Integrated Extraction of Carotenoids, Pectin, and Insoluble-Bound Ferulic Acid from Banana Peel
Abstract
1. Introduction
2. Materials and Methods
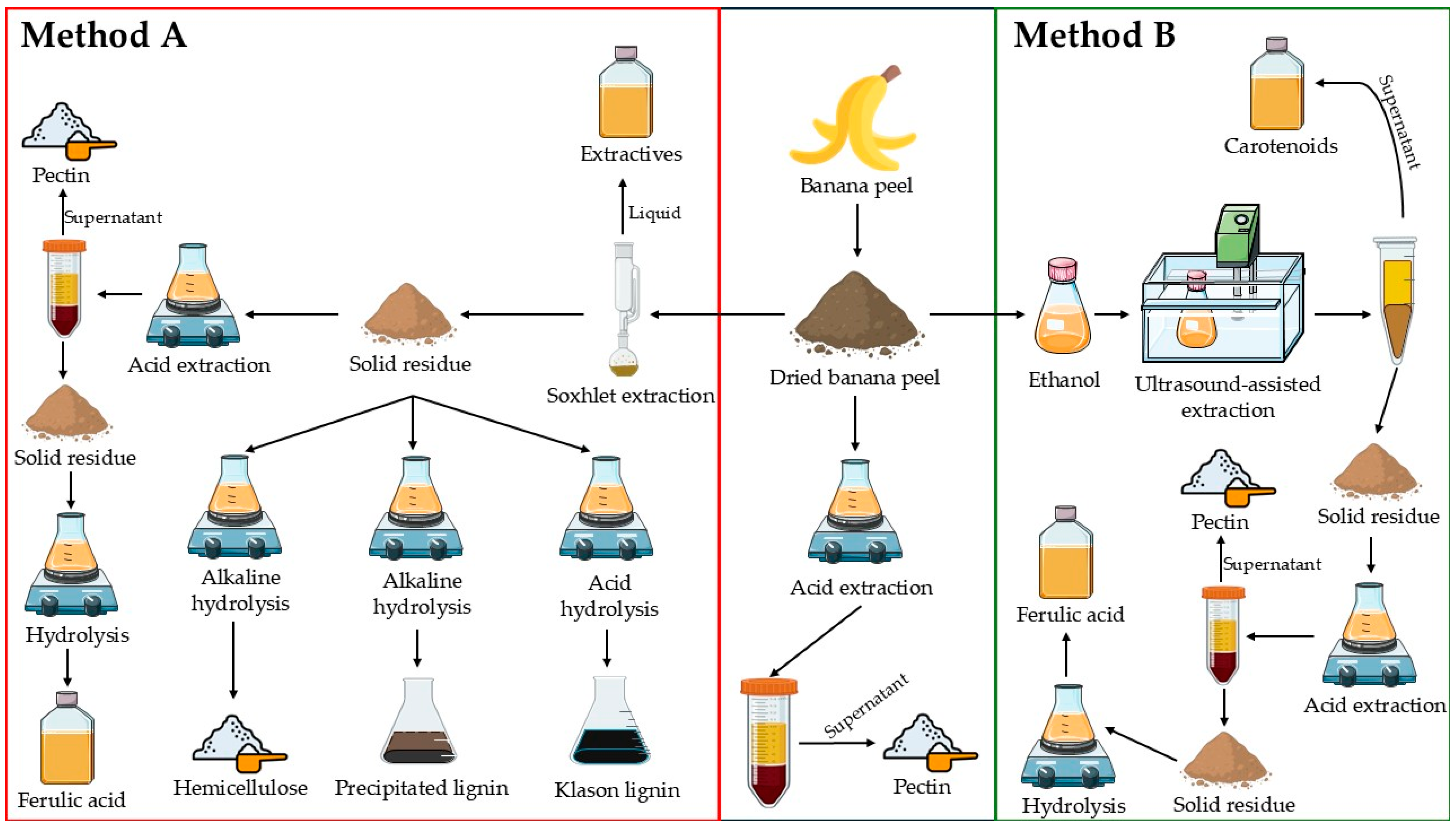
2.1. Biomass Preparation
2.2. Determination of Extractives (Method A)
2.3. Determination of Lignocellulosic Components
2.3.1. Acid Hydrolysis
2.3.2. Determination of Insoluble Lignin (Klason Lignin)
2.3.3. Determination of Lignin by Precipitation
2.3.4. Determination of Hemicellulose
2.3.5. Determination of Cellulose
2.4. Extraction and Determination of Carotenoids
2.4.1. Ultrasound-Assisted Extraction (UAE) of Carotenoid and Extractive Determination (Method B)
2.4.2. Total Carotenoid Determination by Spectrophotometry
2.4.3. Carotenoid Composition by HPLC-DAD
2.5. Pectin Determination
2.6. Determination of Insoluble Phenolic Compounds
2.6.1. Insoluble Phenolic Compounds Extraction
2.6.2. Insoluble Phenolic Compounds Analysis by HPLC-DAD
2.7. Statistical Analyses
3. Results and Discussion
3.1. Analysis of Compounds of Interest in Banana Peel
3.1.1. Extractives and Cell Wall Components
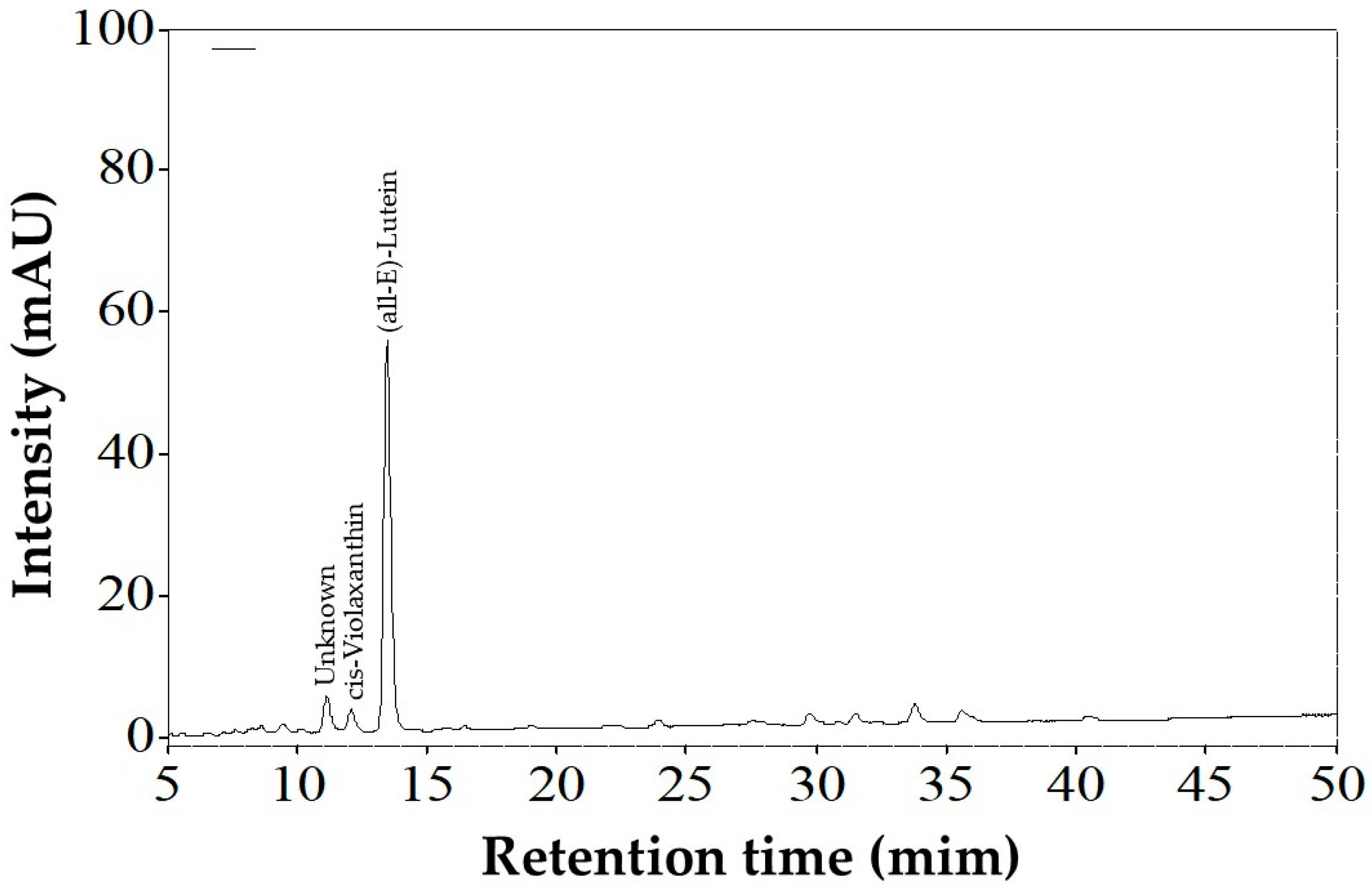
3.1.2. Carotenoids
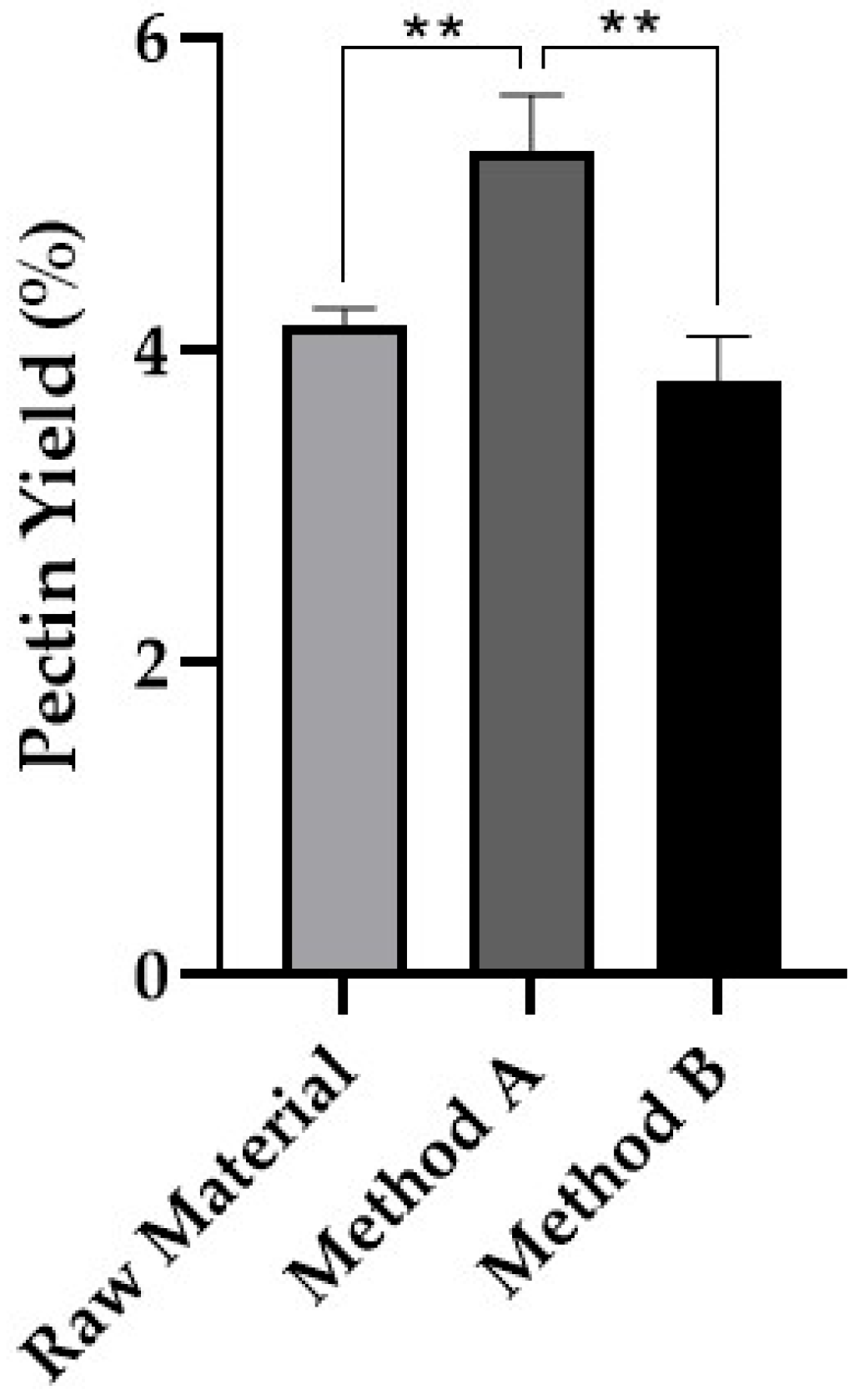
3.1.3. Pectin
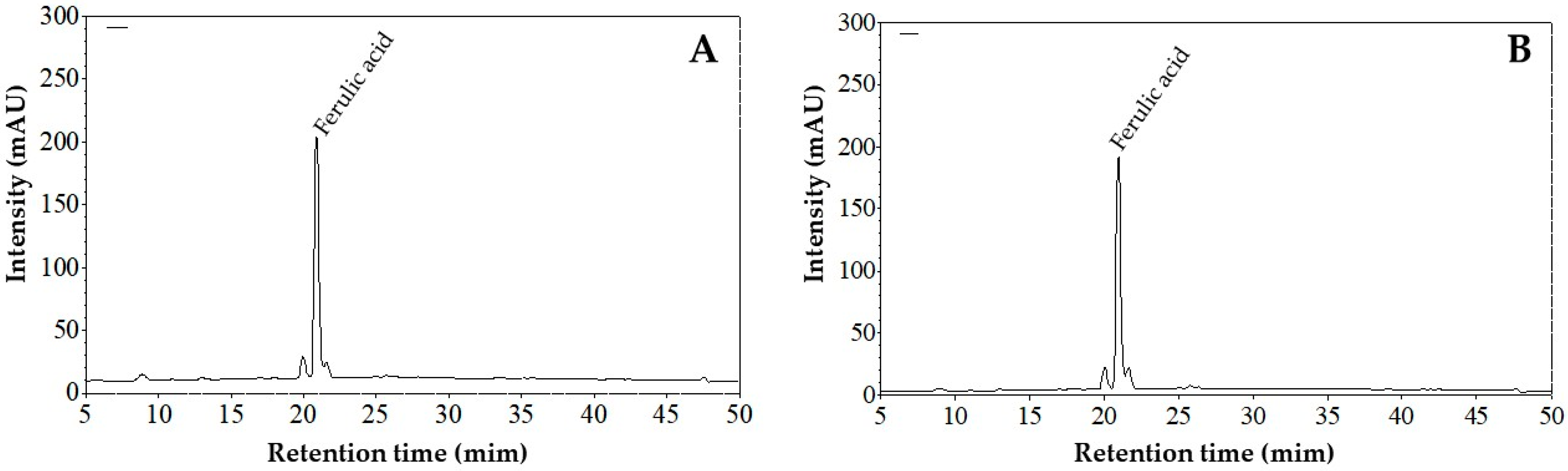
3.1.4. Insoluble Phenolic Compounds
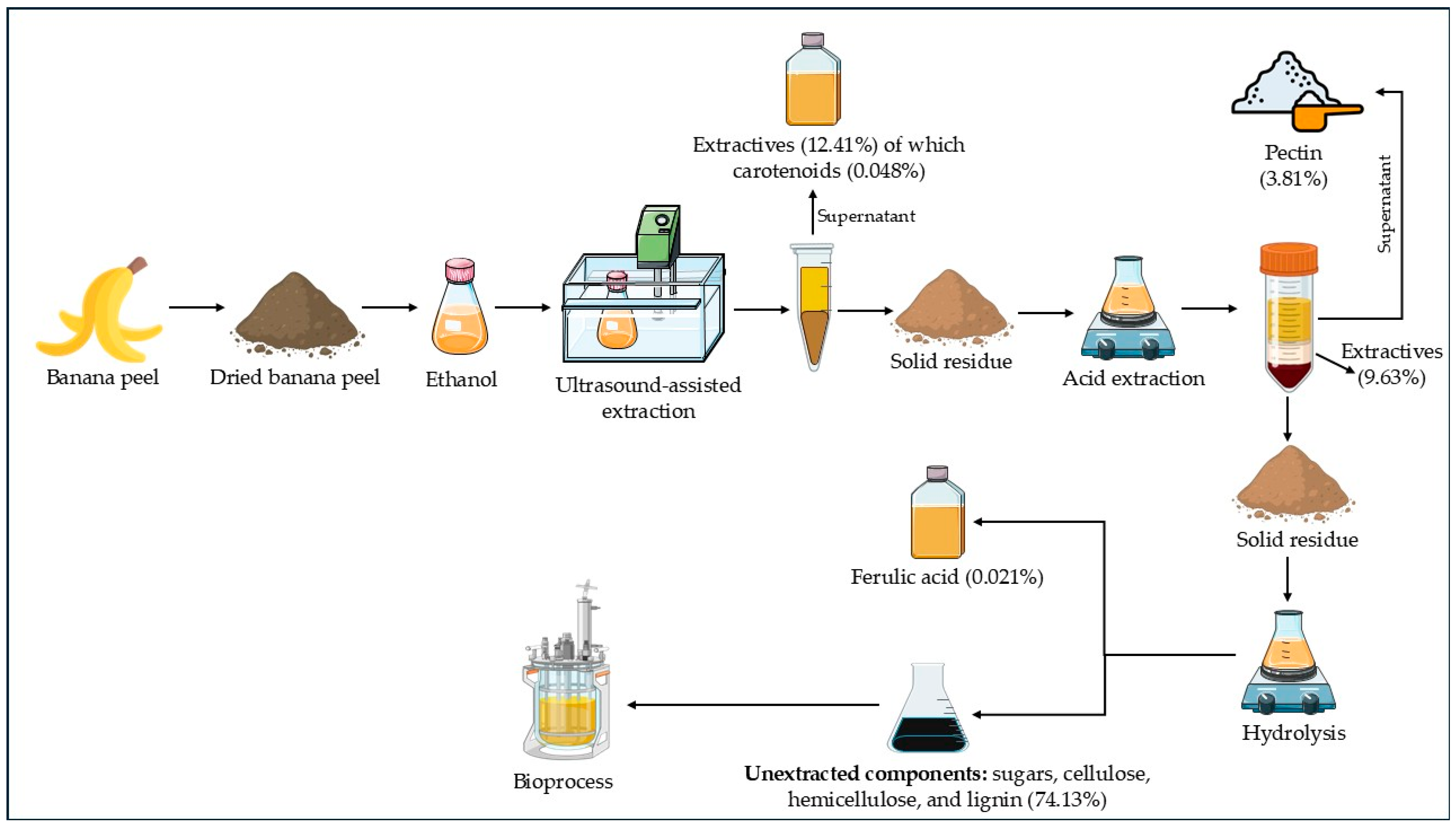
3.2. Integrated Process for Obtaining Various Products from Banana Peel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GRAS | Generally Recognized As Safe |
| HPLC-DAD | High Performance Liquid Chromatography with Diode-Array Detector |
| MeOH | Methanol |
| MTBE | Methyl tert-butyl ether |
| UV/Vis | Ultraviolet/Visible |
| NaClO | Sodium hypochlorite |
| EDTA | Ethylenediaminetetraacetic acid |
| PPO | Polyphenol oxidase |
| ANOVA | Analysis of Variance |
| Tukey’s HSD | Tukey’s Honestly Significant Difference |
| FAO | Food and Agriculture Organization (of the United Nations) |
| AOAC | Association of Official Analytical Chemists |
| NREL | National Renewable Energy Laboratory |
References
- FAO. Banana Market Review 2024; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025. [Google Scholar]
- Evans, E.A.; Ballen, F.H.; Siddiq, M. Banana Production, Global Trade, Consumption Trends, Postharvest Handling, and Processing. In Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition; Siddiq, M., Ahmed, J., Lobo, M.G., Eds.; Wiley: Chichester, UK, 2020; pp. 1–18. ISBN 9781119528265. [Google Scholar]
- Acevedo, S.A.; Carrillo, Á.J.D.; Flórez-López, E.; Grande-Tovar, C.D. Recovery of Banana Waste-Loss from Production and Processing: A Contribution to a Circular Economy. Molecules 2021, 26, 5282. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Kikulwe, E.M.; Kyanjo, J.L.; Kato, E.; Ssali, R.T.; Erima, R.; Mpiira, S.; Ocimati, W.; Tinzaara, W.; Kubiriba, J.; Gotor, E.; et al. Management of Banana Xanthomonas Wilt: Evidence from Impact of Adoption of Cultural Control Practices in Uganda. Sustainability 2019, 11, 2610. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana Peels as a Bioactive Ingredient and Its Potential Application in the Food Industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Berktas, S.; Cam, M. Effects of Acid, Alkaline and Enzymatic Extraction Methods on Functional, Structural and Antioxidant Properties of Dietary Fiber Fractions from Quince (Cydonia oblonga Miller). Food Chem. 2025, 464, 141596. [Google Scholar] [CrossRef]
- Tran, N.T.K.; Nguyen, V.B.; Van Tran, T.; Nguyen, T.T.T. Microwave-Assisted Extraction of Pectin from Jackfruit Rags: Optimization, Physicochemical Properties and Antibacterial Activities. Food Chem. 2023, 418, 135807. [Google Scholar] [CrossRef]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Bux, F. Exploration of Microalgae Biorefinery by Optimizing Sequential Extraction of Major Metabolites from Scenedesmus obliquus. Ind. Eng. Chem. Res. 2017, 56, 3407–3412. [Google Scholar] [CrossRef]
- da Costa Rocha, A.C.; de Andrade, C.J.; de Oliveira, D. Perspective on Integrated Biorefinery for Valorization of Biomass from the Edible Insect Tenebrio molitor. Trends Food Sci. Technol. 2021, 116, 480–491. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sivakumar, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process—A Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Izanlou, Z.; Akhavan Mahdavi, M.; Gheshlaghi, R.; Karimian, A. Sequential Extraction of Value-Added Bioproducts from Three Chlorella Strains Using a Drying-Based Combined Disruption Technique. Bioresour. Bioprocess. 2023, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Wohlt, D.; Schwarz, E.; Schieber, A.; Bader-Mittermaier, S. Effects of Extraction Conditions on Banana Peel Polyphenol Oxidase Activity and Insights into Inactivation Kinetics Using Thermal and Cold Plasma Treatment. Foods 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Khalangre, A.; Mirza, A.; Sharma, A.K.; Shaikh, N.; Shabeer, T.P.A. Effect of Drying Temperature on Preservation of Banana Peel and Its Impact on Degradation of Targeted Phytochemical by LC-Orbitrap-MS Analysis. Biomass Convers. Biorefinery 2025, 15, 30443–30458. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ballus, C.A.; Menezes, C.R.; Wagner, R.; Paniz, J.N.G.; Tischer, B.; Costa, A.B.; Barin, J.S. Green and Fast Determination of the Alcoholic Content of Wines Using Thermal Infrared Enthalpimetry. Food Chem. 2018, 258, 59–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Zimmerman, A.R.; Chen, H.; Hu, X.; Gao, B. Mechanisms and Adsorption Capacities of Hydrogen Peroxide Modified Ball Milled Biochar for the Removal of Methylene Blue from Aqueous Solutions. Bioresour. Technol. 2021, 337, 125432. [Google Scholar] [CrossRef]
- Segura-Badilla, O.; Kammar-García, A.; Mosso-Vázquez, J.; Ávila-Sosa Sánchez, R.; Ochoa-Velasco, C.; Hernández-Carranza, P.; Navarro-Cruz, A.R. Potential Use of Banana Peel (Musa cavendish) as Ingredient for Pasta and Bakery Products. Heliyon 2022, 8, e11044. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- AOAC International. AOAC Official Method 940.26 Ash of Fruits and Fruit Products. In Official Methods of Analysis of AOAC INTERNATIONAL; Latimer, G.W., Jr., Ed.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- Huang, M.; Zhu, L.; Li, J.; Ma, Z. Recovery of Kraft Lignin from Industrial Black Liquor for a Sustainable Production of Value-Added Light Aromatics by the Tandem Catalytic Pyrolysis. J. Clean. Prod. 2024, 446, 141388. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2005, 20, 388–393. [Google Scholar] [CrossRef]
- Silva, J.V.M.; Santos, A.S.; Pereira, G.A.; Chisté, R.C. Ultrasound-Assisted Extraction Using Ethanol Efficiently Extracted Carotenoids from Peels of Peach Palm Fruits (Bactris gasipaes Kunth) without Altering Qualitative Carotenoid Profile. Heliyon 2023, 9, e14933. [Google Scholar] [CrossRef]
- Matos, K.A.N.; Lima, D.P.; Barbosa, A.P.P.; Mercadante, A.Z.; Chisté, R.C. Peels of Tucumã (Astrocaryum vulgare) and Peach Palm (Bactris gasipaes) Are by-Products Classified as Very High Carotenoid Sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of Pectin Extraction from Banana Peels with Citric Acid by Using Response Surface Methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of Free, Esterified, Glycosylated and Insoluble-Bound Phenolics Composition in the Edible Part of Araticum Fruit (Annona crassiflora Mart.) and Its by-Products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Modesto Jr., E.N.; Chaves, R.P.F.; Arruda, H.S.; Borsoi, F.T.; Pastore, G.M.; Pereira, G.A.; Chisté, R.C.; Pena, R.S. Microencapsulation of Anthocyanin-Rich Extract of Grumixama Fruits (Eugenia brasiliensis) Using Non-Conventional Wall Materials and in Vitro Gastrointestinal Digestion. J. Food Eng. 2025, 389, 112393. [Google Scholar] [CrossRef]
- Behera, M.; Ghangrekar, M.M. Performance of Microbial Fuel Cell in Response to Change in Sludge Loading Rate at Different Anodic Feed pH. Bioresour. Technol. 2009, 100, 5114–5121. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.N.; Yusoff, M. Investigation of Chemical Analysis and Physical Properties of Bio-Polymer Waste Banana Peel Fibre Composite. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012042. [Google Scholar] [CrossRef]
- Nascimento, L.S.; Vieira, F.I.D.M.; Horácio, V.; Marques, F.P.; Rosa, M.F.; Souza, S.A.; de Freitas, R.M.; Uchoa, D.E.A.; Mazzeto, S.E.; Lomonaco, D.; et al. Tailored Organosolv Banana Peels Lignins: Improved Thermal, Antioxidant and Antimicrobial Performances by Controlling Process Parameters. Int. J. Biol. Macromol. 2021, 181, 241–252. [Google Scholar] [CrossRef]
- Husni, E.; Ismed, F.; Afriyandi, D. Standardization Study of Simplicia and Extract of Calamondin (Citrus microcarpa Bunge) Peel, Quantification of Hesperidin and Antibacterial Assay. Pharmacogn. J. 2020, 12, 777–783. [Google Scholar] [CrossRef]
- Yin, Q.; Qin, W.; Zhou, Z.; Wu, A.; Deng, W.; Li, Z.; Shan, W.; Chen, J.; Kuang, J.; Lu, W. Banana MaNAC1 Activates Secondary Cell Wall Cellulose Biosynthesis to Enhance Chilling Resistance in Fruit. Plant Biotechnol. J. 2024, 22, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, K. Extraction of Lignin from Sustainable Lignocellulosic Food Waste Resources Using a Green Deep Eutectic Solvent System and Its Property Characterization. Int. J. Biol. Macromol. 2025, 307, 142094. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, P.J.; Velvizhi, G. Substantial Physicochemical Pretreatment and Rapid Delignification of Lignocellulosic Banana, Pineapple and Papaya Fruit Peels: A Study on Physical-Chemical Characterization. Sustain. Chem. Pharm. 2024, 37, 101347. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Rossato, L.A.M.; Strini, A.; Serra, S. From Waste to Value: Recent Insights into Producing Vanillin from Lignin. Molecules 2024, 29, 442. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of Nano Cellulose Fibers from the Banana Peel and Bract for Production of Acetyl and Lauroyl Cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef]
- Odedina, M.J.; Charnnok, B.; Saritpongteeraka, K.; Chaiprapat, S. Effects of Size and Thermophilic Pre-Hydrolysis of Banana Peel during Anaerobic Digestion, and Biomethanation Potential of Key Tropical Fruit Wastes. Waste Manag. 2017, 68, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.F.; Monteiro, C.R.M.; Pereira, G.N.; Júnior, S.E.B.; Zanella, E.; Ávila, P.F.; Stambuk, B.U.; Goldbeck, R.; de Oliveira, D.; Poletto, P. Deconstruction of Banana Peel for Carbohydrate Fractionation. Bioprocess. Biosyst. Eng. 2021, 44, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. [Google Scholar] [CrossRef]
- Rivadeneira, J.P.; Wu, T.; Ybanez, Q.; Dorado, A.A.; Migo, V.P.; Nayve, F.R.P.; Castillo-Israel, K.A.T. Microwave-Assisted Extraction of Pectin from “Saba” Banana Peel Waste: Optimization, Characterization, and Rheology Study. Int. J. Food Sci. 2020, 2020, 879425. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ho, H.T.; Hoang, D.Q.; Nguyen, Q.A.P.; Tran, T. Van Novel Films of Pectin Extracted from Ambarella Fruit Peel and Jackfruit Seed Slimy Sheath: Effect of Ionic Crosslinking on the Properties of Pectin Film. Carbohydr. Polym. 2024, 334, 122043. [Google Scholar] [CrossRef] [PubMed]
- de Rosso, V.V.; Mercadante, A.Z. Identification and Quantification of Carotenoids, By HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Huang, W.; Ma, W.; Lu, Y.; Yan, R. Enzymatic Acylation Improves the Stability and Bioactivity of Lutein: Protective Effects of Acylated Lutein Derivatives on L-O2 Cells upon H2O2-Induced Oxidative Stress. Food Chem. 2023, 410, 135393. [Google Scholar] [CrossRef]
- Aquino, C.F.; Salomão, L.C.C.; Pinheiro-Sant’ana, H.M.; Ribeiro, S.M.R.; De Siqueira, D.L.; Cecon, P.R. Carotenoids in the Pulp and Peel of Bananas from 15 Cultivars in Two Ripening Stages. Rev. Ceres 2018, 65, 217–226. [Google Scholar] [CrossRef]
- Mir, S.A.; Rizwan, D.; Bakshi, R.A.; Wani, S.M.; Masoodi, F.A. Extraction of Carotenoids from Agro-Industrial Waste. In Extraction of Natural Products from Agro-Industrial Wastes; Bhawani, S.A., Khan, A., Ahmad, F.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 157–178. ISBN 9780128233498. [Google Scholar]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Gardea-Béjar, A.A.; Yahia, E.M.; Ornelas-Paz, J.J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Escalante-Minakata, P. Effect of Cultivar on the Content of Selected Phytochemicals in Avocado Peels. Food Res. Int. 2021, 140, 110024. [Google Scholar] [CrossRef] [PubMed]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and Quantification of Bioactive Compounds and Antioxidant Activity in Three Different Varieties of Mango (Mangifera indica L.) Peel from the Ecuadorian Region Using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Dai, Z.; Zhang, Z.; Feng, L.; Nie, M.; Liu, C.; Li, D.; Zhang, M. Study on the Relationship between Lutein Bioaccessibility and in Vitro Lipid Digestion of Nanostructured Lipid Carriers with Different Interface Structures. Food Hydrocoll. 2023, 139, 108569. [Google Scholar] [CrossRef]
- Shi, H.; Nolan, J.M.; Flynn, R.; Prado-Cabrero, A. Beyond Food Colouring: Lutein-Food Fortification to Enhance Health. Food Biosci. 2024, 59, 104085. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, J.; Xiong, R.; Chen, H.; Huang, S.; Li, H.; Pang, J.; Zhang, X.; Zhu, H. Effects and Mechanisms of Lutein on Aging and Age-Related Diseases. Antioxidants 2024, 13, 1114. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef]
- Duan, H.; Yan, X.; Azarakhsh, N.; Huang, X.; Wang, C. Effects of High-pressure Pretreatment on Acid Extraction of Pectin from Pomelo Peel. Int. J. Food Sci. Technol. 2022, 57, 5239–5249. [Google Scholar] [CrossRef]
- Marenda, F.R.B.; Colodel, C.; Canteri, M.H.G.; de Olivera Müller, C.M.; Amante, E.R.; de Oliveira Petkowicz, C.L.; de Mello Castanho Amboni, R.D. Investigation of Cell Wall Polysaccharides from Flour Made with Waste Peel from Unripe Banana (Musa sapientum) Biomass. J. Sci. Food Agric. 2019, 99, 4363–4372. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.W.; Lam, H.M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An Overview of Sources, Extraction and Applications in Food Products, Biomedical, Pharmaceutical and Environmental Issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Lepilova, O.; Aleeva, S.; Koksharov, S.; Lepilova, E. Supramolecular Structure of Banana Peel Pectin and Its Transformations during Extraction by Acidic Methods. Int. J. Biol. Macromol. 2023, 242, 124616. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Mishra, S.; Mohanty, P.; Singh, P.K.; Srivastava, R.K.; Pattnaik, R.; Adhya, T.K.; Das, T.; Lenka, B.; Gupta, V.K.; et al. Food and Fruit Waste Valorisation for Pectin Recovery: Recent Process Technologies and Future Prospects. Int. J. Biol. Macromol. 2023, 235, 123929. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Y.; Tian, J.; Zhao, J.; Ye, N.; Zhang, Y.; Chen, L. Review of Waste Biorefinery Development towards a Circular Economy: From the Perspective of a Life Cycle Assessment. Renew. Sustain. Energy Rev. 2021, 139, 110716. [Google Scholar] [CrossRef]
- Nadar, C.G.; Arora, A.; Shastri, Y. Sustainability Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. Au 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Ghoshal, G.; Negi, P. Isolation of Pectin from Kinnow Peels and Its Characterization. Food Bioprod. Process. 2020, 124, 342–353. [Google Scholar] [CrossRef]
- da Gama, B.V.M.; de Farias Silva, C.E.; da Silva, L.M.O.; de Abud, A.S.K. Extraction and Characterization of Pectin from Citric Waste. Chem. Eng. Trans. 2015, 44, 259–264. [Google Scholar] [CrossRef]
- Phenol-Explorer Database on Polyphenol Content in Foods. Available online: http://phenol-explorer.eu/ (accessed on 26 November 2025).
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid from Plant Biomass: A Phytochemical with Promising Antiviral Properties. Front. Nutr. 2022, 8, 777576. [Google Scholar] [CrossRef]
- Purushothaman, J.R.; Rizwanullah, M. Ferulic Acid: A Comprehensive Review. Cureus 2024, 16, e68063. [Google Scholar] [CrossRef]
- Hikal, W.M.; Said-Al Ahl, H.A.H.; Bratovcic, A.; Tkachenko, K.G.; Sharifi-Rad, J.; Kačániová, M.; Elhourri, M.; Atanassova, M. Banana Peels: A Waste Treasure for Human Being. Evid.-Based Complement. Altern. Med. 2022, 2022, 7616452. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A. Ferulic Acid: Mechanistic Insights and Multifaceted Applications in Metabolic Syndrome, Food Preservation, and Cosmetics. Molecules 2025, 30, 3716. [Google Scholar] [CrossRef]
- Roux, J.; Horton, L.; Babadjouni, A.; Kincaid, C.M.; Mesinkovska, N.A. Ferulic Acid Use for Skin Applications: A Systematic Review. J. Clin. Aesthet. Dermatol. 2025, 18, 38–42. [Google Scholar]
- Moll, E.; Chiralt, A. Active PHBV Films with Ferulic Acid or Rice Straw Extracts for Food Preservation. LWT 2025, 228, 118115. [Google Scholar] [CrossRef]
- Bishnoi, S.; Sharma, S.; Agrawal, H. Exploration of the Potential Application of Banana Peel for Its Effective Valorization: A Review. Indian J. Microbiol. 2023, 63, 398–409. [Google Scholar] [CrossRef]
- Palacios, S.; Ruiz, H.A.; Ramos-Gonzalez, R.; Martínez, J.; Segura, E.; Aguilar, M.; Aguilera, A.; Michelena, G.; Aguilar, C.; Ilyina, A. Comparison of Physicochemical Pretreatments of Banana Peels for Bioethanol Production. Food Sci. Biotechnol. 2017, 26, 993–1001. [Google Scholar] [CrossRef]
- Xiang, T.; Yang, R.; Li, L.; Lin, H.; Kai, G. Research Progress and Application of Pectin: A Review. J. Food Sci. 2024, 89, 6985–7007. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, Q.; Feng, Y.; Xuan, J. Composition of Lignocellulose Hydrolysate in Different Biorefinery Strategies: Nutrients and Inhibitors. Molecules 2024, 29, 2275. [Google Scholar] [CrossRef]
- Klein, G.H.; Longo, V.D.; Romani, L.C.; Saldanha, L.F.; Fornari, A.C.; Bazoti, S.F.; Camargo, A.F.; Alves, S.L.; Treichel, H. Utilization of Banana Peel Waste for the Production of Bioethanol and Other High-Value-Added Compounds. Food Humanit. 2024, 3, 100376. [Google Scholar] [CrossRef]
- Fit, C.G.; Clauser, N.M.; Felissia, F.E.; Area, M.C. Biorefinery Design from Agroindustrial By-Products and Its Scaling-up Analysis. Bioresour. Technol. Rep. 2025, 31, 102175. [Google Scholar] [CrossRef]
- Goula, A.M.; Lazarides, H.N. Integrated Processes Can Turn Industrial Food Waste into Valuable Food By-Products and/or Ingredients: The Cases of Olive Mill and Pomegranate Wastes. J. Food Eng. 2015, 167, 45–50. [Google Scholar] [CrossRef]
| Parameter | Content (g/100 g DW) |
|---|---|
| Extractives (from Method A) | 22.04 ± 1.06 |
| Precipitated lignin | 11.46 ± 0.62 |
| Klason lignin | 29.63 ± 2.73 |
| Hemicellulose | 19.91 ± 1.03 |
| Cellulose 1 | 23.14 ± 2.43 |
| Pectin (from Method A) | 5.28 ± 0.36 |
| Carotenoid 1 | Retention Time (min) 2 | λmax (nm) 3 | %III/II | %AB/AII |
|---|---|---|---|---|
| Unknown | 11.05 | ND | ND | ND |
| cis-Violaxanthin | 12.01 | 269, 410, 347, 466 | 80 | 50 |
| (all-E)-Lutein | 13.15 | 267, 420, 444, 472 | 62 | 0 |
| Carotenoid 1 | Carotenoid Content | |
|---|---|---|
| Banana Peel (µg/g DW) | Ethanolic Extract (µg/mL) | |
| Unknown | 71.64 ± 0.09 | 2.92 ± 0.01 |
| cis-Violaxanthin | 78.37 ± 0.04 | 3.20 ± 0.01 |
| (all-E)-Lutein | 338.05 ± 0.76 | 13.68 ± 0.22 |
| Total carotenoids by chromatography | 476.94 ± 2.95 | 19.47 ± 0.07 |
| Total carotenoids by spectrophotometry | 595.62 ± 4.91 | 24.31 ± 0.08 |
| Ferulic Acid Content | |||
|---|---|---|---|
| Extractive Method | Purified Extract (µg/mL) | Extractive-Free Material (µg/g DW) | Banana Peel (µg/g DW) |
| Method A | 43.65 ± 0.89 | 218.28 ± 4.44 | 158.74 ± 3.23 |
| Method B | 42.50 ± 0.86 | 212.48 ± 0.43 | 178.02 ± 0.36 |
| p-value 1 | 0.175 | 0.175 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Silva, L.d.S.d.; Modesto Junior, E.N.; Arruda, H.S.; Pereira, G.A. Integrated Extraction of Carotenoids, Pectin, and Insoluble-Bound Ferulic Acid from Banana Peel. Processes 2026, 14, 166. https://doi.org/10.3390/pr14010166
Silva LdSd, Modesto Junior EN, Arruda HS, Pereira GA. Integrated Extraction of Carotenoids, Pectin, and Insoluble-Bound Ferulic Acid from Banana Peel. Processes. 2026; 14(1):166. https://doi.org/10.3390/pr14010166
Chicago/Turabian StyleSilva, Larissa de Sousa da, Elivaldo Nunes Modesto Junior, Henrique Silvano Arruda, and Gustavo Araujo Pereira. 2026. "Integrated Extraction of Carotenoids, Pectin, and Insoluble-Bound Ferulic Acid from Banana Peel" Processes 14, no. 1: 166. https://doi.org/10.3390/pr14010166
APA StyleSilva, L. d. S. d., Modesto Junior, E. N., Arruda, H. S., & Pereira, G. A. (2026). Integrated Extraction of Carotenoids, Pectin, and Insoluble-Bound Ferulic Acid from Banana Peel. Processes, 14(1), 166. https://doi.org/10.3390/pr14010166








