Application of a Synthetic Microbial Community to Enhance Pepper Resistance Against Phytophthora capsici
Abstract
1. Introduction
2. Results
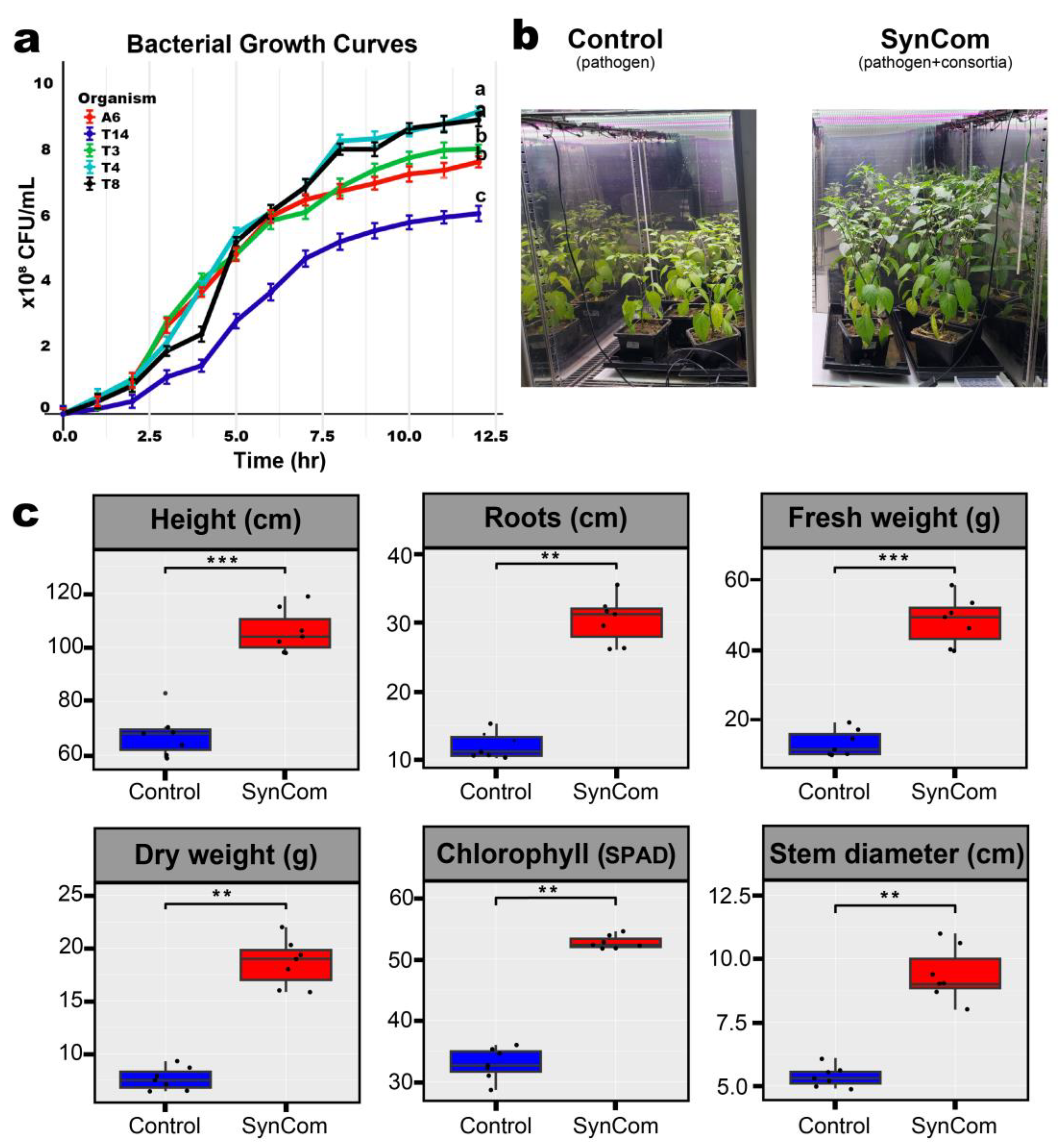
2.1. Identification of Plant-Growth Promoting Rhizobacteria for SynCom Construction
2.2. Changes in the Pepper Growth Through SynCom Application
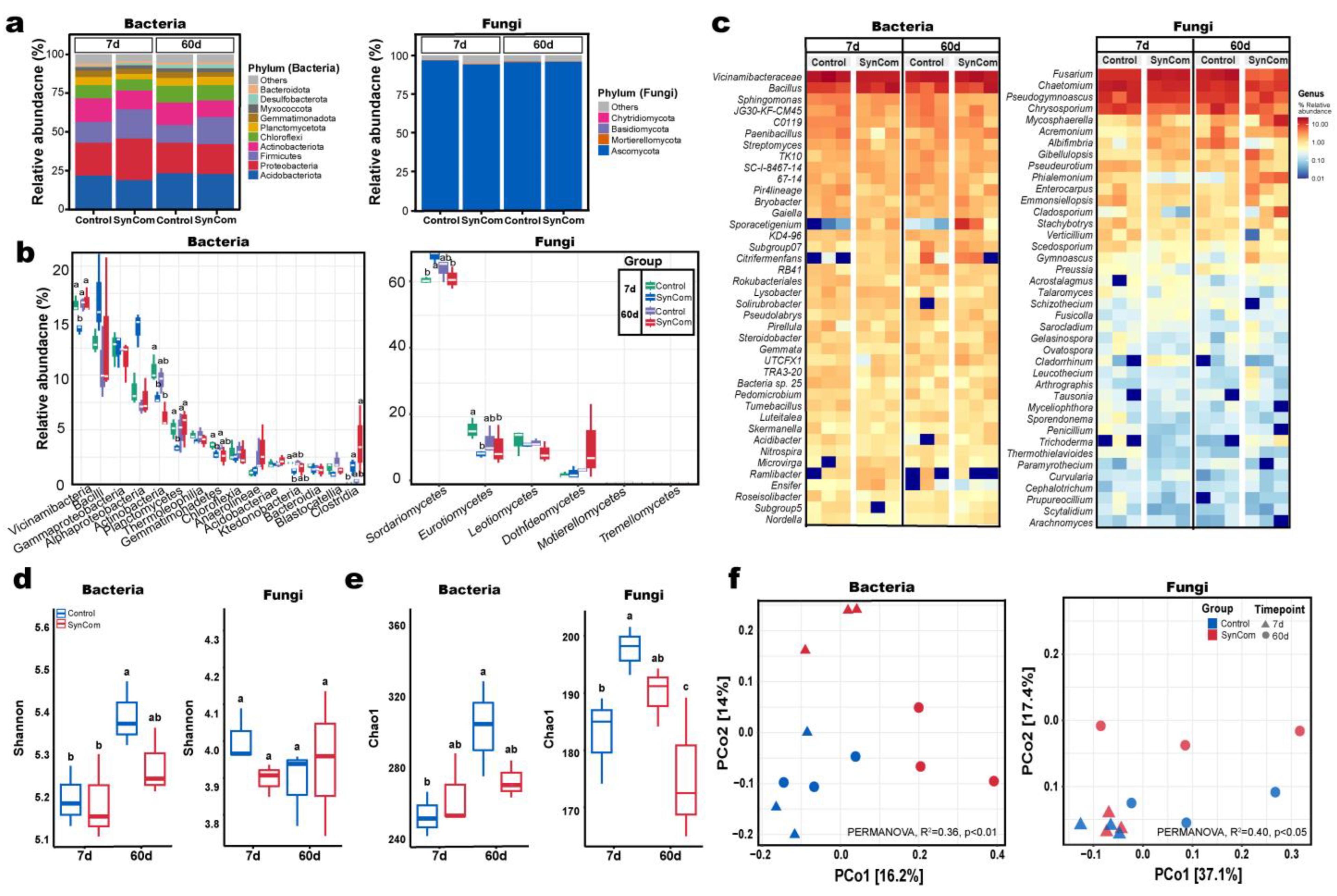
2.3. Microbial Shifts After SynCom Treatment
2.4. Relationships Between Microbial Community and Soil Physicochemical Property
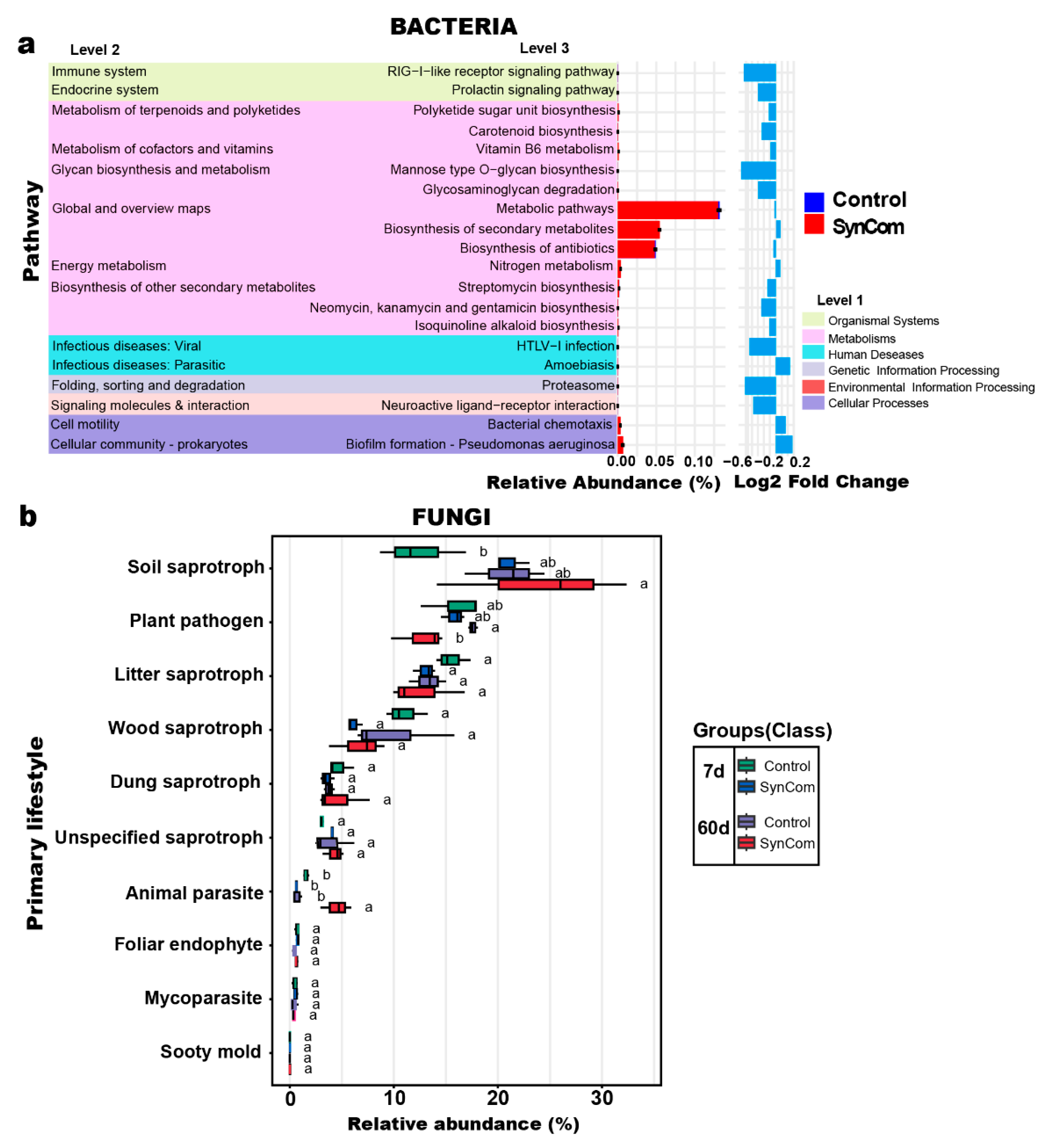
2.5. Microbial Metabolic Changes After Syncom Treatment
3. Discussion
3.1. Effect of SynCom Composed of 5 PGPR on Pepper Plant Resistance to Phytophthora capsici
3.2. Shifts in Rhizosphere Microbial Diversity Under Pathogen Pressure
3.3. Functional Roles of Key Microbial Genera in Disease Resistance
3.4. Metabolic Activities and Transport Mechanisms Supporting Disease Resistance
3.5. Study Limitations and Future Directions
4. Materials and Methods
4.1. Isolation and Characterization of Plant Growth-Promoting Rhizobacteria
4.2. SynCom Design
4.3. Pathogenicity of Phytophthora capsici
4.4. Experimental Design and Soil Sample Collection
4.5. Evaluation of Plant Growth Property
4.6. Bacterial and Fungal Amplicon Sequencing
4.7. Bioinformatics Analysis
4.8. Functional Prediction of Microbial Communities
4.9. Assessment of Soil Physicochemical Properties
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aly, A.A.; Borik, Z.M. Food crops, growth and productivity as an important focus for sustainable agriculture. In Plant Small RNA in Food Crops; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–23. [Google Scholar]
- Patani, A.; Patel, M.; Islam, S.; Yadav, V.K.; Prajapati, D.; Yadav, A.N.; Sahoo, D.K.; Patel, A. Recent advances in Bacillus-mediated plant growth enhancement: A paradigm shift in redefining crop resilience. World J. Microbiol. Biotechnol. 2024, 40, 77. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Di Martino, C.; Torino, V.; Minotti, P.; Pietrantonio, L.; Del Grosso, C.; Palmieri, D.; Palumbo, G.; Crawford Jr, T.W.; Carfagna, S. Mycorrhized Wheat Plants and Nitrogen Assimilation in Coexistence and Antagonism with Spontaneous Colonization of Pathogenic and Saprophytic Fungi in a Soil of Low Fertility. Plants 2022, 11, 924. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, nodule occupancy, and persistence of inoculant strains: Key factors in the rhizobium-legume symbioses. Front. Plant Sci. 2021, 12, 690567. [Google Scholar] [CrossRef]
- Carneiro, B.; Cardoso, P.; Figueira, E.; Lopes, I.; Venâncio, C. Forward-looking on new microbial consortia: Combination of rot fungi and rhizobacteria on plant growth-promoting abilities. Appl. Soil Ecol. 2023, 182, 104689. [Google Scholar] [CrossRef]
- Corozo-Quiñónez, L.; Chirinos, D.T.; Saltos-Rezabala, L.; Monteros-Altamirano, A. Can Capsicum spp. genotypes resist simultaneous damage by both Phytophthora capsici and Bemisia tabaci? Can natural enemies of Bemisia complement plant resistance? Front. Ecol. Evol. 2024, 11, 1275953. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef]
- Abbasi, S.; Safaie, N.; Sadeghi, A.; Shamsbakhsh, M. Tissue-specific synergistic bio-priming of pepper by two Streptomyces species against Phytophthora capsici. PLoS ONE 2020, 15, e0230531. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Spor, A.; Sadeghi, A.; Safaie, N. Streptomyces strains modulate dynamics of soil bacterial communities and their efficacy in disease suppression caused by Phytophthora capsici. Sci. Rep. 2021, 11, 9317. [Google Scholar]
- Thakur, N.; Nigam, M.; Mann, N.A.; Gupta, S.; Hussain, C.M.; Shukla, S.K.; Shah, A.A.; Casini, R.; Elansary, H.O.; Khan, S.A. Host-mediated gene engineering and microbiome-based technology optimization for sustainable agriculture and environment. Funct. Integr. Genom. 2023, 23, 57. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, H.; Wang, L.; Xu, M.; Wan, Y.; Chou, M.; Shi, P.; Wei, G. Multifunctionality and microbial communities in agricultural soils regulate the dynamics of a soil-borne pathogen. Plant Soil 2021, 461, 309–322. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Z.; Sun, H.; Guo, H.; Song, Y.; Zhang, H.; Ruan, Y.; Xu, Q.; Huang, Q.; Shen, Q. Synthetic community derived from grafted watermelon rhizosphere provides protection for ungrafted watermelon against Fusarium oxysporum via microbial synergistic effects. Microbiome 2024, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Koç, E. Physiological responses of resistant and susceptible pepper plants to exogenous proline application under Phytophthora capsicistress. Acta Bot. Croat. 2022, 81, 89–100. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Gu, Y.; Banerjee, S.; Dini-Andreote, F.; Xu, Y.; Shen, Q.; Jousset, A.; Wei, Z. Small changes in rhizosphere microbiome composition predict disease outcomes earlier than pathogen density variations. ISME J. 2022, 16, 2448–2456. [Google Scholar] [CrossRef]
- Nizamani, M.M.; Hughes, A.C.; Qureshi, S.; Zhang, Q.; Tarafder, E.; Das, D.; Acharya, K.; Wang, Y.; Zhang, Z.-G. Microbial biodiversity and plant functional trait interactions in multifunctional ecosystems. Appl. Soil Ecol. 2024, 201, 105515. [Google Scholar] [CrossRef]
- Singh, N.; Das, P.P. Microbial consortium as promising biostimulants for plant health: A future perspective for agriculture. In Microbiome Drivers of Ecosystem Function; Elsevier: Amsterdam, The Netherlands, 2024; pp. 123–143. [Google Scholar]
- da Costa, R.A.; Andrade, I.E.P.C.; Pinto, O.H.B.; de Souza, B.B.P.; Fulgêncio, D.L.A.; Mendonça, M.L.; Kurokawa, A.S.; Ortega, D.B.; Carvalho, L.S.; Krüger, R.H. A novel family of non-secreted tridecaptin lipopeptide produced by Paenibacillus elgii. Amino Acids 2022, 54, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Boubsi, F.; Hoff, G.; Arias, A.A.; Steels, S.; Andrić, S.; Anckaert, A.; Roulard, R.; Rigolet, A.; van Wuytswinkel, O.; Ongena, M. Pectic homogalacturonan sensed by Bacillus acts as host associated cue to promote establishment and persistence in the rhizosphere. iScience 2023, 26, 107925. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol. Rep. 2021, 30, e00613. [Google Scholar] [CrossRef] [PubMed]
- Aroney, S.T.; Poole, P.S.; Sánchez-Cañizares, C. Rhizobial chemotaxis and motility systems at work in the soil. Front. Plant Sci. 2021, 12, 725338. [Google Scholar] [CrossRef]
- Li, Q.; Xiang, P.; Zhang, T.; Wu, Q.; Bao, Z.; Tu, W.; Li, L.; Zhao, C. The effect of phosphate mining activities on rhizosphere bacterial communities of surrounding vegetables and crops. Sci. Total Environ. 2022, 821, 153479. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Alzate Zuluaga, M.Y.; Fattorini, R.; Cesco, S.; Pii, Y. Plant-microbe interactions in the rhizosphere for smarter and more sustainable crop fertilization: The case of PGPR-based biofertilizers. Front. Microbiol. 2024, 15, 1440978. [Google Scholar] [CrossRef]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Ahmad, R.; Alam, M.M.; Hannan, A.; Ahmed, W.; Fatima, N.; Inam-ul-Haq, M. Characterization of native plant growth promoting rhizobacteria and their anti-oomycete potential against Phytophthora capsici affecting chilli pepper (Capsicum annum L.). Sci. Rep. 2020, 10, 13859. [Google Scholar] [CrossRef]
- Thakor, R.; Mistry, H.; Bariya, H. Efficacy of indole-3-acetic acid-producing PGPFs and their consortium on physiological and biochemical parameters of Trigonella foenum-graecum L. Hortic. Environ. Biotechnol. 2023, 64, 533–546. [Google Scholar] [CrossRef]
- Singh, K. Comparative analysis: Physiological and biochemical characterization of phosphate solubilization by Pseudomonas and Bacillus species from various agricultural areas of Dehradun, Uttarakhand, India. Int. J. Life Sci. 2023, 11, 219–227. [Google Scholar] [CrossRef]
- Ehsan, S.; Qureshi, A.; Chaudhary, N.; Ali, A.; Niaz, A.; Javed, H.; Ijaz, F.; Anwar, S.A. Effect of fluorescent-producing rhizobacteria on cereal growth through siderophore exertion. J. Appl. Res. Plant Sci. 2023, 4, 601–611. [Google Scholar] [CrossRef]
- Hamada, M.A.; Soliman, E.R. Characterization and genomics identification of key genes involved in denitrification-DNRA-nitrification pathway of plant growth-promoting rhizobacteria (Serratia marcescens OK482790). BMC Microbiol. 2023, 23, 210. [Google Scholar] [CrossRef]
- John, C.J. PGPR Biotechnology for Management of Biotic and Abiotic Stresses in Agricultural Plants: Recent Developments. In Bioresources and Bioprocess in Biotechnology for a Sustainable Future; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 247–269. [Google Scholar] [CrossRef]
- Utama, S.P.B.; Sulistyowati, L.; Chang, P.P.-C. Characterization of Plant Growth-Promoting Rhizobacteria (PGPR) from Saline Soil in Taiwan. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 012079. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, M.S.; Mosharaf, M.K.; Haque, M.A.; Islam, M.S.; Nahar, K.; Islam, M.M.; Shozib, H.B.; Islam, M.M. Halotolerant biofilm-producing rhizobacteria mitigate seawater-induced salt stress and promote growth of tomato. Sci. Rep. 2022, 12, 5599. [Google Scholar] [CrossRef] [PubMed]
- Mokale Kognou, A.L.; Chio, C.; Khatiwada, J.R.; Shrestha, S.; Chen, X.; Han, S.; Li, H.; Jiang, Z.-H.; Xu, C.C.; Qin, W. Characterization of cellulose-degrading bacteria isolated from soil and the optimization of their culture conditions for cellulase production. Appl. Biochem. Biotechnol. 2022, 194, 5060–5082. [Google Scholar] [CrossRef]
- Yanti, Y.; Hamid, H. Development of the PGPR and cyanobacteria consortium for growth promotion and control Ralstonia syzigii subsp. indonesiensis of tomato. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 012085. [Google Scholar] [CrossRef]
- Sanchez-Gorostiaga, A.; Bajić, D.; Osborne, M.L.; Poyatos, J.F.; Sanchez, A. High-order interactions distort the functional landscape of microbial consortia. PLoS Biol. 2019, 17, e3000550. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Valková, V.; Ďúranová, H.; Vuković, N.; Vukić, M.; Babošová, M.; Kačániová, M. Biological activity of essential oil from. Acta Hortic. Regiotect. 2021, 24, 148–152. [Google Scholar] [CrossRef]
- Ghenu, A.-H.; Marrec, L.; Bank, C. Challenges and pitfalls of inferring microbial growth rates from lab cultures. Front. Ecol. Evol. 2024, 11, 1313500. [Google Scholar] [CrossRef]
- Admassie, M.; González-Pérez, E.; Woldehawariat, Y.; Alemu, T. Screening of potential bacterial isolates against Phytophthora capsici and its plant growth-promoting effect on pepper plants. Physiol. Mol. Plant Pathol. 2023, 127, 102028. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef] [PubMed]
- Kumaishi, K.; Usui, E.; Suzuki, K.; Kobori, S.; Sato, T.; Toda, Y.; Takanashi, H.; Shinozaki, S.; Noda, M.; Takakura, A. High throughput method of 16S rRNA gene sequencing library preparation for plant root microbial community profiling. Sci. Rep. 2022, 12, 19289. [Google Scholar] [CrossRef]
- de Muinck, E.J.; Trosvik, P.; Gilfillan, G.D.; Hov, J.R.; Sundaram, A.Y. A novel ultra high-throughput 16S rRNA gene amplicon sequencing library preparation method for the Illumina HiSeq platform. Microbiome 2017, 5, 68. [Google Scholar] [CrossRef]
- Prodan, A.; Tremaroli, V.; Brolin, H.; Zwinderman, A.H.; Nieuwdorp, M.; Levin, E. Comparing bioinformatic pipelines for microbial 16S rRNA amplicon sequencing. PLoS ONE 2020, 15, e0227434. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 475–490. [Google Scholar]

| Plant Growth Promoting Activity | Bacillus sp. T3 | F. anhuiense T4 | C. firmus T8 | S. roseicoloratus T14 | P. frederiksbergensis A6 |
|---|---|---|---|---|---|
| Antifungal P. capsici (% inhibition) | 0.03 ± 0.01 c | 0.001 ± 0.001 c | 0.007 ± 0.002 c | 58.4 ± 3.2 a | 17.6 ± 2.5 b |
| Chitinase (mm halo zone) | 3.5 ± 0.5 b | 0.005 ± 0.002 c | 0.06 ± 0.001 c | 12.3 ± 1.8 a | 0.002 ± 0.001 c |
| Siderophore (mm reaction zone) | 0.002 ± 0.001 c | 7.2 ± 0.6 b | 0.002 ± 0.001 c | 11.5 ± 0.8 a | 0.003 ± 0.0001 c |
| Protease (mm halo) | 2.1 ± 0.4 b | 0.003 ± 0.001 c | 3.4 ± 0.5 b | 9.7 ± 0.9 a | 0.0001 ± 0.0005 c |
| IAA (μg/mL) | 10.5 ± 1.2 b | 18.7 ± 1.5 a | 7.4 ± 0.8 c | 12.8 ± 1.1 ab | 8.3 ± 0.9 c |
| Phosphate solubilization (PSI, NBRIP) | 4.4 ± 0.7 a | 0.0014 ± 0.0005 c | 0.0012 ± 0.0003 c | 3.8 ± 0.5 ab | 2.7 ± 0.6 b |
| Ammonia production (μg/mL) | 2.0 ± 0.3 b | 2.5 ± 0.4 b | 2.5 ± 0.4 b | 4.8 ± 0.7 a | 2.0 ± 0.3 b |
| Starch (mm reaction zone) | 0.0009 ± 0.0002 c | 4.7 ± 0.8 ab | 5.8 ± 0.9 a | 0.002 ± 0.0003 c | 3.2 ± 0.6 b |
| Cellulose activity (mm halo) | 7.3 ± 0.6 a | 6.9 ± 0.5 a | 6.7 ± 0.6 a | 7.2 ± 0.4 a | 7.5 ± 0.5 a |
| Glucose utilization (OD600) | 0.78 ± 0.2 a | 0.91 ± 0.2 a | 0.52 ± 0.1 a | 0.93 ± 0.2 a | 0.95 ± 0.2 a |
| Urease (mm reaction zone) | 45.6 ± 3.4 a | 0.0 ± 0.0 d | 15.7 ± 1.5 c | 20.4 ± 2.2 b | 12.3 ± 1.4 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashizi, T.F.; Kim, M.-J.; Lim, K.; Lee, G.; Tagele, S.B.; Shin, J.-H. Application of a Synthetic Microbial Community to Enhance Pepper Resistance Against Phytophthora capsici. Plants 2025, 14, 1625. https://doi.org/10.3390/plants14111625
Bashizi TF, Kim M-J, Lim K, Lee G, Tagele SB, Shin J-H. Application of a Synthetic Microbial Community to Enhance Pepper Resistance Against Phytophthora capsici. Plants. 2025; 14(11):1625. https://doi.org/10.3390/plants14111625
Chicago/Turabian StyleBashizi, Tino Flory, Min-Ji Kim, Kyeongmo Lim, GyuDae Lee, Setu Bazie Tagele, and Jae-Ho Shin. 2025. "Application of a Synthetic Microbial Community to Enhance Pepper Resistance Against Phytophthora capsici" Plants 14, no. 11: 1625. https://doi.org/10.3390/plants14111625
APA StyleBashizi, T. F., Kim, M.-J., Lim, K., Lee, G., Tagele, S. B., & Shin, J.-H. (2025). Application of a Synthetic Microbial Community to Enhance Pepper Resistance Against Phytophthora capsici. Plants, 14(11), 1625. https://doi.org/10.3390/plants14111625









