, 8896–8904. [Google Scholar] [CrossRef]
- Yi, J.H.; Ning, J.Q.; Zhu, Z.B.; Cui, L.Q.; Decker, E.A.; McClements, D.J. Impact of interfacial composition on co-oxidation of lipids and proteins in oil-in-water emulsions: Competitive displacement of casein by surfactants. Food Hydrocoll. 2019, 87, 20–28. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zheng, J.B.; Ge, G.; Zhao, M.M.; Sun, W.Z. Impact of heating treatments on physical stability and lipid-protein co-oxidation in oil-in-water emulsion prepared with soy protein isolates. Food Hydrocoll. 2019, 100, 105167. [Google Scholar] [CrossRef]
- Chen, J.X.; Feng, Y.Y.; Kong, B.H.; Xia, X.F.; Liu, Q. An eco-friendly extraction method for adsorbed proteins from emulsions stabilized by whey protein isolate by using Tween 20. Colloid Surf. A Physicochem. Eng. Asp. 2020, 604, 125332. [Google Scholar] [CrossRef]
- Iacazio, G.; Langrand, G.; Baratti, J.; Buono, G.; Triantaphylidès, C. Preparative, enzymic synthesis of linoleic acid (13S)-hydroperoxide using soybean lipoxygenase-1. J. Org. Chem. 1990, 55, 1690–1691. [Google Scholar] [CrossRef]
- Adams, A.; De Kimpe, N.; Van Boekel, M.A.J.S. Modification of casein by the lipid oxidation product malondialdehyde. J. Agric. Food Chem. 2008, 56, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Kazuhiro, M.; Nakauma, M.; Funami, T.; Nambu, Y.; Matsumiya, K.; Matsumura, Y. Stabilization of whey protein isolate-based emulsions via complexation with xanthan gum under acidic conditions. Food Hydrocoll. 2021, 111, 106365. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Geng, R.; Zhao, J.Y.; Chen, Q.; Kong, B.H. Structural and gel textural properties of soy protein isolate when subjected to extreme acid pH-shifting and mild heating processes. J. Agric. Food Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.J.; Wu, W. Effects of oxidative modification by malondialdehyde on the in vitro digestion properties of rice bran protein. J. Cereal Sci. 2021, 97, 103158. [Google Scholar] [CrossRef]
- Domingues, R.M.; Domingues, P.; Melo, T.; Pérez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteom. 2013, 92, 110–131. [Google Scholar] [CrossRef]
- Vandemoortele, A.; De Meulenaer, B. Behavior of malondialdehyde in oil-in-water emulsions. J. Agric. Food Chem. 2015, 63, 5694–5701. [Google Scholar] [CrossRef] [PubMed]
- Nyström, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Ehrenshaft, M.; de Oliveira Silva, S.; Perdivara, I.; Bilski, P.; Sik, R.H.; Chignell, C.F.; Tomer, K.B.; Mason, R.P. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic. Biol. Med. 2009, 46, 1260–1266. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Dalsgaard, T.K.; Otzen, D.; Nielsen, J.H.; Larsen, L.B. Changes in structures of milk proteins upon photo-oxidation. J. Agric. Food Chem. 2007, 55, 10968–10976. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, D.; Pecora, R.P.; Radici, P.M.; Kivatinitz, S.C. Protein oxidative changes in whole and skim milk after ultraviolet or fluorescent light exposure. J. Dairy Sci. 2010, 93, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Wang, W.J.; Ma, X.B.; Lv, R.L.; Watharkar, R.B.; Ding, T.; Ye, X.Q.; Liu, D.H. Effect of pH-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (−)-epigallocatechin-3-gallate. Food Chem. 2018, 274, 234–241. [Google Scholar] [CrossRef]
- Yang, J.Y.; Xiong, Y.L. Comparative time-course of lipid and myofibrillar protein oxidation in different biphasic systems under hydroxyl radical stress. Food Chem. 2018, 243, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Menini, S.; Maineri, E.P.; Patriarca, S.; Odetti, P.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A. Malondialdehyde, a lipoperoxidation-derived aldehyde, can bring about secondary oxidative damage to proteins. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, B890–B895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, M.M.; Qiu, C.Y.; Sun, W.Z. Effect of malondialdehyde modification on the binding of aroma compounds to soy protein isolates. Food Res. Int. 2018, 105, 150–158. [Google Scholar] [CrossRef]
- Yin, Z.C.; Wu, Y.R.; Chen, Y.; Qie, X.J.; Zeng, M.M.; Wang, Z.J.; Qin, F.; Chen, J.; He, Z.Y. Analysis of the interaction between cyanidin-3-O-glucoside and casein hydrolysates and its effect on the antioxidant ability of the complexes. Food Chem. 2021, 340, 127915. [Google Scholar] [CrossRef]
- Claeys, E.; Uytterhaegen, L.; Buts, B.; Demeyer, D. Quantification of beef myofibrillar proteins by SDS-PAGE. Meat Sci. 1995, 39, 177–193. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Maresca, P.; Pataro, G.; Ferrari, G. Influence of pulsed light treatment on the aggregation of whey protein isolate. Food Res. Int. 2017, 99, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Segat, A.; Misra, N.N.; Fabbro, A.; Buchini, F.; Lippe, G.; Cullen, P.J.; Innocente, N. Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Res. Int. 2014, 66, 365–372. [Google Scholar] [CrossRef]

| LPO Products | Addition Levels (μmol/L) | Protein Concentrations (mg/mL Emulsion) | |||
|---|---|---|---|---|---|
| Unadsorbed Protein | Decreasing Rate (%) | Adsorbed Protein | Increasing Rate (%) | ||
| 13-HPODE | 0 | 13.21 ± 0.08 A | - | 7.13 ± 0.10 D | - |
| 1 | 13.09 ± 0.07 A | 0.91 | 7.16 ± 0.06 D | 0.42 | |
| 10 | 13.19 ± 0.06 A | 0.15 | 7.17 ± 0.16 D | 0.56 | |
| 100 | 12.71 ± 0.12 B | 3.79 | 8.16 ± 0.05 C | 14.45 | |
| 1000 | 12.63 ± 0.09 B | 4.39 | 8.27 ± 0.07 BC | 15.99 | |
| MDA | 0 | 13.14 ± 0.15 A | - | 7.23 ± 0.08 D | - |
| 1 | 13.10 ± 0.05 A | 0.30 | 7.24 ± 0.10 D | 0.14 | |
| 10 | 13.09 ± 0.08 A | 0.38 | 7.28 ± 0.09 D | 0.70 | |
| 100 | 12.50 ± 0.14 B | 4.87 | 8.50 ± 0.14 AB | 17.57 | |
| 1000 | 12.14 ± 0.08 C | 7.61 | 8.74 ± 0.08 A | 20.89 | |
| LPO Products | Addition Levels (μmol/L) | Protein Concentrations (mg/mL Emulsion) | |||
|---|---|---|---|---|---|
| Unadsorbed Protein | Decreasing Rate (%) | Adsorbed Protein | Increasing Rate (%) | ||
| 13-HPODE | 0 | 13.46 ± 0.13 AB | - | 6.30 ± 0.11 D | - |
| 1 | 13.53 ± 0.08 A | −0.52 | 6.40 ± 0.04 D | 1.59 | |
| 10 | 13.49 ± 0.09 AB | −0.22 | 6.49 ± 0.08 D | 3.02 | |
| 100 | 13.10 ± 0.05 BC | 2.67 | 7.31 ± 0.05 C | 16.03 | |
| 1000 | 13.04 ± 0.07 CD | 3.12 | 7.44 ± 0.10 BC | 18.10 | |
| MDA | 0 | 13.79 ± 0.10 A | - | 6.23 ± 0.09 D | - |
| 1 | 13.76 ± 0.10 A | 0.22 | 6.24 ± 0.11 D | 0.16 | |
| 10 | 13.65 ± 0.14 A | 1.02 | 6.24 ± 0.13 D | 0.16 | |
| 100 | 12.72 ± 0.24 D | 7.76 | 7.87 ± 0.20 A | 26.32 | |
| 1000 | 12.69 ± 0.17 D | 7.98 | 7.77 ± 0.16 AB | 24.72 | |
| LPO Products | Addition Levels (μmol/L) | Protein Carbonyl (μmol/g Protein) | |||
|---|---|---|---|---|---|
| Unadsorbed Protein | Φ | Adsorbed Protein | Φ | ||
| 13-HPODE | 0 | 2.32 ± 0.05 H | 0.634 | 5.86 ± 0.05 F | 0.979 |
| 1 | 3.00 ± 0.05 G | 5.77 ± 0.04 F | |||
| 10 | 3.59 ± 0.04 F | 5.82 ± 0.04 F | |||
| 100 | 3.91 ± 0.08 E | 6.17 ± 0.07 E | |||
| 1000 | 4.17 ± 0.07 D | 7.05 ± 0.09 C | |||
| MDA | 0 | 3.92 ± 0.18 DE | 0.990 | 6.53 ± 0.09 D | 0.999 |
| 1 | 3.94 ± 0.11 DE | 6.59 ± 0.05 D | |||
| 10 | 4.89 ± 0.08 C | 6.66 ± 0.11 D | |||
| 100 | 7.94 ± 0.07 B | 8.77 ± 0.05 B | |||
| 1000 | 20.52 ± 0.07 A | 22.18 ± 0.04 A | |||
| LPO Products | Addition Levels (μmol/L) | Protein Carbonyl (μmol/g Protein) | |||
|---|---|---|---|---|---|
| Unadsorbed Protein | Φ | Adsorbed Protein | Φ | ||
| 13-HPODE | 0 | 2.95 ± 0.05 H | 0.795 | 6.42 ± 0.06 G | 0.818 |
| 1 | 3.50 ± 0.06 G | 7.38 ± 0.11 F | |||
| 10 | 3.85 ± 0.07 F | 8.02 ± 0.07 E | |||
| 100 | 4.30 ± 0.07 E | 8.83 ± 0.07 D | |||
| 1000 | 4.89 ± 0.06 D | 10.06 ± 0.05 B | |||
| MDA | 0 | 3.80 ± 0.13 F | 0.986 | 6.65 ± 0.10 G | 0.998 |
| 1 | 4.21 ± 0.06 E | 6.66 ± 0.07 G | |||
| 10 | 6.54 ± 0.09 C | 7.23 ± 0.10 F | |||
| 100 | 8.86 ± 0.04 B | 9.42 ± 0.10 C | |||
| 1000 | 23.77 ± 0.05 A | 23.39 ± 0.07 A | |||
| LPO Products | Addition Levels (μmol/L) | NKF | |||
|---|---|---|---|---|---|
| Unadsorbed Protein | Φ | Adsorbed Protein | Φ | ||
| 13-HPODE | 0 | 54.40 ± 0.20 G | 0.906 | 75.97 ± 0.66 F | 0.800 |
| 1 | 58.49 ± 0.40 F | 75.20 ± 0.56 F | |||
| 10 | 59.55 ± 0.38 F | 76.46 ± 0.81 F | |||
| 100 | 67.24 ± 0.69 D | 92.08 ± 0.76 D | |||
| 1000 | 77.81 ± 1.56 C | 97.17 ± 0.63 C | |||
| MDA | 0 | 58.30 ± 0.56 F | 0.923 | 76.94 ± 1.76 EF | 0.917 |
| 1 | 60.29 ± 0.83 F | 77.27 ± 0.71 E | |||
| 10 | 62.63 ± 0.76 E | 78.21 ± 1.91 E | |||
| 100 | 86.27 ± 0.94 B | 121.02 ± 0.75 B | |||
| 1000 | 114.11 ± 0.38 A | 164.64 ± 0.87 A | |||
| LPO Products | Addition Levels (μmol/L) | NKF | |||
|---|---|---|---|---|---|
| Unadsorbed Protein | Φ | Adsorbed Protein | Φ | ||
| 13-HPODE | 0 | 56.09 ± 0.55 H | 0.947 | 76.03 ± 0.69 G | 0.801 |
| 1 | 59.78 ± 0.56 G | 76.84 ± 0.72 G | |||
| 10 | 64.00 ± 0.51 F | 87.66 ± 1.25 F | |||
| 100 | 68.91 ± 0.68 E | 114.58 ± 0.90 D | |||
| 1000 | 87.03 ± 0.80 C | 127.30 ± 0.61 C | |||
| MDA | 0 | 58.53 ± 1.17 GH | 0.931 | 75.38 ± 1.53 G | 0.898 |
| 1 | 65.03 ± 1.44 F | 76.61 ± 1.20 G | |||
| 10 | 73.39 ± 0.86 D | 95.61 ± 1.29 E | |||
| 100 | 94.36 ± 1.01 B | 135.49 ± 0.74 B | |||
| 1000 | 133.12 ± 0.84 A | 184.74 ± 0.64 A | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhao, J.; Kong, B.; Chen, Q.; Liu, Q.; Liu, C. Comparative Study of Oxidative Structural Modifications of Unadsorbed and Adsorbed Proteins in Whey Protein Isolate-Stabilized Oil-in-Water Emulsions under the Stress of Primary and Secondary Lipid Oxidation Products. Foods 2021, 10, 593. https://doi.org/10.3390/foods10030593
Chen J, Zhao J, Kong B, Chen Q, Liu Q, Liu C. Comparative Study of Oxidative Structural Modifications of Unadsorbed and Adsorbed Proteins in Whey Protein Isolate-Stabilized Oil-in-Water Emulsions under the Stress of Primary and Secondary Lipid Oxidation Products. Foods. 2021; 10(3):593. https://doi.org/10.3390/foods10030593
Chicago/Turabian StyleChen, Jiaxin, Jinhai Zhao, Baohua Kong, Qian Chen, Qian Liu, and Chengguo Liu. 2021. "Comparative Study of Oxidative Structural Modifications of Unadsorbed and Adsorbed Proteins in Whey Protein Isolate-Stabilized Oil-in-Water Emulsions under the Stress of Primary and Secondary Lipid Oxidation Products" Foods 10, no. 3: 593. https://doi.org/10.3390/foods10030593
APA StyleChen, J., Zhao, J., Kong, B., Chen, Q., Liu, Q., & Liu, C. (2021). Comparative Study of Oxidative Structural Modifications of Unadsorbed and Adsorbed Proteins in Whey Protein Isolate-Stabilized Oil-in-Water Emulsions under the Stress of Primary and Secondary Lipid Oxidation Products. Foods, 10(3), 593. https://doi.org/10.3390/foods10030593