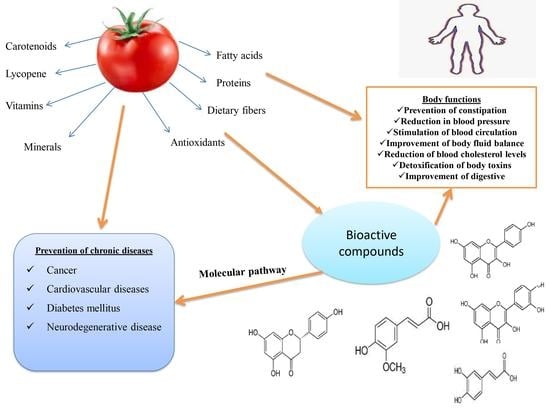
Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review
Abstract
1. Introduction
2. Methodology
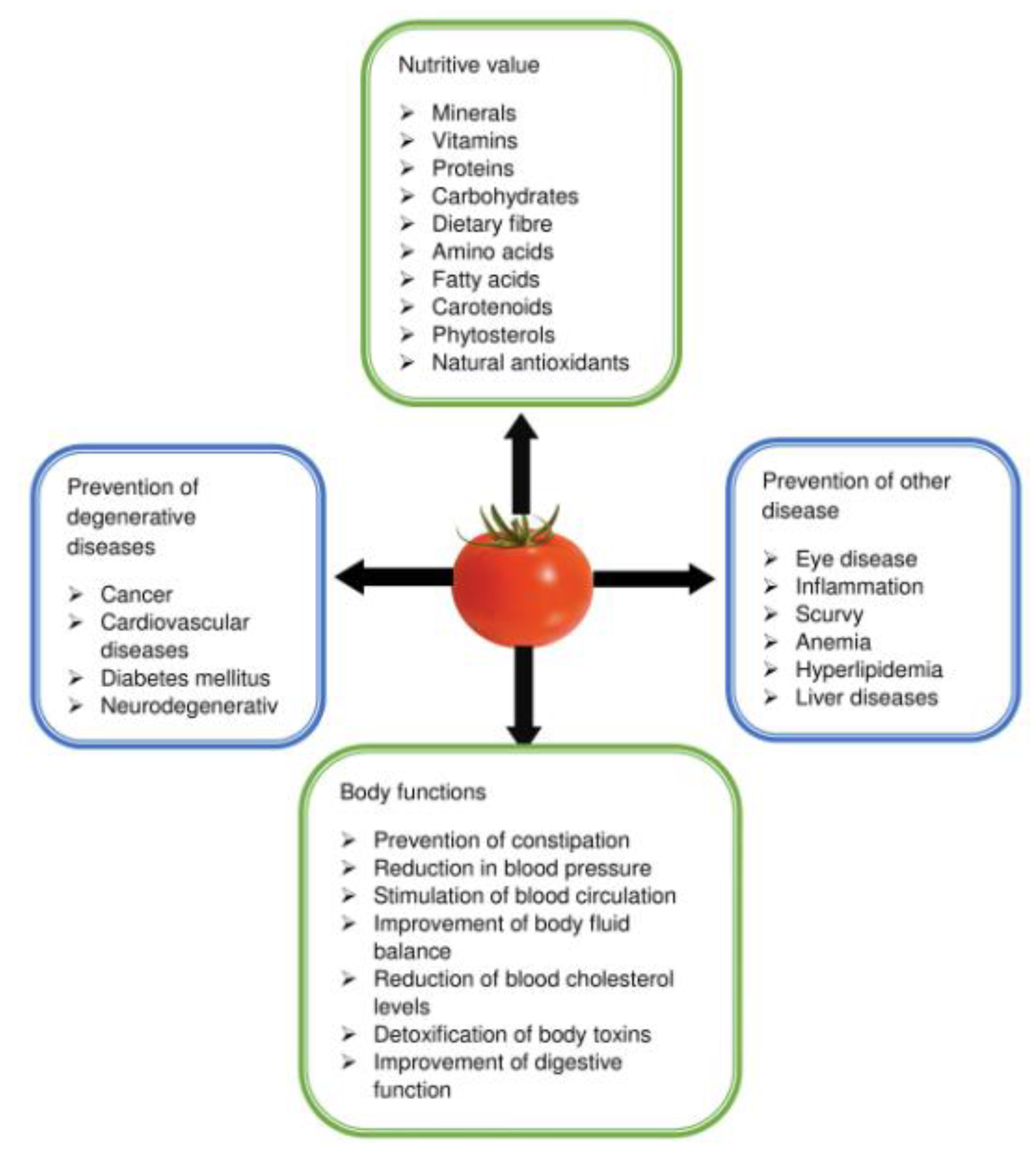
3. Nutritional Composition of Tomato
3.1. Proximate Composition
3.2. Mineral Content
3.3. Vitamin Content
3.4. Fatty Acid Content
3.5. Amino Acid Content
3.6. Carotenoid Content
3.7. Sterol Content
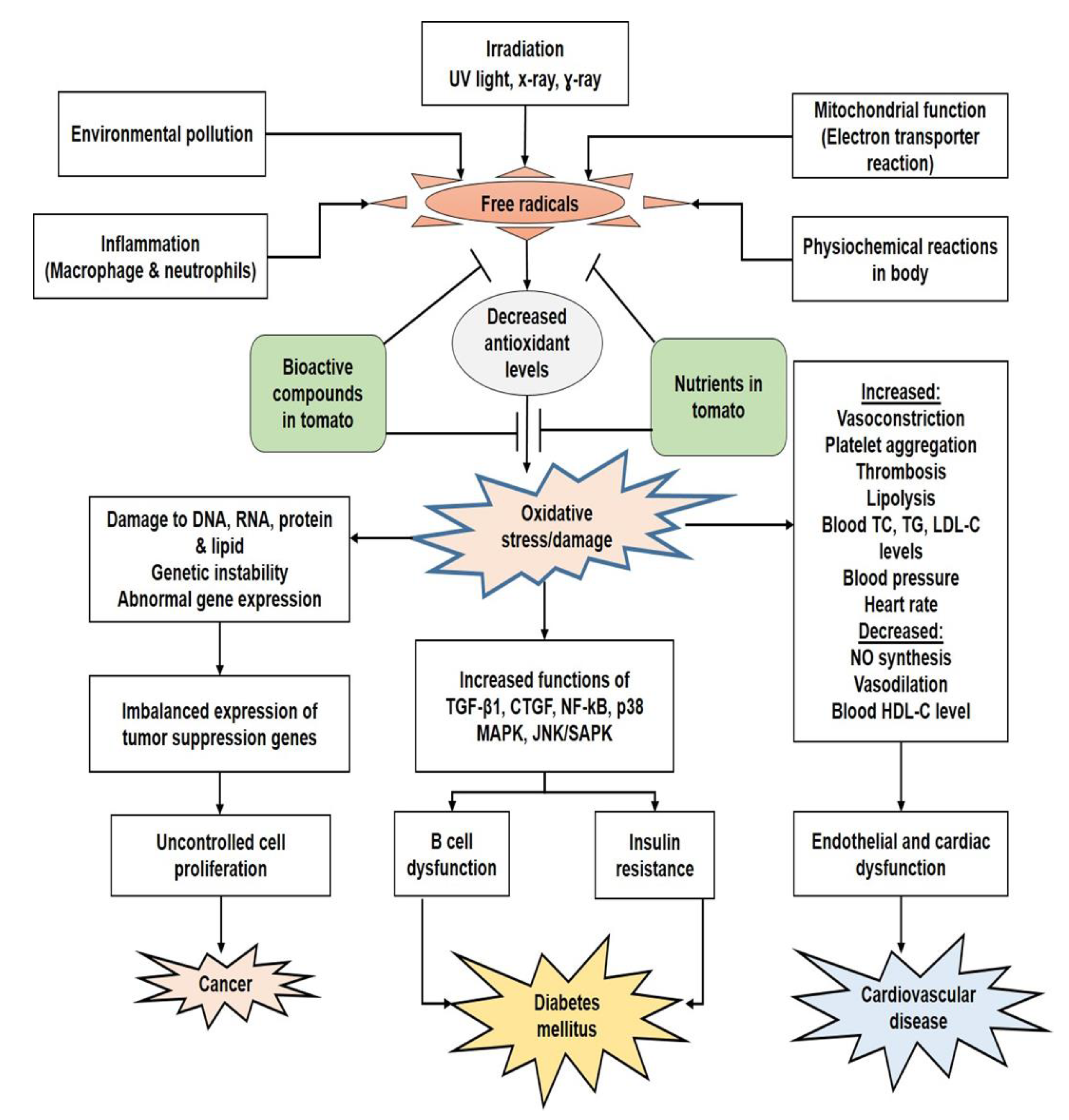
4. Antioxidant Properties and Bioactive Compounds in Tomato
5. Health Benefits of Tomato
6. Effects of Bioactive Compounds of Tomato on Some Human Degenerative Diseases
6.1. Tomato in CVDs
6.2. Tomato in Diabetes
6.3. Tomato against Cancer
7. Limitations and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Abdullahi, I.I.; Abdullahi, N.; Abdu, A.M.; Ibrahim, A.S. Proximate, Mineral and Vitamin Analysis of Fresh and Canned Tomato. Biosci. Biotechnol. Res. Asia 2016, 13, 1163–1169. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Romero-Gonzalez, R.; Gonzalez-Fernandes, M.J.; Guil-Guerrero, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Campestrini, L.H.; Melo, P.S.; Peres, L.E.P.; Calhelha, R.C.; Ferreira, I.C.; De Alencar, S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon 2019, 5, e02831. [Google Scholar] [CrossRef]
- Vats, S.; Bansal, R.; Rana, N.; Kumawat, S.; Bhatt, V.; Jadhav, P.; Kale, V.; Sathe, A.; Sonah, H.; Jugdaohsingh, R.; et al. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food Sci. Nutr. 2020, 1–32. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2020, 128396. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Navarro-González, I.; García-Alonso, J.; Periago, M.J. Bioactive compounds of tomato: Cancer chemopreventive effects and influence on the transcriptome in hepatocytes. J. Funct. Foods 2018, 42, 271–280. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, A.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.-D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Sakemi, Y.; Sato, K.; Hara, K.; Honda, M.; Shindo, K. Biological Activities of Z-Lycopenes Contained in Food. J. Oleo Sci. 2020, 69, 1509–1516. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Metabolomics-inspired insight into developmental, environmental and genetic aspects of tomato fruit chemical composition and quality. Plant Cell Physiol. 2015, 56, 1681–1696. [Google Scholar] [CrossRef]
- Claye, S.S.; Idouraine, A.; Weber, C.W. Extraction and fractionation of insoluble fiber from five fiber sources. Food Chem. 1996, 57, 305–310. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Olivares, M.; Neyrinck, A.M.; Beaumont, M.; Larsen, T.M.; Benitez-Paez, A.; Romani-Perez, M.; Garcia-Campayo, V.; Bosscher, D.; Sanz, Y.; et al. Nutritional interest of dietary fiber and prebiotics in obesity: Lessons from the MyNewGut consortium. Clin. Nutr. 2020, 39, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Merenkova, S.; Zinina, O.; Stuart, M.; Okuskhanova, E.; Androsova, N. Effects of dietary fiber on human health: A review. Чeлoвeк. Cпopт. Meдицинa. 2020, 20, 106–113. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Akanda, J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef] [PubMed]
- Paulino, S.L.J.; Adrián, Á.-T.G.; Gabriela, E.-A.L.; Maribel, V.-M.; Sergio, M.-G. Nutraceutical potential of flours from tomato by-product and tomato field waste. J. Food Sci. Technol. 2020, 57, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Lu, Z.-H.; Maharaj, P.L.; Winberg, J.; Brummer, Y.; Hawke, A. Proximate Analysis and Nutritional Evaluation of Twenty Canadian Lentils by Principal Component and Cluster Analyses. Foods 2020, 9, 175. [Google Scholar] [CrossRef]
- Harris, G.K.; Marshall, M.R. Ash analysis. In Food Analysis; Springer: Cham, Switzerland, 2017; pp. 287–297. [Google Scholar]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.C.; Alché, J.D.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef]
- Nielsen, S.S. Determination of moisture content. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 17–27. [Google Scholar]
- Aurand, L.W. Food Composition and Analysis; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Pomeranz, Y. Food Analysis: Theory and Practice; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Phizicky, E.; Bastiaens, P.I.H.; Zhu, H.; Snyder, M.; Fields, S. Protein analysis on a proteomic scale. Nature 2003, 422, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Advances in Lipid Methodology; Woodhead Publishing Ltd.: Cambridge, UK, 1007; Volume 4. [Google Scholar]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Woodhead Publishing Ltd.: Cambridge, UK, 2019. [Google Scholar]
- BeMiller, J.N. Carbohydrate Analysis. In Food Analysis; Springer: Boston, MA, USA, 2010; pp. 147–177. [Google Scholar]
- Buttriss, J.; Stokes, C.S. Dietary fibre and health: An overview. Nutr. Bull. 2008, 33, 186–200. [Google Scholar] [CrossRef]
- Favier, J.C.; Ireland-Ripert, J.; Toque, C.; Feinberg, M. Repertoire general des aliments, table de composition. Mitt. Geb. Lebensm. Hyg. 1997, 88, 209. [Google Scholar]
- Souci, S.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables, 5th ed.; Medpharm GmbH, Scientific Publishers: Stuttgart, Germany; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- David, H.; Linda, L.; Pamela, P. National Nutrient Database for Standard Reference; Release 24; USDA: Beltsville, MD, USA, 2016.
- Hernández, M.H.; Rodríguez, E.R.; Romero, C.D. Chemical composition of tomato (Lycopersicon esculentum) from Tenerife, the Canary Islands. Food Chem. 2008, 106, 1046–1056. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago-Castón, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Oboulbiga, E.B.; Parkouda, C.; Sawadogo-Lingani, H.; Compaoré, E.W.R.; Sakira, A.K.; Traoré, A.S. Nutritional Composition, Physical Characteristics and Sanitary Quality of the Tomato Variety Mongol F1 from Burkina Faso. Food Nutr. Sci. 2017, 8, 444–455. [Google Scholar] [CrossRef][Green Version]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Rebolloso-Fuentes, M. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J. Food Compos. Anal. 2009, 22, 123–129. [Google Scholar] [CrossRef]
- Opara, U.L.; Al-Ani, M.R.; Al-Rahbi, N.M. Effect of fruit ripening stage on physico-chemical properties, nutritional composition and antioxidant components of tomato (Lycopersicum esculentum) cultivars. Food Bioprocess Technol. 2012, 5, 3236–3243. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef]
- Raffo, A.; Leonardi, C.; Fogliano, V.; Ambrosino, P.; Salucci, M.; Gennaro, L.; Bugianesi, R.; Giuffrida, F.; Quaglia, G. Nutritional value of cherry tomatoes (Lycopersicon esculentum Cv. Naomi F1) harvested at different ripening stages. J. Agric. Food Chem. 2002, 50, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kulshreshtha, K. Nutritional Content and Significance of Tomato Powder. Ann. Arid Zone 2013, 52, 121–124. [Google Scholar]
- Ahmed, M.J.; Iya, I.R.; Dogara, M.F. Proximate, Mineral and Vitamin Content of Flesh, Blanched and Dried Tomatoes (Lycopersicon esculentum). Asian Food Sci. J. 2020, 18, 11–18. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.; Fausto, N.; Aster, J. Robbins and Cotran Pathologic Basis of Disease; Elsevier: Philadelphia, PA, USA, 2005. [Google Scholar]
- Baer, L.; Platman, S.R.; Fieve, R.R. The role of electrolytes in affective disorders: Sodium, potassium, and lithium ions. Arch. Gen. Psychiatry 1970, 22, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ali, Y.; Rumpa, N.-E.-N.; Tanvir, E.M.; Hossen, S.; Saha, M.; Bhoumik, N.C.; Gan, S.H.; Khalil, I. Assessment of toxicity and beneficiary effects of garcinia pedunculata on the hematological, biochemical, and histological homeostasis in rats. Evid. Based Complementary Altern. Med. 2017, 2017, 4686104. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, C.; Sastri, B.R.; Balasubramanian, S. Nutrition Value of Indian Foods; National Institute of Nutrition: Hyderabad, India, 1980. [Google Scholar]
- Ionete, R.E.; Dinca, O.R.; Geana, E.I.; Costinel, D. Macro-and microelements as possible markers of quality and authenticity for fruits and derived products. Prog. Cryog. Isot. Sep. 2016, 19, 55. [Google Scholar]
- Solayman, M.; Islam, A.; Paul, S.; Ali, Y.; Khalil, I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- Nielsen, F.H. Ultratrace elements in nutrition. Annu. Rev. Nutr. 1984, 4, 21–41. [Google Scholar] [CrossRef]
- Zugravu, C.A.; Parvu, M.; Patrascu, D.; Stoian, A. Correlations between lead and cadmium pollution of honey and environmental heavy metal presence in two Romanian counties. Bull. UASVM Agric. 2009, 66, 230–233. [Google Scholar]
- Campbell, J.K.; Canene-Adams, K.; Lindshield, B.L.; Boileau, T.W.-M.; Clinton, S.K.; Erdman, J.J.W. Tomato phytochemicals and prostate cancer risk. J. Nutr. 2004, 134, 3486S–3492S. [Google Scholar] [CrossRef]
- Favier, J.C.; Ireland-Ripert, J.; Toque, C.; Feinberg, M. Répertoire Général des Alimentes: Table de Composition = Composition Tables; INRA: Paris, France, 1995. [Google Scholar]
- Ramesh, K.V.; Paul, V.; Pandey, R. Dynamics of mineral nutrients in tomato (Solanum lycopersicum L.) fruits during ripening: Part I—On the plant. Plant Physiol. Rep. 2020, 1–15. [Google Scholar] [CrossRef]
- Gerald, F. The Vitamins Fundamental Aspects in Nutrition and Health; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Beecher, G.R. Nutrient content of tomatoes and tomato products. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Borguini, R.G.; Torres, E.A.F.D.S. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009, 25, 313–325. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sundquist, A.R. Antioxidant functions of vitamins. Ann. N. York Acad. Sci. 1992, 669, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.J.W. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Sure, B. The vitamins in health and disease. Nature 1933, 132, 732. [Google Scholar]
- Marcus, R.; Coulston, A.M. The vitamin B complex and ascorbic acid. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 10th ed.; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables 1981/82, 2nd ed.; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 1981. [Google Scholar]
- USDA. National Nutrient Database for Standard Reference; Release 20; US Department of Agriculture: Beltsville, MD, USA, 2008.
- Kadiri, M.; Ojewumi, A.; Olawale, S. Minerals, vitamins and chlorophyll contents of fruits, stems and leaves of tomato and garden egg. Pak. J. Food Sci. 2015, 25, 150–154. [Google Scholar]
- Erba, D.; Casiraghi, M.C.; Ribas-Agustí, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Freitas, H.R.; Isaac, A.R.; Malcher-Lopes, R.; Diaz, B.L.; Trevenzoli, I.H.; Reis, R.A.D.M. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2018, 21, 695–714. [Google Scholar] [CrossRef]
- Zárate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Laviano, A. Protein and amino acids: Key players in modulating health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Tsatsaronis, G.C.; Boskou, D.G. Amino acid and mineral salt content of tomato seed and skin waste. J. Sci. Food Agric. 1975, 26, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Woodbridge, C. Effect of maturation, ripening and truss position on the free amino acid content in tomato fruits. Proc. Am. Soc. Hortic. Sci. 1960, 76, 515–523. [Google Scholar]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef]
- Perusek, L.; Maeda, T. Vitamin A derivatives as treatment options for retinal degenerative diseases. Nutrients 2013, 5, 2646–2666. [Google Scholar] [CrossRef]
- Wang, Y.; Chun, O.K.; Song, W.O. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients 2013, 5, 2969–3004. [Google Scholar] [CrossRef]
- Mora-Esteves, C.; Shin, D. Nutrient supplementation: Improving male fertility fourfold. Semin. Reprod. Med. 2013, 31, 293–300. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Domb, A.J.; Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef]
- Sharoni, Y.; Danilenko, M.; Walfisch, S.; Amir, H.; Nahum, A.; Ben-Dor, A.; Hirsch, K.; Khanin, M.; Steiner, M.; Agemy, L.; et al. Role of gene regulation in the anticancer activity of carotenoids. Pure Appl. Chem. 2002, 74, 1469–1477. [Google Scholar] [CrossRef]
- Sayo, T.; Sugiyama, Y.; Inoue, S. Lutein, a nonprovitamin A, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci. Biotechnol. Biochem. 2013, 77, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Salgado, A.C.; Serrano-García, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid.-Based Complementary Altern. Med. 2013, 2013, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Tonucci, L.H.; Holden, J.M.; Beecher, G.R.; Khachik, F.; Davis, C.S.; Mulokozi, G. Carotenoid content of thermally processed tomato-based food products. J. Agric. Food Chem. 1995, 43, 579–586. [Google Scholar] [CrossRef]
- Górecka, D.; Wawrzyniak, A.; Jędrusek-Golińska, A.; Dziedzic, K.; Hamułka, J.; Kowalczewski, P.Ł.; Walkowiak, J. Lycopene in tomatoes and tomato products. Open Chem. 2020, 18, 752–756. [Google Scholar] [CrossRef]
- Ostlund, R.E.; Racette, S.B.; Stenson, W.F. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am. J. Clin. Nutr. 2003, 77, 1385–1389. [Google Scholar] [CrossRef]
- Bradford, P.G.; Awad, A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef]
- Woyengo, T.; Ramprasath, V.; Jones, P. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Demonty, I. Phytosterols: Natural compounds with established and emerging health benefits. Oléagineux Corps Gras Lipides 2007, 14, 259–266. [Google Scholar] [CrossRef]
- Bouic, P.J. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Germán, C.-M.E. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Vlaisavljevic, S.; Martínez, M.C.; Stojanović, A.; Martínez-Huélamo, M.; Grung, B.; Raventós, R.M.L. Characterisation of bioactive compounds and assessment of antioxidant activity of different traditional Lycopersicum esculentum L. varieties: Chemometric analysis. Int. J. Food Sci. Nutr. 2019, 70, 813–824. [Google Scholar] [CrossRef]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Martínez-Huélamo, M.; Jáuregui, O.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Phenolic profile and hydrophilic antioxidant capacity as chemotaxonomic markers of tomato varieties. J. Agric. Food Chem. 2011, 59, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; El-Tawil, O.S.; Bungau, S.; Atanasov, A.G. Applications of antioxidants in metabolic disorders and degenerative diseases: Mechanistic approach. Oxidative Med. Cell. Longev. 2019, 2019, 1–3. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Leonardi, C.; Ambrosino, P.; Esposito, F.; Fogliano, V. Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes. J. Agric. Food Chem. 2000, 48, 4723–4727. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Cichon, M.J.; Riedl, K.M.; Schwartz, S.J. A metabolomic evaluation of the phytochemical composition of tomato juices being used in human clinical trials. Food Chem. 2017, 228, 270–278. [Google Scholar] [CrossRef]
- Periago, M.J.; Garcia-Alonso, J.; Jacob, K.; Olivares, A.B.; Bernal, M.J.; Iniesta, M.D.; Martinez, C.; Ros, G. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int. J. Food Sci. Nutr. 2009, 60, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Erge, H.S.; Karadeniz, F. Bioactive compounds and antioxidant activity of tomato cultivars. Int. J. Food Prop. 2011, 14, 968–977. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease, 9th ed.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Combs, G.F., Jr.; McClung, J.P. The Vitamins: Fundamental Aspects in Nutrition and Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Dutta, D.; Chaudhuri, U.R.; Chakraborty, R. Structure, health benefits, antioxidant property and processing and storage of carotenoids. Afr. J. Biotechnol. 2005, 4, 1510–1520. [Google Scholar] [CrossRef]
- Thompson, G.R.; Grundy, S.M. History and development of plant sterol and stanol esters for cholesterol-lowering purposes. Am. J. Cardiol. 2005, 96, 3–9. [Google Scholar] [CrossRef]
- Quilez, J.; Garcia-Lorda, P.; Salas-Salvado, J. Potential uses and benefits of phytosterols in diet: Present situation and future directions. Clin. Nutr. 2003, 22, 343–351. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Wolak, T.; Sharoni, Y.; Levy, J.; Linnewiel-Hermoni, K.; Stepensky, D.; Paran, E. Effect of Tomato Nutrient Complex on Blood Pressure: A Double Blind, Randomized Dose-Response Study. Nutrients 2019, 11, 950. [Google Scholar] [CrossRef]
- World Health Organization. Prevention of Cardiovascular Disease: Pocket Guidelines for Assessment and Management of Cardiovascular Risk:(WHO/ISH Cardiovascular Risk Prediction Charts for the Europe Region); WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond; American College of Cardiology Foundation: Washington, DC, USA, 2019. [Google Scholar]
- Michaličková, D.; Belović, M.; Ilić, N.; Kotur-Stevuljević, J.; Slanař, O.; Šobajić, S. Comparison of polyphenol-enriched tomato juice and standard tomato juice for cardiovascular benefits in subjects with stage 1 hypertension: A randomized controlled study. Plant Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.-C.; Díaz, L.D.; Kardinaal, A.; Van Lieshout, M. Evidence of antiplatelet aggregation effects from the consumption of tomato products, according to EFSA health claim requirements. Crit. Rev. Food Sci. Nutr. 2020, 60, 1515–1522. [Google Scholar] [CrossRef]
- Gajendragadkar, P.R.; Hubsch, A.; Mäki-Petäjä, K.M.; Serg, M.; Wilkinson, I.B.; Cheriyan, J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomised controlled trial. PLoS ONE 2014, 9, e99070. [Google Scholar] [CrossRef]
- Mordente, A.; Guantario, B.; Meucci, E.; Silvestrini, A.; Lombardi, E.; Martorana, G.E.; Giardina, B.; Bohm, V. Lycopene and cardiovascular diseases: An update. Curr. Med. Chem. 2011, 18, 1146–1163. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Steck, S. Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 2000, 71, 1691S–1695S. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Wang, Y.; Tzu, N.-H.; Fong, T.-H.; Shen, M.-Y.; Lin, K.-H.; Chou, D.-S.; Sheu, J.-R. Inhibitory effects of lycopene on in vitro platelet activation and in vivo prevention of thrombus formation. J. Lab. Clin. Med. 2005, 146, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. DNA 2020, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Zhou, X.-B.; Chen, C.; Mao, W. Systematic investigation of quercetin for treating cardiovascular disease based on network pharmacology. Comb. Chem. High Throughput Screen. 2019, 22, 411–420. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Suhan, T.O.; De Luca, C.; Korkina, L. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011, 658, 248–256. [Google Scholar] [CrossRef]
- Al-Awwadi, N.A.; Araiz, C.; Bornet, A.; Delbosc, S.; Cristol, J.-P.; Linck, N.; Azay, J.; Teissedre, P.-L.; Cros, G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005, 53, 151–157. [Google Scholar] [CrossRef]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef]
- Sánchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef]
- Lafay, S.; Gueux, E.; Rayssiguier, Y.; Mazur, A.; Remesy, C.; Scalbert, A. Caffeic acid inhibits oxidative stress and reduces hypercholesterolemia induced by iron overload in rats. Int. J. Vitam. Nutr. Res. 2005, 75, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ozguner, F.; Altinbas, A.; Ozaydin, M.; Dogan, A.; Vural, H.; Kisioglu, A.N.; Cesur, G.; Yildirim, N.G. Mobile phone-induced myocardial oxidative stress: Protection by a novel antioxidant agent caffeic acid phenethyl ester. Toxicol. Ind. Health 2005, 21, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.P.V. Antihypertensive effect of caffeic acid and its analogs through dual renin-angiotensin-aldosterone system inhibition. Eur. J. Pharmacol. 2014, 730, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, Z.; Yuan, Y.; Li, F.; Liu, Y.; Ma, Z.; Liao, H.; Bian, Z.; Zhang, Y.; Zhou, H.; et al. Naringenin attenuates pressure overload-induced cardiac hypertrophy. Exp. Ther. Med. 2015, 10, 2206–2212. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Xu, X.-R.; Lin, X.-M.; Zhang, H.-B.; Xiao, X.; Ouyang, L.; Huang, Y.-M.; Wang, X.; Liu, Y.-Q. Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2014, 111, 474–480. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Selvaraj, R.K. Dietary lutein and fish oil interact to alter atherosclerotic lesions in a Japanese quail model of atherosclerosis. J. Anim. Physiol. Anim. Nutr. 2011, 95, 762–770. [Google Scholar] [CrossRef]
- Yang, J.-T.; Qian, L.-B.; Zhang, F.-J.; Wang, J.; Ai, H.; Tang, L.-H.; Wang, H.-P. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J. Cardiovasc. Pharmacol. 2015, 65, 349–356. [Google Scholar] [CrossRef]
- Sun, D.; Huang, J.; Zhang, Z.; Gao, H.; Li, J.; Shen, M.; Cao, F.; Wang, H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS ONE 2012, 7, e33491. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, Y.-S.; Shin, C.-H.; Lee, H.-J.; Kim, S. Antithrombotic activities of luteolin in vitro and in vivo. J. Biochem. Mol. Toxicol. 2015, 29, 552–558. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Lozano, M.L.; Castillo, J.; Benavente-Garcia, O.; Vicente, V.; Rivera, J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J. Thromb. Haemost. 2005, 3, 369–376. [Google Scholar] [CrossRef]
- Kumar, S.; Prahalathan, P.; Saravanakumar, M.; Raja, B. Vanillic acid prevents the deregulation of lipid metabolism, endothelin 1 and up regulation of endothelial nitric oxide synthase in nitric oxide deficient hypertensive rats. Eur. J. Pharmacol. 2014, 743, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Hamzavi, G.R.; Badavi, M.; Samarbaf-Zadeh, A. Effect of vanillic acid on ischemia-reperfusion of isolated rat heart: Hemodynamic parameters and infarct size assays. Indian J. Exp. Biol. 2015, 53, 641–646. [Google Scholar] [PubMed]
- Prince, P.S.M.; Dhanasekar, K.; Rajakumar, S. Vanillic acid prevents altered ion pumps, ions, inhibits Fas-receptor and caspase mediated apoptosis-signaling pathway and cardiomyocyte death in myocardial infarcted rats. Chem.-Biol. Interact. 2015, 232, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, D.; Tang, X.; Li, X.; Wo, D.; Yan, H.; Song, R.; Feng, J.; Li, P.; Zhang, J.; et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014, 226, 257–263. [Google Scholar] [CrossRef]
- Alam, M.A.; Sernia, C.; Brown, L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharmacol. 2013, 61, 240–249. [Google Scholar] [CrossRef]
- Roy, A.J.; Prince, P.S.M. Preventive effects of p-coumaric acid on cardiac hypertrophy and alterations in electrocardiogram, lipids, and lipoproteins in experimentally induced myocardial infarcted rats. Food Chem. Toxicol. 2013, 60, 348–354. [Google Scholar] [CrossRef]
- Roy, A.J.; Prince, P.S.M. Preventive effects of p-coumaric acid on lysosomal dysfunction and myocardial infarct size in experimentally induced myocardial infarction. Eur. J. Pharmacol. 2013, 699, 33–39. [Google Scholar] [CrossRef]
- Anandhi, R.; Thomas, P.A.; Geraldine, P. Evaluation of the anti-atherogenic potential of chrysin in Wistar rats. Mol. Cell. Biochem. 2013, 385, 103–113. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.; Ray, R.; Arya, D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem.-Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Hu, T.; Han, Y.; Huang, W.; Yuan, H.; Zhang, Y.-T.; Du, Y.; Jiang, Y.-W. Preventive effects of catechins on cardiovascular disease. Molecules 2016, 21, 1759. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Nocella, C.; Bartimoccia, S.; Bucci, T.; De Falco, E.; Peruzzi, M.; Chimenti, I.; Biondi-Zoccai, G.; Pignatelli, P.; et al. Epicatechin and catechin modulate endothelial activation induced by platelets of patients with peripheral artery disease. Oxidative Med. Cell. Longev. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, H.; Sun, J.; Wang, S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013, 150, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Prince, P.S.M. Protective effects of sinapic acid on lysosomal dysfunction in isoproterenol induced myocardial infarcted rats. Food Chem. Toxicol. 2012, 50, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, T.; Manivannan, J.; Priya, M.K.; Suganya, N.; Chatterjee, S.; Raja, B. Sinapic acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS ONE 2014, 9, e115682. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of caffeine and lycopene in experimentally induced diabetes mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef]
- Bayramoglu, A.; Bayramoglu, G.; Senturk, H. Lycopene partially reverses symptoms of diabetes in rats with streptozotocin-induced diabetes. J. Med. Food 2013, 16, 128–132. [Google Scholar] [CrossRef]
- Ozmutlu, S.; DeDe, S.; Ceylan, E. The effect of lycopene treatment on ACE activity in rats with experimental diabetes. J. Renin-Angiotensin-Aldosterone Syst. 2012, 13, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, G.; Lu, X.; Jiang, Y.-X.; Xu, L.; Zhao, X. Lycopene ameliorates renal function in rats with streptozotocin-induced diabetes. Int. J. Clin. Exp. Pathol. 2014, 7, 5008–5015. [Google Scholar] [PubMed]
- Yildiz, M.; Sandikci, M. Changes in rat ovary with experimentally induced diabetes and the effects of lycopene on those changes. Rom. J. Morphol. Embryol. 2016, 57, 703–713. [Google Scholar] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Gradinaru, D.; Manda, G.; Neagoe, I.; Ilie, M. Membranar effects exerted in vitro by polyphenols—quercetin, epigallocatechin gallate and curcumin—on HUVEC and Jurkat cells, relevant for diabetes mellitus. Food Chem. Toxicol. 2013, 61, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mosawy, S.; Jackson, D.E.; Woodman, O.L.; Linden, M.D. The flavonols quercetin and 3′,4′-dihydroxyflavonol reduce platelet function and delay thrombus formation in a model of type 1 diabetes. Diabetes Vasc. Dis. Res. 2014, 11, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.P.; Momentti, A.C.; Peixoto, F.B.; Baptista, R.D.F.F.; Dos Santos, F.A.; Fava, F.H.; Fernandes, A.A.H. Influence of treatment with quercetin on lipid parameters and oxidative stress of pregnant diabetic rats. Can. J. Physiol. Pharmacol. 2013, 91, 171–177. [Google Scholar] [CrossRef]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Li, Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2013, 31, 265–271. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.-S.; Zhen, W.; Cheng, Z.; Jia, Z.; et al. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J. Diabetes Res. 2015, 2015, 532984. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; AlSaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015, 28, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Veeramani, C.; AlSaif, M.A.; Chandramohan, G. Influence of kaempferol, a flavonoid compound, on membrane-bound ATPases in streptozotocin-induced diabetic rats. Pharm. Biol. 2015, 53, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J.; Chuang, C.-H.; Mong, M.-C.; Kam, W.-Y.; Huang, H.-Y.; Yin, M.-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2011, 60, 514–521. [Google Scholar] [CrossRef]
- Ren, B.; Qin, W.; Wu, F.; Wang, S.; Pan, C.; Wang, L.; Zeng, B.; Ma, S.; Liang, J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016, 773, 13–23. [Google Scholar] [CrossRef]
- Rahigude, A.; Bhutada, P.; Kaulaskar, S.; Aswar, M.; Otari, K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience 2012, 226, 62–72. [Google Scholar] [CrossRef]
- Chao, C.-Y.; Mong, M.-C.; Chan, K.-C.; Yin, M.-C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef]
- Stefanello, N.; Schmatz, R.; Pereira, L.B.; Rubin, M.A.; Rocha, J.B.T.; Facco, G.; Pereira, M.E.; Mazzanti, C.M.D.A.; Passamonti, S.; Rodrigues, M.V.; et al. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Mol. Cell. Biochem. 2014, 388, 277–286. [Google Scholar] [CrossRef]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef]
- Lorenzoni, F.; Giampietri, M.; Ferri, G.; Lunardi, S.; Madrigali, V.; Battini, L.; Boldrini, A.; Ghirri, P. Lutein administration to pregnant women with gestational diabetes mellitus is associated to a decrease of oxidative stress in newborns. Gynecol. Endocrinol. 2013, 29, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Arnal, E.; Miranda, M.; Almansa, I.; Muriach, M.; Barcia, J.M.; Romero, F.J.; Diaz-Llopis, M.; Bosch-Morell, F. Lutein prevents cataract development and progression in diabetic rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L.; Moselhy, S.S. Free fatty acids profiling in response to carnitine synergize with lutein in diabetic rats. Afr. J. Tradit. Complementary Altern. Med. 2016, 13, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, L.; Dong, J.; Zhang, X.; Chen, Y.-G.; Bao, B.; Liu, J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014, 58, 1258–1268. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; Gou, L.; Sun, L.; Ling, X.; Yin, X. Luteolin attenuates diabetes-associated cognitive decline in rats. Brain Res. Bull. 2013, 94, 23–29. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Zhao, Q.; Zhang, J.; Lin, J. Luteolin improves the impaired nerve functions in diabetic neuropathy: Behavioral and biochemical evidences. Int. J. Clin. Exp. Pathol. 2015, 8, 10112–10120. [Google Scholar]
- Vinothiya, K.; AshokKumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Sannigrahi, S.; Rahaman, N.; Ahmed, F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: Effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine 2013, 44, 369–379. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Rahaman, N.; Sannigrahi, S.; Ahmed, F. Ferulic acid in the treatment of post-diabetes testicular damage: Relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling. Cell Biochem. Funct. 2014, 32, 115–124. [Google Scholar] [CrossRef]
- Amalan, V.; Natesan, V.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p -coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; Yousef, A.I.; El-Twab, S.M.A.; Reheim, E.S.A.; Ashour, M.B. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab. Brain Dis. 2017, 32, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Cherng, Y.-G.; Tsai, C.-C.; Chung, H.-H.; Lai, Y.-W.; Kuo, S.-C.; Cheng, J.-T. Antihyperglycemic action of sinapic acid in diabetic rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Deepa, K.; Balakumar, P. Catechin averts experimental diabetes mellitus-induced vascular endothelial structural and functional abnormalities. Cardiovasc. Toxicol. 2014, 14, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, L.; Zhou, Q.; Yan, S.; Li, Z.; Sheng, J.; Zhang, W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol. Nutr. Food Res. 2014, 58, 2249–2260. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Rasedee, A.; Zamri-Saad, M.; Taufiq-Yap, Y.-H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Fu, Z.; Yuskavage, J.; Liu, D. Dietary flavonol epicatechin prevents the onset of type 1 diabetes in nonobese diabetic mice. J. Agric. Food Chem. 2013, 61, 4303–4309. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Gonzalez-Basurto, S.; Coral-Vazquez, R.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and Type 2 diabetes: Restorative effects of (−)-epicatechinrich cocoa. Clin. Sci. 2013, 125, 383–389. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Cheng, N.; Yi, W.-B.; Peng, S.; Zou, X.-Q. Synthesis, nitric oxide release, and α-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorganic Med. Chem. 2014, 22, 1515–1521. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F.; Farkhondeh, T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2015, 94, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zang, A.; Zhang, L.; Zhang, H.; Zhao, L.; Qi, Z.; Wang, H. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol. Sci. 2014, 35, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014, 279, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Franceschi, S.; Parpinel, M.; La Vecchia, C.; Favero, A.; Talamini, R.; Negri, E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology 1998, 9, 338–341. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Wall-Medrano, A.; Olivas-Aguirre, F.J. Antioxidant phytochemicals in cancer prevention and therapy—An update. In Functional Foods in Cancer Prevention and Therapy; Academic Press: Cambridge, MA, USA, 2020; pp. 195–220. [Google Scholar]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessì, M.A.; Spencer, J.P. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem. Biophys. Res. Commun. 2007, 362, 606–611. [Google Scholar] [CrossRef]
- Adams, L.S.; Chen, S. Phytochemicals for breast cancer prevention by targeting aromatase. Front. Biosci. 2009, 14, 3846–3863. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Role of toxicants in oxidative stress mediated DNA damage and protection by phytochemicals. EC Pharmacol. Toxicol. 2019, 7, 1–7. [Google Scholar]
- Ezzat, S.M.; El-Halawany, A.M.; Hamed, A.R.; Abdel-Sattar, E.A. Role Phytochemicals Play in the Activation of Antioxidant Response Elements (AREs) and Phase II Enzymes and Their Relation to Cancer Progression and Prevention. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 60, pp. 345–369. [Google Scholar]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary phytochemicals targeting cancer stem cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Bidoli, E.; La Vecchia, C.; Talamini, R.; D’Avanzo, B.; Negri, E. Tomatoes and risk of digestive-tract cancers. Int. J. Cancer 1994, 59, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Branch, L.G.; Lipnick, R.J.; Willett, W.C.; Rosner, B.A.; Posner, B.M.; Hennekens, C.H. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am. J. Clin. Nutr. 1985, 41, 32–36. [Google Scholar] [CrossRef]
- Freudenhieim, J.L.; Graham, S.; Marshall, J.R.; Haughey, B.P.; Wilkinson, G. A case-control study of diet and rectal cancer in western new york. Am. J. Epidemiol. 1990, 131, 612–624. [Google Scholar] [CrossRef]
- Centonze, S.; Boeing, H.; Leoci, C.; Guerra, V.; Misciagna, G. Dietary habits and colorectal cancer in a low-risk area. Results from a population-based case-control study in southern Italy. Nutr. Cancer 1994, 21, 233–246. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Yu, Y.; Zhao, T.; Liu, S.; Wang, Q. Diet and cancer of the colon and rectum: A case-control study in China. Int. J. Epidemiol. 1991, 20, 362–367. [Google Scholar] [CrossRef]
- Franceschi, S.; Favero, A.; La Vecchia, C.; Negri, E.; Conti, E.; Montella, M.; Giacosa, A.; Nanni, O.; Decarli, A. Food groups and risk of colorectal cancer in Italy. Int. J. Cancer 1997, 72, 56–61. [Google Scholar] [CrossRef]
- Kwatra, B. A review on potential properties and therapeutic applications of lycopene. Int. J. Med. Biomed. Stud. 2020, 4. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Hung, J.C.; Heber, D.; Go, V.L.; Reuter, V.E.; Cordon-Cardo, C.; Scher, H.I.; Marshall, J.R.; Zhang, Z. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 749–756. [Google Scholar]
- Siler, U.; Barella, L.; Spitzer, V.; Schnorr, J.; Lein, M.; Goralczyk, R.; Wertz, K. Lycopene and Vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J. 2004, 18, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Vijayababu, M.; Kanagaraj, P.; Arunkumar, A.; Ilangovan, R.; Aruldhas, M.; Arunakaran, J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J. Cancer Res. Clin. Oncol. 2005, 131, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Laudet, B.; Zohrabian, V.M.; Murali, R.; Jhanwar-Uniyal, M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006, 26, 1177–1181. [Google Scholar] [PubMed]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jin, Y.-R.; Park, B.-S.; Kim, T.-J.; Kim, S.-Y.; Lim, Y.; Hong, J.-T.; Yoo, H.-S.; Yun, Y.-P. Luteolin prevents PDGF-BB-induced proliferation of vascular smooth muscle cells by inhibition of PDGF β-receptor phosphorylation. Biochem. Pharmacol. 2005, 69, 1715–1721. [Google Scholar] [CrossRef]
- Ujiki, M.B.; Ding, X.-Z.; Salabat, M.R.; Bentrem, D.J.; Melstrom, L.G.; Milam, B.; Talamonti, M.S.; Belljr, R.H.; Iwamura, T.; Adrian, T. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol. Cancer 2006, 5, 1–76. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.-F.; Vodnar, D. Bioactive compounds extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Nour, V.; Panainte, T.D.; Ropota, M.; Turcu, R.; Trandafir, I.; Corbu, A.R. Nutritional and bioactive compounds in dried tomato processing waste. CyTA—J. Food 2018, 16, 222–229. [Google Scholar] [CrossRef]
| Parameters | Values | Range | References |
|---|---|---|---|
| Energy (kcal/100 g) | 34.67 ± 18.74 | 18.00–75.00 | [3,4,5,40,41,42,43,44,45,46,47,48,49,50,51,52] |
| Ash (%) | 8.75 ± 1.69 | 5.90–10.60 | |
| Moisture (g/100 g) | 91.18 ± 6.83 | 68.03–96.17 | |
| Total protein (g/100 g) | 17.71 ± 5.40 | 10.50–25.03 | |
| Lipid (g/100 g) | 4.96 ± 1.19 | 3.62–5.39 | |
| Carbohydrates (g/100 g) | 5.96 ± 1.37 | 3.92–8.00 | |
| Total sugar (g/100 g) | 50.60 ± 3.69 | 47.00–56.45 | |
| pH | 3.83 ± 0.21 | 3.61–4.08 | |
| Acidity (%) | 0.48 ± 0.07 | 0.39–0.55 | |
| Reducing sugar (%) | 35.84 ± 4.57 | 30.03–41.21 | |
| Fructose (%) | 2.88 ± 0.49 | 1.15–3.42 | |
| Glucose (%) | 2.45 ± 0.48 | 1.74–3.18 | |
| Sucrose (%) | 0.02 ± 0.05 | 0.01–0.02 | |
| Total fiber (g/100 g) | 11.44 ± 9.31 | 1.32–19.36 |
| Elements | Units | Concentrations | Range | References |
|---|---|---|---|---|
| Sodium (Na) | mg/100 g | 70.38 ± 12.20 | 56.90–80.65 | [3,4,41,42,44,45,47,51,61,62,63] |
| Potassium (K) | mg/100 g | 403.02 ± 254.41 | 16.63–1097.00 | |
| Calcium (Ca) | mg/100 g | 105.21 ± 22.76 | 48.47–162.07 | |
| Magnesium (Mg) | mg/100 g | 172.58 ± 58.92 | 76.87–265.93 | |
| Phosphorus (P) | mg/100 g | 300.99 ± 32.12 | 173.00–379.31 | |
| Chlorine (Cl) | μg/100 g | 517.24 ± 0.00 | 517.24 | |
| Boron (B) | μg/g | 36.83 ± 3.27 | 25.84–48.59 | |
| Nickel (Ni) | mg/100 g | 0.66 ± 0.00 | 0.66 | |
| Nitrate (NO3-) | mg/100 g | 274.37 ± 156.75 | 86.21–459.00 | |
| Iron (Fe) | mg/100 g | 4.55 ± 2.18 | 1.50–6.45 | |
| Zinc (Zn) | mg/100 g | 2.48 ± 1.05 | 0.17–3.17 | |
| Cobalt (Co) | mg/100 g | 19.66 ± 9.66 | 10.00 -29.31 | |
| Copper (Cu) | mg/100 g | 0.67 ± 0.15 | 0.06–1.10 | |
| Manganese (Mn) | mg/100 g | 0.60 ± 0.12 | 0.11–1.88 | |
| Chromium (Cr) | μg/100 g | 193.80 ± 133.80 | 60.00–327.59 | |
| Iodine (I) | mg/100 g | 2.65 ± 1.44 | 0.18–3.97 | |
| Fluorine (F) | μg/100 g | 413.79 ± 0.00 | 413.79 | |
| Aluminum (Al) | μg/100 g | 1241.38 ± 0.00 | 1241.38 | |
| Silicon (Si) | μg/100 g | 46.55 ± 0.00 | 46.55 | |
| Selenium (Se) | μg/100 g | 13.45 ± 3.45 | 10.00–16.90 | |
| Lead (Pb) | μg/ g | 1.21 ± 0.06 | 1.15–1.27 | |
| Cadmium (Cd) | μg/ g | 0.17 ± 0.06 | 0.11–0.22 | |
| Arsenic (As) | μg/ g | 0.20 ± 0.005 | 0.19–0.20 |
| Vitamins | Units | Concentrations | Range | References |
|---|---|---|---|---|
| Vitamin A | IU/100 g | 614.44 ± 248.18 | 267.33–833.00 | [4,46,61,62,65,69,73,74,75,76] |
| Vitamin E | μg/100 g | 15.08 ± 1.06 | 14.02–16.13 | |
| Vitamin K | μg/100 g | 98.28 ± 0.00 | 98.28 | |
| Vitamin C | mg/100 g | 36.16 ± 29.64 | 10.86–85.00 | |
| Thiamine | mg/100 g | 0.66 ± 0.44 | 0.04–0.98 | |
| Riboflavin | mg/100 g | 0.48 ± 0.34 | 0.02–0.81 | |
| Niacin | mg/100 g | 9.68 ± 0.00 | 9.68 | |
| Pantothenic Acid | mg/100 g | 4.93 ± 0.41 | 4.52–5.34 | |
| Vitamin B6 | mg/100 g | 1.51 ± 0.22 | 1.29–1.72 | |
| Biotin | μg/100 g | 68.97 ± 0.00 | 68.97 | |
| Folate | mg/100 g | 14.00 ± 1.00 | 13.00–15.00 |
| Fatty Acids | Concentrations (g/100 g) | Range | References |
|---|---|---|---|
| Myristic acid | 0.56 ± 0.22 | 0.32–0.93 | [5,41,47,49,62] |
| Palmitic acid | 18.07 ± 2.90 | 12.40–22.50 | |
| Stearic acid | 4.81 ± 1.50 | 2.80–6.84 | |
| Palmitoleic acid | 0.25 ± 0.10 | 0.03–0.32 | |
| Oleic acid | 14.24 ± 3.50 | 9.00–19.14 | |
| Linoleic acid | 49.40 ± 4.16 | 46.33–54.10 | |
| Linolenic acid | 10.17 ± 4.46 | 4.26–15.53 | |
| Caproic acid | 0.03 ± 0.02 | 0.01–0.05 | |
| Caprylic acid | 0.06 ± 0.04 | 0.02–0.10 | |
| Capric acid | 0.04 ± 0.03 | 0.01–0.07 | |
| Heptadecanoic acid | 0.26 ± 0.05 | 0.18–0.13 | |
| Lauric acid | 0.09 ± 0.05 | 0.04–0.15 | |
| Pentadecanoic acid | 0.12 ± 0.03 | 0.08–0.15 | |
| Arachidic acid | 0.88 ± 0.24 | 0.61–1.26 | |
| Eicosadienoic acid | 0.04 ± 0.02 | 0.02–0.06 | |
| Arachidonic acid | 0.04 ± 0.02 | 0.01–0.06 | |
| Eicosapentaenoic acid | 0.05 ± 0.01 | 0.03–0.06 | |
| Erucic acid | 0.02 ± 0.01 | 0.01–0.03 | |
| Docosadienoic acid | 0.07 ± 0.03 | 0.03–0.10 | |
| Behenic acid | 0.59 ± 0.19 | 0.31–0.82 | |
| Tricosanoic acid | 0.68 ± 0.54 | 0.16–1.52 | |
| Lignoceric acid | 0.74 ± 0.20 | 0.45–1.01 | |
| Saturated fatty acid | 27.40 ± 3.74 | 22.37–33.22 | |
| Monounsaturated fatty acid | 13.80 ± 2.42 | 11.00–17.66 | |
| Polyunsaturated fatty acid | 57.55 ± 23.51 | 55.78–58.63 | |
| Vaccenic acid | 0.53 ± 0.05 | 0.50–0.60 | |
| Eicosanoic acid | 0.10 ± 0.03 | 0.05–0.12 |
| Amino Acids | Concentrations (g/100 g Protein) | Range | References |
|---|---|---|---|
| Threonine * | 1.37 ± 0.97 | 0.40–2.34 | [3,41,62,80,81] |
| Valine * | 2.49 ± 2.09 | 0.40–2.49 | |
| Methionine * | 0.57 ± 0.45 | 0.12–1.02 | |
| Isoleucine * | 2.13 ± 1.73 | 0.40–3.86 | |
| Leucine * | 2.80 ± 2.28 | 0.52–5.07 | |
| Phenylalanine * | 1.77 ± 1.36 | 0.41–13.12 | |
| Histidine * | 1.93 ± 1.71 | 0.22–3.64 | |
| Lysine * | 2.45 ± 1.95 | 0.50–4.40 | |
| Arginine * | 2.33 ± 2.02 | 0.31–4.34 | |
| Aspartic Acid ** | 1.40 ± 0.70 | 0.70–2.09 | |
| Serine ** | 1.78 ± 1.30 | 0.48–3.08 | |
| Glutamic Acid ** | 10.13 ± 4.44 | 5.69–14.56 | |
| Proline ** | 1.53 ± 1.25 | 0.28–2.78 | |
| Glycine ** | 2.30 ± 1.99 | 0.31–4.29 | |
| Alanine ** | 2.74 ± 2.29 | 0.45–5.02 | |
| Cystine ** | 0.21 ± 0.19 | 0.02–0.39 | |
| Tyrosine ** | 1.82 ± 1.61 | 0.21–3.42 |
| Units | Concentrations | Range | References | |
|---|---|---|---|---|
| β-carotene | μg/100 g | 9942.16 ± 264.74 | 3677.42–10,206.90 | [5,7,46,47,74,90,91,92,93,94] |
| α-carotene | μg/100 g | 101.00 | 101.00 | |
| Lycopene | μg/100 g | 8002.50 ± 243.54 | 5020.00–11,110.00 | |
| Lutein + zeaxanthin | μg/100 g | 60.67 ± 43.86 | 18.07–123.00 | |
| Phytoene | μg/100 g | 668.33 ± 361.95 | 430.00–1860.00 | |
| Phytofluene | μg/100 g | 500.00 ± 100.49 | 390.00–820.00 | |
| All trans-lutein | mg/kg | 5.00 ± 0.82 | 4.00–6.00 | |
| All trans-β carotene | mg/kg | 29.25 ± 27.26 | 4.00–75.00 | |
| 9-cis-β carotene | mg/kg | 6.50 ± 2.29 | 3.00–9.00 |
| Concentrations (mg/kg) | Range | References | |
|---|---|---|---|
| Campesterol | 147.50 ± 31.13 | 100.00–18.00 | [5,92] |
| Stigmasterol | 387.50 ± 88.71 | 260.00–510.00 | |
| Stigmastanol | 28.25 ± 10.92 | 10.00–38.00 | |
| β-sitosterol | 720.00 ± 175.64 | 520.00–1000.00 | |
| Δ5-Avenasterol | 62.30 ± 2.21 | 10.00–65.87 | |
| Cholestanol | 9.70 ± 1.80 | 2.10–11.54 | |
| Cholest-7-en-3-ol | 3.60 ± 0.13 | 0.42–4.40 | |
| Cholesterol | 41.90 ± 2.10 | 8.40–43.45 | |
| Lanost-8-en-3-β-ol | 52.40 ± 6.80 | 4.50–60.65 | |
| 24-Oxocholesterol | 67.50 ± 3.20 | 14.20–70.69 | |
| Total | 1283.25 ± 239.39 | 918.00–1570.00 |
| Units | Concentrations | Range | References | |
|---|---|---|---|---|
| α-tocopherol | mg/100 g | 0.701 ± 0.110 | 0.59–0.88 | [3,5,49,50,61,65,90,113] |
| β-tocopherol | mg/100 g | 0.030 ± 0.004 | 0.02–0.03 | |
| γ-tocopherol | mg/100 g | 0.810 ± 0.720 | 0.40–2.24 | |
| δ-tocopherol | mg/100 g | 0.020 ± 0.010 | 0.01–0.02 | |
| Total tocopherol | mg/100 g | 1.200 ± 0.150 | 1.02–1.44 | |
| Vitamin C | mg/100 g | 36.160 ± 29.640 | 10.86–85.00 | |
| β-carotene | mg/100 g | 9.420 ± 2.640 | 3.67–10.21 | |
| Lycopene | mg/100 g | 7.960 ± 1.780 | 5.02–9.49 | |
| Phenolic acids | mg CIAE/g extract | 25.500 ± 3.590 | 21.34–31.23 | |
| Flavonoids | mg QE/g extract | 4.230 ± 1.280 | 3.06–6.36 | |
| Anthocyanins | mg ME/g extract | 0.870 ± 0.470 | 0.23–1.36 |
| Name of Bioactive Compound | IUPAC Name | Molecular Formulas | Structure | Other Sources | References |
|---|---|---|---|---|---|
| Quercetin | 3,5,7,3′,4′-pentahydroxyflavone | C15H10O7 | 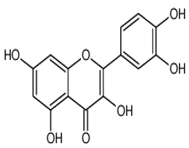 | Onion family, chocolate, fortified wine, berry wine, grape wine, sparkling wine, cereals, herbs, fruit juices, tea infusions, spices, nuts, fruit | [3,5,13,44,92,104,114,115,116,117,118,119] |
| Kaempferol | 3,5,7,4′-tetrahydroxyflavone | C15H10O6 | 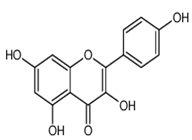 | Pod vegetables, pulses, wine, fruit juices, spices, tea infusions, nuts, fruit | |
| Naringenin | 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one | C15H12O5 | 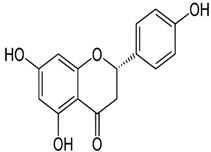 | Herbs, fruits, vegetables, beans, drynaria | |
| Caffeic acid | 3,4-dihydroxycinnamic acid | C9H8O4 | 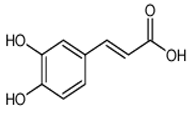 | Grape wine, beer, fortified wine, cereal products, sparkling wine, cereals, tubers, berries, dried drupes, other dried fruits, citrus fruits, pomes, unknown coffee beverages, drupe juices, berry juices, pome juices, vegetable oils, herbs, other seasonings, tropical fruit juices, other types of seed oils, spices, fruit vegetables, cabbages, leafy vegetables, root vegetables | |
| Rutin | Rutoside; quercetin 3-rutinoside | C27H30O16 | 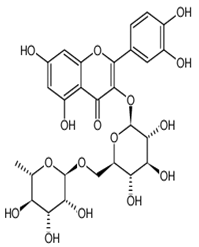 | Invasive plant species (Carpobrotusedulis) | |
| Chlorogenic acid | 3-caffeoylquinic and neochlorogenic acid | C16H18O9 | 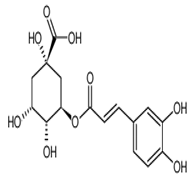 | Grape wines, tea infusions, pomes, dried drupes, pome jams, tropical fruits, robusta coffee beverages, root vegetables, unknown coffee beverages, cabbages | |
| Ferulic acid | Transferulic acid; 4-hydroxy-3-methoxycinnamic acid | C10H10O4 |  | Cabbages, fruit vegetables, beer, fortified wine, sparkling wine, other dried fruits, cereals, soy based products, grape wine, cereal products, pome juices, chocolate, berries, citrus fruits, other fruits, pomes, berry juices, tropical fruit juices, fruit vegetable oils, other seed oils, herbs, nuts, beans and lentils | |
| P-coumaric acid | p-hydroxycinnamic acid | C9H8O3 |  | Sparkling wines, citrus fruits, beers, nut liquors, grape wines, fortified wines, dried drupes, other dried fruits, lentils, berries, other fruits, pomes, berry juices, pome juices, tropical fruit juices, fruit vegetable oils, other seed oils, herbs, other seasonings, spices, nuts, fruit vegetables | |
| Lycopene | lycopene, (7-cis,7’-cis 9-cis,9’-cis)-isomer | C40H56 | 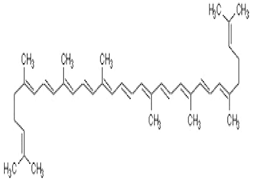 | autumn olive, guac, guava, papaya, grapefruit, sea buckthorn, wolfberry, watermelon | |
| Resveratrol | 3,5,4′-trihydroxy-trans-stilbene | C14H12O3 | 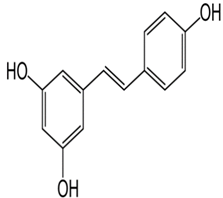 | Grapes, wine, peanuts and soy | |
| Chrysin | C15H10O4 | 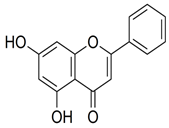 | Fruit juices, especially citrus | ||
| Epicatechin | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | C15H14O6 |  | Tea, cocoa, grapes | |
| Catechin | (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | C15H14O6 | 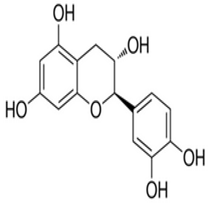 | Tea, cocoa, grapes | |
| Luteolin | 2-(3,4-dihydroxyphenyl)- 5,7-dihydroxy-4-chromenone | C15H10O6 | 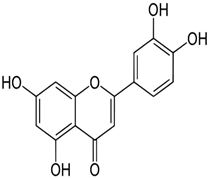 | Honey, fruit, vegetable oils, nuts, herbs, shoot vegetables, fruit, vegetables, lentils, carrots, olive oil | |
| Cinnamic acid | 2-propenoic acid | C9H8O2 | 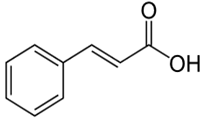 | Propolis, fruit and vegetable oils, berries, citrus juices, fruit, vegetables, spices | |
| Phloretic acid | 3-(4-hydroxyphenyl) propanoic acid | C9H10O3 | 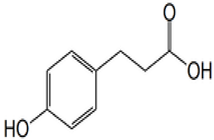 | Dried grass | |
| Sinapic acid | 3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid | C11H12O5 | 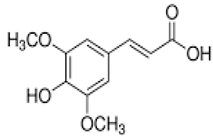 | Black mustard seeds | |
| Vanillic acid | 4-hydroxy-3-methoxybenzoic acid | C8H8O4 |  | Rum, beer, brandy, wine, herbs, grapes, citrus fruit, whiskey |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 45. https://doi.org/10.3390/foods10010045
Ali MY, Sina AAI, Khandker SS, Neesa L, Tanvir EM, Kabir A, Khalil MI, Gan SH. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods. 2021; 10(1):45. https://doi.org/10.3390/foods10010045
Chicago/Turabian StyleAli, Md Yousuf, Abu Ali Ibn Sina, Shahad Saif Khandker, Lutfun Neesa, E. M. Tanvir, Alamgir Kabir, Md Ibrahim Khalil, and Siew Hua Gan. 2021. "Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review" Foods 10, no. 1: 45. https://doi.org/10.3390/foods10010045
APA StyleAli, M. Y., Sina, A. A. I., Khandker, S. S., Neesa, L., Tanvir, E. M., Kabir, A., Khalil, M. I., & Gan, S. H. (2021). Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods, 10(1), 45. https://doi.org/10.3390/foods10010045