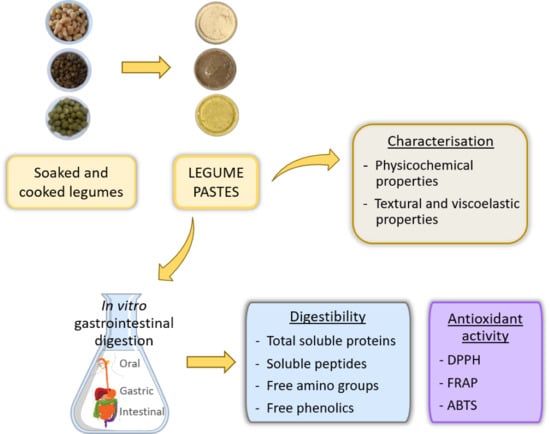
Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Legume Pastes
2.3. Characterization of Legume Pastes
2.3.1. Physicochemical Parameters
2.3.2. Texture and Viscoelastic Behavior
2.4. In Vitro Gastrointestinal Digestion
2.5. Total Soluble Proteins, TCA-Soluble Peptides, and Free Amino Groups
2.6. Total Free Phenolics
2.7. Antioxidant Activity
2.7.1. DPPH Radical Scavenging Activity
2.7.2. Ferric-Reducing Antioxidant Power (FRAP)
2.7.3. ABTS Radical Scavenging Capacity
2.8. Statistical Analysis
3. Results and Discussion
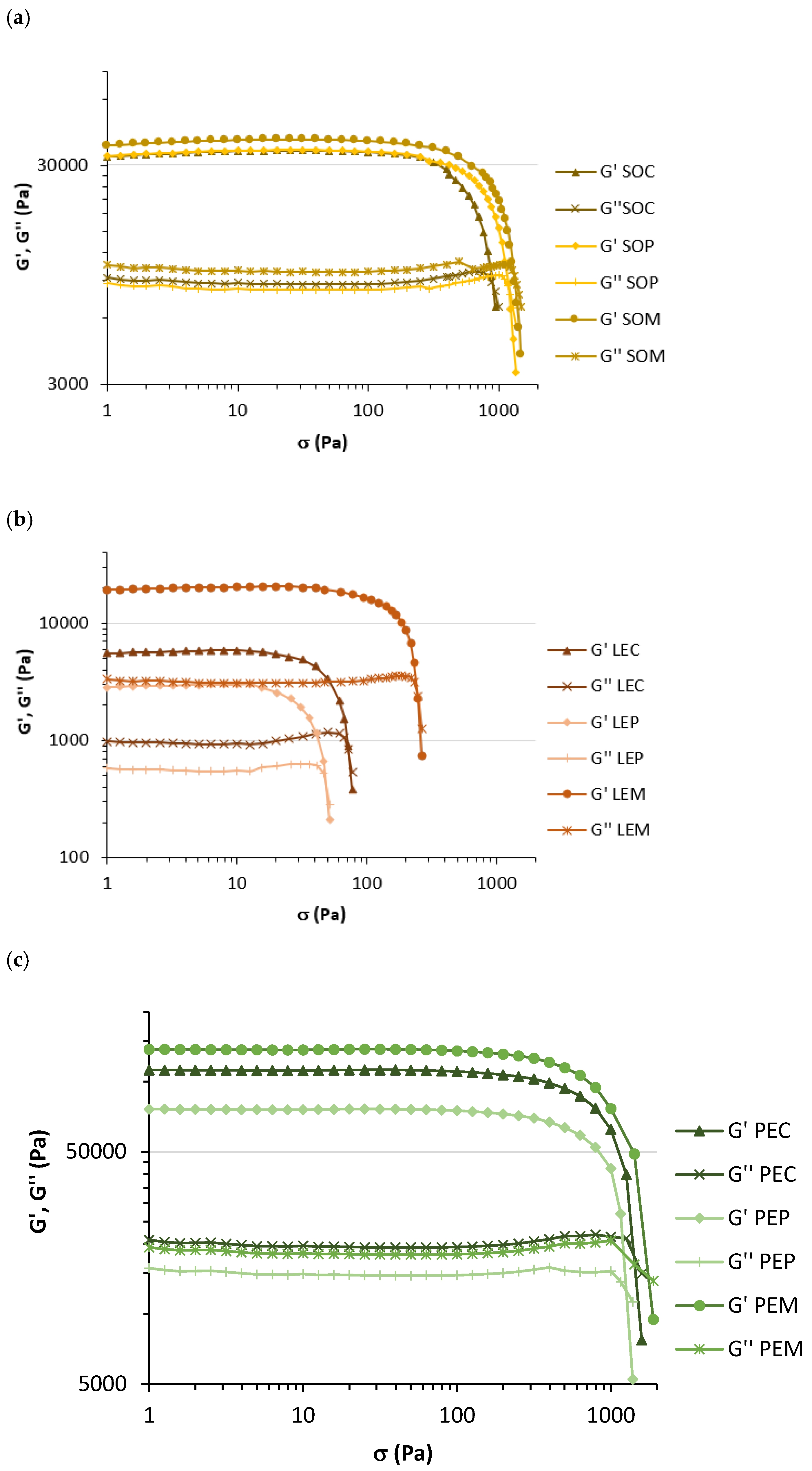
3.1. Physicochemical, Textural, and Rheological Characteristics of Legume Pastes
3.2. Protein Digestibility of Legume Pastes
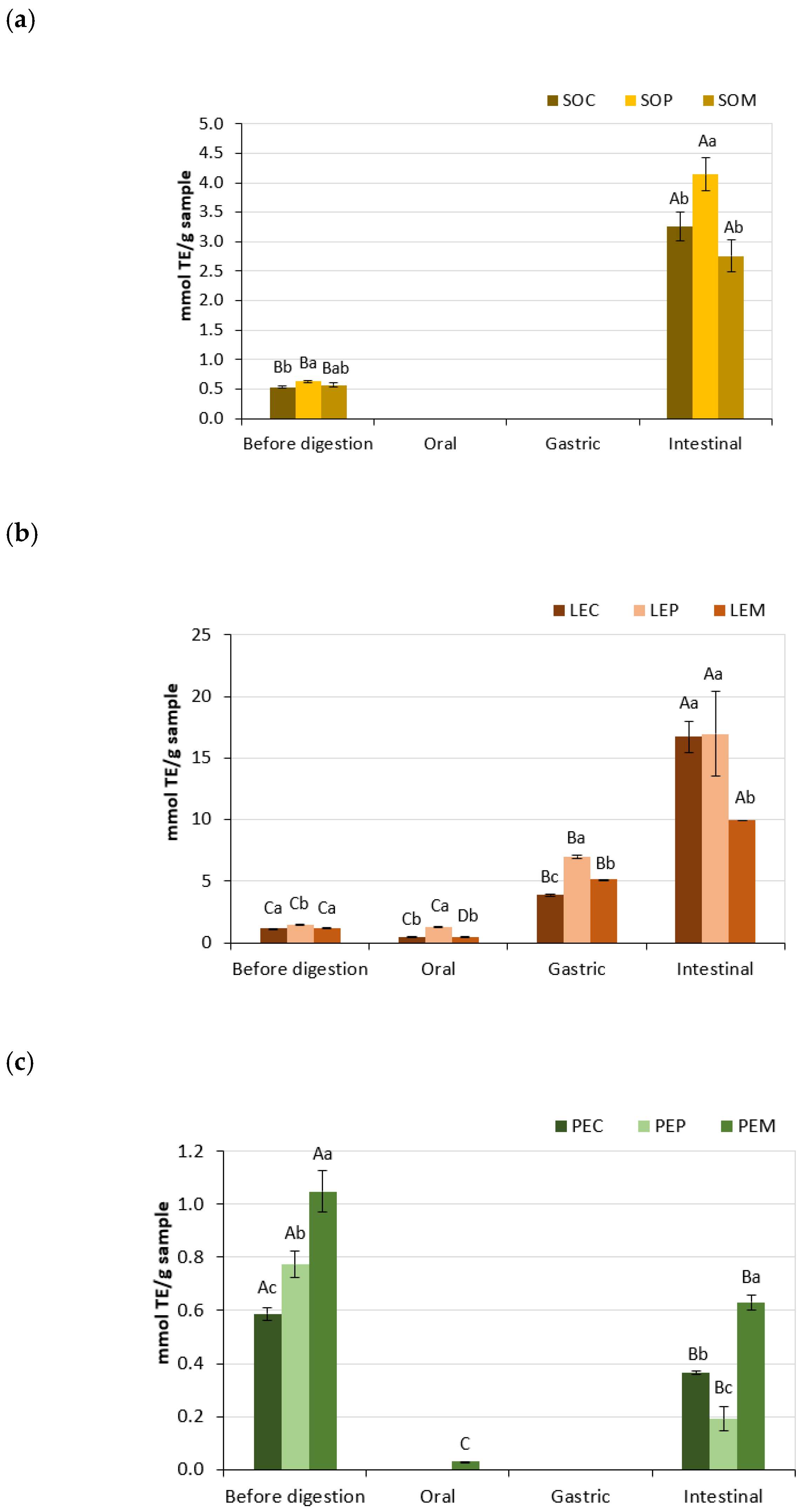
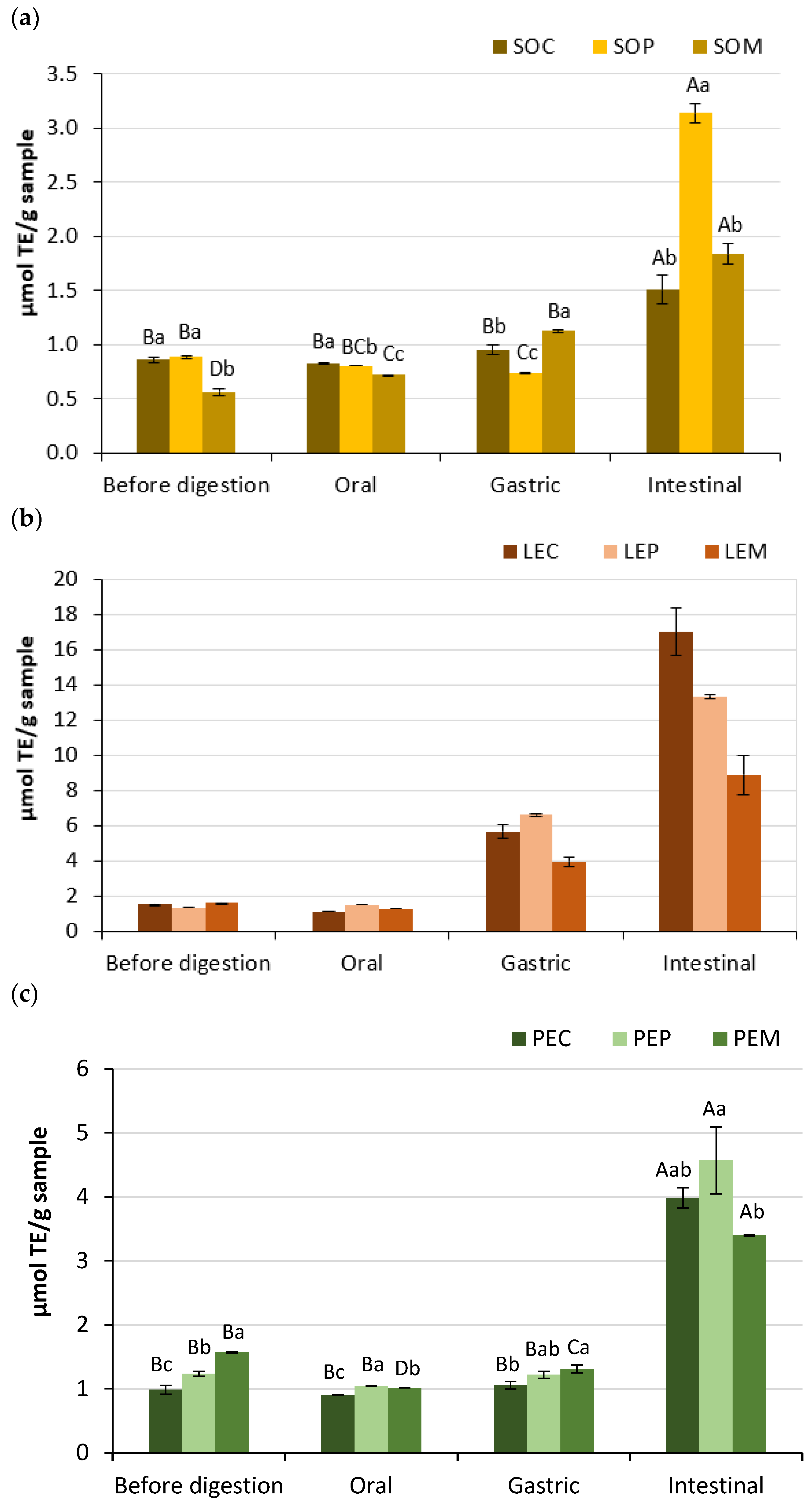
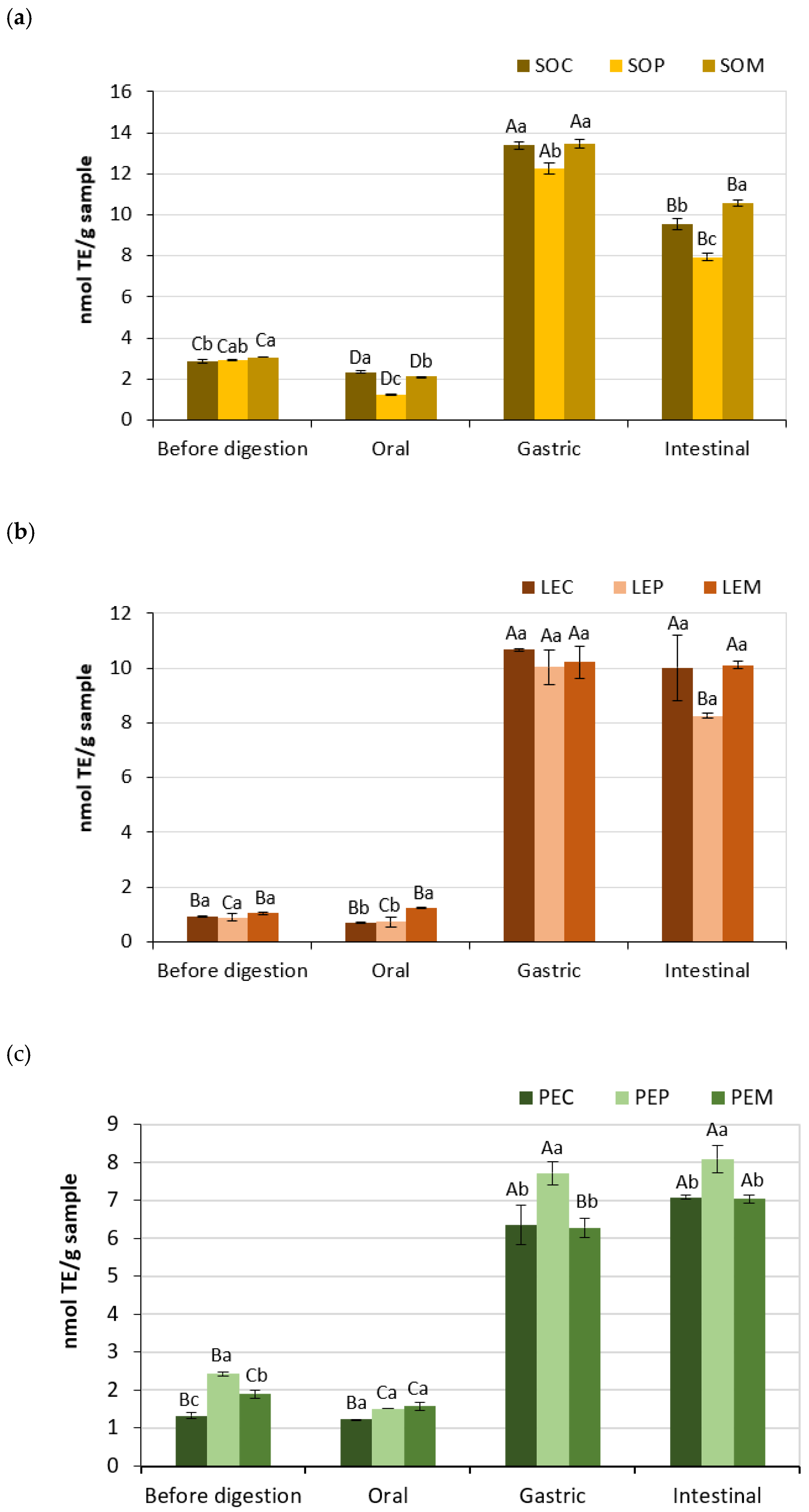
3.3. Antioxidant Activity of Legume Pastes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gueguen, J.; Cerletti, P. Proteins of some legume seeds: Soybean, pea, fababean and lupin. In New and Developing Sources of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1994; pp. 145–193. [Google Scholar]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Park, D.J. Texture-modified foods for the elderly: Status, technology and opportunities. Trends Food Sci. Technol. 2016, 57, 156–164. [Google Scholar] [CrossRef]
- Lutz, M.; Petzold, G.; Albala, C. Considerations for the development of innovative foods to improve nutrition in older adults. Nutrients 2019, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawy, A.A.; Sobihah, T.Y. Effect of soaking process on nutritional quality and protein solubility of some legume seeds. Nahrung 2000, 44, 339–343. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldivar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Drulyte, D.; Orlien, V. The effect of processing on digestion of legume proteins. Foods 2019, 8, 224. [Google Scholar] [CrossRef]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive compounds in legumes: Pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Wang, S.; Melnyk, J.P.; Tsao, R.; Marcone, M.F. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Patil, S.S.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. The effects of fortification of legumes and extrusion on the protein digestibility of wheat-based snack. Foods 2016, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Talens, P.; Castells, M.L.; Verdú, S.; Barat, J.M.; Grau, R. Flow, viscoelastic and masticatory properties of tailor made thickened pea cream for people with swallowing problems. J. Food Eng. 2021, 292, 110265. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ketnawa, S.; Ogawa, Y. Evaluation of protein digestibility of fermented soybeans and changes in biochemical characteristics of digested fractions. J. Funct. Foods 2019, 52, 640–647. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Donlao, N.; Ogawa, Y. Impacts of processing conditions on digestive recovery of polyphenolic compounds and stability of the antioxidant activity of green tea infusion during in vitro gastrointestinal digestion. LWT 2018, 89, 648–656. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Huang, S.J.; Mau, J.L. Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem. 2006, 98, 670–677. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Barampama, Z.; Simard, R.E. Effects of soaking, cooking and fermentation on composition, in-vitro starch digestibility and nutritive value of common beans. Plant Foods Hum. Nutr. 1995, 48, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Alajaji, S.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compos. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Güzel, D.; Sayar, S. Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. J. Food Sci. Technol. 2012, 49, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, T.H. Effect of processing methods on nutritional composition and anti-nutritional factors in lentils (Lens culinaris). Ann. Agric. Sci. 2011, 56, 57–61. [Google Scholar] [CrossRef]
- Bayram, M.; Oner, M.D.; Kaya, A. Influence of soaking on the dimensions and colour of soybean for bulgur production. J. Food Eng. 2004, 61, 331–339. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Fuentes, R.; Guerrero, G.; Canet, W. Characterization of commercial Spanish hummus formulation: Nutritional composition, rheology, and structure. Int. J. Food Prop. 2017, 20, 845–863. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Tovar, C.A.; Borderías, J. Effect of several cryoprotectants on the physicochemical and rheological properties of suwari gels from frozen squid surimi made by two methods. J. Food Eng. 2010, 97, 457–464. [Google Scholar] [CrossRef]
- Cho, H.M.; Yoo, B. Rheological characteristics of cold thickened beverages containing xanthan gum–based food thickeners used for dysphagia diets. J. Acad. Nutr. Diet. 2015, 115, 106–111. [Google Scholar] [CrossRef]
- Le Gall, M.; Guéguen, J.; Séve, B.; Quillien, L. Effects of grinding and thermal treatments on hydrolysis susceptibility of pea proteins (Pisum sativum L.). J. Agric. Food Chem. 2005, 53, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Rutherfurd, S.M.; Muñoz, L.S.; Peters, M.; Montoya, C.A. The impact of heating and soaking on the in vitro enzymatic hydrolysis of protein varies in different species of tropical legumes. Food Chem. 2016, 194, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility- digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Deshpande, R.; Marathe, S.A. Effect of processing conditions and in vitro protein digestion on bioactive potentials of commonly consumed legumes. Food Biosci. 2017, 20, 1–11. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6326–6336. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism, 6th ed.; Wadsworth Publishing: Belmont, CA, USA, 2013. [Google Scholar]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The role of dietary phenolic compounds in protein digestion and processing technologies to improve their antinutritive properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef]
- Chen, C.C.; Shih, Y.C.; Chiou, P.W.S.; Yu, B. Evaluating nutritional quality of single stage-and two stage-fermented soybean meal. Asian-Australas. J. Anim. Sci. 2010, 23, 598–606. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein-polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Liao, W.; Gu, L.; Zheng, Y.; Zhu, Z.; Zhao, M.; Liang, M.; Ren, J. Analysis of the quantitative structure–activity relationship of glutathione-derived peptides based on different free radical scavenging systems. Med. Chem. Commun. 2016, 7, 2083–2093. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of Alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Soybean | |||
| Ordinary Cooking | Pressure Cooking | Microwave Cooking | ||
| Moisture content (%) | 71.40 ± 1.20 a | 70.85 ± 0.37 a | 71.15 ± 0.91 a | |
| aw | 0.99 ± 0.00 a | 0.99 ± 0.00 a | 0.99 ± 0.00 a | |
| pH | 6.58 ± 0.01 a | 6.50 ± 0.04 a | 6.38 ± 0.03 b | |
| Total soluble solids (° Brix) | 13.13 ± 0.09 c | 13.27 ± 0.04 b | 13.78 ± 1.16 a | |
| Color | L* | 79.11 ± 0.48 b | 79.09 ± 0.16 b | 80.42 ± 0.38 a |
| C* | 20.78 ± 0.68 a | 21.32 ± 0.49 a | 21.44 ± 0.34 a | |
| h* | 83.23 ± 0.13 c | 84.17 ± 0.16 b | 87.45 ± 0.35 a | |
| Parameter | Lentil | |||
| Ordinary cooking | Pressure cooking | Microwave cooking | ||
| Moisture content (%) | 76.40 ± 0.23 b | 78.79 ± 0.43 a | 73.57 ± 0.62 c | |
| aw | 0.99 ± 0.00 a | 0.99 ± 0.00 a | 0.99 ± 0.00 a | |
| pH | 7.11 ± 0.04 a | 6.98 ± 0.02 ab | 6.78 ± 0.12 b | |
| Total soluble solids (° Brix) | 3.48 ± 0.02 b | 2.77 ± 0.38 b | 7.80 ± 0.33 a | |
| Color | L* | 48.42 ± 0.47 b | 47.24 ± 0.43 b | 50.63 ± 0.62 a |
| C* | 10.94 ± 0.82 b | 12. 70 ± 0.02 a | 12.16 ± 0.11 ab | |
| h* | 68.19 ± 0.76 b | 64.66 ± 1.06 c | 77.85 ± 0.03 a | |
| Parameter | Pea | |||
| Ordinary cooking | Pressure cooking | Microwave cooking | ||
| Moisture content (%) | 66.56 ± 0.20 b | 68.60 ± 0.30 a | 65.40 ± 0.07 c | |
| aw | 0.99 ± 0.00 a | 0.99 ± 0.00 a | 0.99 ± 0.00 a | |
| pH | 6.03 ± 0.08 a | 5.96 ± 0.09 a | 6.06 ± 0.04 a | |
| Total soluble solids (° Brix) | 6.85 ± 1.47 b | 5.85 ± 0.14 b | 13.49 ± 0.08 a | |
| Color | L* | 61.33 ± 0.45 a | 61.99 ± 0.30 a | 62.06 ± 0.21 a |
| C* | 28.87 ± 0.40 b | 29.58 ± 0.33 ab | 30.20 ± 0.10 a | |
| h* | 88.31 ± 0.30 a | 87.52 ± 0.05 b | 88.53 ± 0.13 a | |
| Parameter | Soybean | |||
| Ordinary Cooking | Pressure Cooking | Microwave Cooking | ||
| Back extrusion | Max. force (N) | 20.83 ± 2.87 a | 18.79 ± 1.69 a | 23.03 ± 3.70 a |
| AUC (N·mm) | 543 ± 50 a | 512 ± 57 a | 606 ± 100 a | |
| LAOS | G′LVR (Pa) | 39757 ± 8421 a | 35077 ± 1051 a | 45944 ± 11974 a |
| StressLVR (Pa) | 158 ± 0 a | 115 ± 16 b | 158 ± 0 a | |
| Flow point (Pa) | 9463 ± 82 b | 8495 ± 7 c | 9839 ± 128 a | |
| SAOS | G* (Pa) | 41955 ± 8676 a | 36905 ± 1435 a | 48645 ± 11802 a |
| G′ (Pa) | 40860 ± 8655 a | 35955 ± 1435 a | 47490 ± 11922 a | |
| G′′ (Pa) | 9489 ± 1105 a | 8330 ± 182 a | 10445 ± 729 a | |
| ƞ* (Pa · s) | 6678 ± 1381 a | 5874 ± 228 a | 7743 ± 1877 a | |
| δ (°) | 13.20 ± 1.23 a | 13.05 ± 0.23 a | 12.68 ± 2.24 a | |
| Tan δ | 0.23 ± 0.02 a | 0.23 ± 0.00 a | 0.22 ± 0.04 a | |
| Parameter | Lentil | |||
| Ordinary cooking | Pressure cooking | Microwave cooking | ||
| Back extrusion | Max. force (N) | 2.16 ± 0.14 b | 1.08 ± 0.13 b | 7.12 ± 1.58 a |
| AUC (N·mm) | 48 ± 2 b | 26 ± 4 b | 163 ± 31 a | |
| LAOS | G′LVR (Pa) | 4722 ± 982 b | 2869 ± 85 b | 24300 ± 7071 a |
| StressLVR (Pa) | 18 ± 3 b | 15 ± 0 b | 47 ± 0 a | |
| Flow point (Pa) | 735 ± 238 b | 487 ± 41 b | 3365 ± 306 a | |
| SAOS | G* (Pa) | 5159 ± 1086 b | 3125 ± 90 b | 25975 ± 7573 a |
| G′ (Pa) | 5090 ± 1078 b | 3073 ± 86 b | 25750 ± 7594 a | |
| G′′ (Pa) | 839 ± 136 b | 569 ± 26 b | 3350 ± 316 a | |
| ƞ* (Pa · s) | 821 ± 173 b | 497 ± 14 b | 4134 ± 1205 a | |
| δ (°) | 9.41 ± 0.46 a | 10.48 ± 0.19 a | 7.63 ± 1.54 a | |
| Tan δ | 0.17 ± 0.01 a | 0.19 ± 0.00 a | 0.13 ± 0.03 a | |
| Parameter | Pea | |||
| Ordinary cooking | Pressure cooking | Microwave cooking | ||
| Back extrusion | Max. force (N) | 60.59 ± 1.92 a | 42.99 ± 5.27 b | 66.85 ± 6.80 a |
| AUC (N·mm) | 1696 ± 77 a | 1119 ± 98 b | 1827 ± 179 a | |
| LAOS | G′LVR (Pa) | 115464 ± 14617 ab | 84243 ± 14930 b | 132086 ± 1720 a |
| StressLVR (Pa) | 251 ± 0 a | 158 ± 0 b | 158 ± 0 b | |
| Flow point (Pa) | 21351 ± 239 b | 16668 ± 725 c | 28959 ± 377 a | |
| SAOS | G* (Pa) | 124400 ± 15698 ab | 87735 ± 14927 b | 137082 ± 1671 a |
| G′ (Pa) | 122850 ± 15910 ab | 86580 ± 15344 b | 135750 ± 1768 a | |
| G′′ (Pa) | 19320 ± 523 a | 13905 ± 1393 b | 18628 ± 441 a | |
| ƞ* (Pa · s) | 19795 ± 2496 ab | 13960 ± 2376 b | 21815 ± 275 a | |
| δ (°) | 9.03 ± 1.40 a | 9.34 ± 2.53 a | 7.80 ± 0.28 a | |
| Tan δ | 0.16 ± 0.02 a | 0.16 ± 0.04 a | 0.14 ± 0.01 a | |
| Soybean | ||||
|---|---|---|---|---|
| Digestion Phase | Ordinary Cooking | Pressure Cooking | Microwave Cooking | |
| Soluble proteins (mg/g) | Before | 15.04 ± 0.62 Aa | 12.71 ± 1.48 Ab | 9.62 ± 0.55 Bc |
| Oral | 1.03 ± 0.46 Cb | 1.46 ± 0.66 Cb | 3.39 ± 0.33 Da | |
| Gastric | 1.48 ± 0.23 Cb | 2.75 ± 1.17 Cb | 5.88 ± 0.40 Ca | |
| Intestinal | 9.23 ± 1.04 Bb | 7.67 ± 0.46 Bb | 23.05 ± 2.42 Aa | |
| TCA-soluble peptides (mg/g) | Before | 8.31 ± 0.25 Ca | 8.19 ± 0.16 Ca | 7.83 ± 0.02 Ca |
| Oral | 5.92 ± 0.02 Db | 7.87 ± 0.53 Ca | 7.94 ± 0.02 Ca | |
| Gastric | 24.36 ± 0.69 Bc | 33.45 ± 0.39 Bb | 39.63 ± 0.98 Ba | |
| Intestinal | 73.13 ± 0.46 Aa | 80.08 ± 3.17 Aa | 81.56 ± 5.04 Aa | |
| Free amino groups (mg/g) | Before | 2.50 ± 0.02 Cb | 2.52 ± 0.03 Cb | 3.38 ± 0.09 Ca |
| Oral | 6.00 ± 0.09 Ca | 3.82 ± 0.02 Cb | 6.00 ± 0.11 Ca | |
| Gastric | 40.41 ± 1.24 Bb | 41.78 ± 0.34 Bab | 44.02 ± 0.05 Ba | |
| Intestinal | 128.76 ± 5.48 Ab | 148.96 ± 13.59 Aab | 165.28 ± 10.59 Aa | |
| Total free phenolics (mg/g) | Before | 1.16 ± 0.02 Ba | 1.09 ± 0.02 Bb | 1.07 ± 0.01 Cb |
| Oral | 0.84 ± 0.07 Ba | 0.61 ± 0.00 Cb | 0.94 ± 0.06 Ca | |
| Gastric | 3.87 ± 0.35 Aa | 3.81 ± 0.17 Aa | 4.16 ± 0.12 Aa | |
| Intestinal | — | 0.23 ± 0.19 Db | 1.63 ± 0.27 Ba | |
| Lentil | ||||
| Digestion phase | Ordinary cooking | Pressure cooking | Microwave cooking | |
| Soluble proteins (mg/g) | Before | 9.09 ± 0.77 Aa | 8.71 ± 0.60 Aa | 7.17 ± 0.64 Ab |
| Oral | 1.13 ± 0.10 Ba | 1.31 ± 0.15 Ca | 1.23 ± 0.09 Ca | |
| Gastric | 0.26 ± 0.14 Ba | 0.38 ± 0.13 Ca | 0.33 ± 0.19 Ca | |
| Intestinal | 8.87 ± 0.79 Aa | 7.33 ± 1.36 Ba | 2.83 ± 0.71 Bb | |
| TCA-soluble peptides (mg/g) | Before | 2.76 ± 0.20 Cb | 3.20 ± 0.05 Ba | 3.21 ± 0.02 Ca |
| Oral | 1.35 ± 0.08 Db | 1.02 ± 0.04 Bc | 1.88 ± 0.04 Da | |
| Gastric | 11.53 ± 0.12 Bb | 14.05 ± 0.02 Bb | 13.78 ± 0.11 Ba | |
| Intestinal | 77.93 ± 0.28 Aa | 52.55 ± 11.22 Ab | 41.64 ± 0.25 Ab | |
| Free amino groups (mg/g) | Before | 2.86 ± 0.05 Cb | 3.03 ± 0.02 Cb | 3.73 ± 0.08 Ca |
| Oral | 6.20 ± 0.08 Ca | 6.63 ± 0.21 Ca | 6.43 ± 0.24 Ca | |
| Gastric | 30.97 ± 0.55 Ba | 25.08 ± 0.97 Bb | 29.71 ± 0.33 Ba | |
| Intestinal | 74.90 ± 4.55 Aa | 40.13 ± 7.00 Ab | 73.95 ± 11.56 Aa | |
| Total free phenolics (mg/g) | Before | 0.56 ± 0.00 Cb | 0.64 ± 0.02 Ba | 0.63 ± 0.01 Ba |
| Oral | 0.59 ± 0.05 Cb | 0.77 ± 0.01 Ba | 0.54 ± 0.04 Bb | |
| Gastric | 3.16 ± 0.01 Aa | 3.00 ± 0.22 Aa | 2.95 ± 0.21 Aa | |
| Intestinal | 2.21 ± 0.60 Ba | — | — | |
| Pea | ||||
| Digestion phase | Ordinary cooking | Pressure cooking | Microwave cooking | |
| Soluble proteins (mg/g) | Before | 6.28 ± 0.41 Ba | 3.15 ± 0.25 Bb | 5.82 ± 0.19 Ba |
| Oral | 3.76 ± 0.31 Ca | 1.18 ± 0.17 Cc | 2.23 ± 0.17 Cb | |
| Gastric | 0.79 ± 0.10 Da | 0.67 ± 0.02 Ca | 0.88 ± 0.24 Da | |
| Intestinal | 8.16 ± 1.04 Aa | 9.05 ± 1.31 Aa | 9.07 ± 0.79 Aa | |
| TCA-soluble peptides (mg/g) | Before | 2.58 ± 0.03 Cb | 2.27 ± 0.13 Cc | 3.79 ± 0.09 Ca |
| Oral | 1.26 ± 0.05 Cb | 2.64 ± 0.11 Ca | 2.49 ± 0.14 Ca | |
| Gastric | 5.02 ± 0.42 Bc | 11.02 ± 0.72 Ba | 7.86 ± 0.41 Bb | |
| Intestinal | 33.75 ± 0.79 Ac | 59.76 ± 4.21 Aa | 45.05 ± 2.29 Ab | |
| Free amino groups (mg/g) | Before | 2.06 ± 0.06 Dc | 3.61 ± 0.02 Ca | 3.26 ± 0.04 Db |
| Oral | 4.62 ± 0.25 Cb | 5.89 ± 0.08 Ca | 6.17 ± 0.02 Ca | |
| Gastric | 17.18 ± 0.72 Ba | 19.84 ± 2.86 Ba | 17.31 ± 0.10 Ba | |
| Intestinal | 61.11 ± 1.19 Ab | 74.00 ± 5.47 Aa | 51.77 ± 0.76 Ab | |
| Total free phenolics (mg/g) | Before | 0.59 ± 0.02 Bb | 0.71 ± 0.01 Ca | 0.71 ± 0.01 Ba |
| Oral | 0.59 ± 0.04 Ba | 0.68 ± 0.00 Ca | 0.65 ± 0.05 Ba | |
| Gastric | 1.87 ± 0.09 Ab | 2.30 ± 0.08 Aa | 1.84 ± 0.05 Ab | |
| Intestinal | 0.31 ± 0.04 Cb | 1.15 ± 0.22 Ba | — | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, M.; Arnal, M.; Barat, J.M.; Talens, P. Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods 2021, 10, 47. https://doi.org/10.3390/foods10010047
Gallego M, Arnal M, Barat JM, Talens P. Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods. 2021; 10(1):47. https://doi.org/10.3390/foods10010047
Chicago/Turabian StyleGallego, Marta, Milagros Arnal, José Manuel Barat, and Pau Talens. 2021. "Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes" Foods 10, no. 1: 47. https://doi.org/10.3390/foods10010047
APA StyleGallego, M., Arnal, M., Barat, J. M., & Talens, P. (2021). Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods, 10(1), 47. https://doi.org/10.3390/foods10010047