Secondary Volatile Metabolite Composition in Scorzonera pseudolanata Grossh. Plant Parts
Abstract
1. Introduction
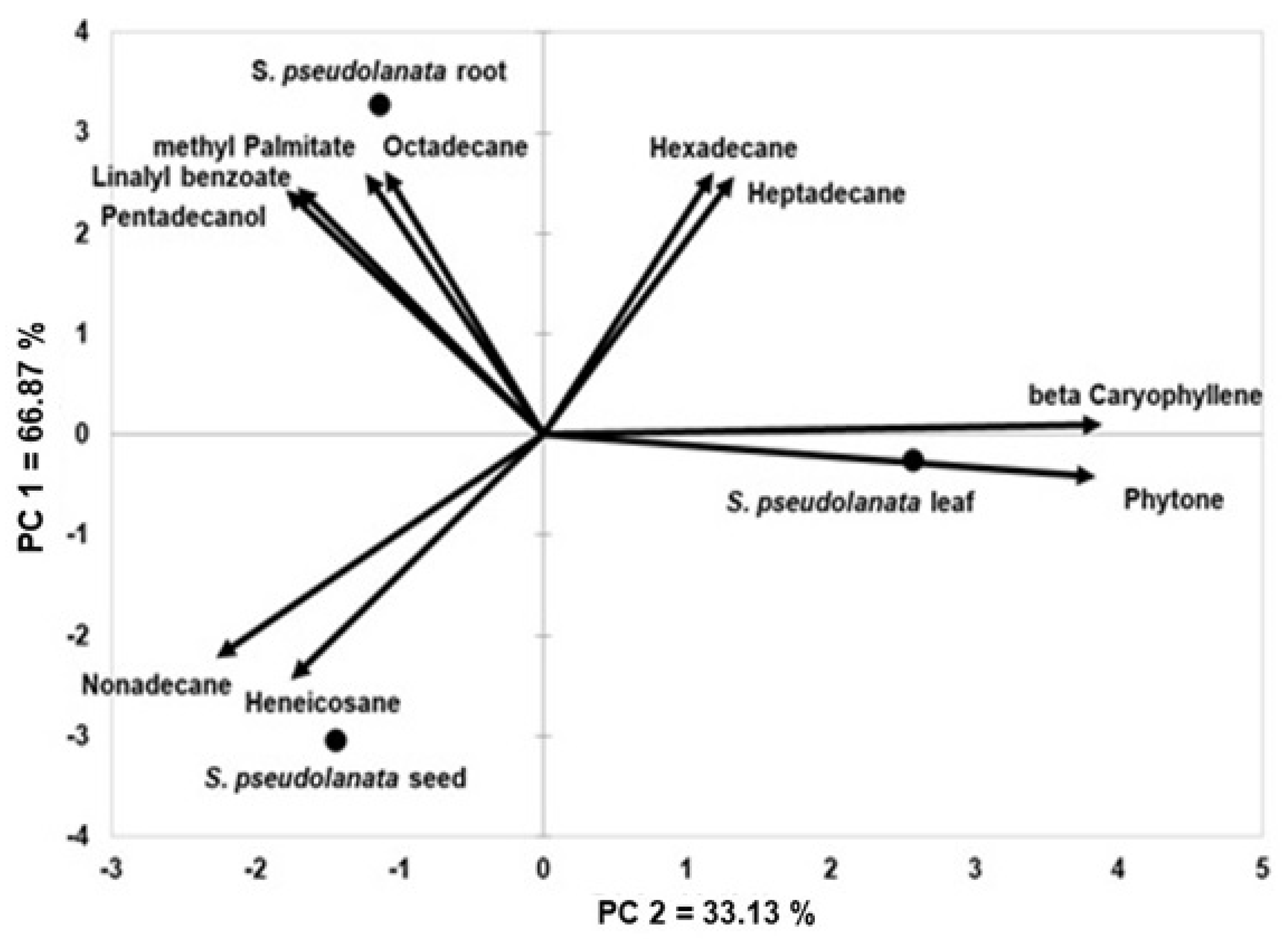
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Secondary Metabolite Composition Analysis
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-MS | gas chromatography–mass spectrometry |
References
- Kamelin, R.V.; Tagaev, I.U. Survey of species of the genus Scorzonera (Asteracea). Bot. Journ. 1986, 71, 1672–1682. (In Russian) [Google Scholar]
- Bremer, K. Asteraceae, Cladistics & Classification, 1st ed.; Timber Press: Portland, OR, USA, 1994; p. 752. [Google Scholar]
- Nazarova, E.A. Karyosystematic investigation of the genus Scorzonera L. s.l. (Lactuceae, Asteraceae). Caryologia 1997, 50, 239–261. [Google Scholar] [CrossRef]
- Zaika, M.A.; Killian, N.; Katy, J.; Krinitsina, A.A.; Nilova, M.V.; Speranskaya, A.S.; Sukhorukov, A.P. Scorzonera sensu lato (Asteraceae, Cichorieae)—Taxonomic reassessment in the light of new molecular phylogenetic and carpological analyses. PhytoKeys 2020, 137, 1–85. [Google Scholar] [CrossRef]
- Coşkunçelebi, K.; Makbul, S.; Gültepe, M.; Okur, S.; Güzel, M.E. A conspectus of Scorzonera s.l. in Turkey. Turk. J. Bot. 2015, 39, 76–87. [Google Scholar] [CrossRef]
- Zidorn, C.; Ellmerer-Müller, E.P.; Stuppner, H. Sesquiterpenoids from Scorzonera hispanica L. Pharmazie 2000, 55, 550–551. [Google Scholar]
- Zidorn, C.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Tyrolobibenzyls E and F from Scorzonera humilis and distribution of caffeic acid derivatives, lignans and tyrolobibenzyls in European taxa of the subtribe Scorzonerinae (Lactuceae, Asteraceae). Phytochemistry 2003, 63, 61–67. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Kalpoutzakis, E.; Harvala, C.; Skaltsounis, A.L. Three new dihydroisocoumarins from the Greek endemic species Scorzonera cretica. J. Nat. Prod. 2001, 64, 1585–1587. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Hu, P.; Wu, W. Biguaiascorzolides A and B, Two novel dimeric guaianolides with a rare skeleton, from Scorzonera austriaca. Food Chem. 2009, 114, 1316–1320. [Google Scholar] [CrossRef]
- Tsevegsuren, N.; Edrada, R.A.; Lin, W.; Ebel, R.; Torre, C.; Ortlepp, S.; Wray, V.; Proksch, P. Four new natural products from Mongolian medicinal plants Scorzonera divaricata and Scorzonera pseudodivaricata (Asteraceae). Planta Medica 2006, 72, 967. [Google Scholar]
- Wang, Y.; Edrada-Ebel, R.A.; Tsevegsuren, N.; Sendker, J.; Braun, M.; Wray, V.; Wenhan, L.; Proksch, P. Dihydrostilbene derivatives from the Mongolian medicinal plant Scorzonera radiata. J. Nat. Prod. 2009, 72, 671–675. [Google Scholar] [CrossRef]
- Ekim, T.; Koyuncu, M.; Vural, M.; Duman, H.; Aytaç, Z.; Adıgüzel, N. Red Book of Plant of Turkey; 100th Year University and Turkish Nature Conservation Association: Ankara, Türkiye, 2000; p. 246. [Google Scholar]
- Ben Miri, Y. Essential Oils: Chemical Composition and Diverse Biological Activities: A Comprehensive Review. Nat. Prod. Commun. 2025, 20. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Réthy, B.; Zupkó, I.; Máthé, I.; Rédei, T.; Falkay, G.; Hohmann, J. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part. II. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1109–1115. [Google Scholar] [CrossRef]
- Harkati, B.; Akkal, S.; Bayat, C.; Laouer, H.; Franca, M.D. Secondary metabolites from Scorzonera undulata ssp. deliciosa (Guss.) Maire (Asteracae) and their antioxidant activities. Rec. Nat. Prod. 2010, 4, 171. [Google Scholar]
- Bahadır-Acıkara, Ö.; Özbilgin, S.; Saltan-İşcan, G.; Dall’Acqua, S.; Rjašková, V.; Özgökçe, F.; Suchý, V.; Šmejkal, K. Phytochemical Analysis of Podospermum and Scorzonera n-Hexane Extracts and the HPLC Quantitation of Triterpenes. Molecules 2018, 23, 1813. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Mittal, C.; Rana, V.; Dabral, K.; Parveen, G. Role of essential oil used pharmaceutical cosmetic product. J. Res. Appl. Sci. Biotechnol. 2023, 2, 147–157. [Google Scholar] [CrossRef]
- Gong, Y.; Shi, Z.N.; Yu, J.; He, X.F.; Meng, X.H.; Wu, Q.X.; Zhu, Y. The genus Scorzonera L.(Asteraceae): A comprehensive review on traditional uses, phytochemistry, pharmacology, toxicology, chemotaxonomy, and other applications. J. Ethnopharmacol. 2024, 320, 116787. [Google Scholar] [CrossRef]
- Sarı, A.; Zidorn, C.; Ellmerer, E.P.; Özgökce, F.; Ongania, K.H.; Stuppner, H. Phenolic Compounds from Scorzonera tomentosa. Helv. Chim. Acta 2007, 90, 311–317. [Google Scholar] [CrossRef]
- Harkati, B.; Akkal, S.; Franca, M.G.D. Composition of the Volatile Components Extracted from the Roots of Scorzonera undulata ssp. deliciosa (Guiss) Maire. From Algeria. Green. Sustain. Chem. 2012, 2, 59–61. [Google Scholar] [CrossRef]
- Boussaada, O.; Ammar, S.; Saidana, D.; Chriaa, J.; Chraif, I.; Daami, M. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule and Scorzonera undulata growing wild in Tunisia. Microbiol. Res. 2008, 163, 87–95. [Google Scholar] [CrossRef]
- Ugur, A.; Sarac, N.; Ceylan, O.; Duru, M.E.; Beyatlı, Y. Chemical Composition of Endemic Scorzonera sandrasica and Studies on the Antimicrobial Activity Against Multiresistant Bacteria. J. Med. Food. 2010, 13, 635–639. [Google Scholar] [CrossRef]
- Ayromlou, A.; Masoud, S.; Mirzaie, A. Chemical composition, antioxidant, antibacterial, and anticancer activities of Scorzonera calyculata Bboiss. and Centaurea irritans Wwagenitz. Extracts, endemic to Iiran. J. Rep. Pharm. Sci. 2019, 79, 118–127. [Google Scholar]
- Acıkara, Ö.B.; Citoglu, G.S.; Coban, T. Phytochemical Screening and Antioxidant Activities of Selected Scorzonera Species. Turk. J. Pharm. Sci. 2013, 10, 453–462. [Google Scholar]
- Granica, S.; Zidorn, C. Phenolic compounds from aerial parts as chemosystematic markes in the Scorzonerinae (Asteraceae). Biochem. Syst. Ecol. 2015, 25, 102–113. [Google Scholar] [CrossRef]
- Şahin, H.; Sarı, A.; Özsoy, N.; Celik, B.Ö.; Koyuncu, O. Two new phenolic compounds and some biological activities of Scorzonera pygmaea Sibth. & Sm subaerial parts. Nat. Prod. Res. 2020, 34, 621–628. [Google Scholar]
- Bader, A.; De Tommasi, N.; Cotugno, R.; Braca, A. Phenolic compounds from the roots of Jordanian viper’s grass, Scorzonera judaica. J. Nat. Prod. 2011, 74, 1421–1426. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Ak, G.; Sut, S.; Ferrerese, I.; Zengin, G.; Yıldıztugay, E.; Mahomoodally, M.F.; Sinan, K.I.; Lobine, D. Phenolics from Scorzonera tomentosa L.: Exploiring the potential use in industrial applications via an integrated approach. Ind. Crops Prod. 2020, 154, 112751. [Google Scholar] [CrossRef]
- Erden, Y.; Kırbag, S.; Yılmaz, Ö. Phytochemical composition and antioxidant activity of some Scorzonera species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 271–276. [Google Scholar] [CrossRef]
- MacLeod, G.; Ames, J. Gas chromatography-mass spectrometry of the volatile components of cooked Scorzonera. Phytochem 1991, 3, 833–8898. [Google Scholar] [CrossRef]
- Temiz, M.A. Antioxidant and antihyperglycemic activities of Scorzonera cinerea radical leaves in streptozocin-induced diabetic rats. Acta Pharmac. 2021, 71, 603–617. [Google Scholar] [CrossRef]
- Jehle, M.; Bano, J.; Ellmerer, E.P.; Zidorn, C. Natural Products from Scorzonera aristata (Astereaceae). Nat. Prod. Commun. 2010, 55, 725–727. [Google Scholar]
- Senol, F.S.; Acikara, O.B.; Citoglu, G.S.; Orhan, İ.E.; Dall’Acqua, S.; Özgökce, F. Prospective neurobiological effects of the aerial and root extracts and some pure compounds of randomly selected Scorzonera species. Pharm. Biol. 2014, 52, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Sarı, A.; Özsoy, N.; Celik, B.Ö.; Koyuncu, O. Phenolic compounds and bioactivity of Scorzonera pygmaea Sibth. & Sm. aerial parts: In vitro antioxidant, anti-inflammatory and antimicrobial activities. Istanb. J. Pharm. 2020, 50, 294–299. [Google Scholar]
- Sakul, A.A.; Kurtul, E.; Ozbek, H.; Kirmizi, N.İ.; Bahtiyar, B.C.; İscan, G.S.; Acikara, O.B. Evaluation of antidiabetic activities of Scorzonera species on Alloxan-induced diabetic mice. Clin. Exp. Health Sci. 2021, 11, 74–80. [Google Scholar] [CrossRef]
- Kargol, H.S.; Elgadi, H.M.; Gadamsi, M.T.; Shubar, H.M.; Geroush, A.M. Pharmacognostical, antimicrobial and laxative study of Scorzonera undulata in Libya. Int. Res. J. Pharm. 2013, 4, 96–99. [Google Scholar]
- Petkova, N. Characterization of inulin from black salsify (Scorzonera hispanica L.) for food and pharmaceutical purposes. Asian J. Pharm. Clin. Res. 2018, 11, 221–225. [Google Scholar] [CrossRef]
- Sweidan, A.; El-Mestrah, M.; Kanaan, H.; Dandache, I.; Merhi, F.; Chokr, A. Antibacterial and antibiofilm activities of Scorzonera mackmeliana. Pak. J. Pharm. Sci. 2020, 33, 199–206. [Google Scholar]
- Abdelkader, H.B.; Salah, K.B.H.; Liouane, K.; Boussasa, O.; Gafsi, K.; Mahjoub, M.A.; Aouni, M.; Hella, A.N.; Mighri, Z. Antimicrobial activity of Rhaponticum acaule and Scorzonera undulata growing wild in Tunisia. J. Microb. Res. 2010, 4, 1954–1958. [Google Scholar]
- Akkol, E.K.; Acıkara, B.; Süntar, I.; Çitoglu, S.G.; Keles, H.; Ergene, B. Enhancement of wound healing by topical application of Scorzonera species: Determination of the constituents by HPLC with new validated reverse phase method. J. Ethnopharmac. 2011, 137, 1018–1027. [Google Scholar] [CrossRef]
- Süntar, İ.; Acıkara, Ö.B.; Citoglu, G.S.; Keles, H.; Eregene, B.; Akkol, E.K. In vivo and in vitro evaluation of the therapeutic potential of some Turkish Scorzonera species as wound healing agent. Curr. Pharm. Des. 2012, 18, 1421–1433. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Bigy, S.S.; Allahresani, A.; Malekaneh, M. Assesment of antioxidant activity, chemical characterization and evaluation of fatty acid compositions of Scorzonera paradoxa Fisch and CA Mey. J. Nat. Pharm. Prod. 2014, 10, 19781. [Google Scholar]
- Meng, X.H.; Yang, Y.J.; Gong, Y.; Zhu, Y. Chemical constituents of the roots of Scorzonera divaricata and their chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 93, 104135. [Google Scholar] [CrossRef]
- Sarialtin, S.Y.; Acikara, O.B. Assessment of Correlation Analysis, Phytochemical Profile, and Biological Activities of Endemic Scorzonera Species from Turkey. Chem. Biodivers. 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Zengin, G.; Dall’Acqua, S.; Ferrarese, I.; Sut, S.; Glamočlija, J.; Soković, M.; Nenadić, M.; Chiavaroli, A.; Recinella, L.; et al. A new step on the chemical profiles and pharmacological effects of three Scorzonera species (S. hieraciifolia, S. hispanica and S. tomentosa). Plant Biosyst. Int. J. Deal. All. Asp. Plant Biol. 2022, 157, 119–128. [Google Scholar] [CrossRef]
- Guclu, G.; Eruygur, N.; Ucar, E.; Ozbek, D.U.; Bal, O.; Akpulat, H.A.; Kahrizi, D. Biological Activity Evaluation of Scorzonera tomentosa L. J. Turk. Agric. Res. 2023, 10, 162–167. [Google Scholar]
- Sahin, H.; Demir, S.; Boga, M.; Sarı, A.; Makbul, S.; Coskuncelebi, K. A Comprehensive Study on a Medicinal and Edible Plant Scorzonera incisa DC., Discussing the Natural Limits of Phytochemical Profiling. Chem. Sel. 2023, 8, e202302173. [Google Scholar]
- Yurteri, E.; Makbul, S.; Çoşkunçelebi, K.; Seyis, F. Essential Oil Composition In Different Plant Parts of Scorzonera acuminata. In New Development on Medicinal and Aromatic Plants; Gülen, Ö., Yayınevi, N., Eds.; IKSAD: Ankara, Türkiye, 2021; pp. 243–264. [Google Scholar]
- Yurteri, E.; Makbul, S.; Coskuncelebi, K.; Gultepe, M.; Seyis, F. Evaluation of the chemical composition in different plant parts of Scorzonera papposa. Fresenius Environ. Bull. 2022, 31, 3460–3468. [Google Scholar]
- Yurteri, E.; Makbul, S.; Gültepe, M.; Seyis, F. Detrmination of the chemical composition in different plant parts of S. mollis taxa. Stud. Chem. 2023, 3, 161–178. [Google Scholar]
- Ercan, S.; Kurtul, E.; Yilmaz, Ö.; Bahadır Acıkara, Ö. Validated Hplc Method To Analyze Phytochemical Structure of Scorzonera Species Grown In Türkiye. J. Fac. Pharm. Ank. Univ. 2024, 48, 32. [Google Scholar]
- Mondello, L. FFNSC3. In Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley: Hoboken, NJ, USA, 2025; Available online: https://www.chromaleont.it/ffnsc-3-wiley-library/ (accessed on 25 March 2025).
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Abu-Shanab, B.; Adwan, G.M.; Safiya, D.A.; Jarrar, N.; Adwan, K. Antibacterial activities of some plant extracts utilized in popular medicine in Palestine. Turk. J. Biol. 2005, 28, 99–102. [Google Scholar]
- Lendzion, K.; Gornowicz, A.; Bielawski, K.; Bielawska, A. Phytochemical Composition and Biological Activities of Scorzonera Species. Int. J. Mol. Sci. 2021, 22, 5128. [Google Scholar] [CrossRef] [PubMed]
- Cosima, C.H.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar]
- Armaka, M.; Papanikolaou, E.; Sivropoulou, A.; Arsenakis, M. Antiviral properties of isoborneol, a potent inhibitor of Herpes simplex type 1. Antivir. Res. 1999, 43, 79–92. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and Biological Activities of Decanal, Linalool, Valencene, and Octanal from Sweet Orange Oil. J. Food Sci. 2012, 77, 1750–3841. [Google Scholar] [CrossRef]
- Bettarini, F.; Borgonovi, G.E.; Fioranti, T.; Gagliardi, I.; Capriolo, V.; Massardo, P.; Ogoche, J.I.J.; Hassanali, A.; Nyandat, E.; Chapya, A. Antiparasitic compounds from East-African plants—Isolation and biological-activity of anonaine, matriacarianol, canthin-6-one and caryophyllene oxide. Int. J. Trop. Insect Sci. 1993, 14, 93–99. [Google Scholar] [CrossRef]
- Langenheim, J.H.; Foster, C.E.; McGinley, C.A. Inhibitory effects of different quantitative compositions of Hymenaea leaf resins on a generalist herbivore Spodoptera exigua. Biochem. Syst. Ecol. 1980, 8, 385–396. [Google Scholar] [CrossRef]
- Bommareddy, A.; Brozena, S.; Steigerwalt, J.; Landis, T.; Hughes, S.; Mabry, E.; Knopp, A.; VanWert, A.L.; Dwivedi, C. Medicinal properties of alpha-santalol, a naturally occurring constituent of sandalwood oil: Review. Nat. Product. Res. 2017, 33, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Togar, B.; Di Stefano, A.; Taspınar, N.; Sozio, P. Protective effects of cyclosativene on H2O2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2015, 67, 299–309. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Tatar, A.; Geyikoğlu, F.; Hacımüftüoğlu, A. Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biologia 2014, 69, 936–942. [Google Scholar] [CrossRef]
- Alimoradi, Z.; Taghian, F.; Jalali Dehkordi, K. Effect of Linalool, Cineole, and β-Bourbonene Coupled with Aerobic Training on the Improvement of Presenilin-1/Amyloid Protein Precursor/Interleukin-1 beta/CASPASE 1 Network, Oxidative Capacity, and miRNA-210 in Mice with Alzheimer’s Disease. Arch. Razi Inst. J. 2024, 79, 629–638. [Google Scholar]
- Paula-Freire, L.I.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine 2014, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.M.L.; de Oliveira Barbosa, M.; Pereira Lopes, M.J.; de Araújo Delmondes, G.; Bezerra, D.S.; Araújo, I.M.; Carvalho de Alencar, C.D.; Coutinho, H.D.Ö.; Peixoto, L.R.; Barbosa-Filho, J.M.; et al. Pharmacological and toxicological activities of α-humulene and its isomers: A systematic review. Trends Food Sci. Technol. 2021, 115, 255–274. [Google Scholar] [CrossRef]
- Sahin, F.; Gulluc, M.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M.; Agar, G.; Ozer, H. Biological activities of the essential oils and methanol extract of Orignum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Olajuyigbe, O.; Ashafa, A. Chemical Composition and Antibacterial Activity of Essential Oil of Cosmos bipinnatus Cav. Leaves South. Africa. Iran. J. Pharm. Res. 2014, 13, 1417–1423. [Google Scholar]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Štefániková, J.; Čmiková, N.; Vukic, M.; Vukovic, N.L.; Kowalczewski, P.Ł. Chemical Composition, Antioxidant, In Vitro and In Situ Antimicrobial, Antibiofilm, and Anti-Insect Activity of Cedar atlantica Essential Oil. Plants 2022, 11, 358. [Google Scholar] [CrossRef]
- Ishak, E.; Kiliç, G.; Öztürk, E.; Karaoğlu, Ş.A.; Yayli, N. Chemical composition, antimicrobial, and lipase enzyme activity of essential oil and solvent extracts from Serapias orientalis subsp. Orientalis. Turk. J. Chem. 2020, 44, 18. [Google Scholar]
- Pradhan, S.; Rituparna, S.; Dehury, H.; Dhall, M.; Singh, Y.D. Nutritional profile and pharmacological aspect of Houttuynia cordata Thunb. and their therapeutic applications. Pharmacol. Res. Mod. Chin. Med. 2023, 9, 100311. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 1, 123–129. [Google Scholar] [CrossRef]
- Zavala-Sanchez, M.A.; Pérez-Gutiérrez, S.; Perez-González, C.; Sánchez-Saldivar, D.; Arias-García, L. Antidiarrhoeal activity of nonanal, an aldehyde isolated from Artemisia ludoviciana. Pharm. Biol. 2002, 40, 263–268. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Zeng, L.; Wang, T.T. Antifungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef] [PubMed]
- Boutebouhert, H.; Didaoui, L. Study of the chemical composition and antioxidant activity of olive leaves of different varieties (Olea europaea L.) growing in Algeria. In CIPAM 2014; International Congress on Aromatic and Medicinal Plants: Zarzis, Tunisia, 2014. [Google Scholar]
- Dengle-Pulate, V.; Chandorkar, P.; Bhagwat, S.; Prabhune, A. Antimicrobial and SEM Studies of Sophorolipids Synthesized Using Lauryl Alcohol. J. Surfactants Deterg. 2013, 17, 11743. [Google Scholar] [CrossRef]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef]
- Thanusha, M.; Mohanalakshmi, M.; Rajasree, V.; Renuka, R.; Meenakshi, P. Assessment of growth and essential oil profiling in leafy coriander (Coriandrum sativum L.) genotypes. Plant Sci. Today 2024, 11, 4961. [Google Scholar] [CrossRef]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of aegle marmelos. BioMed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef]
- Chatterjee, S.; Karmakar, A.; Azmi, S.A.; Barik, A. Antibacterial Activity of Long- Chain Primary Alcohols from Solena amplexicaulis Leaves. Proc. Zool. Soc. 2017, 71, 313–319. [Google Scholar] [CrossRef]
- Endallew, S.A.; Dagne, E. Isolation, Characterization and Quantification of Civetone from Civet Musk. Chem. Sci. J. 2020, 11, 1000204. [Google Scholar]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Nasr, Z.; El-shershaby, H.; Sallam, K.; Abed, N.; Abd-El ghany, I.; Sidkey, N. Evaluation of Antimicrobial Potential of Tetradecane Extracted from Pediococcus acidilactici DSM: 20284—CM Isolated from Curd Milk. Egypt. J. Chem. 2022, 65, 705–713. [Google Scholar] [CrossRef]
- Ozdemir, G.; Karabay, N.U.; Dalay, M.C.; Pazarbasi, B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother. Res. 2004, 18, 754–757. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.-Y.; Wen, S.-Y. Exploring the caste-specific multi-layer defense mechanism of formosan subterranean termites, Coptotermes formosanus Shiraki. Int. J. Mol. Sci. 2017, 18, 2694. [Google Scholar] [CrossRef] [PubMed]
- Abdul, K.; Arumugasamy, K.; Jemimma, H.L.; Nantha Kumar, R. GC-MS Analysis of Root and Aerial Parts Ethanolic Extract of Phyllanthus vasukii Parthipan et al., Sp. Nov. (Phyllanthaceae). Int. J. Ayurvedicand Herbal. Med. 2017, 7, 2672–2684. [Google Scholar]
- Yogeswari, S.; Ramalakshmi, S.; Neelavathy, R.; Muthumary, J. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob. J. Pharmacol. 2012, 6, 65–71. [Google Scholar]
- Arora, S.; Kumar, G.; Meena, S. Gas chromatography-mass spectroscopy analysis of root of an economically important plant, Cenchrus ciliaris L. from Thar desert, Rajasthan (India). Asian J. Pharm. Clin. Res. 2017, 10, 64–69. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Bains, A.; Chawla, P.; Sridhar, K.; Sharma, M.; Inbaraj, B.S. Antimicrobial and Anti-Inflammatory Activity of Low-Energy Assisted Nanohydrogel of Azadirachta indica Oil. Gels 2022, 8, 434. [Google Scholar] [CrossRef]
- Wijayanti, D.R.; Dewi, A.P. Extraction and Identification Potent Antibaterial Bioactive Compound of Streptomyces sp. MB 106 from Euphorbia sp. Rhizosphere. Bioeduscience 2022, 6, 84–88. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Expr. 2017, 7, 54. [Google Scholar] [CrossRef]
- Tunca-Pinarli, Y.; Benek, A.; Turu, D.; Bozyel, M.E.; Canli, K.; Altuner, E.M. Biological Activities and Biochemical Composition of Endemic Achillea fraasii. Microorganisms 2023, 11, 978. [Google Scholar] [CrossRef]
- Manjunath, A.; Chinmayi, G.V.A.; Renganathan, S.; Chandramohan, V.; Sabat, S. Antimicrobial activity of Geranyl acetate against cell wall synthesis proteins of P. aeruginosa and S. aureus using molecular docking and simulation. J. Biomol. Struct. Dyn. 2024, 42, 3030–3050. [Google Scholar] [CrossRef]
- Widiyarti, G.; Megawati, M.; Hanafi, M. The Potential use of Geraniol Esters from Citronella Oil as Anticancer Agents. Orient. J. Chem. 2019, 35, 987–996. [Google Scholar] [CrossRef]
- Neto, G.; Chaves, C.A. Biopesticide Potential of Terpenic Esters Enzymatically Synthesized by Lipases (CAL-B) Immobilized on Magnetic Cashew Apple Bagasse Lignin. 2024. 117 f. Tese (Doutorado em Engenharia Química)—Centro de Tecnologia; Universidade Federal do Ceará: Fortaleza, Brazil, 2024. [Google Scholar]
- Kishimoto, N.; Sugihara, S.; Mochida, K.; Fujita, T. In Vitro Antifungal and Antiviral Activities of γ-and δ-Lactone Analogs Utilized as Food Flavoring. Biocontrol Sci. 2005, 10, 31–36. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Markowska, A. Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes. Molecules 2021, 26, 2901. [Google Scholar] [CrossRef]
- Henderson, G.; Wells, J.D.; Jeanne, R.L. Methyl Palmitate and Methyl Myristate Repel Flies. Florid Entomol. 1991, 74, 365–368. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, Y.S.; Chang, H.C. Purification and characterization of antifungal δ-dodecalactone from Lactobacillus plantarum AF1 isolated from kimchi. J. Food Prot. 2011, 74, 651–657. [Google Scholar] [CrossRef]
- Wu, K.H.; Lee, W.J.; Cheng, T.C.; Chang, H.W.; Chen, L.C.; Chen, C.C.; Lien, H.M.; Lin, T.N.; Ho, Y.S. Study of the antitumor mechanisms of apiole derivatives (AP-02) from Petroselinum crispum through induction of G0/G1 phase cell cycle arrest in human COLO 205 cancer cells. BMC Complement. Altern. Med. 2019, 19, 188. [Google Scholar] [CrossRef]
- ToMoo, M.S.A.T. Anti-inflammatory constituents of toplcall aypplied crude drugs. V. comstituents and anti-inflammatory effect of Aoki, Aucuba japonica THUNB. Biol. Pharm. Bull. 1994, 17, 665–667. [Google Scholar]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E. Formagio ASN. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Hardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata suppresses LPS-induced inflammatory response in RAW 264.7 Macrophages and sprague dawley rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- de Almeida Júnior, J.S.; da Silva, É.B.S.; Moraes, T.M.P.; Kasper, A.A.M.; Sartoratto, A.; Baratto, L.C.; de Oliveira, E.C.P.; Oliveira, E.; Barata, L.E.S.; Minervino, A.H.H.; et al. Anti-inflammatory potential of the oleoresin from the Amazonian Tree Copaifera reticulata with an unusual chemical composition in rats. Vet. Sci. 2021, 8, 320. [Google Scholar] [CrossRef]
- Sheela, D.; Uthayakumari, F. GC-MS Analysis Of Bioactive Constituents From Coastal Sand Dune Taxon –Sesuvium Portulacastrum (L.). Biosci. Discov. 2013, 4, 47–53. [Google Scholar]
- Pinto, M.E.A.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A.R.S. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Ann. Braz. Acad. Sci. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Prasanna, B.N. Analysis of genetic diversity in crop plants-salient statistical tools and considerations. Crop Sci. 2003, 43, 1235–1248. [Google Scholar] [CrossRef]
- Peeters, J.P.; Martinelli, J.A. Hierarchical cluster analysis as a tool to manage variation in germplasm collections. Theor. Appl. Genet. 1989, 78, 42–48. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Zuehlke, S.; Spiteller, M.; Raabe, N.; Ozen, T. Phenolic Cons-tituents of 17 Hypericum species from Turkey. Biochem. Syst. Ecol. 2008, 36, 316–319. [Google Scholar] [CrossRef]
- Bertoli, A.; Cirak, C.; Leonardi, M.; Seyis, F.; Pistelli, L. Morphogenetic changes in essential oil composition of Hypericum perforatum during the course of ontogenesis. Pharm. Biol. 2011, 49, 741–751. [Google Scholar] [CrossRef]
- Rachovska, G.; Dimova, D.; Bojinov, B. Application of cluster analysis and principal component analysis for evaluation of common winter wheat genotypes. Aust. J. of Plant Sci. 2010, 4, 505–514. [Google Scholar]
- Aghaee, M.; Mohammadi, R.; Nabovati, S. Agro-morphological characterization of durum 16 wheat accessions using pattern analysis. Aust. J. Plant Sci. 2010, 4, 505–514. [Google Scholar]
- Hattori, E.K.; Nakajima, J.N. A família Asteraceae na estação de pesquisa e desenvolvimento ambiental galheiro, perdizes, Minas Gerais, Brasil. Rodriguésia 2008, 59, 687–749. [Google Scholar] [CrossRef]
- Funk, V.A.; Anderberg, A.A.; Baldwin, B.G.; Bayer, R.J.; Bonifacino, J.M.; Breitwieser, I.; Crawford, D.J. Compositae Metatrees: The Next Generation; Systematics, Evolution, and Biogeography of Compositae; Institute of Botany, University of Vienna: Wien, Austria, 2009; p. 965. [Google Scholar]
- Babakr, S.H.; Erez, E.; Mükemre, M.; Dalar, A. The phenolic profile and biological activities of common Scorzonera species from Eastern Anatolia. Int. J. Second. Metab. 2022, 9, 538–550. [Google Scholar] [CrossRef]
- Backhaus, K.; Erichson, B.; Plinke, W.; Weiber, R. Multivariate Analysis Methods; Springer: Berlin/Heidelberg, Germany, 1989; pp. 453–516. [Google Scholar]


| Investigated Components | Investigated Part | Species | Reference |
|---|---|---|---|
| Phenolic compounds | Subaerial parts | S. tomentosa Siev. ex Ledeb. | [19] |
| Volatile secondary metabolite composition and phenolic content | Roots [20], capitula and aerial parts [21], aerial parts [22,23], | S. Scorzonera undulata ssp. deliciosa (Guiss) Maire., Scorzonera undulata Vahl, Scorzonera sandrasica Hartvig & Strid, Scorzonera calyculata Boiss. | [20,21,22,23] |
| Phenolic content | Flowering parts | S. undulata ssp. deliciosa, S. undulata, S. sandrasica, S. calyculata Boiss. | [24] |
| Chemosystematic studies | Aerial parts [25], subaerial parts [26] | S. cinerea Boiss., S. incisa DC., S. eriophora DC., S. laciniata Jacq., S. parviflora Jacq., S. cana (C.A. Meyer) Hoffm. var. alpina (Boiss.) D.F.Chamb., S. cana (C.A. Meyer) Hoffm. var. jacquiniana (W.Koch) D.F.Chamb. | [25,26] |
| Phenolic components and in vitro antioxidant, antibacterial, anti-inflammatory | Roots and leaves | S. austriaca Balb., S. aristata Ramond ex DC., S. montana var. boetica Boiss. ex DC., S. hispanica L., S. crispatula Boiss., S. trachysperma Günther ex Spreng., S. villosa Scop. | [18] |
| Phenolic compounds | Subaerial parts [19], roots [27], aerial parts and roots [28] | S. hieraciifolia Hayek | [19,27,28] |
| Phytochemical components and antioxidant activity | Aerial parts | S. judaica Eig and S. tomentosa | [29] |
| Chemical composition | Dried roots [20], aerial parts [21,22], whole plant [30] | S. sandrasica, S. undulata, S. undulata ssp. deliciosa, S. hispanica | [20,21,22,30] |
| Antioxidant and antihyperglycemic activity | Leaves | S. cinerea | [31] |
| Chemical components (GC-MS), antioxidant, anticancer and antibacterial activity | Aerial parts | S. calyculata | [23] |
| Anti-antinociceptive action and natural compounds | Leaves and rootstocks | S. latifolia DC., S. mollis ssp. szowitzii Chamb., S. suberosa K.Koch, S. tomentosa, S. aristata | [32] |
| Biologically active natural compounds | Aerial parts | S. divaricata Aucher ex DC., S. pseudodivaricata Lipsch. | [10] |
| Prospective neurobiological effect | Aerial parts and roots | 27 different Scorzonera species including S. pseudolanata Grossh. | [33] |
| Phenolic compounds and certain biological activities | Aerial parts and roots | S. pygmaea Sm. | [34] |
| Antidiabetic effects | Aerial parts | S. tomentosa, S. mollis ssp. szowitzii, S. suberosa, S. eriophora, S. acuminata Boiss., S. sublanata Lipsch., S. cana var. jacquiniana | [35] |
| Pharmacognostic, antibacterial, and laxative investigation | Aerial parts and roots | S. undulata | [36] |
| Phenolic compounds | Aerial parts | S. aristata, S. austriaca, S. montana var. boetica, S. crispatula, S. hispanica, S. trachysperma, and S. villosa | [25] |
| Inulin | Roots and leaves | S. hispanica | [37] |
| Antibacterial and antibiofilm activity | Whole plant, flowers, stems, leaves and roots | S. mackmeliana Boiss. | [38] |
| Antibacterial potential | Whole plant | S. undulata | [39] |
| Wound healing | Aerial parts and roots Aerial parts and roots | S. cinerea, S. latifolia, S. incisa, S. mollis ssp. szowitzii, S. parviflora, S. tomentosa S. acuminata, S. cana var. alpina, S. cana var. jacquiniana, S. cana (C.A Meyer) Hoffm. var. radicosa (Boiss.) Chamberlain, S. eriophora, S. suberosa and S. sublanata | [40,41] |
| Fatty acid compositions, chemical content, and antioxidant activity | Leaves and roots | S. paradoxa Fisch and C.A. Mey | [42] |
| Chemical constituents | Roots | Scorzonera divaricata | [43] |
| Phytochemical profile and biological Activities | Aerial parts and roots | S. sandrasica, S. coriacea A. Duran and Aksoy, and S. ahmet-duranii Makbul and Coskuncelebi | [44] |
| Chemical profiles and pharmacological effects | Aerial parts and roots | S. hieraciifolia, S. hispanica, S. tomentosa | [45] |
| Biological activity | Leaves | S. tomentosa | [46] |
| Phenolics, terpenoids, and potential bioactivities | Aerial parts | S. incisa | [47] |
| Secondary volatile metabolite composition and phenolic content | Root, stem, leaf, and seed | S. acuminata | [48] |
| Root, stem, leaf, and seed | S. papposa DC. | [49] | |
| Root, stem, and leaves | S. mollis M.Bieb. ssp. mollis and S. mollis ssp. szowitzii | [50] | |
| Phenolic content | Root, stem, leaf, and seed | 25 Scorzonera species, including S. pseudolanata | [51] |
| No | RI * | RI in Library ** | Component | Root | Leaf | Seed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area % | Measured RI Value | Similarity % | Area % | Measured RI Value | Similarity % | Area % | Measured RI Value | Similarity % | ||||
| 1 | 801 | 801 | Capronaldehyde | 2.38 | 801 | 96 | 1.73 | 801 | 96 | 0.39 | 801 | 97 |
| 2 | 1003 | 1006 | Caprylaldehyde | - | - | - | - | - | - | 0.17 | 1008 | 94 |
| 3 | 1032 | 1032 | Eucalyptol | - | - | - | 2.00 | 1032 | 95 | - | - | - |
| 4 | 1042 | 1045 | Phenylacetaldehyde | 3.51 | 1043 | 95 | 1.08 | 1043 | 95 | 0.3 | 1043 | 97 |
| 5 | 1107 | 1107 | Pelargonaldehyde | - | - | - | 2.33 | 1105 | 96 | 0.52 | 1105 | 97 |
| 6 | 1167 | 1165 | Isoborneol | 1.94 | 1168 | 94 | - | - | - | - | - | - |
| 7 | 1206 | 1208 | Decanal | 1.90 | 1211 | 89 | - | - | - | - | - | - |
| 8 | 1367 | 1367 | Cyclosativene | 5.81 | 1380 | 89 | - | - | - | - | - | - |
| 9 | 1375 | 1375 | α Copaene | - | - | - | 2,40 | 1376 | 95 | - | - | - |
| 10 | 1389 | 1382 | β Bourbonene | - | - | - | 1.95 | 1385 | 95 | - | - | - |
| 11 | 1400 | 1400 | Tetradecane | 3.05 | 1402 | 89 | 1.29 | 1402 | 97 | 0.32 | 1402 | 98 |
| 12 | 1418 | 1424 | β Caryophyllene | 2.18 | 1456 | 91 | 8.28 | 1425 | 98 | 1.39 | 1425 | 97 |
| 13 | 1454 | 1450 | Geranyl acetone | - | - | - | 2.30 | 1452 | 96 | 0.17 | 1452 | 98 |
| 14 | 1456 | 1459 | Geranyl butyrate | 1.82 | 1462 | 92 | - | - | - | - | - | - |
| 15 | 1458 | 1454 | α Humulene | - | - | - | 1.90 | 1456 | 98 | - | - | - |
| 16 | 1465 | 1447 | Theaspirane | - | - | - | 1.42 | 1449 | 93 | - | - | - |
| 17 | 1485 | 1480 | Germacrene D | 3.34 | 1484 | 92 | 2.63 | 1484 | 92 | - | - | - |
| 18 | 1490 | 1490 | β Ionone | - | - | - | 1.88 | 1490 | 92 | - | - | - |
| 19 | 1493 | 1476 | Lauryl alcohol | - | - | - | 1.87 | 1477 | 96 | 1.88 | 1477 | 96 |
| 20 | 1500 | 1500 | Pentadecane | 3.94 | 1502 | 90 | 1.69 | 1502 | 96 | 0.39 | 1502 | 98 |
| 21 | 1510 | 1516 | Tridecylaldehyde | - | - | - | 2.23 | 1519 | 94 | 0.18 | 1518 | 95 |
| 22 | 1529 | 1518 | δ Cadinene | - | - | - | 1.57 | 1520 | 90 | - | - | - |
| 23 | 1532 | 1529 | Citronellyl butyrate | - | - | - | 2.44 | 1530 | 99 | 0.27 | 1530 | 99 |
| 24 | 1577 | 1602 | Undecalactone | - | - | - | - | - | - | 0.16 | 1603 | 97 |
| 25 | 1589 | 1587 | Caryophyllene oxide | - | - | - | 1.60 | 1588 | 92 | 0.73 | 1588 | 91 |
| 26 | 1600 | 1600 | Hexadecane | 13.06 | 1602 | 95 | 9.76 | 1602 | 98 | 152 | 1602 | 95 |
| 27 | 1615 | 1614 | Tetradecanal | - | - | - | 1.82 | 1615 | 97 | 0.5 | 1615 | 93 |
| 28 | 1620 | 1708 | Dodecalactone | 2.76 | 1709 | 9 | - | - | - | 0.3 | 1709 | 98 |
| 29 | 1657 | 1653 | Dihydrojasmonate | - | - | - | 0.97 | 1658 | 95 | 0.27 | 1658 | 91 |
| 30 | 1666 | 1727 | Myristate | - | - | - | - | - | - | 0.16 | 1729 | 97 |
| 31 | 1671 | 1676 | α Santalol | - | - | - | 2.44 | 1678 | 95 | 0.16 | 1678 | 92 |
| 32 | 1687 | 1683 | Apiole | 3.78 | 1687 | 87 | 2.77 | 1687 | 94 | 0.25 | 1687 | |
| 33 | 1695 | 1680 | Myristic alcohol | 2.79 | 1682 | 96 | 1.48 | 1682 | 95 | 0.18 | 1682 | 95 |
| 34 | 1700 | 1700 | Heptadecane | 6.55 | 1702 | 97 | 5.37 | 1702 | 96 | 1.83 | 1702 | 98 |
| 35 | 1784 | 1784 | Pentadecanol | 6.73 | 1786 | 91 | - | - | - | - | - | - |
| 36 | 1792 | 1796 | Linalyl benzoate | 7.26 | 1798 | 97 | - | - | - | - | - | - |
| 37 | 1800 | 1800 | Octadecane | 8.11 | 1802 | 97 | 2.66 | 1802 | 98 | 1.46 | 1802 | 99 |
| 38 | 1841 | 1841 | Phytone | - | - | - | 16.36 | 1848 | 99 | 1.57 | 1848 | 97 |
| 39 | 1901 | 1900 | Nonadecane | 3.65 | 1903 | 92 | 1.69 | 1903 | 90 | 56.45 | 1903 | 98 |
| 40 | 1922 | 1977 | Hexadecenoic acid | - | - | - | 1.02 | 1978 | 94 | - | - | - |
| 41 | 1925 | 1925 | methyl Palmitate | 6.75 | 1929 | 93 | 2.47 | 1929 | 97 | 1.75 | 1929 | 96 |
| 42 | 1972 | 1968 | Geranyl benzoate | 4.63 | 1968 | 94 | - | - | - | - | - | - |
| 43 | 2001 | 2000 | Eicosane | - | - | - | 1.49 | 2002 | 95 | 2.19 | 2002 | 98 |
| 44 | 2020 | 2016 | Civetone | 4.06 | 2020 | 95 | - | - | - | - | - | - |
| 45 | 2100 | 2100 | Heneicosane | - | - | - | 3.1 | 2103 | 95 | 23.82 | 2103 | 98 |
| 46 | 2115 | 2106 | Phytol | - | - | - | 1.29 | 1402 | 97 | 0.32 | 1402 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özcan Aykutlu, A.; Makbul, S.; Coşkunçelebi, K.; Seyis, F. Secondary Volatile Metabolite Composition in Scorzonera pseudolanata Grossh. Plant Parts. Plants 2025, 14, 1624. https://doi.org/10.3390/plants14111624
Özcan Aykutlu A, Makbul S, Coşkunçelebi K, Seyis F. Secondary Volatile Metabolite Composition in Scorzonera pseudolanata Grossh. Plant Parts. Plants. 2025; 14(11):1624. https://doi.org/10.3390/plants14111624
Chicago/Turabian StyleÖzcan Aykutlu, Aysel, Serdar Makbul, Kamil Coşkunçelebi, and Fatih Seyis. 2025. "Secondary Volatile Metabolite Composition in Scorzonera pseudolanata Grossh. Plant Parts" Plants 14, no. 11: 1624. https://doi.org/10.3390/plants14111624
APA StyleÖzcan Aykutlu, A., Makbul, S., Coşkunçelebi, K., & Seyis, F. (2025). Secondary Volatile Metabolite Composition in Scorzonera pseudolanata Grossh. Plant Parts. Plants, 14(11), 1624. https://doi.org/10.3390/plants14111624








