Optimization and Validation of a Method Based on QuEChERS Extraction and Gas Chromatographic-Mass Spectrometric Analysis for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Olive Fruits Irrigated with Treated Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Olive Fruit Samples
2.3. Chromatographic Analysis
2.4. QuEChERS Extraction and Clean-Up Procedure
2.5. Method Validation
2.6. Apparent Recovery
2.7. Matrix Effect
2.8. Method Detection Limits (MDL) and Method Quantitation Limits (MQL)
2.9. Linearity
2.10. Intra-Day and Inter-Day Precision
3. Results and Discussion
3.1. Optimization of QuEChERS Extraction: Effects of the Main Parameters
3.1.1. Extractions in Dichloromethane
3.1.2. Extractions in Cyclohexane
3.1.3. Extractions in Acetonitrile
| Analytes | AR (%) of the QuEChERS Trials | ||
|---|---|---|---|
| #9 | #10 | #11 | |
| BaA-d12 | 43 | 99 | 122 |
| Chr-d12 | 43 | 91 | 94 |
| BbFl-d12 | 44 | 100 | 102 |
| BkFl-d12 | 53 | 99 | 102 |
| BaP-d12 | 54 | 96 | 98 |
| Ind-d12 | 43 | 98 | 102 |
| DBA-d14 | 46 | 98 | 97 |
| BP-d12 | 30 | 72 | 101 |
3.2. Validation of the Optimized QuEChERS Procedure
3.2.1. Linearity
3.2.2. Method Detection Limits (MDL), Method Quantitation Limits (MQL), and Precision
3.2.3. Matrix Effect
3.3. Determination of PAHs and PCBs in Olive Crops Irrigated with Treated Wastewaters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivoira, L.; Castiglioni, M.; Kettab, A.; Ouazzani, N.; Al-Karablieh, E.; Boujelben, N.; Fibbi, D.; Coppini, E.; Giordani, E.; Del Bubba, M. Impact of effluents from wastewater treatments reused for irrigation: Strawberry as case study. Environ. Eng. Manag. J. (EEMJ) 2019, 18, 2133–2143. [Google Scholar]
- Renai, L.; Tozzi, F.; Scordo, C.V.; Giordani, E.; Bruzzoniti, M.C.; Fibbi, D.; Mandi, L.; Ouazzani, N.; Del Bubba, M. Productivity and nutritional and nutraceutical value of strawberry fruits (Fragaria × ananassa Duch.) cultivated under irrigation with treated wastewaters. J. Sci. Food Agric. 2021, 101, 1239–1246. [Google Scholar] [CrossRef]
- Mcheik, M.; Toufaily, J.; Haj Hassan, B.; Hamieh, T.; Abi Saab, M.T.; Rouphael, Y.; Ferracin, E.; da Shio, B.; Bashabshah, I.; Al Hadidi, L. Reuse of treated municipal wastewater in irrigation: A case study from Lebanon and Jordan. Water Environ. J. 2017, 31, 552–558. [Google Scholar] [CrossRef]
- Djillali, Y.; Chabaca, M.N.; Benziada, S.; Bouanani, H.; Mandi, L.; Bruzzoniti, M.; Boujelben, N.; Kettab, A. Effect of treated wastewater on strawberry. Desalin. Water Treat. 2020, 181, 338–345. [Google Scholar] [CrossRef]
- Scordo, C.V.A.; Checchini, L.; Renai, L.; Orlandini, S.; Bruzzoniti, M.C.; Fibbi, D.; Mandi, L.; Ouazzani, N.; Del Bubba, M. Optimization and validation of a method based on QuEChERS extraction and liquid chromatographic-tandem mass spectrometric analysis for the determination of perfluoroalkyl acids in strawberry and olive fruits, as model crops with different matrix characteristics. J. Chromatogr. A 2020, 1621, 461038. [Google Scholar] [PubMed]
- Jaramillo, M.F.; Restrepo, I. Wastewater Reuse in Agriculture: A Review about Its Limitations and Benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S.; Shahid, M.; Natasha; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [Green Version]
- Renai, L.; Tozzi, F.; Checchini, L.; Del Bubba, M. Impact of the use of treated wastewater for agricultural need: Behavior of organic micropollutants in soil, transfer to crops, and related risks. Adv. Chem. Pollut. Environ. Manag. Prot. 2020, 6, 103–135. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Rivoira, L.; Castiglioni, M.; El Ghadraoui, A.; Ahmali, A.; El Mansour, T.E.H.; Mandi, L.; Ouazzani, N.; Del Bubba, M. Extraction of polycyclic aromatic hydrocarbons and polychlorinated biphenyls from urban and olive mill wastewaters intended for reuse in agricultural irrigation. J. AOAC Int. 2020, 103, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Diez, C.M.; Trujillo, I.; Martinez-Urdiroz, N.; Barranco, D.; Rallo, L.; Marfil, P.; Gaut, B.S. Olive domestication and diversification in the Mediterranean Basin. New Phytol. 2015, 206, 436–447. [Google Scholar] [CrossRef]
- Gharbi, I.; Moret, S.; Chaari, O.; Issaoui, M.; Conte, L.S.; Lucci, P.; Hammami, M. Evaluation of hydrocarbon contaminants in olives and virgin olive oils from Tunisia. Food Control 2017, 75, 160–166. [Google Scholar] [CrossRef]
- Costopoulou, D.; Vassiliadou, I.; Chrysafidis, D.; Bergele, K.; Tzavara, E.; Tzamtzis, V.; Leondiadis, L. Determination of PCDD/F, dioxin-like PCB and PAH levels in olive and olive oil samples from areas affected by the fires in summer 2007 in Greece. Chemosphere 2010, 79, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Altunoğlu, Y.; Yemişçioğlu, F. Determination of polycyclic aromatic hydrocarbons in olives exposed to three different industrial sources and in their respective oils. Food Addit. Contam. Part A 2021, 38, 439–451. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Current trends in QuEChERS method. A versatile procedure for food, environmental and biological analysis. TrAC Trends Anal. Chem. 2019, 116, 214–235. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the term” recovery” and” apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 2201–2205. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.; Fajgelj, A.; Willetts, P.; Wood, R. Harmonized guidelines for the use of recovery information in analytical measurement. Pure Appl. Chem. 1999, 71, 337–348. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Guidance, A.D.L. Laboratory Guide for Determining Method Detection Limits; Laboratory Certification Program April; Wisconsin Department of Natural Resources: Madison, WI, USA, 1996.
- Anastassiades, M.; Scherbaum, E.; Tasdelen, B.; Stajnbaher, D. Recent developments in QuEChERS methodology for pesticide multiresidue analysis. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Wiley: Hoboken, NJ, USA, 2007; Volume 46, pp. 439–458. [Google Scholar]
- Rouvière, F.; Buleté, A.; Cren-Olivé, C.; Arnaudguilhem, C. Multiresidue analysis of aromatic organochlorines in soil by gas chromatography-mass spectrometry and QuEChERS extraction based on water/dichloromethane partitioning. Comparison with accelerated solvent extraction. Talanta 2012, 93, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, J.; Dong, F.; Liu, X.; Zhang, Y.; Tao, Y.; Wu, X.; Zheng, Y. Simultaneous determination of three strobilurin fungicide residues in fruits, vegetables and soil by a modified quick, easy, cheap, effective, rugged (QuEChERS) method coupled with gas chromatography-tandem mass spectrometry. Anal. Methods 2013, 5, 7102–7109. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Products from Olive Tree; IntechOpen: London, UK, 2016; pp. 67–98. [Google Scholar]
- Castiglioni, M.; Onida, B.; Rivoira, L.; Del Bubba, M.; Ronchetti, S.; Bruzzoniti, M.C. Amino groups modified SBA-15 for dispersive-solid phase extraction in the analysis of micropollutants by QuEchERS approach. J. Chromatogr. A 2021, 1645, 462107. [Google Scholar] [CrossRef] [PubMed]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Rajski, Ł.; Lozano, A.; Uclés, A.; Ferrer, C.; Fernández-Alba, A.R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2013, 1304, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Chan, C.; Abd Shukor, S.; Mashitah, M. Adsorption kinetics and thermodynamics of β-carotene on silica-based adsorbent. Chem. Eng. J. 2009, 148, 378–384. [Google Scholar] [CrossRef]
- Lucas, D.; Zhao, L. PAH analysis in salmon with enhanced matrix removal. In Agilent Technologies Application Note; Agilent Technologies: Palo Alto, CA, USA, 2015. [Google Scholar]
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, J.S.; Mitic, V.D.; Jovanovic, V.P.S.; Dimitrijevic, M.V.; Petrovic, G.M.; Nikolic-Mandic, S.D.; Stojanovic, G.S. Optimization of the QuEChERS extraction procedure for the determination of polycyclic aromatic hydrocarbons in soil by gas chromatography-mass spectrometry. Anal. Methods 2016, 8, 1711–1720. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Ekner, H.; Dreij, K.; Sadiktsis, I. Determination of polycyclic aromatic hydrocarbons in commercial olive oils by HPLC/GC/MS–Occurrence, composition and sources. Food Control 2022, 132, 108528. [Google Scholar] [CrossRef]
- Pandey, M.K.; Mishra, K.K.; Khanna, S.K.; Das, M. Detection of polycyclic aromatic hydrocarbons in commonly consumed edible oils and their likely intake in the Indian population. J. Am. Oil Chem. Soc. 2004, 81, 1131–1136. [Google Scholar] [CrossRef]
- Jimenez, A.; Adisa, A.; Woodham, C.; Saleh, M. Determination of polycyclic aromatic hydrocarbons in roasted coffee. J. Environ. Sci. Health Part B 2014, 49, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Masuda, M.; Wang, Q.; Tokumura, M.; Miyake, Y.; Amagai, T. Simultaneous determination of polycyclic aromatic hydrocarbons and their chlorinated derivatives in grilled foods. Ecotoxicol. Environ. Saf. 2019, 178, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Centre for the Promotion of Imports from Developing Countries (CBI). The European Market Potential for Table Olives. 2022. Available online: https://www.cbi.eu/market-information/processed-fruit-vegetables-edible-nuts/table-olives/market-potential (accessed on 13 March 2022).
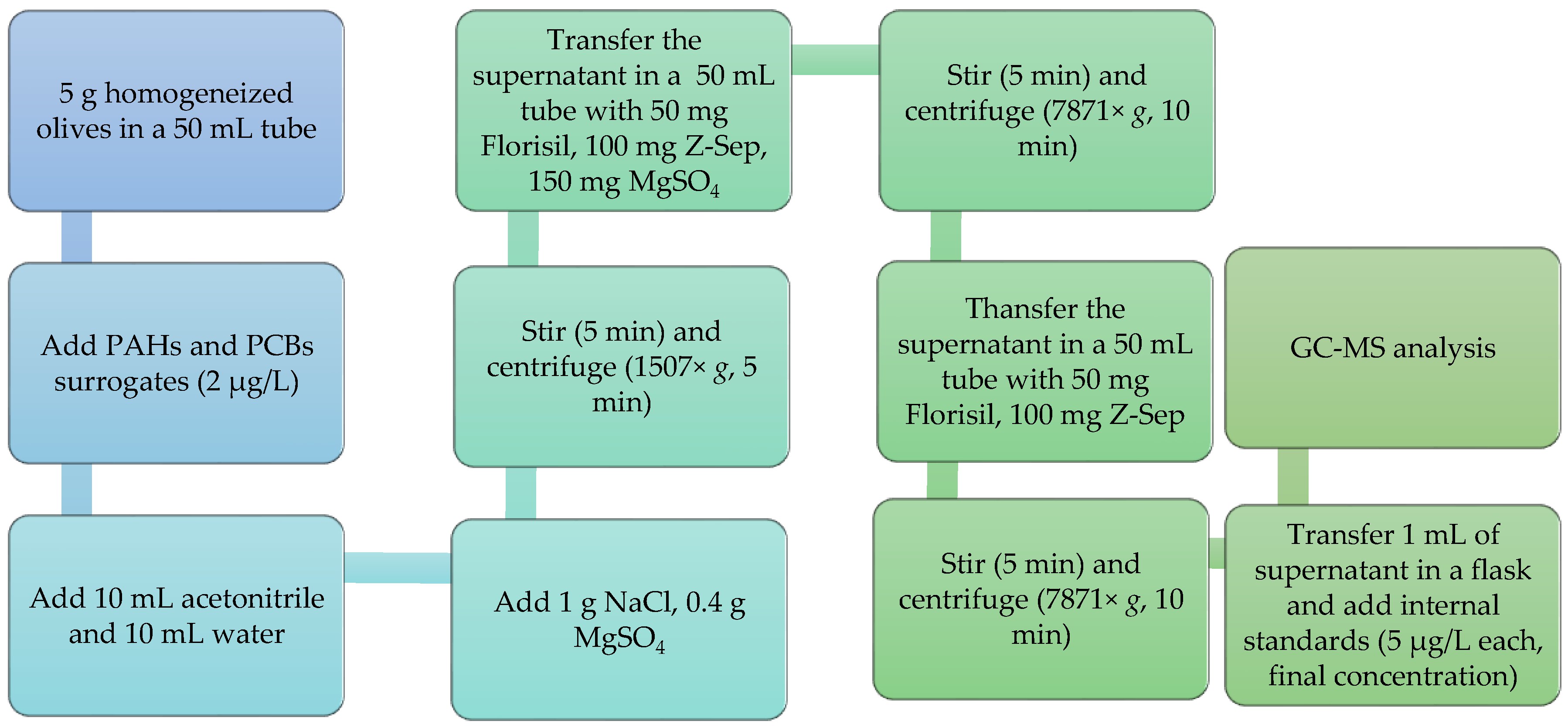

| QuEChERS Trial | Conditions | |
|---|---|---|
| Extraction | Clean Up | |
| #1 | 10 mL dichloromethane:water a | 100 mg PSA b |
| #2 | 300 mg PSA b | |
| #3 | 300 mg PSA b, 2 mL 96% H2SO4 c | |
| #4 | 300 mg PSA, 50 mg C18 b | |
| #5 | 100 mg PSA, 50 mg Florisil b | |
| #6 | 10 mL cyclohexane:water a | 100 mg PSA b |
| #7 | 100 mg Z-Sep, 50 mg Florisil b | |
| #8 | 100 mg Z-Sep b, 2 mL 96%H2SO4 c | |
| #9 | 10 mL acetonitrile:water a | 100 mg PSA, 50 mg Florisil b |
| #10 | 100 mg PSA, 50 mg Florisil b, 100 mg PSA, 50 mg Florisil d | |
| #11 | 100 mg Z-Sep, 50 mg Florisil b, 100 mg Z-Sep, 50 mg Florisil d | |
| Analytes | AR (%) of the QuEChERS Trials | ||||
|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | |
| BaA-d12 | 49 | 95 | * | 32 | 67 |
| Chr-d12 | 55 | 101 | * | 24 | 65 |
| BbFl-d12 | 24 | 77 | 92 | 27 | 52 |
| BkFl-d12 | 30 | 94 | 74 | 24 | 46 |
| BaP-d12 | 25 | 64 | ** | 30 | 38 |
| Ind-d12 | 27 | 21 | ** | 16 | 13 |
| DBA-d14 | 19 | 31 | 22 | 28 | 20 |
| BP-d12 | 18 | 41 | ** | 48 | 25 |
| Analytes | AR (%) of the QuEChERS Trials | ||
|---|---|---|---|
| #6 | #7 | #8 | |
| BaA-d12 | 73 | 87 | * |
| Chr-d12 | 71 | 94 | * |
| BbFl-d12 | 40 | 53 | 61 |
| BkFl-d12 | 52 | 51 | 34 |
| BaP-d12 | 32 | 122 | ** |
| Ind-d12 | 36 | 42 | ** |
| DBA-d14 | 33 | 91 | 105 |
| BP-d12 | 24 | 32 | ** |
| Compound | MDL | MQL | Compound | MDL | MQL |
|---|---|---|---|---|---|
| Naph | 0.73 | 2.2 | PCB 11 | 0.60 | 1.8 |
| AcPY | 0.43 | 1.3 | PCB 15 | 0.17 | 0.52 |
| AcPh | 0.17 | 0.52 | PCB 28 | 0.20 | 0.60 |
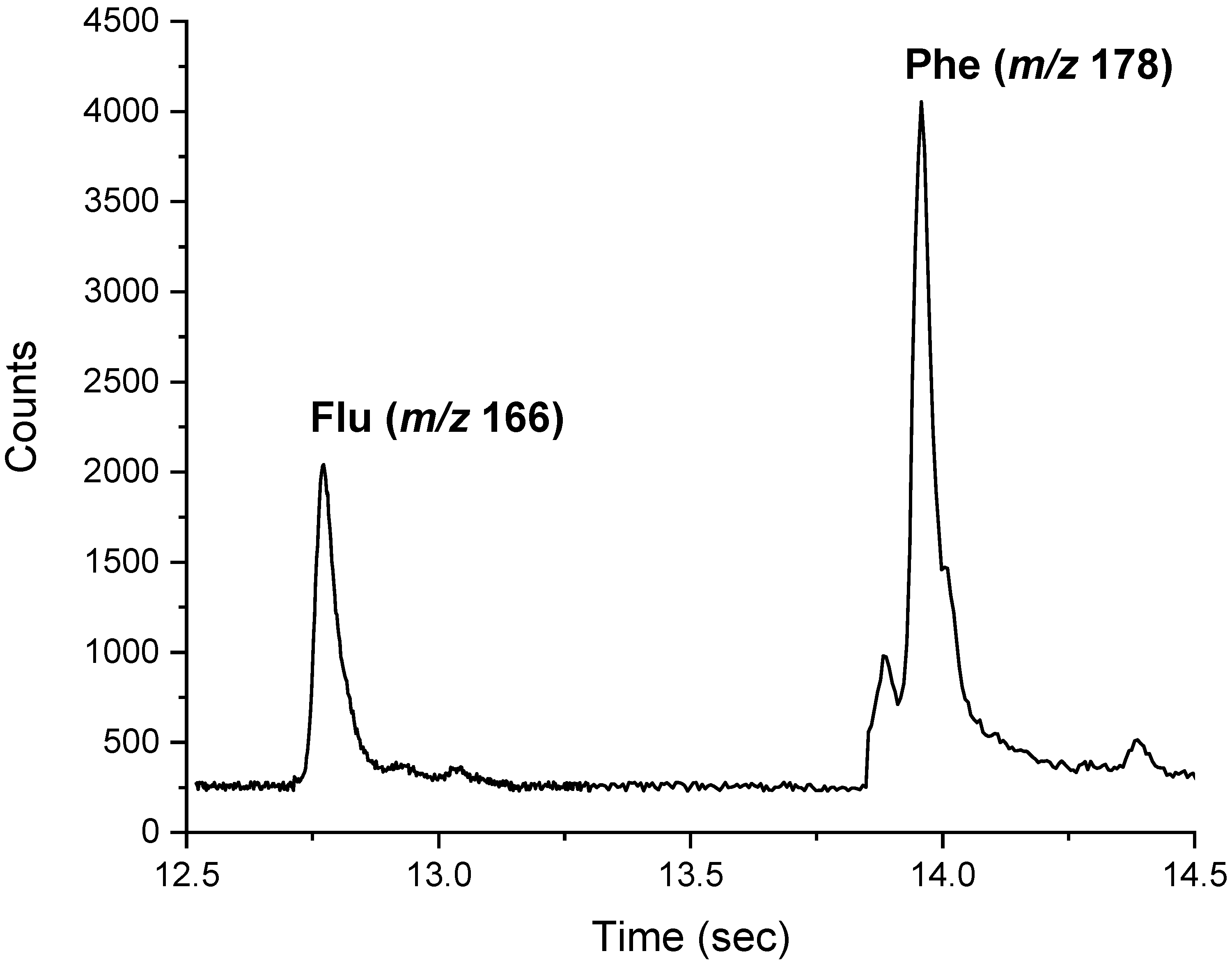
| Flu | 0.43 | 1.3 | PCB 52 | 0.18 | 0.54 |
| Phe | 0.97 | 2.9 | * PCB 81 | 0.37 | 1.1 |
| Ant | 0.97 | 2.9 | PCB 101 | 0.14 | 0.42 |
| Flth | 0.57 | 1.7 | * PCB 118 | 0.30 | 0.91 |
| Pyr | 0.53 | 1.6 | * PCB 123 | 0.31 | 0.93 |
| BaA | 0.83 | 2.5 | PCB 138 | 0.30 | 1.2 |
| Chr | 1.0 | 3.1 | PCB 153 | 0.12 | 0.36 |
| BbFl | 0.87 | 2.6 | * PCB 167 | 0.37 | 1.1 |
| BkFl | 0.83 | 2.5 | * PCB 169 | 0.80 | 2.4 |
| BaP | 0.60 | 1.8 | PCB 180 | 0.18 | 0.54 |
| Ind | 0.77 | 2.3 | * PCB 189 | 0.43 | 1.3 |
| DBA | 1.1 | 3.3 | |||
| BP | 0.70 | 2.1 |
| Compound | Concentration (µg/kg) (a) | ||||
|---|---|---|---|---|---|
| FW | TWW1 | TWW2 | TWW3 | TWW4 | |
| Flu | 2.0 ± 0.1 | 5.5 ± 0.7 | 3.9 ± 0.1 | 3.3 ± 0.1 | 1.3 ± 0.1 |
| Phe | <MQL | 6.4 ± 1 | <MQL | <MQL | <MQL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzzoniti, M.C.; Rivoira, L.; Castiglioni, M.; Cagno, E.; Kettab, A.; Fibbi, D.; Del Bubba, M. Optimization and Validation of a Method Based on QuEChERS Extraction and Gas Chromatographic-Mass Spectrometric Analysis for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Olive Fruits Irrigated with Treated Wastewaters. Separations 2022, 9, 82. https://doi.org/10.3390/separations9030082
Bruzzoniti MC, Rivoira L, Castiglioni M, Cagno E, Kettab A, Fibbi D, Del Bubba M. Optimization and Validation of a Method Based on QuEChERS Extraction and Gas Chromatographic-Mass Spectrometric Analysis for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Olive Fruits Irrigated with Treated Wastewaters. Separations. 2022; 9(3):82. https://doi.org/10.3390/separations9030082
Chicago/Turabian StyleBruzzoniti, Maria Concetta, Luca Rivoira, Michele Castiglioni, Enrico Cagno, Ahmed Kettab, Donatella Fibbi, and Massimo Del Bubba. 2022. "Optimization and Validation of a Method Based on QuEChERS Extraction and Gas Chromatographic-Mass Spectrometric Analysis for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Olive Fruits Irrigated with Treated Wastewaters" Separations 9, no. 3: 82. https://doi.org/10.3390/separations9030082
APA StyleBruzzoniti, M. C., Rivoira, L., Castiglioni, M., Cagno, E., Kettab, A., Fibbi, D., & Del Bubba, M. (2022). Optimization and Validation of a Method Based on QuEChERS Extraction and Gas Chromatographic-Mass Spectrometric Analysis for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Olive Fruits Irrigated with Treated Wastewaters. Separations, 9(3), 82. https://doi.org/10.3390/separations9030082








