Quality Traits of Dry-Cured Loins from Iberian Pigs Reared in Montanera System as Affected by Pre-Freezing Cure
Abstract
1. Introduction
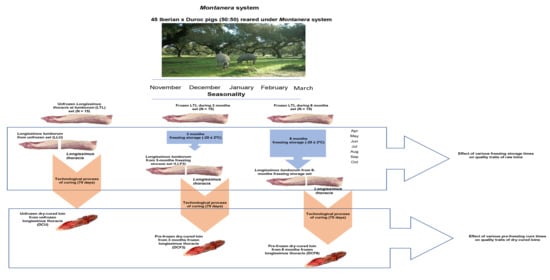
2. Materials and Methods
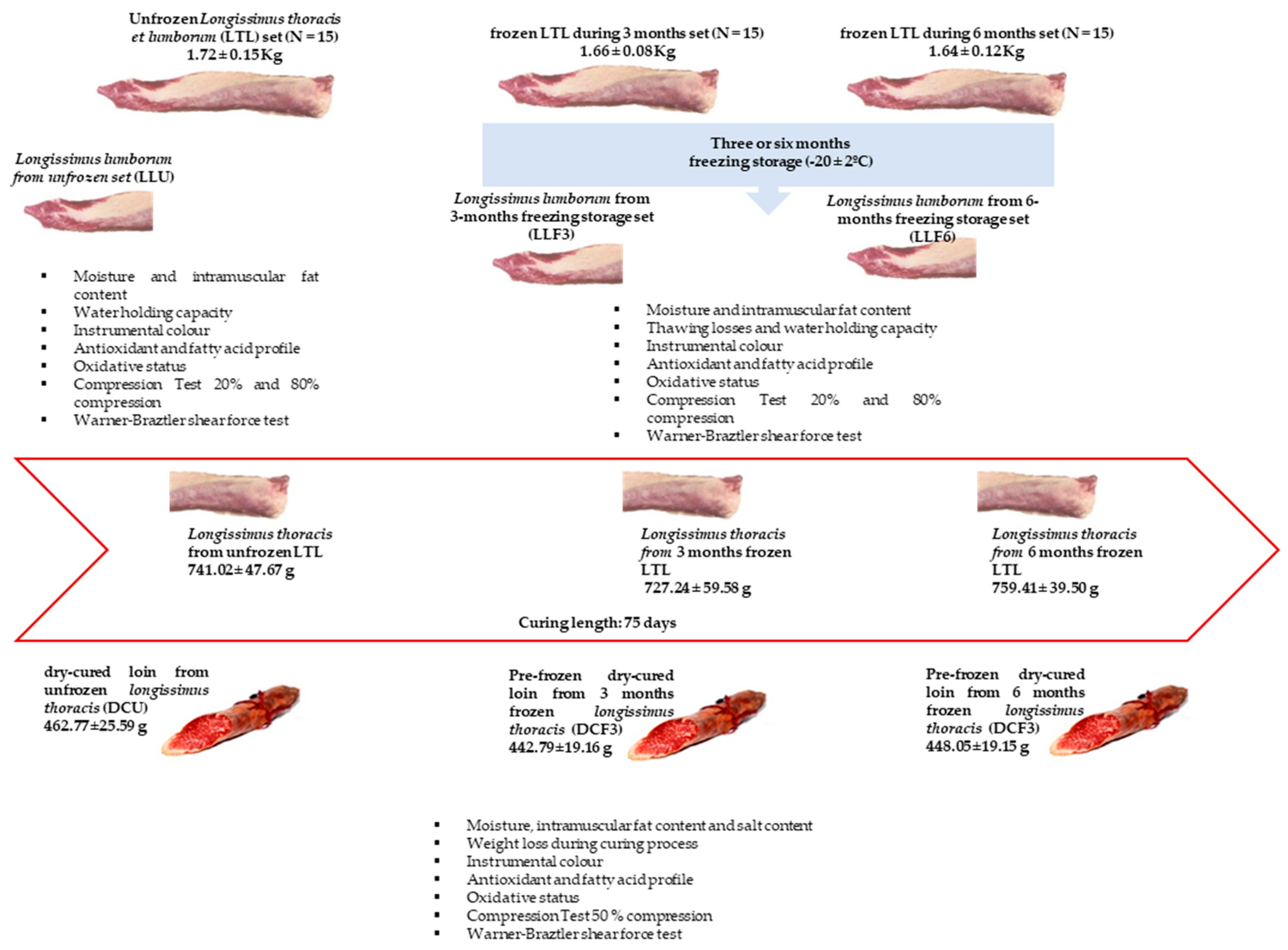
2.1. Meat Sampling
2.2. Freezing and Thawing Process
2.3. Technological Process of Curing
2.4. Methods
2.4.1. Proximate Composition
2.4.2. Thawing Loss (TL) and Water Holding Capacity (WHC)
2.4.3. Weight Loss in Dry-Cured Loins
2.4.4. Colour Measurement
2.4.5. Determination of α and γ-tocopherol
2.4.6. Lipid and Protein Oxidation
2.4.7. Determination of Fatty Acid Profile
2.4.8. Texture Analysis
2.4.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Pre-Freezing Cure Time on Proximate Composition, Water Losses and Instrumental Colour
3.2. Effect of Pre-Freezing Cure Time on Antioxidant Content, Oxidative Status, and Fatty Acids Profile
3.3. Effect of Pre-Freezing Cure Time on Textural Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Caro, C.; García-Torres, S.; Elghannam, A.; Tejerina, D.; Mesias, F.J.; Ortiz, A. Is production system a relevant attribute in consumers’ food preferences? The case of Iberian dry-cured ham in Spain. Meat Sci. 2019, 158. [Google Scholar] [CrossRef]
- RD 4/2014 Real Decreto por el Que se Aprueba la Norma de Calidad Para la Carne, el Jamón, la Paleta y la Caña de Lomo Ibérico; Spanish Ministry of Agriculture, Fisheries and Food: Madrid, Spain, 2014.
- Pérez-Palacios, T.; Ruiz, J.; Martín, D.; Barat, J.M.; Antequera, T. Pre-cure freezing effect on physicochemical, texture and sensory characteristics of Iberian ham. Food Sci. Technol. Int. 2011. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Parra, V.; Estévez, M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014, 96, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Palacios, T.; Ruiz, J.; Grau, R.; Flores, M.; Antequera, T. Influence of pre-cure freezing of Iberian hams on lipolytic changes and lipid oxidation. Int. J. Food Sci. Technol. 2009, 44, 2287–2295. [Google Scholar] [CrossRef]
- Soyer, A.; Hultin, H.O. Kinetics of oxidation of the lipids and proteins of cod sarcoplasmic reticulum. J. Agric. Food Chem. 2000, 48, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Soler, C.; Aristoy, M.C.; Toldrá, F. Effect of brine thawing/salting for time reduction in Spanish dry-cured ham manufacturing on proteolysis and lipolysis during salting and post-salting periods. Eur. Food Res. Technol. 2006, 222, 509–515. [Google Scholar] [CrossRef]
- Bañón, S.; Cayuela, J.M.; Granados, M.V.; Garrido, M.D. Pre-cure freezing affects proteolysis in dry-cured hams. Meat Sci. 1999, 51, 11–16. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Ruiz, J.; Barat, J.M.; Aristoy, M.C.; Antequera, T. Influence of pre-cure freezing of Iberian ham on proteolytic changes throughout the ripening process. Meat Sci. 2010, 85, 121–126. [Google Scholar] [CrossRef]
- Lorido, L.; Ventanas, S.; Akcan, T.; Estévez, M. Effect of protein oxidation on the impaired quality of dry-cured loins produced from frozen pork meat. Food Chem. 2016, 196, 1310–1314. [Google Scholar] [CrossRef]
- Abellán, A.; Salazar, E.; Vázquez, J.; Cayuela, J.M.; Tejada, L. Changes in proteolysis during the dry-cured processing of refrigerated and frozen loin. LWT 2018, 96, 507–512. [Google Scholar] [CrossRef]
- European Union. Regulation No 16/2012 Concerning Requirements for Frozen Food of Animal Origin Intended for Human Consumption; European Commission: Luxembourg, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Association of Analytical: Washington, DC, USA, 2003. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Tejerina, D.; García-Torres, S.; Cava, R. Water-holding capacity and instrumental texture properties of m. Longissimus dorsi and m. Serratus ventralis from Iberian pigs as affected by the production system. Livest. Sci. 2012, 148, 46–51. [Google Scholar] [CrossRef]
- Liu, Q.; Scheller, K.; Schaeffer, D. Technical note: A simplified procedure for vitamin E determination in beef muscle. J. Anim. Sci. 1996, 74, 2406–2410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Batifoulier, F.; Mercier, Y.; Gatellier, P.; Renerre, M. Influence of vitamin E on lipid and protein oxidation induced by H2O2-activated MetMb in microsomal membranes from turkey muscle. Meat Sci. 2002, 61, 389–395. [Google Scholar] [CrossRef]
- Lepetit, J.; Culioli, J. Mechanical properties of meat. Meat Sci. 1994, 36, 203–237. [Google Scholar] [CrossRef]
- Bourne, M. Texture Profile Analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Ramírez, M.R.; Cava, R. Effect of Iberian x Duroc genotype on dry-cured loin quality. Meat Sci. 2007, 76, 333–341. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Heinonen, M.; Puolanne, E. Protein carbonilation and water-holding capacity of pork subjected to frozen storage. Effect of muscle type, premincing, and packaging. J. Agric. Food Chem. 2011, 59, 5435–5443. [Google Scholar] [CrossRef]
- Kim, G.-D.; Jung, E.-Y.; Lim, H.-J.; Yang, H.-S.; Joo, S.-T.; Jeong, J.-Y. Influence of meat exudates on the quality characteristics of fresh and freeze-thawed pork. Meat Sci. 2013, 95, 323–329. [Google Scholar] [CrossRef]
- Grau, R.; Albarracín, W.; Toldrá, F.; Antequera, T.; Barat, J. Study of salting and postsalting of fresh and thawed iberian hams. Meat Sci. 2008, 79, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, J.; Sayas-Barberá, M.; Fernández-López, J.; Gago-Gago, M.; Pagán-Moreno, M.; Aranda-Catalá, V. Spanish Dry-Cured Ham Aging Process: Colour Characteristics. In Proceedings of the 44 th ICOMST, Barcelona, Spain, 1 September 1998; pp. 984–985. [Google Scholar]
- Martín Mateos, M. Aptitud de Distintos Sistemas de Conservación Para la Prolongación de la Vida Útil de Carne Fresca de Cerdo Ibérico Para el Consumo Directo y de Productos Derivados; University of Extremadura-España: Badajoz, Spain, 2013. [Google Scholar]
- Carrapiso, A.I.; García, C. Instrumental colour of Iberian ham subcutaneous fat and lean (biceps femoris): Influence of crossbreeding and rearing system. Meat Sci. 2005, 71, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, D.; García-Torres, S.; Cabeza de Vaca, M.; Vázquez, F.M.; Cava, R. Acorns (Quercus rotundifolia Lam.) and grass as natural sources of antioxidants and fatty acids in the “Montanera” feeding of Iberian pig: Intra- and inter-annual variations. Food Chem. 2011, 124, 997–1004. [Google Scholar] [CrossRef]
- Isabel, B.; Lopez-Bote, C.J.; Rey, A.I.; Sanz Arias, R. Influence of dietary α-tocopheryl acetate supplementation of pigs on oxidative deterioration and weight loss in sliced dry-cured ham. Meat Sci. 1999, 51, 227–232. [Google Scholar] [CrossRef]
- Rey, A.I.; López-Bote, C.J.; Daza, A.; Lauridsen, C. Accumulation and evolution of tocopherols in dry-cured hams from Iberian pigs as affected by their feeding and rearing system. Food Chem. 2010, 123, 1170–1175. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Cabeza de Vaca, M.C.; Vázquez, F.M.; Cava, R. Study of variability in antioxidant composition and fatty acids profile of Longissimus dorsi and Serratus ventralis muscles from Iberian pigs reared in two different Montanera seasons. Meat Sci. 2012, 90, 414–419. [Google Scholar] [CrossRef]
- Gilles, G. Dry cured ham quality as related to lipid quality of raw material and lipid changes during processing: A review. Grasas Aceites 2009, 60, 297–307. [Google Scholar] [CrossRef]
- Hernández, P.; Navarro, J.L.; Toldrá, F. Effect of frozen storage on lipids and lipolytic activities in the Longissimus dorsi muscle of the pig. Z. Leb. Forsch. A 1999, 208, 110–115. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012, 90, 917–924. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.; Hoffman, L. Impact of Freezing and Thawing on the Quality of Meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, J.; Arnau, J.; Serra, X.; Gou, P. Relationship between water content, NaCl content, pH and texture parameters in dry-cured muscles. Meat Sci. 2005, 70, 579–587. [Google Scholar] [CrossRef] [PubMed]
| Fresh Loin | Dry-Cured Loin | |||||||
|---|---|---|---|---|---|---|---|---|
| LLU | LLF3 | LLF6 | Sig | DCU | DCF3 | DCF6 | Sig | |
| Proximate composition (g/100 g) | ||||||||
| Moisture | 70.1 ± 1.28 | 70.7 ± 0.70 | 70.1 ± 0.74 | ns | 44.2 a ± 2.09 | 41.9 b ± 3.21 | 42.4 b ± 2.28 | * |
| IMF 1 | 12.1 ± 1.08 | 13.5 ± 1.55 | 12.7 ± 2.75 | ns | 16.4 ± 1.08 | 17.6 ± 0.97 | 17.3 ± 2.44 | ns |
| NaCl | - | - | - | 5.6 a ± 0.66 | 4.1 b ± 0.43 | 4.1 b ± 0.50 | *** | |
| Water losses (g water/100 g muscle) | ||||||||
| TL | - | 2.5 ± 0.54 | 2.7 ± 0.62 | ns | - | - | - | |
| WL | - | - | - | 37.6 c ± 1.78 | 39.2 b ± 1.47 | 41.3 a ± 1.97 | *** | |
| WHC | 29.0 a ± 0.50 | 26.4 c ± 1.39 | 28.0 b ± 0.46 | *** | - | - | - | - |
| Instrumental Color coordinates | ||||||||
| L * | 47.9 a ± 2.48 | 44.9 b ± 2.51 | 45.4 b ± 2.67 | *** | 35.3 ± 2.17 | 36.7 ± 3.87 | 36.3 ± 3.29 | ns |
| a * | 14.0 a ± 1.51 | 12.5 b ± 1.20 | 12.0 b ± 1.24 | *** | 12.6 a ± 1.04 | 11.0 b ± 0.50 | 12.6 a ± 0.85 | ns |
| b * | 7.5 ± 1.28 | 7.4 ± 0.70 | 7.5 ± 0.54 | ns | 6.3 a ± 0.54 | 4.5 b ± 0.19 | 3.5c ± 0.39 | *** |
| C * | 16.2 a ± 1.70 | 14.3 b ± 1.47 | 13.8 b ± 1.66 | *** | 14.2 ± 1.12 | 12.0 ± 0.93 | 12.8 ± 0.85 | ns |
| H° | 20.0 b ± 1.32 | 30.0 a ± 1.55 | 30.4 a ± 1.20 | *** | 26.3 a ± 1.47 | 22.0 a ± 2.05 | 15.4 b ± 1.08 | *** |
| Fresh Loin | Dry-Cured Loin | |||||||
|---|---|---|---|---|---|---|---|---|
| LLU | LLF3 | LLF6 | Sig | DCU | DCF3 | DCF6 | Sig | |
| Antioxidant Composition and Oxidative status | ||||||||
| α-Tocopherol 1 | 16.4 ± 1.43 | 16.6 ± 2.28 | 16.3 ± 2.21 | ns | 10.7 ± 0.89 | 11.1 ± 2.67 | 11.7 ± 0.97 | ns |
| γ-Tocopherol 1 | 2.6 ± 0.43 | 2.9 ± 0.50 | 2.9 ± 0.46 | ns | 1.6 b ± 0.31 | 1.9 a ± 0.38 | 2.0 a ± 0.31 | ** |
| mg MDA/kg | 0.1 b ± 0.04 | 0.2 a ± 0.08 | 0.2 a ± 0.04 | *** | 1.0 b ± 0.19 | 1.3 a ± 0.31 | 1.2 a ± 0.36 | * |
| nmol thiol/mg prot | 166.7 a ± 21.98 | 127.4 b ± 14.20 | 88.1 c ± 16.69 | *** | 210.0 a ± 16.87 | 147.8 b ± 33.59 | 138.8 b ± 16.18 | *** |
| Fatty acid composition (g/100 g FAMEs) | ||||||||
| C16:0 | 23.0 ± 1.20 | 23.6 ± 1.78 | 23.4 ± 2.32 | ns | 23.8 ± 2.05 | 23.5 ± 1.27 | 23.9 ± 1.90 | ns |
| C16:1 | 3.8 ± 0.35 | 3.9 ± 0.43 | 3.8 ± 0.69 | ns | 3.9 ± 0.69 | 3.7 ± 0.38 | 3.8 ± 0.34 | ns |
| C18:0 | 11.0 ± 0.81 | 10.3 ± 1.12 | 10.8 ± 1.54 | ns | 11.6 ± 1.78 | 12.2 ± 0.97 | 11.2 ± 1.63 | ns |
| C18:1 n-9 | 52.1 ± 1.39 | 52.1 ± 0.77 | 52.3 ± 1.35 | ns | 51.6 ± 2.83 | 50.3 ± 1.89 | 51.0 ± 1.32 | ns |
| C18:2 n-6 | 6.2 a ± 0.31 | 6.0 a b ± 0.54 | 5.6 b ± 0.81 | * | 5.6 a b ± 0.62 | 5.7 a ± 0.43 | 5.2 b ± 0.54 | * |
| C18:3 n-3 | 0.5 a ± 0.01 | 0.3 b ± 0.04 | 0.4 b ± 0.07 | *** | 0.3 a ± 0.07 | 0.2 b ± 0.04 | 0.2 b ± 0.04 | *** |
| SFA | 35.5 ± 1.90 | 36.4 ± 2.36 | 36.7 ± 3.72 | ns | 36.8 ± 3.98 | 37.9 ± 2.39 | 38.0 ± 2.59 | ns |
| MUFA | 56.9 ± 1.66 | 56.9 ± 0.93 | 56.9 ± 1.74 | ns | 56.2 ± 1.89 | 56.0 ± 2.01 | 56.1 ± 1.35 | ns |
| PUFA | 7.7 a ± 0.43 | 6.7 b ± 0.58 | 6.4 b ± 0.85 | *** | 7.1 a ± 0.74 | 6.1 b ± 0.74 | 5.9 b ± 0.54 | *** |
| n-6/n-3 | 11.9 c ± 0.77 | 18.3 a ± 2.28 | 16.4 b ± 2.78 | *** | 17.6 b ± 2.47 | 22.5 a ± 3.63 | 21.9 a ± 2.40 | *** |
| Fresh Loin | ||||
|---|---|---|---|---|
| LLU | LLF3 | LLF6 | Sig | |
| Compression Test I (TPA-20% compression) | ||||
| Hardness (N/cm2) | 1.9 b ± 0.50 | 5.2 a ± 1.47 | 5.5 a ± 1.47 | *** |
| Springiness (cm) | 0.8 ± 0.04 | 0.8 ± 0.04 | 0.9 ± 0.04 | ns |
| Cohesiveness | 0.7 ± 0.04 | 0.7 ± 0.04 | 0.7 ± 0.04 | ns |
| Gumminess (N cm s2) | 1.2 b ± 0.19 | 3.7 a ± 1.16 | 4.1 a ± 1.16 | *** |
| Chewiness (N cm s2) | 1.0 b ± 0.27 | 3.0 a ± 1.08 | 3.5 a ± 1.08 | *** |
| Resilience | 0.5 b ± 0.04 | 0.5 a ± 0.08 | 0.5 a ± 0.04 | ns |
| Compression Test II (TPA-80% compression) | ||||
| Hardness (N/cm2) | 100.0 b ± 10.02 | 134.3 a ± 12.93 | 132.1 a ± 12.03 | *** |
| Springiness (cm) | 0.5 b ± 0.04 | 0.5 a ± 0.04 | 0.5 a ± 0.04 | *** |
| Cohesiveness | 0.4 b ± 0.04 | 0.5 a ± 0.04 | 0.5 a ± 0.04 | *** |
| Gumminess (N cm s2) | 41.4 b ± 8.05 | 61.6 a ± 11.14 | 62.8 a ± 12.54 | *** |
| Chewiness (N cm s2) | 17.8 b ± 4.02 | 32.7 a ± 6.85 | 33.3 a ± 7.93 | *** |
| Resilience | 0.2 ± 0.04 | 0.3 ± 0.04 | 0.3 ± 0.04 | ns |
| Warner—Braztler shear force test (WBSF) | ||||
| WBSF (N/cm2) | 72.0 a ± 13.43 | 46.2 b ± 9.79 | 49.3 b ± 11.22 | *** |
| Dry-Cured Loin | ||||
|---|---|---|---|---|
| DCU | DCF3 | DCF6 | Sig | |
| Compression Test (TPA-50% compression) | ||||
| Hardness (N/cm2) | 30.9 b ± 2.97 | 33.2 a b ± 1.70 | 36.0 a ± 2.36 | * |
| Springiness (cm) | 0.6 b ± 0.04 | 0.7 a ± 0.08 | 0.7 a ± 0.04 | *** |
| Cohesiveness | 0.6 ± 0.04 | 0.6 ± 0.04 | 0.6 ± 0.04 | ns |
| Gumminess (N cm s2) | 18.5 ± 4.91 | 20.1 ± 3.44 | 21.8 ± 3.64 | ns |
| Chewiness (N cm s2) | 10.7 b ± 3.02 | 13.9 a ± 1.97 | 14.2 a ± 2.17 | *** |
| Resilience | 2.1 a ± 0.31 | 0.2 b ± 0.04 | 0.2 b ± 0.04 | *** |
| Warner–Braztler shear force test | ||||
| WBSF (N/cm2) | 44.1 a ± 5.68 | 33.5 b ± 5.46 | 32.6 b ± 3.48 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, A.; Tejerina, D.; Contador, R.; de Andrés, A.I.; Petrón, M.J.; Cáceres-Nevado, J.M.; García-Torres, S. Quality Traits of Dry-Cured Loins from Iberian Pigs Reared in Montanera System as Affected by Pre-Freezing Cure. Foods 2021, 10, 48. https://doi.org/10.3390/foods10010048
Ortiz A, Tejerina D, Contador R, de Andrés AI, Petrón MJ, Cáceres-Nevado JM, García-Torres S. Quality Traits of Dry-Cured Loins from Iberian Pigs Reared in Montanera System as Affected by Pre-Freezing Cure. Foods. 2021; 10(1):48. https://doi.org/10.3390/foods10010048
Chicago/Turabian StyleOrtiz, Alberto, David Tejerina, Rebeca Contador, Ana Isabel de Andrés, María Jesús Petrón, Juan Manuel Cáceres-Nevado, and Susana García-Torres. 2021. "Quality Traits of Dry-Cured Loins from Iberian Pigs Reared in Montanera System as Affected by Pre-Freezing Cure" Foods 10, no. 1: 48. https://doi.org/10.3390/foods10010048
APA StyleOrtiz, A., Tejerina, D., Contador, R., de Andrés, A. I., Petrón, M. J., Cáceres-Nevado, J. M., & García-Torres, S. (2021). Quality Traits of Dry-Cured Loins from Iberian Pigs Reared in Montanera System as Affected by Pre-Freezing Cure. Foods, 10(1), 48. https://doi.org/10.3390/foods10010048