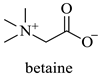
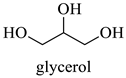
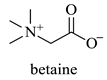
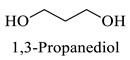
Abstract
Medicinal and aromatic plants continue to attract attention as rich sources of natural bioactive compounds with potential health benefits. Among them, Greek mountain tea (Sideritis scardica) is widely recognized for its high content of phytochemicals, which have been associated with various biological activities. In this study, Natural Deep Eutectic Solvents (NADESs) were investigated as a sustainable and efficient alternative to conventional solvents for the extraction of such compounds, aiming to the development of a more efficient extraction process. Six task-specific designed NADESs were prepared and evaluated for their extraction efficiency, based on the Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) of the extract. The most promising NADES, comprising betaine and 1,3-propandeiol, was selected for process optimization using a Box–Behnken design and key extraction parameters were systematically examined to maximize TPC and TFC. The extract obtained under the proposed conditions (S/L = 20 mg/g, 240 min, 40% water as cosolvent) exhibited a TPC and TFC value of 49.2 mgGAE/g and 45.9 mgCAE/g, respectively, approximately two times higher than the values of a hydroethanolic extract, obtained under the same conditions (TPC = 26.6 mgGAE/g, TF = 19.9 mgCAE/g). The optimum extract was further analyzed using HPLC-DAD to determine its phytochemical profile and was compared with the conventional hydroethanolic extract, revealing the role of the selected media on the extracted compounds.
1. Introduction
In recent years, medicinal and aromatic plants have been exploited as a natural source of bioactive compounds with therapeutic applications in cosmetics, pharmaceuticals, and nutraceuticals. According to the International Union for the Conservation of Nature (IUCN) and the Worldwide Fund for Nature (WWF), 50,000–80,000 plant species are being harnessed for their medicinal potential [1]. Among these plants, Sideritis scardica is a perennial herb that is endemic to the Balkan region and has attracted the interest of the scientific community due to its use in traditional medicine to treat the common cold and respiratory, gastrointestinal, inflammatory, and rheumatic conditions [2,3]. In antiquity, Sideritis was utilized to treat soldiers’ wounds from iron weapons, leading to its name that derives from the Greek word “σίδηρος” (sidēros), meaning iron [2,4]. Nowadays, S. scardica is commonly known as “Mursalski tea”, “Pirinski tea”, or “Alibotushki tea”, depending on the region and the mountain that is being harvested from [5,6]. In Greece, it is referred to as “Greek Mountain tea” or “Greek Olympus tea”.
S. scardica extract has been extensively studied both in terms of its phytochemical profile as well as its medicinal properties. Phenolic compounds (e.g., chlorogenic acid, 3-caffeoylquinic acid, feruloylquinic acid) and their glycosides, flavonoids (e.g., apigenin, luteolin, chrysoeriol) and their glycosides, phenylethanoid glycosides (e.g., echinacoside, lavandulifolioside, verbascoside), and terpenoids (e.g., siderol, sideridiol, isolinearol) have been identified in S. scardica extracts [3,5,7]. Owing to the plethora of pharmaceutically active constituents present in the S. scardica, a variety of biological activities of its extracts have been investigated, such as antioxidant, antimicrobial, anti-inflammatory, neuroprotective, and gastroprotective activity [1,4,5,8,9,10,11,12,13].
Different extraction techniques and extraction solvents have been implemented so far for the acquisition of the plant extracts, including Natural Deep Eutectic Solvents (NADESs) [6,10,11]. Deep Eutectic Solvents (DESs) are a class of solvents formed by mixing at least two compounds in specific molar ratios, that interact with each other, forming an extensive hydrogen bond network. This interaction leads to a significant decrease in the melting point of the individual components and in the formation of a mixture that is liquid in a wide range of temperatures. Natural Deep Eutectic Solvents (NADESs) are a specific type of DES composed of naturally occurring compounds which also form a strong hydrogen bond network and, in addition, can offer increased biocompatibility to the resulting solvent [14,15].
Due to their favorable properties, such as low volatility, chemical and thermal stability, and biodegradability, which can be tailor-made to meet the criteria of the desired application, NADESs have found numerous applications, including the extraction of bioactive compounds from plants and biomass [16,17,18]. Moreover, NADESs as extraction media possess several advantages over conventional organic solvents. Their low volatility renders them safer to use and store, aligning with the principles of Green Chemistry, while they can be customized to be non-toxic and biodegradable, allowing the incorporation of the NADES-extract directly in the final product, eliminating the need to isolate the extracted compounds [19]. Furthermore, a noteworthy asset of NADESs is that their extracting capability allows them to extract compounds with a wider range of polarity than conventionally used solvents, as well as their ability to protect and preserve the extracted compounds and their activity for a prolonged period of time [20,21].
To our knowledge, there are only two reports concerning the extraction of S. scardica using NADESs. Grozdanova et al. assessed the extracting ability of four NADESs in terms of Total Phenolic Content and the antimicrobial activity of the extracts [11]. The results indicated choline chloride/glucose 5:2 with 30% of water as the most promising extracting solvent of phenolic compounds, and extracts with citric acid/1,2-propanediol 1:4 and choline chloride/glycerol 1:2 as the extracts with the best antimicrobial activity. On the other hand, Vasileva et al. utilized the NADES citric acid/1,2-propanediol 1:4 as the extracting medium and investigated the anti-aging properties of the NADES-extract [10]. Despite these encouraging results, the use of NADES for the extraction of S. scardica has been explored only in a limited and non-systematic manner. The need for a wider screening of more effective NADES systems, a study of the extraction process, as well as the optimization of the extraction of phytochemicals from S. scardica using NADES represent a significant gap in the development of a sustainable and high-performance extraction method for this important medicinal plant.
In this context, and as a continuation of our work [20,22], the aim of the present study is the development and optimization of the extraction of bioactive compounds from Greek mountain tea S. scardica using NADES as the extraction solvent. More specifically, six task-specific designed NADESs were prepared and studied for their extracting ability. The obtained NADES-extracts were evaluated in terms of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC). Upon selection of the most potent NADES, the extraction process was optimized using a symmetrical three-level Box–Behnken design. As independent variables, extraction time, % (w/w) of water in the NADES extraction system as cosolvent, and solid-to-liquid ratio were selected as three of the most important factors in the extraction efficiency. The NADES-extract obtained under the selected optimal conditions in terms of TPC and TFC values was evaluated regarding its phytochemical profile using HPLC-DAD, and the results were compared with the extract obtained using a hydroethanolic solution as the solvent. Overall, this work provides a green, optimized extraction process for S. scardica, highlighting the effectiveness of NADESs compared to conventional extraction media.
2. Materials and Methods
2.1. Materials
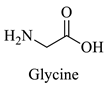
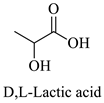
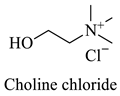
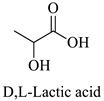
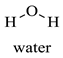
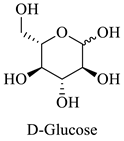
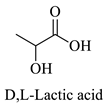
Greek mountain tea (Sideritis scardica) was collected from Mount Olympus in Greece and was kindly provided by Korres Natural Products S.A. For the NADES preparation. The reagents utilized were glycine (Sigma-Aldrich, St. Luis, MO, USA), D,L-lactic acid (80–85% aq. sol.) (Alfa Aesar, Ward Hill, MA, USA), choline chloride (Glentham, Unit 5 Leafield Way, Corsham, UK), D-(+)-glucose (Sigma-Aldrich, St. Luis, MO, USA), fructose (Labbox Labware S.L, Barcelona, Spain), glycerol (Kalo Chem, Piraeus, Greece), anhydrous betaine (Fluorochem, Hadfield, UK), and 1,3-propanediol (Sigma-Aldrich, St. Luis, MO, USA). Folin–Ciocalteu reagent was acquired from Merck Millipore (Darmstadt, Germany), and ethanol from Sigma-Aldrich (St. Luis, MO, USA). Additionally, double-deionized water was used for all the experiments. For the HPLC analysis, the reagents utilized were methanol for HPLC analysis from Fischer Scientific (Hampton, VA, USA), gallic acid, benzoic acid, 4-hydroxy-benzoic acid, vanillic acid, salicylic acid from Fluka (Dresden, Germany), catechin, trans-cinnamic acid, resveratrol, tyrosol from Sigma-Aldrich (St. Luis, MO, USA), caffeic acid, ferulic acid, vanillin, apigenin, chicoric acid from Glentham (Unit 5 Leafield Way, Corsham, UK), coumaric acid, chlorogenic acid, quercetin from Fluorochem (Hadfield, UK) and syringic acid, naringin, rosmarinic acid and kampherol from Alfa Aesar (Ward Hill, MA, USA).
2.2. NADES Preparation
For the NADES preparation, the heating and stirring method was used, as previously described by Tzani et al. [23]. The applied conditions for each NADES are presented in Table 1.

Table 1.
NADES Preparation Conditions.
2.3. pH Determination
The pH of each NADES was evaluated following dilution with double-deionized water, resulting in aqueous solutions of NADES (100%, 75%, 50%, 25%, and 12.5% w/w). These measurements were performed using a calibrated pH meter (Metrohm 744 pH meter) [24].
2.4. Polarity Determination
The polarity of the synthesized NADES was measured using the solvatochromic dye Nile Red, as previously described by Karadendrou et al. [24]. This solvatochromic indicator is sensitive to the solvent’s polarity, exhibiting color changes in response to the surrounding environment, which results in different interactions and shifts in light absorption behavior. The maximum absorption (λmax) of each NADES was determined using a Jasco V-770 UV–Vis/NIR spectrometer (Jasco, Portland, OR, USA). Afterwards, the molecular transition energy (ENR) was calculated according to Equation (1).
ENR (kcal·mol−1) = 28,591/λmax
2.5. Plant Material Preparation
The plant material (all the parts of the collected mountain tea) (Figure 1) was initially ground using a domestic mechanical grinder and was stored in the dark at room temperature until further use.

Figure 1.
All parts of Greek mountain tea before grinding.
2.6. Extraction Process Using NADES–Water System
A specific amount of ground mountain tea was dispersed in the appropriate amount of the selected NADES–water system (in a solid-to-liquid ratio between 20 and 80 mg/g), with water serving as a cosolvent (10–40% w/w), as shown in Table 2. The extraction process was carried out at 45 °C (using an oil bath) under continuous stirring, for the selected extraction period (30–240 min) (Table 2). Also, after the extraction, vacuum filtration was performed in order to separate the liquid NADES-extract from the solid residue, and finally, all obtained NADES-extracts were subsequently stored at 4 °C until further analysis.

Table 2.
Raw and coded values of independent variables for the NADES extraction process.
All six NADESs presented in Table 1 were tested as extraction solvents, based on the TPC and TFC response of the resulting NADES-extracts, and the most effective medium was then used for the optimization process.
2.7. Extraction Process Using a Hydroethanolic Solution
For comparison reasons, the extraction efficiency of a conventional solvent system, EtOH-Water (60:40 w/w), was also evaluated. The extraction was performed under selected conditions, as indicated by the optimization process through the experimental design.
The hydroethanolic solution was added to a round-bottom flask, and the appropriate amount of the raw material was suspended in it. A reflux condenser was adjusted to the flask, and the mixture was heated at 45 °C through continuous stirring for the appropriate amount of time. Upon completion of the extraction, the extract was separated from the solid residue by vacuum filtration, and then the extraction solvent was removed through rotary vacuum evaporation. The resulting dry extract was stored in the dark at 4 °C until further analysis.
2.8. Extraction Process Optimization
The interactions between the extraction parameters and the preferable conditions for the extraction process of phenolic and flavonoid compounds using the optimum among the studied NADESs (Bet/1,3-PDO) were determined by response surface methodology (RSM). To this end, a three-level, 15-run Box–Behnken Design (BBD) with three replicates at the center point was employed using the trial version of the Design-Expert 12.0 software (Stat-Ease Inc., Minneapolis, MN, USA).
The chosen independent variables were the % (w/w) water within the NADES–water solvent system (A), the extraction time (min) (B), and the raw mountain tea-to-solvent ratio (mg/g) (C), since they are considered the most important parameters in an extraction process. The experimental ranges of each factor were determined based on values commonly reported in the literature for the extraction of mountain tea. The TPC and TFC were selected as response variables for these factors, with the statistical significance level set at p < 0.05. The extraction parameters and conditions are shown in Table 2.
The experimental data of the extractions were statistically analyzed using ANOVA and fitted to second-order polynomial equations (Equation (2)) to provide a correlation between the factors and to verify the accuracy of the predicted model.
where Y is the response, A, B, C are the coded independent variables, β0 is the intercept, β1, β2, β3 are the linear coefficients, β11, β22, β33 are the quadratic coefficients, and β12, β13, β23 are the interaction coefficients.
Y = β0 + β1A + β2B + β3C + β11A2 + β22B2 + β33C2 + β12AB + β13AC + β23BC
The optimization of the process was based on the maximization of the TPC and TFC responses, while a confirmatory experiment within the design boundaries was conducted in duplicate to verify the results of the developed model. The predicted values of TPC and TFC, as well as the 95% low and high intervals of the model, were calculated from the RSM models and are presented in Table 3.

Table 3.
Predicted response values and 95% prediction intervals.
2.9. Determination of the Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) of the Extracts
For each extract (both NADES-extracts and the hydroethanolic extract), the TPC was evaluated using the Folin–Ciocalteu method, as reported before in our previously published research work, and the results are expressed as gallic acid equivalents (GAEs) per gram of mountain tea [19]. Regarding the TFC analysis for the same extracts, the aluminum chloride colorimetric method was used as previously described, and the results are presented as catechin equivalents (CAEs) per gram of raw material [25].
All obtained extracts used for the above measurements were first diluted with double-deionized water (stock solution). Each dilution was prepared by mixing 30 μL of NADES-extract with double-deionized water to a final volume of 90 μL, yielding a final concentration of 33.3% v/v. All measurements were repeated in triplicate using the BioTek Epoch 2 spectrophotometer.
2.10. High-Pressure Liquid Chromatography (HPLC-DAD)
The identification and qualitative assessment of the phenolic compounds present in S. scardica extracts obtained through both conventional extraction and extraction using NADES were performed via HPLC analysis utilizing a developed library of 22 reference standard compounds. The chromatographic conditions were applied as reported in our previously published research work by Karadendrou et al. [20]. For the HPLC analysis, the studied extracts were diluted to 40% (v/v) concentration using ultrapure water.
3. Results and Discussion
3.1. Task-Specific Design of NADES and Properties Determination
The selected NADESs were task-specifically designed using starting materials such as glycine, betaine, and choline chloride as hydrogen bond acceptors and lactic acid, glucose, fructose, glycerol, and 1,3-propanediol as hydrogen bond donors, all of which are well-established ingredients in the food, pharmaceutical, and cosmetic industries. All the NADES were studied for two of their most important properties for extraction processes, their pH and polarity.
3.1.1. pH
The pH physicochemical property is fundamental in the design, operation, and optimization of experimental and industrial processes, while, regarding extraction processes, pH significantly influences the extraction efficiency of plant-derived metabolites.
The pH values of the six NADESs’ aqueous solutions (with varying concentrations of water in the NADES–water system) were also determined, as water is commonly used in combination with NADES in extraction processes. The pH values as a function of the %water (w/w) in the NADES–water systems are presented in Figure 2.
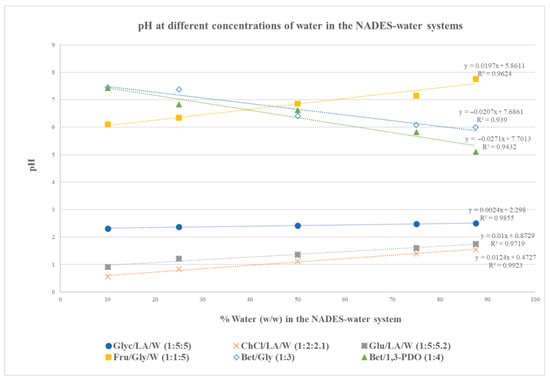
Figure 2.
pH at different concentrations of water in the studied NADES–water systems at 25 °C.
The data presented in Figure 2 show that the three NADESs that contain lactic acid have a strong acidic character (pH). A slight increase in the pH is observed with increasing amounts of water in the NADES system, as is also mentioned by Pozharitskaya et al. and Mitar et al. [26,27].
The Fru/Gly/W NADES is only slightly acidic, as could be expected by the weak acidic character of both fructose and glycerol. This is in accordance with the results of other reports measuring the pH of fructose-containing NADESs [28,29]. Increasing the water content increases the pH of this NADES, as was observed in the case of the three acidic NADESs.
An exception is noted in the case of the two betaine-based NADES, where the pH was decreased after water dilution. A possible explanation could be the strong intramolecular hydrogen bonding between betaine (characterized by its zwitterionic nature) and polyols that makes the release of free protons limited. Upon dilution with water, these strong interactions are disrupted [30].
3.1.2. Polarity
The polarity of a solvent is a key parameter in extraction processes, as it can significantly influence the solubility and extraction efficiency of specific bioactive compounds [21]. The Nile Red polarity scale was chosen due to its widespread use in investigating the polarity of DESs, and the results for each NADES are presented in Table 4.

Table 4.
Polarity of the studied NADESs and water measured at 25 °C.
The polarity of NADESs is closely related to hydrogen bonding interactions between hydrogen bond donors and acceptors, both intermolecular and intramolecular. Therefore, a higher number of hydrogen bonds is expected to increase solvent polarity [31]. It can thus be concluded that the composition of the NADES components and the interactions between them strongly influence solvent polarity. For reference and comparison, the polarity of water was also measured, and showed an ENR value of 48.71 kcal/mol. This indicates that the molar transition energy values of the prepared NADESs are comparable to those of water.
3.2. NADES Screening
In order to determine the extraction conditions for the screening experiments, parameters such as extraction time, extraction temperature, the % w/w water content in the solvent system used, and the solid-to-solvent ratio were taken into consideration. The initial selection of these conditions was guided by the literature’s data and previous studies conducted by our research team.
According to the literature, the extraction temperature when NADESs are used as solvents ranges mostly from room temperature up to 60 °C [32]. Elevated temperatures not only increase energy consumption but may also degrade thermally sensitive extracted compounds. For this reason, in this study, the temperature was set at 45 °C for all the extraction experiments.
Regarding the extraction time, extended extraction durations increase operational costs, whereas shorter times may result in incomplete recovery of target compounds. The extraction times usually range from 20 min to a few hours (e.g., 4 h). However, it must be noted that the extraction time reported in the literature for the extraction of various plant sources using NADESs varies depending on the methodology employed (e.g., heating and stirring, ultrasound, microwave-assisted extraction, etc.).
The solid-to-liquid (S/L) ratio is also a very important parameter in the extraction process, since it affects the dispersion of the solvent around the raw material. In the case of Sideritis herbs extraction processes, the S/L ratio reported in the literature varies between 1:30 and 1:80 [10,33,34]. For the screening process, the solid-to-liquid (S/L) ratio was set at 1:33.
Finally, regarding the water as a cosolvent in the NADES-based extraction processes of plant sources, a range of 20% all the way up to 80% w/w can be found [32]. Elevated water content may result in a disruption of the hydrogen bonding network of the NADES (potentially reducing even its extraction efficiency). Dai et al. mention in their study that the interactions of the NADES system weakened with water dilution and even disappeared when the water content was above 50% (v/v) [35]. Nevertheless, this does not necessarily mean that higher water levels are entirely ineffective or unsuitable.
According to the aforementioned studies, the NADES screening was performed under the following conditions: 120 min, 30% w/w of water in the NADES–water system, and 30 mg/g solid-to-liquid ratio. The TPC and TFC values were determined for each NADES-extract, and the results are shown in Table 5.

Table 5.
NADES screening for use as extraction media.
The results indicate that NADESs derived from betaine and polyols generally yield higher TPC and TFC values. The Bet/Gly system showed the highest TPC (55.8 mgGAE/g) and TFC (33.3 mgCAE/g), followed closely by Bet/1,3-PDO (TPC = 52.0 mgGAE/g; TFC = 30.5 mgCAE/g) (Table 5). Nevertheless, the NADES Bet/1,3-PDO has been chosen as a suitable candidate for subsequent optimization experiments, since it possesses some important advantages over the Bet/Gly. Bet/1,3-PDO can be prepared by heating under lower temperatures and possesses considerably lower viscosity at room temperature compared to Bet/Gly, thus facilitating the handling.
3.3. Box–Behnken Experimental Design
In order to investigate the effect of the extraction parameters and optimize the mountain tea extraction process, a three-level symmetrical Box–Behnken experimental design was conducted, as mentioned previously in Section 2.8. The TPC and TFC of the NADES extracts were used as the response variables, and the results are presented in Table 6.

Table 6.
Experimental results of the experimental design.
The experimental results were evaluated by analysis of variance (ANOVA). The key statistical parameters in order to obtain a well-fitted model included the p-value, which reflects the significance of each factor and their interactions, and the lack of fit (F-value), which reflects the extent to which the model predictions miss the observations.
In order to obtain a model that can be used to predict outcomes and optimize the process, the model has to be significant (p-value less than 0.05), the lack of fit insignificant, and finally, a good agreement between adjusted and predicted R2 should be observed (a difference lower than 0.2 is desired). p-values greater than 0.10 indicate these models’ terms are not significant, and in that case, a model reduction may improve the model.
3.4. Optimization of the Extraction Methodology
3.4.1. TPC of the NADES-Extracts
Based on the ANOVA statistical evaluation, the reduced cubic model was applied to describe the TPC response, excluding the variables that did not show statistical significance.
Equation in terms of Actual Factors (Equation (3)) allows the prediction of TPC values for specific input conditions.
TPC = 19.19 − 0.384 (water) + 0.134 (Time) + 0.530 (S/L) + 0.00619 (water × Time) + 0.00259 (water × S/L) − 0.00162 (Time × S/L) + 0.00871 (water2) − 0.00034 (Time2) − 0.00553 (S/L2) − 0.000114 (water2 × Time)
According to the analysis of variance results (Table 7), the model F-value of 68.00 and p-value of 0.0005 indicate the model’s significance. Moreover, the lack of fit is non-significant (lack of fit F-value = 1.16), while the coefficient of determination R2 and adjusted R2 reached values of 99.42 and 97.95%, respectively, whereas the predicted R2 reached 86.82%. The close agreement between adjusted and predicted R2 confirms the adequacy of the model, supporting its use for navigating the design space.

Table 7.
Significance of each factor equation model term for the TPC.
Initially, the individual effect plots for each parameter were examined to assess the separate influence of each factor on the system. Subsequently, the parameter values associated with an increasing trend in TPC were identified and retained for further analysis using the 3D response surface plots, as depicted in Figure 3.
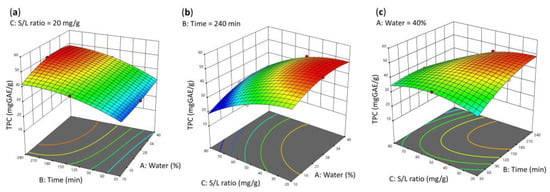
Figure 3.
RSM plots for the TPC response showing the correlation between TPC and the factors (a) time and % water in the NADES–water system (in a solid-to-liquid ratio of 20 mg/g) and (b) solid-to-liquid ratio and % water in the NADES–water system (for extraction time = 240 min), and (c) solid-to-liquid ratio and time (for 40% of water in the NADES–water system).
The response surface analysis indicates that, with the solid-to-liquid ratio maintained at 20 mg/g (Figure 3a), increasing both the extraction time above 180 min and the water (as a cosolvent) above 30% w/w positively influences the extraction of phenolic compounds (TPC values greater than 40 mgGAE/g). Similarly, in Figure 3b, with the extraction time fixed at 240 min, higher TPC values are observed when the water content exceeds 30% w/w and the solid-to-solvent ratio is at its lowest level.
3.4.2. TFC of the NADES-Extracts
In the case of the TFC values of the NADES-extracts, according to the ANOVA statistical analysis, the reduced quadratic model was applied to describe this response (excluding the variables that did not show statistical significance).
Equation in terms of Actual Factors (Equation (4)) allows prediction of TFC values for specific input conditions.
TFC = 17.46194 + 0.206417 (water) + 0.229389 (Time) − 0.040595 (S/L) − 0.001058 (Time × S/L) − 0.000517 (Time2)
As shown by the analysis of variance results (Table 8) the proposed model is considered significant, with an F-value of 21.27 and a p-value of 0.0001. The lack of fit was found to be non-significant (lack of fit F-value = 0.73). The coefficient of determination (R2) and adjusted R2 were 92.20% and 87.86%, respectively, while the predicted R2 reached 73.50%. The close agreement between the adjusted and predicted R2 values further confirms the reliability and adequacy of the model.

Table 8.
Significance of each factor equation model term for the TFC.
As illustrated in Figure 4, the interaction between extraction time and solid-to-liquid ratio is depicted using the 3D response surface plot.
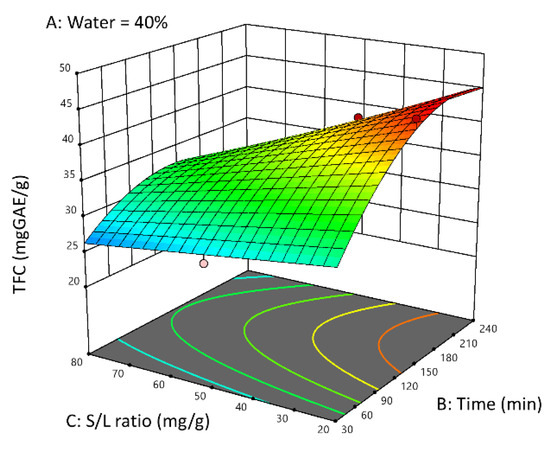
Figure 4.
RSM plot for the TFC response showing the correlation between TFC and the factors solid-to-liquid ratio and time (for 40% of water in the NADES–water system).
According to the response surface analysis, it can be concluded that, to obtain NADES-extracts rich in flavonoid compounds (TFC values greater than 40 mgCAE/g) while keeping the water content of the extraction solvent system fixed at 40% w/w, the extraction time should exceed 180 min, whereas the solid-to-liquid ratio should be maintained at lower values.
3.5. Model Confirmation
Based on the aforementioned analysis for the two selected responses, the following can be considered as preferable extraction conditions within the studied boundaries, aiming to achieve simultaneously high TPC and TFC values: 20 mg of mountain tea per gram of extraction solvent, an extraction time of 240 min, and 40% w/w water in the NADES–water system.
To confirm the validity of the proposed model, two additional experiments were performed under these specific extraction conditions (Table 9). The experimental values of the TPC (49.2 mgGAE/g) and TFC (45.9 mgCAE/g) responses were in excellent agreement with the predicted values, falling within the 95% low and high prediction intervals and thus validating the proposed model.

Table 9.
Proposed model confirmation.
3.6. Comparative Analysis of Phytochemical Profiles in NADES and Hydroethanolic Extracts
For comparison purposes, under the aforementioned conditions (240 min, 40% (w/w) of water as a cosolvent, 20 mg mountain tea per gram of extraction solvent), a conventional extraction of mountain tea was carried out using a hydroethanolic solvent system. The hydroethanolic extract was characterized for its total phenolic and flavonoid content, and the results are presented in Table 10.

Table 10.
Comparison of hydroethanolic extract and NADES-extract prepared using the optimum conditions (240 min, 40% (w/w) of water as a cosolvent, 20 mg mountain tea per gr of extraction solvent).
It is noteworthy that the NADES-extracts exhibited considerably higher TPC (49.2 mg GAE/g) and TFC (45.9 mgCAE/g) values compared to the hydroethanolic extract (26.6 and 19.9 mg/g, respectively), with an 86 and 131% increase, respectively, highlighting the superior extraction efficiency of the NADES-based system for phenolic and flavonoid compounds in the applied conditions.
Moreover, the NADES-water extract may be considered superior, not only due to the higher TPC and TFC values, but also because NADES can serve as a protective medium for the extracted compounds. Since the solvent is not removed at the end of the extraction, it remains in the system, allowing the extract to be used directly in subsequent applications, thereby simplifying the overall process.
To obtain a more detailed insight into the compounds extracted from mountain tea, HPLC analysis was performed using the NADES (Bet/1,3-PDO, 1:4)–water system (40% w/w) and the hydroethanolic solution as extraction solvents (Figure 5a,b). The conducted analysis targeted common phenolic compounds for which reference standards were available, employing a developed library of 22 reference standards. The corresponding chromatographs and identified compounds of this analysis are presented in Figure 5 and Table 11, respectively.
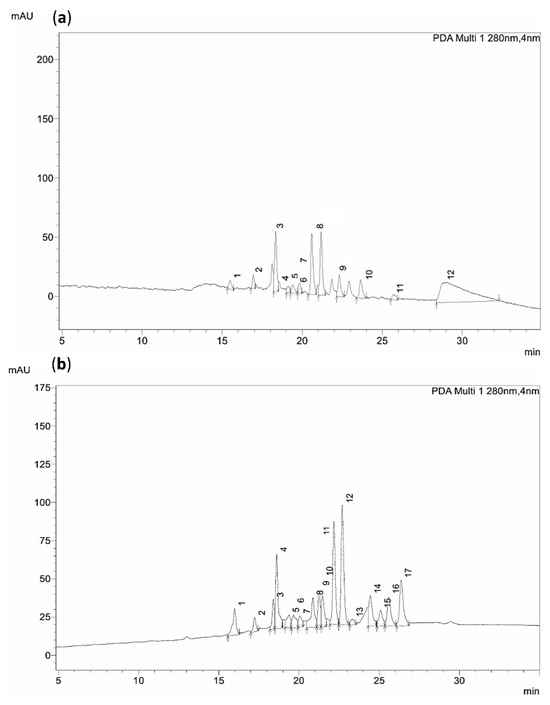
Figure 5.
(a) Chromatogram of NADES-extract. Identified peaks: 1. Chlorogenic acid, 2. Syringic acid, 3. Coumaric acid, 4. Ferullic Acid, 5. Naringin, 6. Benzoic acid, 7. Resveratrol, 8. Salicylic acid, 9. Cinnamic acid, 10. Hesperetin, 11. Apigenin, 12. Unknown. (b) Chromatogram of hydroethanolic extract. Identified peaks: 1. Vanillinic acid, 2. Vanillin, 3. Unidentified, 4. Coumaric acid, 5. Ferullic Acid, 6. Naringin, 7. Rosmarinic acid, 8. Salicylic acid, 9–11. Unidentified, 12. Cinnamic acid, 13. Hesperetin, 14–16. Unknown, 17. Apigenin.

Table 11.
Tentative phenolic compounds and flavonoids detected at 280 and 330 nm in extracts based on the available library of reference standard phenolic compounds.
According to the obtained data (Table 11), it can be concluded that certain phenolic compounds and flavonoids are selectively extracted depending on the solvent system employed. Specifically, benzoic acid, syringic acid, resveratrol, vanillic acid, and chlorogenic acid were detected exclusively in the NADES-extract, whereas kampherol, chicoric acid, and vanillin can be detected only in the hydroethanolic extract. Additionally, according to a first attempt of a qualitative evaluation of the extracted compounds identified in the two extracts (Figure S1), it can be observed that the hydroethanolic extract showed higher levels of coumaric and ferulic acids, while the NADES-extract showed higher levels of naringin, salicylic acid, and hesperetin.
4. Conclusions
This study highlights the effectiveness of NADES as a sustainable and efficient extraction medium for S. scardica. Among the six tested NADES systems, betaine:1,3-propanediol was identified as the most suitable and the extraction using the selected NADES was optimized through a Box–Behnken design. Response surface analysis demonstrated that extraction performance is enhanced at a solid-to-liquid ratio of 20 mg/g, with extraction times above 180 min and water content exceeding 30% w/w. Under specific selected conditions in order to achieve simultaneous maximization of TPC and TFC (S/L = 20 mg/g, 240 min, 40% water as cosolvent), the NADES system with water as cosolvent yielded extracts with substantially higher TPC (49.2 mg GAE/g) and TFC (45.9 mg CAE/g) compared to the hydroethanolic extract (26.6 and 19.9 mg/g, respectively). HPLC profiling further confirmed qualitative differences between the two solvent systems, indicating several compounds were detected exclusively in each extract.
Future research should focus on the industrial scalability of the proposed process, including the long-term stability of the NADES-extract, as well as its economic assessment. Additionally, further studies on the medicinal profile and the toxicity of the extract are of high importance in order to evaluate the potential use of the extract in cosmetic and nutraceutical applications. Expanding this NADES-based strategy to other medicinal plants rich in phenolic compounds may also broaden the applicability of these solvents, leading to a promising direction for future work.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13123945/s1, Figure S1: HPLC chromatographic profiles of the hydroethanolic extract and the NADES (Bet/1,3-PDO) extract.
Author Contributions
Conceptualization, A.T. and A.D.; methodology, M.-A.K., A.K., I.P., and A.T.; validation, M.-A.K., I.P., and K.S.; formal analysis, A.K. and A.T.; investigation, A.K.; resources, G.S. and A.D.; data curation, M.-A.K., I.P., and K.S.; writing—original draft preparation, M.-A.K., A.K., and A.T.; writing—review and editing, M.-A.K., I.P., K.S., G.S., A.T., and A.D.; visualization, A.T.; supervision, A.D.; project administration, A.D.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further enquiries can be directed to the corresponding author.
Acknowledgments
M.-A. Karadendrou gratefully acknowledges financial support from the Research Committee of the National Technical University of Athens (scholarship for postgraduate studies).
Conflicts of Interest
Author Georgios Stavropoulos is employed by Korres S.A.-Natural Products. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NADES | Natural Deep Eutectic Solvents |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| GAE | Gallic Acid Equivalents |
| CAE | Catechin Equivalents |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| Glyc | Glycine |
| LA | D,L-Lactic Acid |
| ChCl | Choline Chloride |
| Glu | Glucose |
| Fru | Fructose |
| Gly | Glycerol |
| Bet | Betaine |
| 1,3-PDO | 1,3 Propanediol |
| W | Water |
| RSM | Response Surface Methodology |
| HPLC | High-Performance Liquid Chromatography |
| BBD | Box–Behnken Design |
| ANOVA | Analysis of Variance |
References
- Walasek-Janusz, M.; Papliński, R.; Mysiak, B.; Nurzyńska-Wierdak, R. Phenolic Profile and Antioxidant Activity of Extracts from Aerial Parts of Thymus vulgaris L. and Sideritis scardica Griseb. Appl. Sci. 2025, 15, 3842. [Google Scholar] [CrossRef]
- Papanikolaou, K.; Kouloridas, K.; Rosvoglou, A.; Gatsas, A.; Georgakouli, K.; Deli, C.K.; Draganidis, D.; Argyropoulou, A.; Michailidis, D.; Fatouros, I.G.; et al. Characterization of the Sideritis scardica Extract SidTea+TM and Its Effect on Physiological Profile, Metabolic Health and Redox Biomarkers in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Molecules 2024, 29, 1113. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Voynikov, Y.; Gevrenova, R.; Balabanova, V. A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules 2024, 29, 204. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef]
- Mróz, M.; Malinowska-Pańczyk, E.; Bartoszek, A.; Kusznierewicz, B. Comparative Study on Assisted Solvent Extraction Techniques for the Extraction of Biologically Active Compounds from Sideritis raeseri and Sideritis scardica. Molecules 2023, 28, 4207. [Google Scholar] [CrossRef]
- Sarrou, E.; Tsivelika, N.; Martens, S.; Irakli, M.; Bletsaki, F.; Broufa, S.; Panajiotidis, S.; Chatzopoulou, P.S.; Abraham, E.M. First Steps towards Pre-Breeding of Sideritis scardica: A Phenotypic, Agronomic, and Phytochemical Profiling Approach. Agronomy 2024, 14, 1448. [Google Scholar] [CrossRef]
- Karapandzova, M.; Qazimi, B.; Stefkov, G.; Andonovska, K.B.; Stafilov, T.; Panovska, T.K.; Kulevanova, S. Chemical Characterization, Mineral Content and Radical Scavenging Activity of Sideritis scardica and S. raeseri from R. Macedonia and R. Albania. Nat. Prod. Commun. 2013, 8, 639–644. [Google Scholar] [CrossRef]
- Tasheva, K.; Georgieva, A.; Denev, P.; Dimitrova, L.; Dimitrova, M.; Misheva, S.; Petkova-Kirova, P.; Lazarova, M.; Petrova, M. Antioxidant and Antitumor Potential of Micropropagated Balkan Endemic Sideritis scardica Griseb. Plants 2023, 23, 3924. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, B.; Staneva, D.; Grozdanova, T.; Petkov, H.; Trusheva, B.; Alipieva, K.; Popova, M.; Miloshev, G.; Bankova, V.; Georgieva, M. Natural Deep Eutectic Extracts of Propolis, Sideritis scardica, and Plantago major Reveal Potential Antiageing Activity During Yeast Chronological Lifespan. Oxidative Med. Cell. Longev. 2022, 2022, 8368717. [Google Scholar] [CrossRef] [PubMed]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef]
- Tadić, V.M.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovic, I.; Trajkovic, V.; Bojovic, D.; Arsic, I. Anti-inflammatory, Gastroprotective, and Cytotoxic Effects of Sideritis scardica Extracts. Planta Med. 2012, 78, 415–427. [Google Scholar] [CrossRef]
- Ververis, A.; Ioannou, K.; Kyriakou, S.; Violaki, N.; Panayiotidis, M.I.; Plioukas, M.; Christodoulou, K. Sideritis scardica Extracts Demonstrate Neuroprotective Activity against Aβ25–35 Toxicity. Plants 2023, 12, 1716. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Liu, Y.; Wu, K.; Zhu, Y.; Lu, H.; Liang, B. Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents. Environ. Sci. Pollut. Res. 2021, 28, 35537–35563. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Bubalo, M.C.; Redovniković, I.R. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar] [CrossRef]
- Karadendrou, M.-A.; Botsi, Y.; Detsi, A.; Tzani, A. Investigation of the Ability of Natural Deep Eutectic Solvents to Act as Efficient Extraction Media for Chamomille (Matricaria chamomilla L.). Food Bioprocess Technol. 2024, 18, 4010–4024. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Tzani, A.; Lymperopoulou, T.; Pitterou, I.; Karetta, I.; Belfquih, F.; Detsi, A. Development and optimization of green extraction process of spent coffee grounds using natural deep eutectic solvents. Sustain. Chem. Pharm. 2023, 34, 101144. [Google Scholar] [CrossRef]
- Tzani, A.; Pitterou, I.; Divani, F.; Tsiaka, T.; Sotiroudis, G.; Zoumpoulakis, P.; Detsi, A. Green Extraction of Greek Propolis Using Natural Deep Eutectic Solvents (NADES) and Incorporation of the NADES-Extracts in Cosmetic Formulation. Sustain. Chem. 2023, 4, 8–25. [Google Scholar] [CrossRef]
- Karadendrou, M.-A.; Kostopoulou, I.; Kakokefalou, V.; Tzani, A.; Detsi, A. L-Proline-Based Natural Deep Eutectic Solvents as Efficient Solvents and Catalysts for the Ultrasound-Assisted Synthesis of Aurones via Knoevenagel Condensation. Catalysts 2022, 12, 249. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline Chloride and Tartaric Acid, a Natural Deep Eutectic Solvent for the Efficient Extraction of Phenolic and Carotenoid Compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikova, V.A.; Flisyuk, E.V.; Vishnyakov, E.V.; Makarevich, E.V.; Shikov, A.N. Physicochemical and Antimicrobial Properties of Lactic Acid-Based Natural Deep Eutectic Solvents as a Function of Water Content. Appl. Sci. 2024, 14, 10409. [Google Scholar] [CrossRef]
- Mitar, A.; Panić, M.; Prlić Kardum, J.; Halambek, J.; Sander, A.; Zagajski Kučan, K.; Radojčić Redovniković, I.; Radošević, K. Physicochemical Properties, Cytotoxicity, and Antioxidative Activity of Natural Deep Eutectic Solvents Containing Organic Acid. Chem. Biochem. Eng. Q. 2019, 33, 1–18. [Google Scholar] [CrossRef]
- Juric, T.; Uka, D.; Hollo, B.B.; Jovic, B.; Kordic, B.; Popovic, B.M. Comprehensive physicochemical evaluation of choline chloride-based natural deep eutectic solvents. J. Mol. Liq. 2021, 343, 116968. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Monteiro, H.; Paiva, A.; Duarte, A.R.C.; Galamba, N. Structure and Dynamic Properties of a Glycerol-Betaine Deep Eutectic Solvent: When Does a DES Become an Aqueous Solution? ACS Sustain. Chem. Eng. 2022, 10, 3501–3512. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep eutectic solvents in separations: Methods of preparation, polarity, and applications in extraction and capillary electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as Extraction Media for Valuable Flavonoids from Natural Sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Koskeridou, A.; Irakli, M.; Karioti, A.; Tsivelika, N.; Chatzopoulou, P. Bioactive Compounds of Green Phenolic Extracts Obtained via Microwave-Assisted Extraction of Sideritis Species Grown in Greece. Molecules 2024, 29, 5612. [Google Scholar] [CrossRef] [PubMed]
- Zissi, L.; Dimaki, V.D.; Birba, V.S.; Galani, V.C.; Magafa, V.; Hatziantoniou, S.; Lamari, F.N. Natural Deep Eutectic Solvents as Green Alternatives for Extracting Bioactive Compounds from Sideritis taxa with Potential Cosmetic Applications. Antioxidants 2025, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).