Abstract
Many gluten-free products are deficient in amino acids, especially in essential amino acids (EAA). Therefore, the incorporation of additives rich in free amino acids (FAA) into gluten-free products can be a promising strategy to alleviate certain symptoms of celiac disease associated with EAA deficiencies. This study aimed to evaluate the effect of the incorporation of broccoli leaf powder (BLP) into gluten-free mini sponge cakes (GFS) on the profile of FFA. BLP replaced an equivalent amount (2.5%, 5%, 7.5%; w/w) of corn and potato starches in GFS formulation, resulting in B1-B3 formulations. The first step was the selection of the most efficient method for extraction of FAA. Extraction based on 50% methanol (method 1) was compared to extraction by 25% of acetonitrile in 0.1 M hydrochloric acid (method 2). In total, 26 and 14 FAA were found in BLP after extraction using methods 1 and 2, respectively. Moreover, considering the total content of FAA, method 1 was more efficient, reaching a 14-fold higher concentration of FFA in BLP compared to method 2. The incorporation of BLP resulted in a significant increase in FAA, irrespective of the applied extraction method. The total concentrations of NEAA and EAA increased significantly in B3 compared to control GFS. In summary, this study showed that 50% methanol was more efficient for the extraction of FFA from plant and bakery matrices. Moreover, BLP was found as a good source of FFA, including EAA, and the obtained experimental GFS could be considered a promising product for individuals on a gluten-free diet.
1. Introduction
Amino acids play important role in the metabolism of bacteria, plants, and humans. They are involved in the formation of protein structure, regulation of anabolic and catabolic metabolism, and detoxification processes. Moreover, amino acids are precursors for the synthesis of important mediators, such as glutathione and serotonin. In food, amino acids contribute to the formation of the pleasant aroma of products, i.e., the baked and roasted aroma caused by pyrazines formed from lysine in Maillard reaction [1] or the rose-like aroma formed in the Ehrlich pathway from L-phenylalanine [2]. Free amino acids (FAA) are an important class of metabolites of plants that provide indicative information about biological responses to environmental or physiological metabolism changes caused by fluctuation in the nitrogen status, water shortage, or other stress [3].
The content of FAA in plants and food products can be analyzed using a broad range of methods. The most commonly used are high-performance liquid chromatography (HPLC) and gas chromatography (GC) followed by derivatization. The crucial step in the FAA analysis is extraction. Different solvents at different concentrations were used for the extraction of FFA, including methanol, ethanol, acetone, acetonitrile, hydrochloric acid, water, and others [3,4,5,6,7]. However, there is no consensus on which method is the most suitable for certain matrices.
Wheat contains all the essential amino acids (EAA), in most cases present in adequate concentrations to meet the daily intake values suggested by WHO [8]. Additionally, barley, commonly used for the production of bakery goods, is a good source of amino acids [9]. The gluten-free diet, based mainly on corn and rice starches, does contain amino acids too; however, compared to a wheat-based diet, the amounts of several EAA such lysine and tryptophan are limited [10]. A reduced intake of tryptophan, leading to the reduced synthesis of monoamines, including serotonin, was suggested to be the cause of the psychological symptoms accompanying celiac disease such as anxiety, irritability, and depression [10,11,12]. In fact, patients with celiac disease following a gluten-free diet for a long time were found to have a lower level of EAA in biological fluids compared with healthy subjects [10]. Diet was found to have a substantial impact on the amino acid status in individuals with celiac disease [13]; thus, the incorporation of novel, functional food products with improved amino acid profiles could be beneficial to alleviate nutritional deficiencies. Pseudocereals flours usually have better FAA profiles compared to corn and rice [14] and thus can be considered an alternative for gluten-free products. Importantly, considering the absorption of the amino acids, food products rich in FAA are preferred. Weijzen et al. [15] showed that the ingestion of FAA results in more rapid absorption and higher postprandial amino acids availability compared to intact protein. Furthermore, formulations rich in FAA were found to stimulate gut microbiota development in monkey infants better than formulations that contained more protein [16]. It is of great importance considering the dysbiosis of gut microbiota commonly observed in celiac disease [17].
Cruciferous plants are a good source of bioactive compounds, antioxidants, vitamins, and FAA [18,19,20]. For broccoli, florets are the main edible part, which means that more than 70% of the plant (mainly leaves) is discarded. The broccoli leaves, considered a by-product, have a similar chemical profile to florets and have been found to be rich in bioactive and nutritive compounds [21,22]. Broccoli leaves were suggested to be an interesting source of FAA, including EAA, mainly phenylalanine, isoleucine, leucine, lysine, valine, and tryptophan [23]. Nowadays, there is a growing interest in the utilization of plant-based by-products to recover bioactive compounds, vitamins, and protein [24,25]. Notably, broccoli leaf powder (BLP), obtained from freeze-dried broccoli leaves, has been successfully applied as an additive of gluten-free products [26,27,28]. However, to date, the effect of BLP incorporation on the profile of FFA in bakery goods was not analyzed. Therefore, the aim of this study was to select the most effective method for the extraction of FAA from the BLP and bakery matrix and then to evaluate the effect of the incorporation of BLP into a gluten-free bakery product on the profile of FAA. In this study, gluten-free mini sponge cake (GFS) was used as a model bakery product.
2. Materials and Methods
2.1. Chemicals
Ultra-pure water was obtained with a mili-Q-system (Millipore, Bedford, MA, USA). Methanol and acetonitrile were purchased in Sigma Aldrich (Steinheim, Switzerland). Hydrochloric acid was from POCh (Gliwice, Poland). EZ: faast™ kit for free (physiological) amino acids containing all the reagents, calibration standards, and separation column was purchased in Phenomenex (Aschaffenburg, Germany).
2.2. Preparation of Gluten-Free Sponge Cakes Fortified with Broccoli By-Products
The preparation of BLP and GFS was described previously [26]. Briefly, BLP was prepared from fresh, undamaged broccoli leaves, which underwent freeze-drying and pulverization. GFS were prepared using potato and corn starch, eggs, sugar, oil, salt, and baking powder with proportions described previously [26]. All ingredients were purchased in local stores in Olsztyn (Poland). An equivalent of corn and potato starch in the control formulation was replaced with BLP with the following percentages: control–0%, B1–2.5%, B2–5% and B3–7.5% (w/w). GFS were baked for 25 min at 180 °C in the laboratory oven (SVEBA DAHLEN, AB model DC-21, Fristad, Sweden). After cooling, GFS were freeze-dried and grounded to a fine powder, which was stored in −20 °C until analyses. The pictures presenting the obtained GFS are shown in Figure 1.

Figure 1.
The presentation of the experimental pasta fortified with BLP. C—control, B1—2.5% starch replacement with BLP, B2—5% starch replacement with BLP, and B3—7.5% starch replacement with BLP.
2.3. Extraction of Amino Acids
In the literature, various methods were used to extract the free amino acids [3,4,29,30,31,32]. Since there is no consensus on which extraction method is the most suitable for vegetables, two methods based on the two the most commonly reported solvent were compared in this study.
2.3.1. Method 1
The first method of extraction was based on the 50% methanol, as described elsewhere [33]. Approx. 100 mg of the freeze-dried sample was placed in a 5 mL Eppendorf vial. Then, 3 mL of 50% methanol (v/v) at a temperature of 50 °C was added and incubated for 20 min at 50 °C with shaking of 500 rpm using MultiTherm shaker (Benchmark Scientific, Edison, NJ, USA). After that, samples were centrifuged for 15 min at 10,000 rpm, and the supernatant was collected for analysis.
2.3.2. Method 2
The second extraction was based on the method described by Barba et al. [32]. Approx. 100 g of freeze-dried sample was placed in a 5 mL Eppendorf vial. Then, 3 mL of a solution containing 25% of acetonitrile in 0.1 M hydrochloric acid was added. Extraction was performed at room temperature for 1 h with shaking of 500 rpm using a MultiTherm shaker (Benchmark Scientific, Edison, NJ, USA). After that, samples were centrifuged for 15 min at 10,000 rpm and the supernatant was collected for analysis.
2.4. Analysis of Amino Acids
The analysis of amino acids was performed as previously described by Drabińska et al. [33]. Briefly, 100 µL of the extract was analyzed using the EZ: faast™ kit for free (physiological) amino acids (Phenomenex, Aschaffenburg, Germany) according to the producer’s recommendations. The identification of individual FAA was performed using external standards, and quantification was performed using calibration curves and normalized according to the internal standard (norvaline).
Amino acids were analyzed in an Agilent 7890A gas chromatograph coupled with a 5975C mass selective detector, 7683B autoinjector (Agilent Technologies, Santa Clara, CA, USA), and a data station containing the NIST/EPA/NIH Mass Spectral Library (Version 2). The compounds were separated in a ZB-AAA EZ: faast™ capillary column (10 m 0.25 mm, (Phenomenex, Aschaffenburg, Germany)). The carrier gas was helium (1.5 mL/min). The samples (2 µL) were injected in split mode (1:15). The oven temperature was initially set at 110 °C and then increased to 320 °C (30 °C/min). Injector and ion source temperatures were 250 °C and 240 °C, respectively.
2.5. Statistical Analysis
All analytical measurements were performed in triplicates. The content of individual amino acids was compared between the GFS using a one-way analysis of variance (ANOVA), separately for each extraction method. The significance of differences between the samples was determined by Fisher‘s LSD test at p-value < 0.05. All statistical analyses were performed using STATISTICA version 13.3 (Statsoft, Tulsa, OK, USA) and GraphPad Prism version 8.0.0 for Windows (San Diego, CA, USA) software.
3. Results
In total, 26 and 14 FAA were found in BLP after extraction using methods 1 and 2, respectively (Table 1 and Table 2). Moreover, considering the total content of FAA, method 1 was more efficient, reaching a 14-fold higher value compared to method 2. Interestingly, the profile of FAA differs between the extraction methods. In method 1, the dominant FAA in BLP were glutamine, aspartic acid, serine, and alanine, whereas in the extracts obtained by method 2, glutamic acid, proline and alanine had the highest abundance. Control GFS had relatively low FAA content, irrespective of the used extraction method (Table 1 and Table 2). Method 1 allowed us to extract 20 FAA, with glutamic acid being the dominant one. At the same time, only eight FAA could be extracted using method 2. Contrary to method 1, valine was found to be the dominant FAA in control GFS extracted using method 2.

Table 1.
Amino acid profile in BLP and BLP fortified GFM extracted using method 1. Data expressed as mean ± SD in nmol/g DM. Different letters in superscript in the same line indicate a significant difference (p < 0.05) (Fisher’s LSD. ANOVA). C—control, B1—2.5% starch replacement with BLP, B2—5% starch replacement with BLP, and B3—7.5% starch replacement with BLP.

Table 2.
Amino acid profile in BLP and BLP fortified GFM extracted using method 2. Data expressed in nmol/g DM. (ND—not detected). Different letters in superscript in the same line indicate a significant difference (p < 0.05) (Fisher’s LSD. ANOVA). C—control, B1—2.5% starch replacement with BLP, B2—5% starch replacement with BLP, and B3—7.5% starch replacement with BLP.
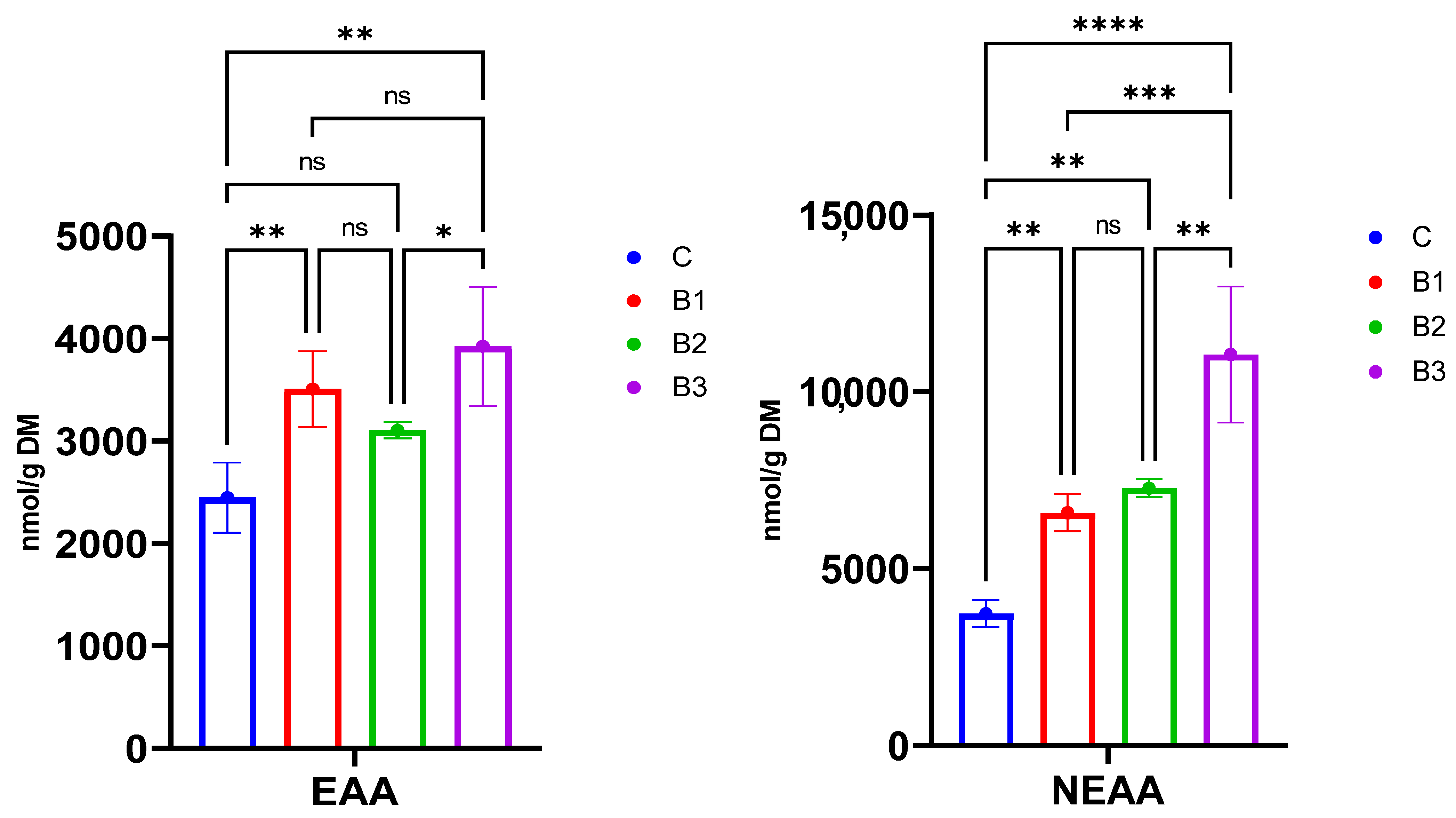
The incorporation of BLP resulted in a significant increase in FAA, irrespective of the applied extraction method (Table 1 and Table 2). Since method 1 was found to be more efficient in the extraction of FAA, both in terms of the concentration and the number of extracted FAA, further comparison of the experimental GFS was performed based on method 1. Looking at individual FAA, the concentrations of all non-essential amino acids (NEAA) increased with increasing BLP addition, except for tyrosine, which remained unchanged. The biggest increases after BLP incorporation were noted for alanine, serine, aspartic, and glutamic acid, which were dominant NEAA in BLP, whereas among EAA, the biggest increases were noticed for leucine, threonine, valine, and lysine, which were also detected in high concentrations in BLP. Consequently, the total concentration of NEAA increased significantly in B3 compared to C (Figure 2). The total content of essential amino acids (EAA) also increased after the incorporation of BLP (Figure 2). Notably, the significant increase was noted only in B1 and B3, whereas in B2, the total content of EAA was statistically similar to the C; however, the increasing trend was observed. It is mainly related to the lower contents of methionine, tryptophan, and histidine, which were detected at lower values than C (Table 1).
Figure 2.
The total concentrations of essential (EAA) and non-essential amino acids (NEAA) in gluten-free mini sponge cakes fortified with broccoli leaf powder (BLP) extracted by method 1. C—control, B1—2.5% starch replacement with BLP, B2—5% starch replacement with BLP, and B3—7.5% starch replacement with BLP, ns—not significant. (*)—p-value < 0.05, (**)—p-value < 0.01, (***)—p-value < 0.001, (****)—p-value < 0.0001.
4. Discussion
Amino acids are the main form of nitrogen transport in plants; thus, they are involved in all physiological processes, including growth, the storage of nutrients, and transportation [34]. In humans, disturbance in amino acid metabolism was associated with the incidence of several health conditions such as autism spectrum disorders, depression, cancer, and celiac disease [10,13,35,36]. On the one hand, the pathogenesis of these diseases can be associated with the disturbance of amino acid metabolism, but on the other hand, the elimination diets used as treatment can lead to amino acid deficits [10]. Therefore, it is important to develop novel products, which can provide deficient amino acids, especially in a gluten-free diet.
In this study, the profile of FAA in BLP and fortified GFS was assessed. To do so, two extraction methods were compared. There is certainly a wider disparity of opinion among scientists as to the most suitable methods for extraction of FAA from plant matrices. In this study, two simple extraction techniques successfully applied previously were compared [32,33]. The results showed that extraction with 50% methanol was much more efficient compared to acetonitrile-HCl solution. The discrepancy between the amount of extracted FAA, both in terms of number of FAA and their concentration, between these two methods was surprising; however, it can be associated with the pH of the solvents. The organic solvents were previously reported to extract better neutral and acidic amino acids, whereas acids were more effective in the extraction of basic FAA [7]. It has to be kept in mind that amino acids are a big group of chemicals, with varying properties related to different polarities, ionization, and the character of side-chain groups. Therefore, the selection of the optimal extraction conditions can be challenging. Arnáiz et al. [20] found that water gave the best yield of extraction of FAA from broccoli leaves, mainly due to its higher polarity. However, although the highest total concentration was obtained, the water did not allow the extraction of some of the individual compounds. The authors found that proline and glutamine were more efficiently extracted by methanol-water solutions and supercritical fluid extraction [20]. In this study, the method based on acetonitrile and low-molar acid did not extract amino acid derivatives, glutamine, and asparagine. Therefore, method 1 was again better, especially since glutamine is also involved in gut barrier integrity and inflammation state, which are of particular importance in celiac disease [13].
Taking into account the results obtained by method 1, the presence of 26 FAA could be detected in BLP. This is more than in Arnáiz et al. [20], who detected 20 FAA by supercritical fluid extraction. The authors applied the same type of derivatization, using a commercial EZ: faast™ kit. Previously, 20 FAA were also detected in broccoli florets using different analytical approaches, such as HPLC and ion-exchange chromatography [37,38]. Therefore, the results of this study showed that broccoli leaves are a good source of FAA. The comparison of the different aerial parts of cauliflower showed that, among them, leaves are the richest source of FAA [33]. It could be explained by the higher assimilation of nitrogen in photosynthetic leaves [39]. It can also suggest that outer leaves of other vegetables, which are considered by-products, can be used for the extraction of FAA or can be applied as nutritive additives for food products.
Raw materials used for baking gluten-free products differ in terms of amino acids profile, especially EAA. In general, cereal proteins are usually deficient in lysine, tryptophan, and threonine [40]. In this study, GFS was characterized by the presence of 20 FAA, including lysine, tryptophan, and threonine. It can be explained by the fact that, in this study, the mixture of corn and potato starch was used for the GFS formulations. Notably, comparing the total FAA, control GFS had a much lower concentration than BLP. The incorporation of BLP significantly increased the content of FAA in GFS; however, the profile of FAA remained similar to the control GFS. It can be explained by the small amount of BLP added to each formulation. However, due to the high content of NEAA in BLP, their concentrations increased successively by two and three times in B1 and B3 formulation, respectively. Importantly, a lower level of glutamine was observed in children with celiac disease following a gluten-free diet [41]. BLP-enriched GFS were found to be a very good source of this FAA, which can contribute to the improvement of amino acids status. The content of EAA also increased in BLP-enriched GFS; however, this increase was smaller than NEAA. Unfortunately, the level of tryptophan was not affected by BLP incorporation, which was of primary importance considering the physiological issues related to celiac disease [10].
Importantly, FAA also affect the technological properties of bakery goods. Amino acids have an impact on the swelling power of starch, which can affect the texture of starch-based products [42]. For instance, charge-carrying amino acids, such as lysine, arginine, aspartic acid, and glutamic acid, were found to influence the gelatinization and retrogradation properties of potato starch [43]. As reported previously [28], BLP addition reduced the viscosity of the GFS batters and increased firmness of the GFS, which could be associated with the observed change in FAA profile. It is also of great importance considering the challenges in the formulations of gluten-free products [44].
5. Conclusions
In conclusion, this study showed that the by-products of broccoli processing are a good source of FAA. The most suitable method for the extraction of FAA was extraction with 50% methanol, allowing for the detection of 26 FAA in BLP. The incorporation of BLP into GFS resulted in a significant increase in both EAA and NEAA. The biggest increases after BLP incorporation were noted for alanine, serine, aspartic, and glutamic acid, which were dominant NEAA in BLP, whereas among EAA, the biggest increases were noticed for leucine, threonine, valine, and lysine, which were also detected in high concentrations in BLP. These findings suggest that BLP can be a valuable supplement for GFS. The incorporation of BLP into GFS allowed compensating the FAA deficits of gluten-free products. In conclusion, the obtained added-value baked product could provide health-promoting benefits for subjects on a gluten-free diet. Furthermore, FAA-rich BLP could be used for the development of new functional products for subjects on the elimination diets, helping, at the same time, to eliminate the by-products of the vegetable industry.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The study was supported by the research funds of the Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences. The author would like to thank Urszula Krupa-Kozak for support in materials.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hwang, H.-I.; Hartman, T.G.; Rosen, R.T.; Lech, J.; Ho, C.-T. Formation of Pyrazines from the Maillard Reaction of Glucose and Lysine-.Alpha.-Amine-15N. J. Agric. Food Chem. 1994, 42, 1000–1004. [Google Scholar] [CrossRef]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Majcher, M.A. Analysis of the Ability to Produce Pleasant Aromas on Sour Whey and Buttermilk By-Products by Mold Galactomyces Geotrichum: Identification of Key Odorants. Molecules 2021, 26, 6239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Xiao, H.; Zhang, Z.; Gao, X.; Zhao, J. Rapid and Sensitive Method for Determining Free Amino Acids in Plant Tissue by High-Performance Liquid Chromatography with Fluorescence Detection. Acta Geochim. 2017, 36, 680–696. [Google Scholar] [CrossRef]
- Antoine, F.R.; Wei, C.I.; Littell, R.C.; Marshall, M.R. HPLC Method for Analysis of Free Amino Acids in Fish Using O-Phthaldialdehyde Precolumn DerivatizationJ. Agric. Food Chem. 1999, 47, 5100–5107. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Barroso, M.A.; Ruiz, J.; Antequera, T. A Rapid and Accurate Extraction Procedure for Analysing Free Amino Acids in Meat Samples by GC-MS. Int. J. Anal. Chem. 2015, 2015, 209214. [Google Scholar] [CrossRef]
- Shafaei, A.; Halim, N.H.A.; Zakaria, N.; Ismail, Z. Analysis of Free Amino Acids in Different Extracts of Orthosiphon Stamineus Leaves by High-Performance Liquid Chromatography Combined with Solid-Phase Extraction. Pharmacogn. Mag. 2017, 13, S385–S391. [Google Scholar] [CrossRef] [PubMed]
- Saifer, A. Comparative Study of Various Extraction Methods for the Quantitative Determination of Free Amino Acids from Brain Tissue. Anal. Biochem. 1971, 40, 412–423. [Google Scholar] [CrossRef]
- Sabença, C.; Ribeiro, M.; de Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef]
- Šterna, V.; Zute, S.; Jansone, I.; Kantane, I. Chemical Composition of Covered and Naked Spring Barley Varieties and Their Potential for Food Production. Pol. J. Food Nutr. Sci. 2017, 67, 151–158. [Google Scholar] [CrossRef]
- van Hees, N.J.M.; Giltay, E.J.; Tielemans, S.M.A.J.; Geleijnse, J.M.; Puvill, T.; Janssen, N.; van der Does, W. Essential Amino Acids in the Gluten-Free Diet and Serum in Relation to Depression in Patients with Celiac Disease. PLoS ONE 2015, 10, e0122619. [Google Scholar] [CrossRef]
- Carta, M.G.; Hardoy, M.C.; Boi, M.F.; Mariotti, S.; Carpiniello, B.; Usai, P. Association between Panic Disorder, Major Depressive Disorder and Celiac Disease: A Possible Role of Thyroid Autoimmunity. J. Psychosom. Res. 2002, 53, 789–793. [Google Scholar] [CrossRef]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Abenavoli, L.; Vonghia, L.; Cardone, S.; Leso, V.; Cossari, A.; et al. State and Trait Anxiety and Depression in Patients Affected by Gastrointestinal Diseases: Psychometric Evaluation of 1641 Patients Referred to an Internal Medicine Outpatient Setting. Int. J. Clin. Pract. 2008, 62, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Krupa-Kozak, U.; Ciska, E.; Jarocka-Cyrta, E. Plasma Profile and Urine Excretion of Amino Acids in Children with Celiac Disease on Gluten-Free Diet after Oligofructose-Enriched Inulin Intervention: Results of a Randomised Placebo-Controlled Pilot Study. Amino Acids 2018, 50, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Paucar-Menacho, L.M.; Dueñas, M.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Effect of Dry Heat Puffing on Nutritional Composition, Fatty Acid, Amino Acid and Phenolic Profiles of Pseudocereals Grains. Pol. J. Food Nutr. Sci. 2018, 68, 289–297. [Google Scholar] [CrossRef]
- Weijzen, M.E.G.; van Gassel, R.J.J.; Kouw, I.W.K.; Trommelen, J.; Gorissen, S.H.M.; van Kranenburg, J.; Goessens, J.P.B.; van de Poll, M.C.G.; Verdijk, L.B.; van Loon, L.J.C. Ingestion of Free Amino Acids Compared with an Equivalent Amount of Intact Protein Results in More Rapid Amino Acid Absorption and Greater Postprandial Plasma Amino Acid Availability Without Affecting Muscle Protein Synthesis Rates in Young Adults in a Double-Blind Randomized Trial. J. Nutr. 2022, 152, 59–67. [Google Scholar] [CrossRef]
- He, X.; Sotelo-Orozco, J.; Rudolph, C.; Lönnerdal, B.; Slupsky, C.M. The Role of Protein and Free Amino Acids on Intake, Metabolism, and Gut Microbiome: A Comparison between Breast-Fed and Formula-Fed Rhesus Monkey Infants. Front. Pediatrics 2020, 7, 563. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Jarocka-Cyrta, E.; Markiewicz, L.H.; Krupa-Kozak, U. The Effect of Oligofructose-Enriched Inulin on Faecal Bacterial Counts and Microbiota-Associated Characteristics in Celiac Disease Children Following a Gluten-Free Diet: Results of a Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 201. [Google Scholar] [CrossRef]
- Park, S.; Valan Arasu, M.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Lee, S.W.; Al-Dhabi, N.A.; Kim, S.J. Quantification of Glucosinolates, Anthocyanins, Free Amino Acids, and Vitamin C in Inbred Lines of Cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Al-Dhabi, N.A.; Kim, S.J. Analysis and Metabolite Profiling of Glucosinolates, Anthocyanins and Free Amino Acids in Inbred Lines of Green and Red Cabbage (Brassica oleracea L.). LWT—Food Sci. Technol. 2014, 58, 203–213. [Google Scholar] [CrossRef]
- Arnáiz, E.; Bernal, J.; Martín, M.T.; Nozal, M.J.; Bernal, J.L.; Toribio, L. Supercritical Fluid Extraction of Free Amino Acids from Broccoli Leaves. J. Chromatogr. A 2012, 1250, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Campas-Baypoli, O.N.; Snchez-Machado, D.I.; Bueno-Solano, C.; Núñez-Gastélum, J.A.; Reyes-Moreno, C.; López-Cervantes, J. Biochemical Composition and Physicochemical Properties of Broccoli Flours. Int. J. Food Sci. Nutr. 2009, 60, 163–173. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D. A Broccoli-Derived by-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef] [PubMed]
- Arnáiz, E.; Bernal, J.; Martín, M.T.; García-Viguera, C.; Bernal, J.L.; Toribio, L. Supercritical Fluid Extraction of Lipids from Broccoli Leaves. Eur. J. Lipid Sci. Technol. 2011, 113, 479–486. [Google Scholar] [CrossRef]
- Kalaydzhiev, H.; Ivanova, P.; Silva, C.L.M.; Chalova, V.I. Functional Properties of Protein Isolate and Acid Soluble Protein-Rich Ingredient Co-Produced from Ethanol-Treated Industrial Rapeseed Meal. Pol. J. Food Nutr. Sci. 2019, 69, 129–136. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary Fibre and Phytochemical Characteristics of Fruit and Vegetable By-Products and Their Recent Applications as Novel Ingredients in Food Products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli By-Products Improve the Nutraceutical Potential of Gluten-Free Mini Sponge Cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Bączek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jeliński, T.; Ostaszyk, A. Broccoli Leaf Powder as an Attractive By-Product Ingredient: Effect on Batter Behaviour, Technological Properties and Sensory Quality of Gluten-Free Mini Sponge Cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef]
- Mncwangi, N.P.; Viljoen, A.M. Quantitative Variation of Amino Acids in Sutherlandia Frutescens (Cancer Bush)-towards Setting Parameters for Quality Control. S. Afr. J. Bot. 2012, 82, 46–52. [Google Scholar] [CrossRef]
- Pournamdari, M.; Saadi, A.; Ellis, E.; Andrew, R.; Walker, B.; Watson, D.G. Development of a Derivatisation Method for the Analysis of Aldehyde Modified Amino Acid Residues in Proteins by Fourier Transform Mass Spectrometry. Anal. Chim. Acta 2009, 633, 216–222. [Google Scholar] [CrossRef]
- Starkute, V.; Bartkiene, E.; Bartkevics, V.; Rusko, J.; Zadeike, D.; Juodeikiene, G. Amino Acids Profile and Antioxidant Activity of Different Lupinus Angustifolius Seeds after Solid State and Submerged Fermentations. J. Food Sci. Technol. 2016, 53, 4141–4148. [Google Scholar] [CrossRef][Green Version]
- Barba, F.J.; Poojary, M.M.; Wang, J.; Olsen, K.; Orlien, V. Effect of High Pressure Processing and Storage on the Free Amino Acids in Seedlings of Brussels Sprouts. Innov. Food Sci. Emerg. Technol. 2017, 41, 188–192. [Google Scholar] [CrossRef]
- Drabińska, N.; Jeż, M.; Nogueira, M. Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxidants 2021, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential Distribution of Amino Acids in Plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Dunstan, R.H.; Rothkirch, T.; Roberts, T.K.; Reichelt, K.L.; Cosford, R.; Deed, G.; Ellis, L.B.; Sparkes, D.L. Altered Amino Acid Excretion in Children with Autism. Nutr. Neurosci. 2008, 11, 9–17. [Google Scholar] [CrossRef]
- Dereziński, P.; Klupczynska, A.; Sawicki, W.; Pałka, J.A.; Kokot, Z.J. Amino Acid Profiles of Serum and Urine in Search for Prostate Cancer Biomarkers: A Pilot Study. Int. J. Med. Sci. 2017, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; López-Ayerra, B.; Martínez-Tomé, M.; García-Carmona, F. Effect of Industrial Processing on Amino Acid Content of Broccoli. J. Sci. Food Agric. 2001, 81, 1299–1305. [Google Scholar] [CrossRef]
- Hansen, M.E.; Sørensen, H.; Cantwell, M. Changes in Acetaldehyde, Ethanol and Amino Acid Concentrations in Broccoli Florets during Air and Controlled Atmosphere Storage. Postharvest Biol. Technol. 2001, 22, 227–237. [Google Scholar] [CrossRef]
- Yoneyama, T.; Suzuki, A. Light-Independent Nitrogen Assimilation in Plant Leaves: Nitrate Incorporation into Glutamine, Glutamate, Aspartate, and Asparagine Traced by (15)N. Plants 2020, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Šmídová, Z.; Rysová, J. Gluten-Free Bread and Bakery Products Technology. Foods 2022, 11, 480. [Google Scholar] [CrossRef]
- Sevinc, E.; Akar, H.H.; Sevinc, N.; Arslan, D.; Sezgin, G.C. Amino Acid Levels in Children with Celiac Disease. Nutr. Hosp. 2015, 32, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Fang, L.; Zhou, H.; Yang, H. Effects of Amino Acids on the Physiochemical Properties of Potato Starch. Food Chem. 2014, 151, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, H.; Yang, H.; Cui, M. Effects of Charge-Carrying Amino Acids on the Gelatinization and Retrogradation Properties of Potato Starch. Food Chem. 2015, 167, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and Nutritional Challenges, and Novelty in Gluten-Free Breadmaking: A Review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).