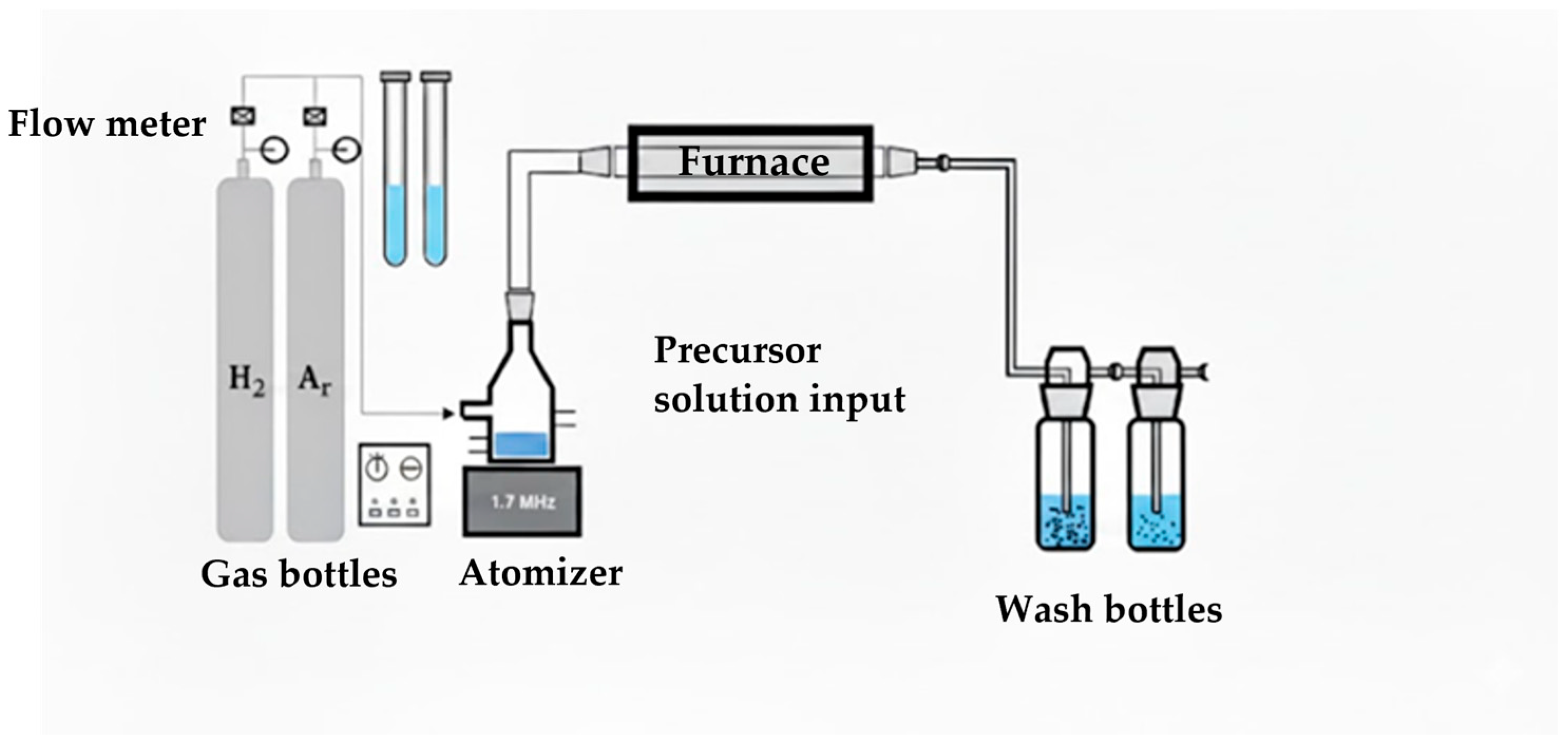
1. Introduction
Copper (Cu) and silver (Ag) are technologically important metals owing to their high electrical and thermal conductivity, chemical stability, and broad applicability in electronics, catalysis, and functional coatings [
1]. In recent years, Cu–Ag bimetallic systems have attracted increasing attention because the combination of these two metals can result in synergistic properties that differ from those of the individual components, such as enhanced catalytic selectivity, improved oxidation resistance, and tunable electrical behavior. These characteristics make Cu–Ag materials promising candidates for applications in conductive inks, electrocatalysis, plasmonic devices, and energy-related technologies [
2,
3,
4,
5].
Despite strong interest in Cu, Ag, and Cu–Ag powders for conductive inks and catalytic systems, practical implementation is often limited by (i) oxidation and conductivity loss of Cu during storage and processing, (ii) particle sintering/agglomeration at elevated temperatures, (iii) surface contamination from organic reagents used in wet-chemical routes, and (iv) scalability and batch-to-batch reproducibility challenges. These issues motivate scalable gas-phase synthesis routes capable of producing clean surfaces and controllable morphologies under continuous conditions.
A wide range of synthesis methods has been reported for Cu, Ag, and Cu–Ag powders, including wet chemical reduction, electrodeposition, solvothermal routes, and physical vapor-based techniques. While these approaches allow fine control over particle size and composition, they often rely on organic reducing agents, surfactants, or stabilizers, which can introduce impurities, complicate post-treatment, and limit scalability. In addition, batch-type synthesis routes may suffer from poor reproducibility and limited throughput, restricting their suitability for large-scale production [
3,
6,
7,
8,
9,
10,
11,
12,
13].
Ultrasonic spray pyrolysis (USP) represents an alternative gas-phase synthesis technique that enables continuous production of fine powders from liquid precursor solutions. In USP, precursor droplets generated by ultrasonic atomization undergo solvent evaporation, thermal decomposition, and particle formation within a high-temperature reactor. Compared to wet chemical methods, USP offers several advantages, including a continuous and scalable process, relatively short residence times, precise control over precursor composition, and reduced risk of contamination from organic additives. When combined with a reducing atmosphere, USP can be adapted for the synthesis of metallic and bimetallic particles with controlled morphology and composition [
14,
15,
16].
Prior USP and spray-based studies have demonstrated the feasibility of producing metallic Cu and Ag powders and, in some cases, Ag–Cu composites; however, most reports focus on single-metal systems or do not systematically compare the coupled effects of temperature, gas atmosphere, and Cu:Ag precursor ratio under hydrogen-assisted conditions. As a result, parameter–morphology–stability relationships for USP–HR-derived Cu–Ag powders remain insufficiently established.
Despite these advantages, systematic studies on the synthesis of Cu, Ag, and Cu–Ag powders via USP—particularly under hydrogen-assisted reduction conditions—remain limited. Existing reports often focus on single-metal systems or rely on inert atmospheres, while the combined influence of synthesis temperature, gas atmosphere, and precursor ratio on particle morphology and compositional stability has not been thoroughly examined for Cu–Ag systems produced by USP. In particular, understanding how process parameters affect particle size distribution, agglomeration behavior, and susceptibility to post-synthesis oxidation is essential for tailoring materials for practical applications.
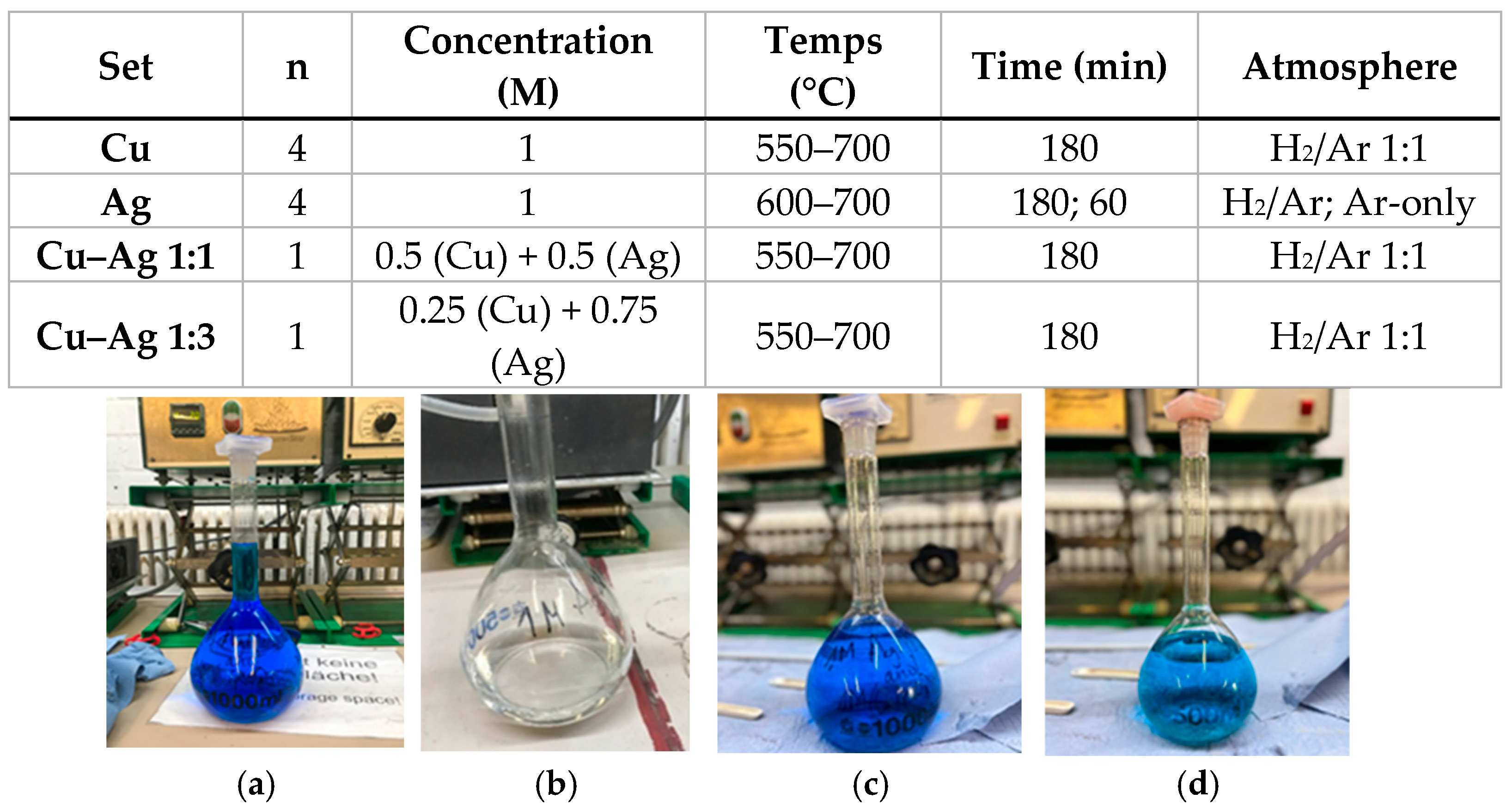
In this work, Cu, Ag, and Cu–Ag powders are synthesized using ultrasonic spray pyrolysis combined with hydrogen reduction (USP–HR). The effects of reduction temperature, gas atmosphere, and precursor molar ratio on particle morphology, size distribution, and elemental composition are systematically investigated using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). Rather than proposing new formation mechanisms, this study aims to provide process-level insight into how USP–HR parameters influence the characteristics of mono- and bimetallic Cu–Ag powders. The results contribute to the development of scalable synthesis strategies for Cu-based and Ag-based materials with tunable properties, relevant for applications in electronics, catalysis, and energy-related technologies.
It should be emphasized that the objective of the present work is not to propose new fundamental nucleation or growth mechanisms for Cu–Ag bimetallic systems, which have been extensively investigated in wet chemical and electrochemical synthesis routes. Instead, this study focuses on evaluating how processing parameters in a gas-phase ultrasonic spray pyrolysis–hydrogen reduction (USP–HR) system influence particle morphology, size distribution, and compositional stability under continuous synthesis conditions.
3. Discussion
3.1. Influence of Temperature on Particle Morphology and Purity
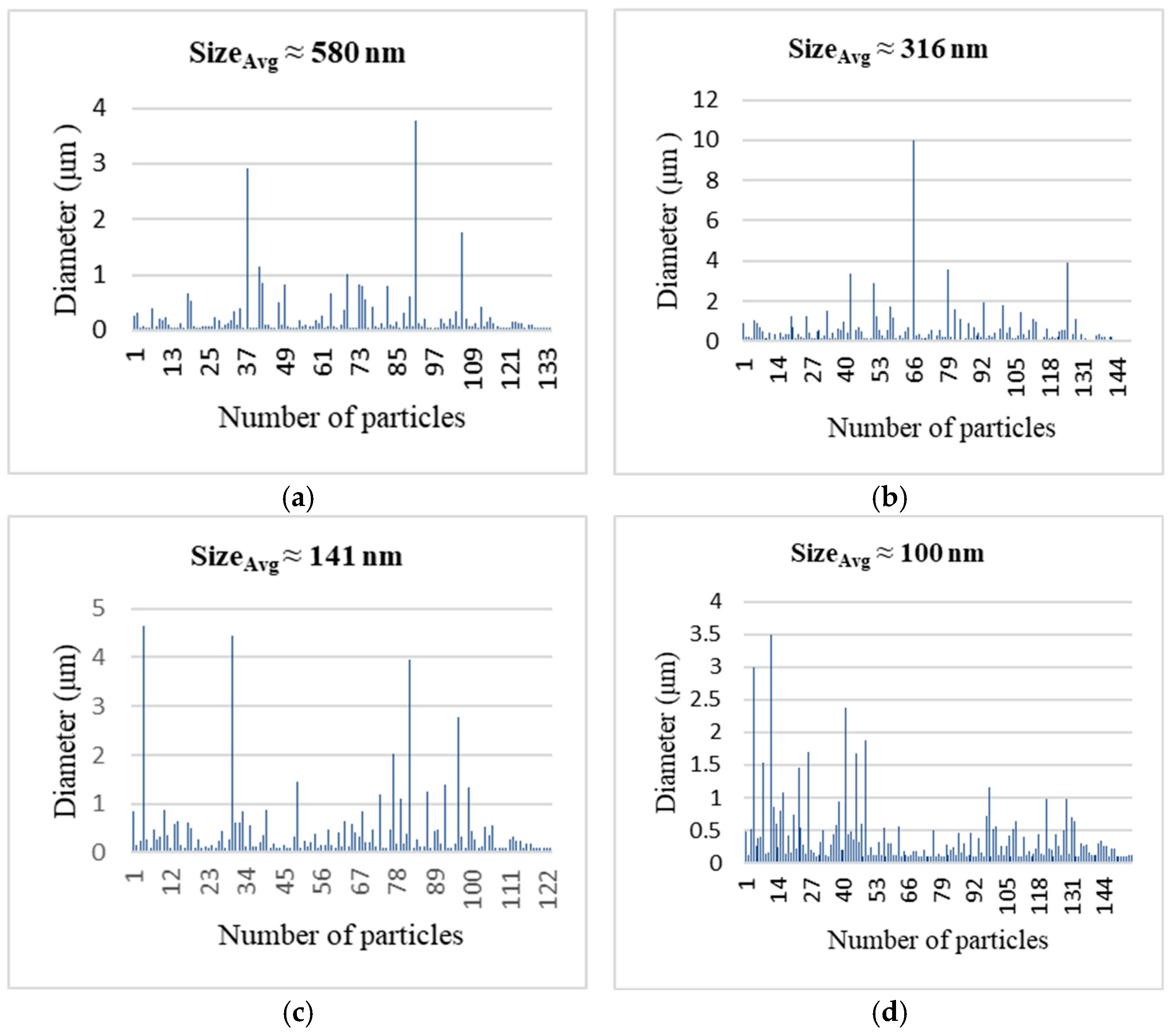
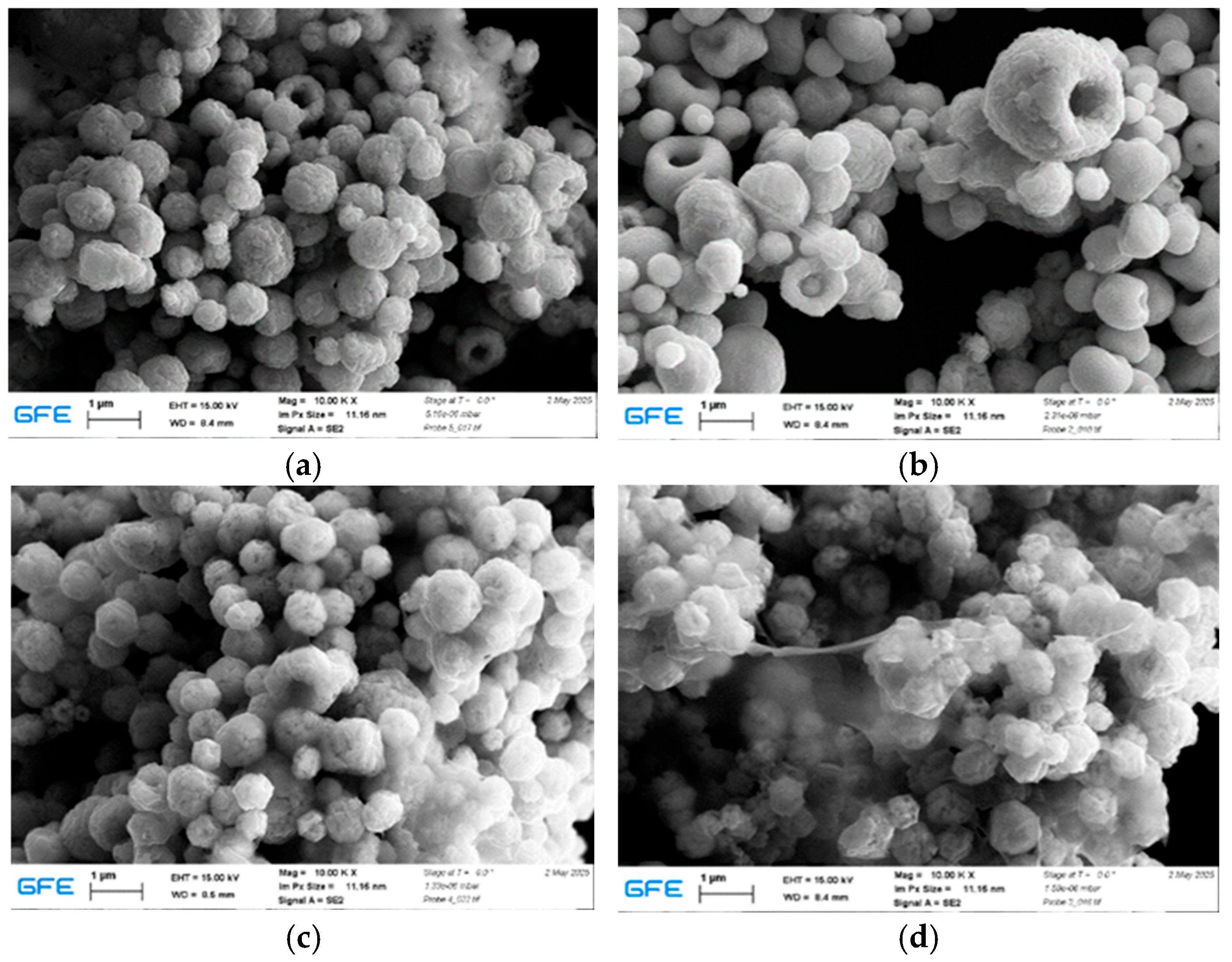
Higher synthesis temperatures accelerate solvent evaporation and reduction kinetics, promoting rapid nucleation and limiting particle growth during the early stages of particle formation. However, excessive heating, especially at temperatures around 700 °C, enhances interparticle interactions and sintering, which explains the increased agglomeration observed at the highest temperature. The formation of smaller and more uniform particles at elevated temperatures under a hydrogen-containing atmosphere suggests that hydrogen facilitates reduction kinetics and promotes burst nucleation, leading to the rapid formation of numerous fine nuclei. In contrast, at lower temperatures such as 600 °C, reduced nucleation rates allow fewer nuclei to grow for longer periods before precursor depletion, resulting in larger particle sizes.
Comparable temperature-dependent trends in particle morphology have been reported in previous spray-based and thermal synthesis studies, although the dominant mechanisms strongly depend on the synthesis route and operating conditions [
10]. Additionally, literature reports on copper oxide systems indicate that elevated temperatures can influence the oxidation state of copper, as evidenced by residual oxygen contents after thermal treatment, which is consistent with the compositional trends observed in the present work [
11].
3.2. Influence of Gas Atmosphere on Particle Formation
The influence of gas atmosphere on particle size and morphology in the present study is interpreted on a phenomenological basis, considering differences in reduction kinetics, nucleation rates, and particle growth behavior under hydrogen-containing and inert atmospheres.
From a thermodynamic perspective, hydrogen provides a strongly reducing environment that favors conversion of transient oxide or nitrate-derived intermediates to the metallic state at elevated temperatures, thereby shifting reactions toward metal formation. Under argon, metal formation relies on thermal decomposition pathways and transient oxide intermediates, which alters the nucleation–growth balance and can lead to different coalescence behavior. In the present work, these thermodynamic tendencies are used only to support a qualitative, process-level interpretation consistent with SEM/EDS trends.
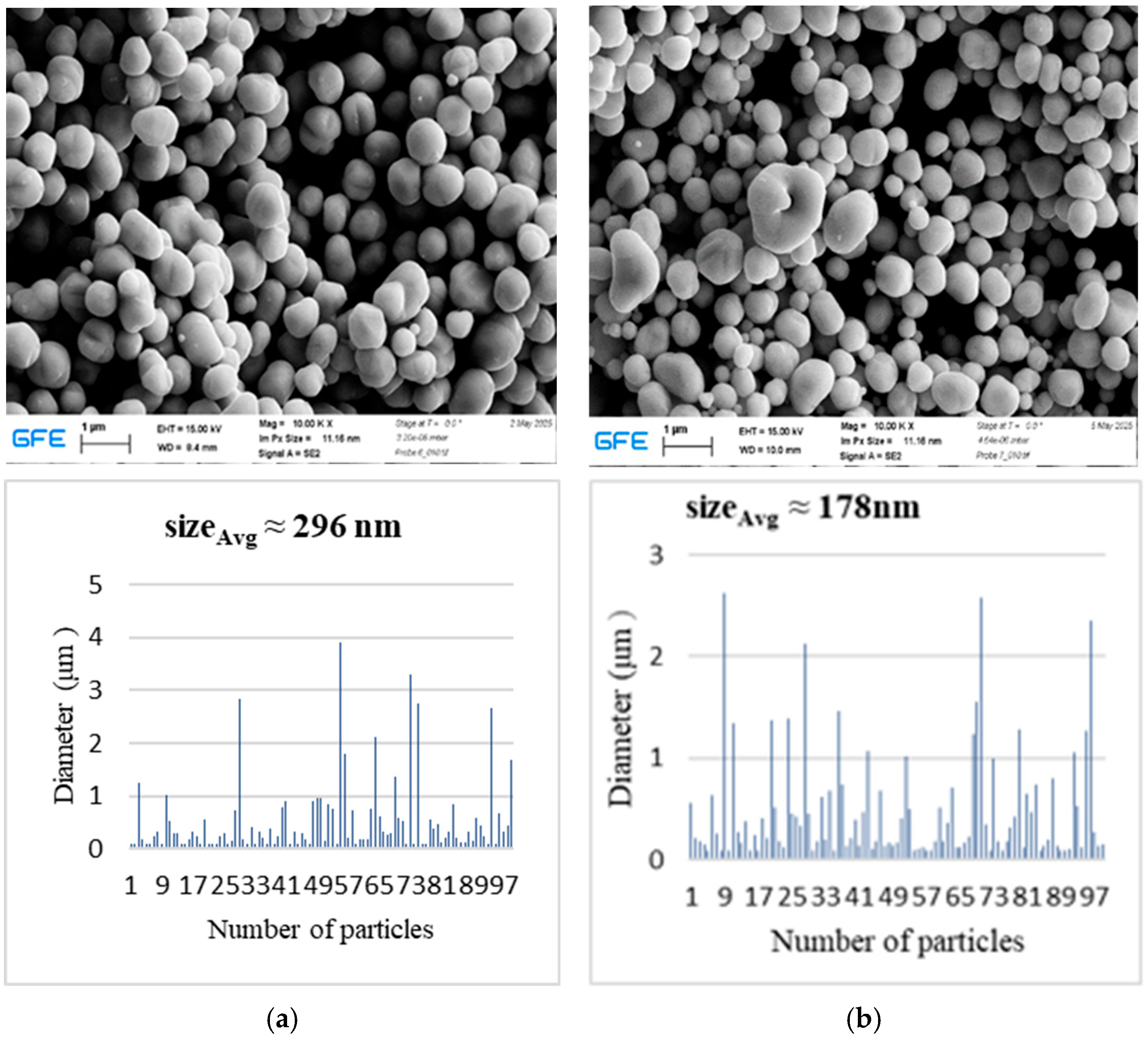
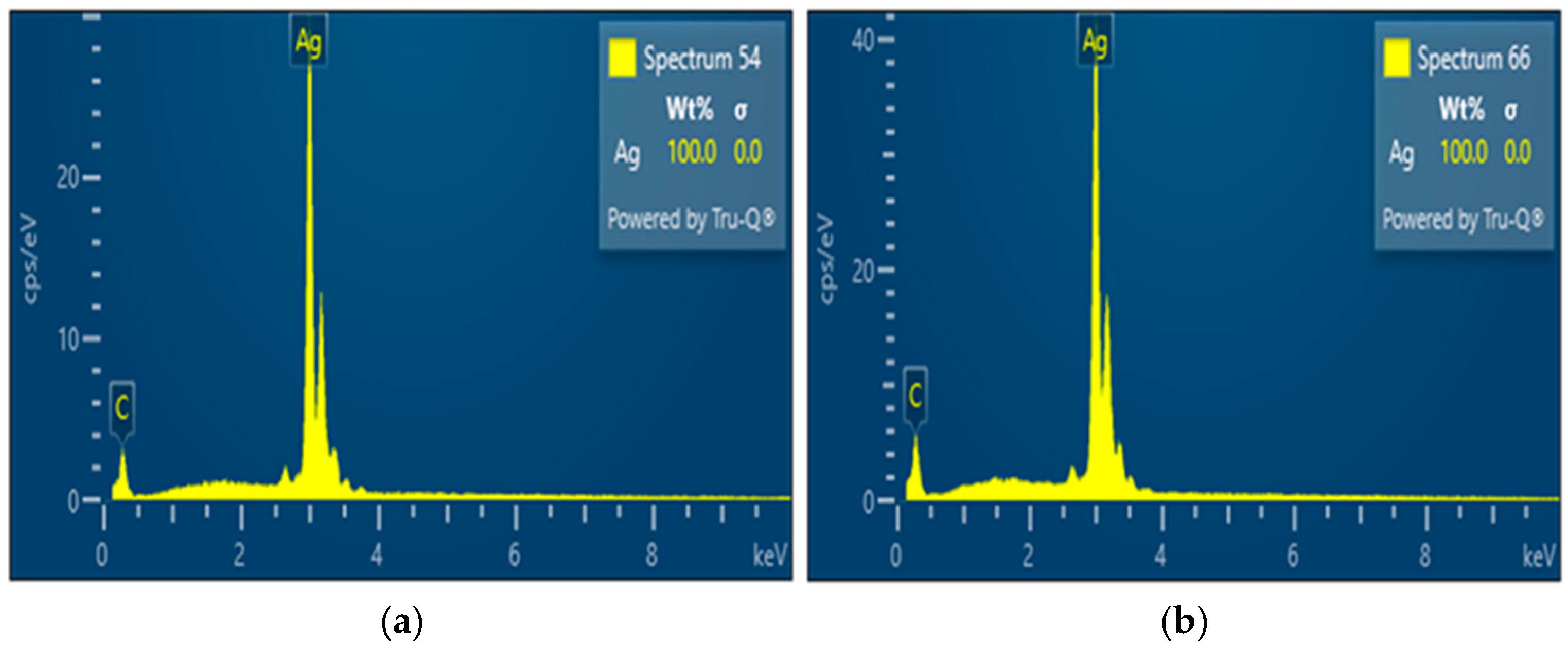
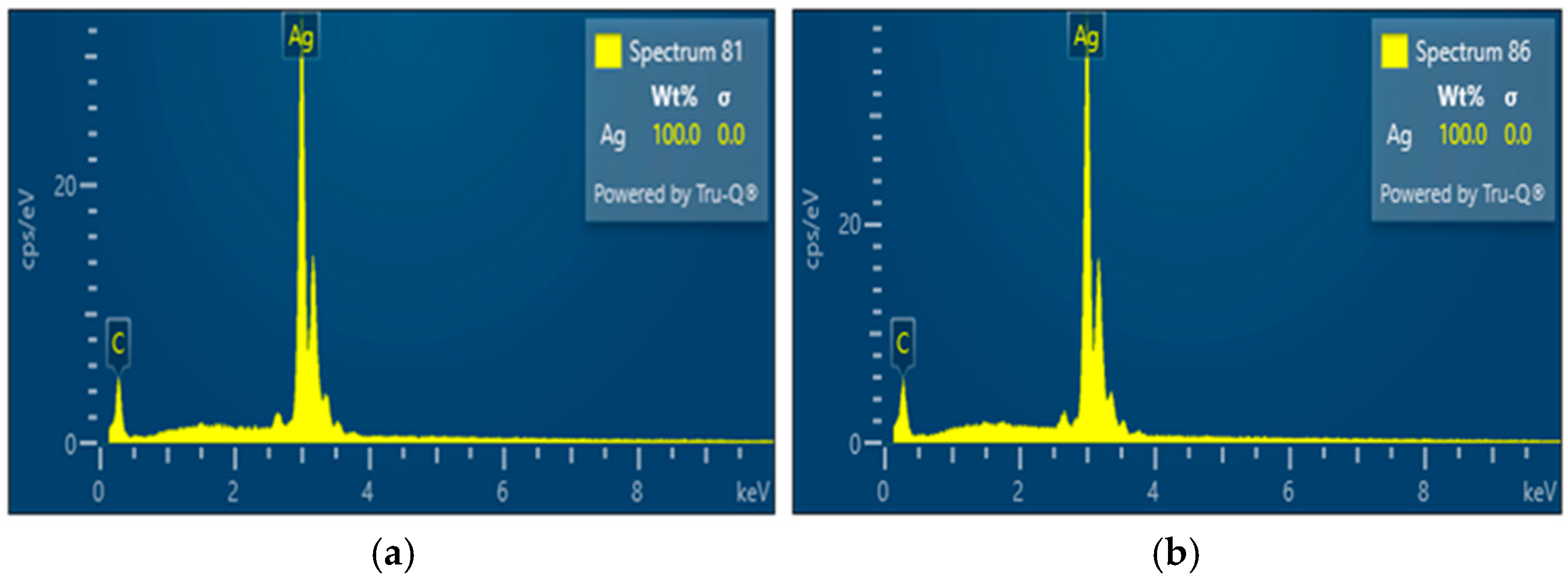
The SEM and EDS results for silver powders synthesized under different gas atmospheres highlight the critical role of the reaction environment in determining particle morphology and compositional trends. EDS spectra showed only Ag-related signals within the detection limits of the technique, indicating effective reduction of the silver nitrate precursor under both hydrogen-containing and argon atmospheres. Such compositional purity is particularly important because the electrical, catalytic, and plasmonic properties of silver particles are highly sensitive to oxide formation and surface contamination [
4].
In hydrogen-assisted synthesis, the reducing atmosphere is expected to enhance reduction kinetics, leading to rapid conversion of the precursor and promoting metallic silver formation. The accelerated reduction in hydrogen leads to rapid formation of metallic nuclei at an early stage, which favors subsequent particle growth and coalescence within the high-temperature zone, resulting in larger average particle sizes. In contrast, under argon, silver nitrate reduction proceeds primarily through thermally driven decomposition, with Ag2O acting as a transient intermediate before conversion to metallic Ag. As argon does not provide reactive reducing species, metal formation relies on thermally driven decomposition, which delays nucleation and increases supersaturation within droplets, promoting the formation of finer and more uniform particles. At lower temperatures, slower nucleation rates favor the formation of fewer nuclei and consequently larger particles with broader size distributions. Increasing the synthesis temperature enhances evaporation rates and supersaturation within the droplets, generating more nucleation sites and resulting in finer and more uniform particles.
The reduction of silver under argon conditions can also be described by an autocatalytic deposition mechanism, in which initially formed silver nuclei catalyze further reduction of remaining silver species [
12]. In the present study, silver powders produced under hydrogen-containing and argon atmospheres exhibited comparable compositional trends within the semi-quantitative limits of EDS analysis, suggesting that sufficiently high synthesis temperatures enable effective silver reduction even in the absence of hydrogen. Nevertheless, the observed differences in particle size and morphology indicate that the gas atmosphere remains an important parameter for tailoring particle characteristics in USP-based synthesis.
3.3. Influence of Precursor Ratio on Morphology and Stability
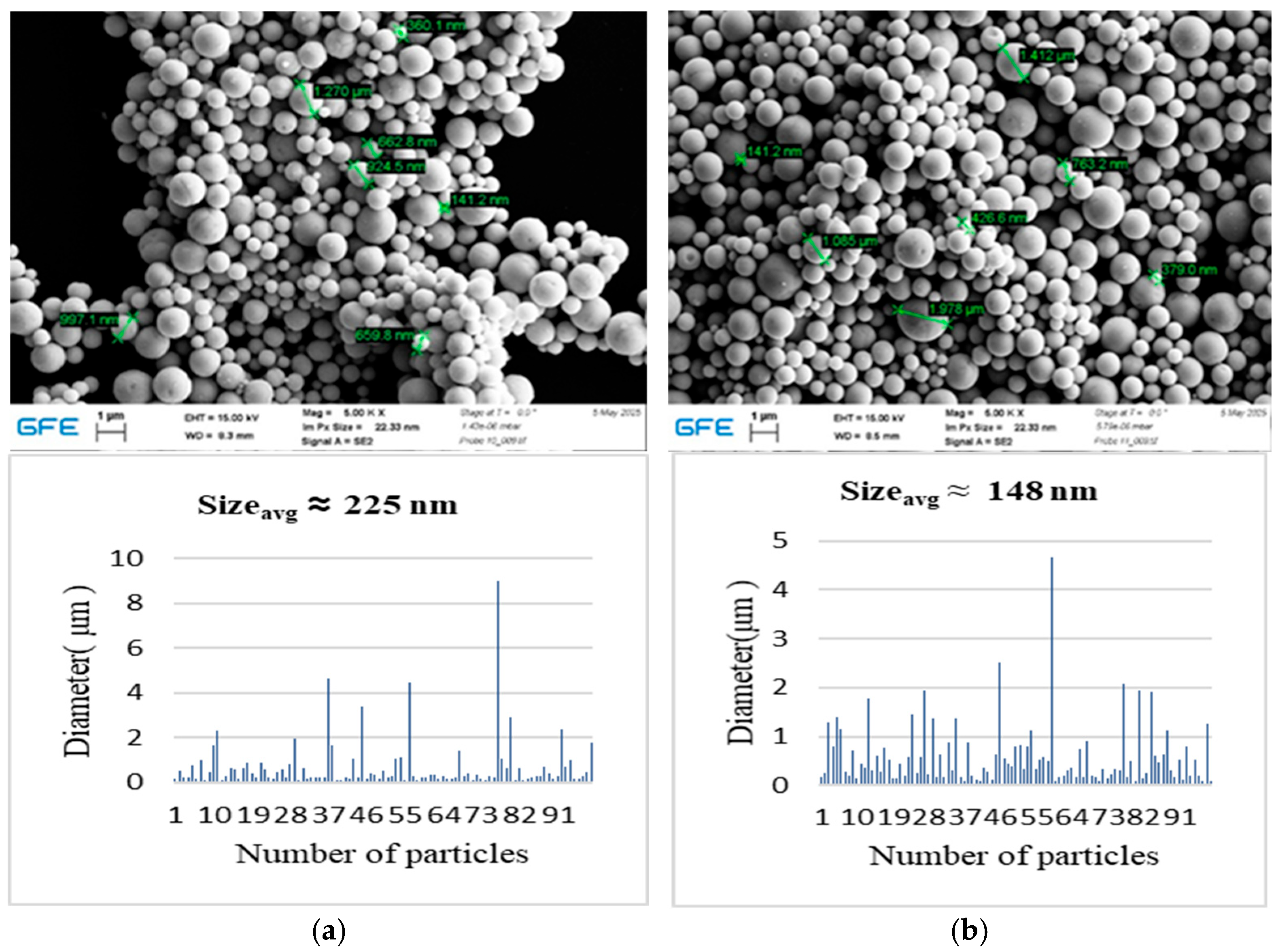
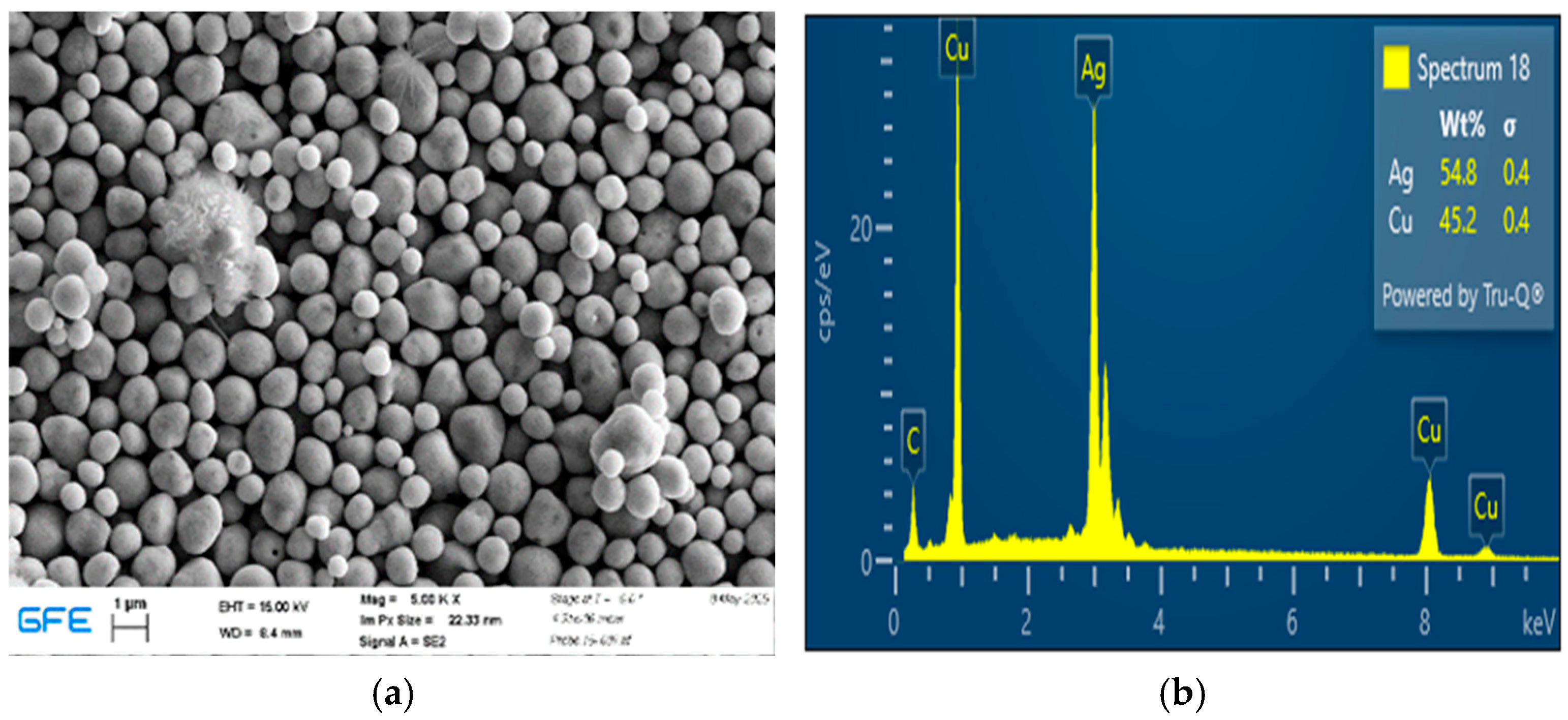
Cu–Ag composite powders synthesized at 650 °C exhibited distinct morphological and compositional differences depending on the Cu:Ag precursor ratio. These observations are consistent with the well-documented immiscibility of copper and silver, which promotes phase segregation tendencies commonly associated with core–shell-type configurations rather than homogeneous solid solutions [
13]. During hydrogen-assisted reduction, silver atoms—owing to their lower surface energy—tend to migrate toward the particle surface, resulting in Ag-enriched outer regions relative to the copper-rich interior.
Such Ag-enriched surface configurations have been reported in the literature to enhance oxidation resistance by shielding the copper component and may impart favorable surface characteristics for catalytic or plasmonic applications [
17]. The effective reduction achieved at 650 °C demonstrates that the USP–HR process is capable of producing metallic Cu–Ag composites under continuous synthesis conditions.
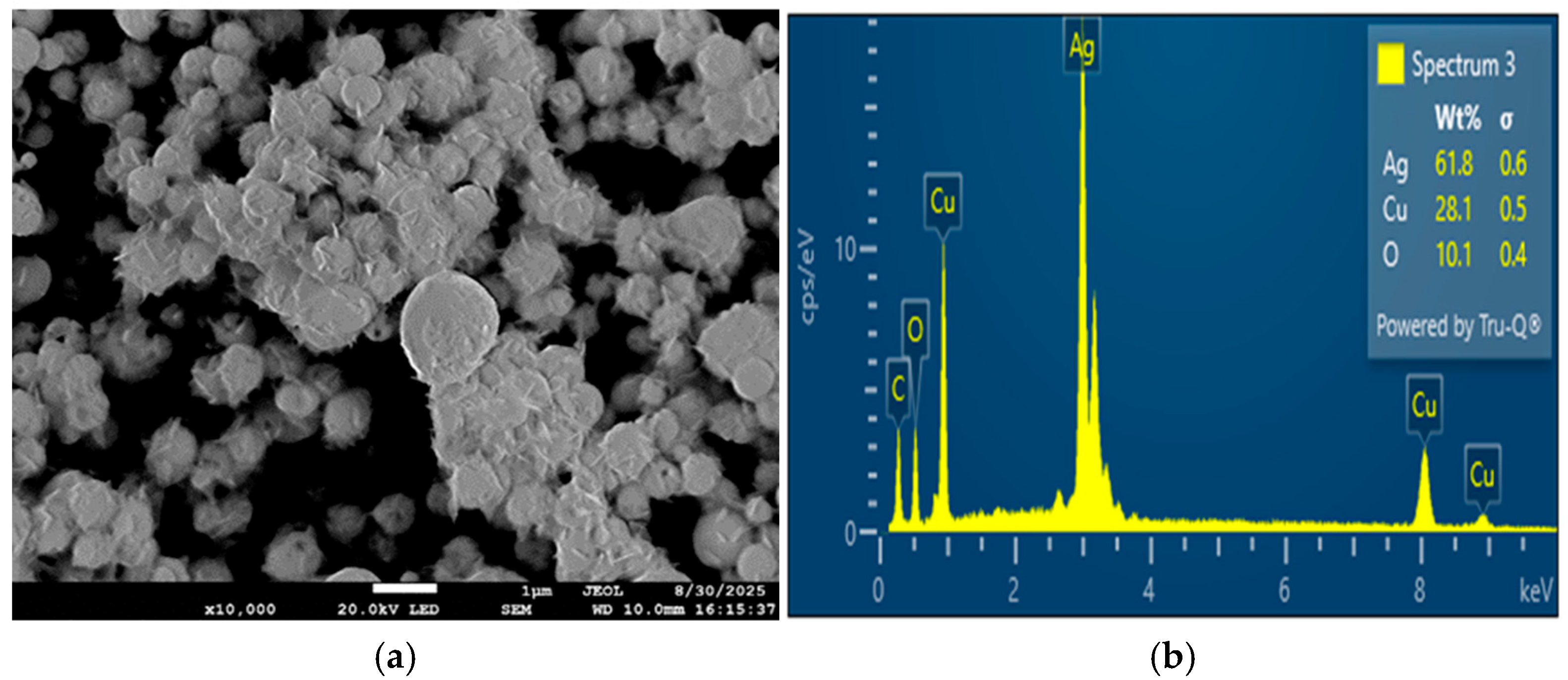
The Ag-rich (1:3 Cu:Ag) sample exhibited larger particle sizes and a higher degree of clustering compared to the equimolar composition. This behavior can be attributed to the higher silver content, which influences particle growth dynamics and promotes aggregation during synthesis. Excess silver enhances surface diffusion and coalescence, leading to localized particle growth and denser packing. At the same time, Ag-rich particles showed a greater tendency toward post-synthesis oxidation, as indicated by the oxygen detected by EDS. In contrast, the 1:1 Cu:Ag sample displayed improved morphological uniformity and compositional stability, suggesting a more favorable balance between homogeneity, oxidation resistance, and structural integrity.
Overall, these results indicate that increasing silver content can enhance surface mobility but may compromise long-term stability, whereas an equimolar Cu:Ag ratio provides more uniform and stable particles under the investigated conditions. While Cu–Ag bimetallic systems have shown promising performance in applications such as electrochemical CO2 reduction, further optimization and comprehensive characterization are required to address remaining challenges related to stability and performance.
The Ag-enriched surface features observed in this study are consistent with prior reports on Cu–Ag systems synthesized via wet chemical and electrochemical methods, where differences in surface energy and reduction kinetics favor silver segregation. No new nucleation or shell-formation mechanisms are proposed here. Rather, the present results demonstrate that similar compositional tendencies can be achieved under gas-phase USP–HR conditions without the use of surfactants or post-synthesis treatments, underscoring the capability of this approach to reproduce known structural features in a scalable and continuous manner.
It should be emphasized that, in the absence of X-ray diffraction and transmission electron microscopy, the present discussion is limited to compositional and morphological trends inferred from SEM and EDS analysis. Consequently, definitive determination of crystallographic structure, phase composition, and internal particle architecture is beyond the scope of this study.
5. Conclusions
This study demonstrates that gas atmosphere, synthesis temperature, and precursor molar ratio have a pronounced influence on the morphology and elemental composition of Ag, Cu, and Cu–Ag powders produced by ultrasonic spray pyrolysis combined with hydrogen reduction. Variations in processing conditions resulted in clear differences in particle size distribution, agglomeration behavior, and susceptibility to surface oxidation. For copper powders, synthesis at intermediate temperatures (600–650 °C) led to more uniform particle morphologies, whereas higher temperatures promoted finer particles but with increased agglomeration. In Cu–Ag systems, the precursor ratio played a critical role, with Ag-rich compositions exhibiting enhanced particle coalescence and a higher tendency toward post-synthesis oxidation compared to equimolar Cu–Ag powders.
Overall, the results highlight the strong sensitivity of USP-based synthesis to processing parameters and demonstrate that controlled adjustment of temperature, gas atmosphere, and precursor composition provides an effective means of tailoring particle characteristics. The conclusions drawn in this work are based on morphological and compositional trends observed by SEM and EDS and are intended to provide process-oriented insight rather than mechanistic interpretation at the atomic scale.
The present study does not constitute a full parametric optimization of the USP–HR process, and temperature-dependent effects in Cu–Ag bimetallic systems were not systematically explored. These aspects will be addressed in future work through expanded experimental matrices.
Future work will focus on incorporating complementary characterization techniques to overcome the limitations of the present analysis. X-ray diffraction (XRD) will be employed to assess crystallinity and phase composition, while TEM/HRTEM will be used to resolve internal particle structures; additionally, X-ray photoelectron spectroscopy (XPS) will be considered to evaluate surface oxidation states where relevant. Additional techniques, such as UV–Vis spectroscopy, will be considered where optical properties are relevant. Furthermore, systematic investigations of oxidation stability, particle growth behavior, and application-oriented performance—such as catalytic or plasmonic activity—are required to establish robust structure–property relationships. Thermogravimetric (TGA) and differential scanning calorimetry (DSC) analyses will also be incorporated in future studies to enable a more precise correlation between precursor decomposition behavior and particle formation during the USP–HR process.