Abstract
Fucoxanthin is a bioactive compound that is a kind of natural carotenoid. Fucoxanthin is known to protect against UV-B-induced cell damage in hairless mice, even though it is physiochemically unstable to heat and acid due to its polyunsaturated structure, indicating that fucoxanthin possesses a low bioavailability, and this disadvantage limits its application in the cosmetic industry. Solid lipid nanoparticle (SLN) systems are known to be suitable as carriers for sunscreen agents. In this research work, the sunscreen-boosting effect of SLN, as a deliverer of functional ingredient, especially fucoxanthin, has been developed and evaluated by comparing the sunburn protection factors (SPF) of macroemulsion (cream and lotion type) and an SLN formula containing various kinds of sunscreen agents, respectively. Several results such as stability test, particle size, DSC analysis, and X-ray analysis show that the SLN formula loading fucoxanthin has the possibility of being a stable and high-functioning ingredient delivery system. Moreover, the SLN formula has shown a higher SPF value than others, meaning that the SLN formula exhibits a good sunscreen-boosting effect. This study indicates that the use of SLN as a carrier enhanced the bioavailability of fucoxanthin and shows that SLN could be a promising carrier for the production of sunscreen products by allowing the scaling-up of production.
1. Introduction
The skin is the primary defensive barrier of our body that prevents the invasion of external environmental pollutants, including UV radiation and environmental chemicals [1]. Oxidative stress is the primary cause of extrinsic aging or photoaging, caused mainly by UV radiation. The principal effects of UV radiation in the skin are DNA damage, oxidative stress, deleterious impact on the extracellular matrix, inflammation, and immunosuppression [2]. Therefore, to prevent UV-induced damage, the treatment of the skin with products containing functional antioxidant ingredients may be one of the useful strategies. Furthermore, the use of formulations containing both sunscreen chemicals and naturally occurring antioxidants may be the most instructive for more effective protection of skin photodamage [3].
Fucoxanthin is a bioactive compound contained in some seaweeds, for instance, tangle and brown algae. It is a kind of natural carotenoid that has a dark yellowish or reddish color. Fucoxanthin is known for several functions that are beneficial to one’s health, such as having anti-obesity, anti-diabetic, anti-cancer, anti-oxidation (anti-aging), and anti-angiogenic effects, among others [4]. Moreover, fucoxanthin has also been successfully used topically to prevent against UV-B-induced cell damage in hairless mice and in human fibroblast cell lines as an antioxidant against skin aging caused by free radical damage [5,6].
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are dispersion systems that have 1~1000 nm particle size with SLN or NLC at room temperature. SLN can be a successful drug delivery agent for several reasons: (1) SLN has a high drug loading capacity and can increase the possibility of drug targeting so that we have better control over release kinetics. (2) toxicity is so acute and chronic SLN has outstanding physical and chemical storage stability [7,8,9]. NLC is also used for the storage of bioactive compounds due to possessing a high loading capacity and a lower water content of the particle suspension [10].
The aim of this study was to assess the scale-up feasibility for the production of sunscreen products, together with the enhancement of bioactive bioavailability and stability using lipid nanoparticles. Therefore, the physical stability of the SLN and NLC dispersion as a pre-requisite for sunscreen formulations, together with the sunscreen-boosting effects of the SLN and NLC, loaded with or without fucoxanthin, were investigated.
2. Materials and Methods
2.1. SLN and NLC Manufacturing Process
The manufacturing processes of both SLN and NLC are shown in Figure 1. Each oil and water phase was mixed and melted well at elevated temperatures. Coarse pre-emulsion preparation by homogenization of two phases with a high-speed homogenizer was performed using a Robomics (Tokushu Kika Kogyo, Osaka, Japan) at 75 °C. Then, SLN and NLC were homogenized with a high-pressure homogenizer (HPH) and microfluidizer (Microfluidics, Westwood, MA, USA).
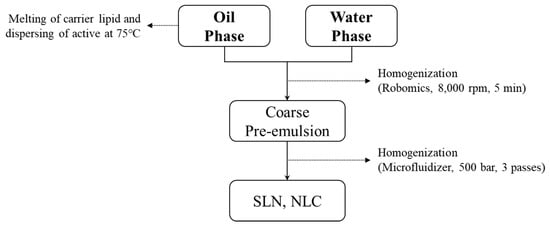
Figure 1.
The solid lipid nanoparticle (SLN) and nanostructured lipid carrier (NLC) manufacturing processes.
2.2. Formulation of Fucoxanthin-Loaded SLN and NLC
The SLN and NLC samples were prepared according to the formulation shown in Table 1. A total of four samples were prepared, two of which were in the form of SLN including controls that did not contain fucoxanthin, and the rest in the form of NLC, respectively. The solvent extract of 95% fucoxanthin powder was purchased from Xi’an Geekee Biotech Co., Ltd. (Shanxi, China).

Table 1.
Formulation of SLN and NLC with and without fucoxanthin (SLN1: SLN without fucoxanthin, SLN2: SLN with fucoxanthin, NLC1: NLC without fucoxanthin, NLC2: NLC with fucoxanthin).
2.3. Analysis of Particle Size and Stability
The particle sizes of the SLN and NLC samples were analyzed by photon correlation spectroscopy (PCS) using a Mastersizer 2000 (Malvern Panalytical, Malvern, UK), respectively. PCS shows the data for the mean diameter of the bulk population and for the width of the distribution via the polydispersity index (PI). The measuring range was 3 nm to approximately 3 μm. PI values were the averages of results obtained for 3 replicates.
For the analysis of stability, the SLN and NLC samples were incubated for 4 weeks at room temperature and 40 °C, respectively. The stability of samples was measured by Turbiscan (Formulation, France).
2.4. Differential Scanning Calorimetry (DSC) Analysis
For estimating the heat capacity of SLN and NLC samples, DSC analysis was conducted by a differential scanning calorimeter, model 4207 (Hart Scientific, American Fork, Utah, USA). The calorimetric system was calibrated, in temperature and enthalpy changes, following the procedure of the instrument. In all the experiments, the reference pan was filled with the same buffer present in the sample under investigation.
2.5. X-Ray Diffraction Analysis
To ensure that the SLN and NLC containing fucoxanthin were well prepared, X-ray diffraction patterns were obtained using a D/MAX-2200V diffractometer (Rigaku, Tokyo, Japan) with a copper anode (Cu Kα radiation, = 0.15418 nm) equipped with a sample spinner operating at a current of 30 mA and a voltage of 40 kV. The measurements were performed at room temperature, scanning at 2θ from 1° to 100°, with a 0.05° step size and a 1 s step time.
2.6. Sunburn Protection Factors (SPF) Analysis
In order to evaluate the sunburn protection efficacy and the physical stability of the SLN samples, including 3 sunscreen chemical agents with or without fucoxanthin, SLN, lotion, and cream samples were prepared as shown in Tables 3 and 4. The sunburn protection factors (SPF) of the samples were measured by using an SPF-290s analyzer (Optometrics, Littleton, MA, USA).
3. Results and Discussion
3.1. Size Analysis of SLN and NLC and Stability Test
Two important standards, namely particle size and size distribution of the nanoparticles, affect the drug release rate, bio-distribution, mucoadhesion, cellular uptake of water and buffer exchange to the interior of the nanoparticles, and protein diffusion [11]. Moreover, the particle size and size distribution are important for the indices to evaluate a colloidal dosage form upon storage. Due to the unsaturated structure, fucoxanthin is sensitive to heat, light, and oxidative degradation during the processing and storage stages. Therefore, in this study, to confirm the storage stability of SLN and NLC, four kinds of samples were made with or without fucoxanthin according to the formulation shown in Table 1. As shown in Table 2, fucoxanthin-loaded SLN (SLN2) and NLC (NLC2) were smaller than the control samples (SLN1 and NLC1). The particle sizes and polydispersity index of SLN and NLC did not change dramatically during storage for 4 weeks both at room temperature and 40 °C, indicating that the SLN and NLC particles had good physical stability.

Table 2.
Particle sizes of SLN and NLC.
The physical stability of the vesicles and nanoparticle dispersion of the SLN and NLC samples were assessed using the optical analyzer Turbiscan; the backscattering change was measured after 4 weeks of storage. As shown in Figure 2, on the upper side of the figure, the backscattering intensity (%) of both SLN1 and SLN2 kept at room temperature for 28 days did not differ in the range 36% and 37%, respectively. However, the backscattering intensity (%) of both SLN1 and SLN2 kept at 40 °C for 28 days was found to have changed to around 40%, respectively. On the other hand, in the case of NLC samples, the backscattering intensity (%) had increased at both RT and 40 °C, respectively, meaning that the stability of the SLN samples was much better than that of NLC samples in room temperature conditions.
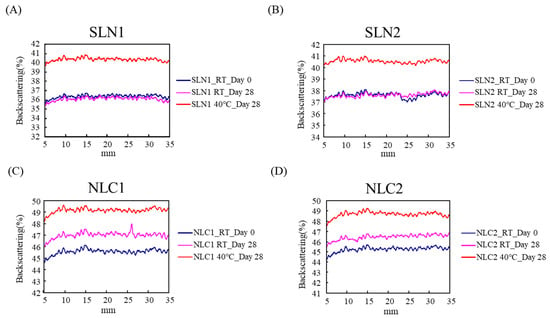
Figure 2.
Stability of SLN and NLC with and without fucoxanthin. The backscattering intensity (%) of SLN (A,B) and NLC (C,D) samples were assessed using the optical analyzer Turbiscan.
3.2. DSC and X-Ray Diffraction Analysis
Differential scanning calorimetry (DSC) was used to characterize drug delivery systems and to investigate their interactions with bio-membranes. In this work, we compared SLN and NLC interactions, loaded with or without fucoxanthin, and assessed by DSC. As shown in Figure 3, the SNL samples presented the peak of heat capacity change, and although there were some differences between SLN1 and SLN2, peak range and shape were approximately the same. On the other hand, there was no peak in the NLC samples. This result indicates that the structure of the SLN sample formed successfully and that there were no crystal formations in NLC samples. In addition, the peak of the heat capacity change had not disappeared after the long-term storage of the SLN samples, shown in Figure 4, meaning that the structure of the SLN samples was maintained so that the SLN nanoparticle system was expected to be applicable to the active preservation of cosmetic functional materials.
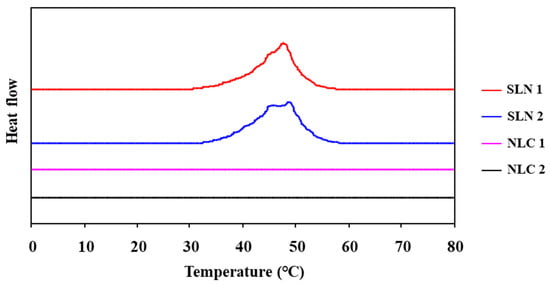
Figure 3.
DSC heating curves of SLN and NLC dispersions.
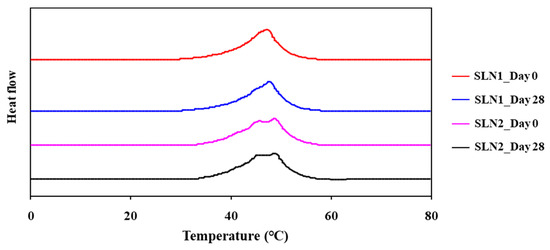
Figure 4.
DSC heating curves of SLN dispersion for long-term storage.
X-ray diffraction (XRD) was used to study the crystalline structure of SLN and NLC loaded with or without fucoxanthin. The diffractograms of samples are displayed in Figure 5. The diffraction curve of SLN samples shows differences in comparing with the NLC samples. Compared with the SNL samples, the peak intensities of NLC samples were much weaker, which indicates these differences were derived from the presence of a mixture of polymorphs in the nanoparticles. It can also be seen that the diffractograms of SLN2 after the addition of fucoxanthin had minor changes. The low intensity of peaks made it difficult to distinguish events related to each polymorph, compared to the available data in the literature. Thus, the results seem to indicate that SLN samples were crystallized and well-formed, and that fucoxanthin did not affect the stability of the crystal structure of SLN, as observed previously by the DSC analysis.
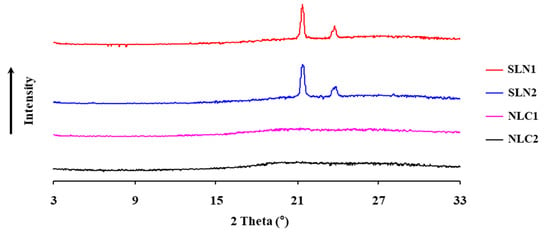
Figure 5.
X-ray diffraction patterns of SLN and NLC with and without fucoxanthin. Fucoxanthin was loaded to form SLN2 and NLC2, respectively. SLN1 and NLC1 were formed without fucoxanthin, respectively.
3.3. SPF Analysis
Recently, Netto and Jose (2018) reported that sunscreen containing silymarin solid nanoparticles exhibited excellent photoprotective action [12]. Therefore, in order to confirm the sunscreen-boosting effect of the SNL samples, the anti-UV radiation activity was assessed by the determination of the SPF value. As shown in Table 3, the SLN samples, including general chemical agents of sunscreen products, were prepared with or without fucoxanthin, respectively. In addition, the samples made in the form of a common sunscreen product for comparison with SLN samples, and cream and lotion samples, were also prepared, including general chemical agents of sunscreen products with or without fucoxanthin, as shown in Table 4, respectively. As can be seen in Figure 6, the SPF value of the SLN-T sample was higher than the others, indicating that the SLN containing 2% fucoxanthin had higher SPF values and was significantly higher (1.85-fold) than the SLN-F sample. These data indicate that fucoxanthin is a potential UV-blocking agent for cosmeceutical products. Moreover, these findings indicate that the SLN formulation of fucoxanthin provides a more efficient UV-blocking property than the cream and lotion types, meaning that the SLN formula exhibits a good sunscreen-boosting effect, and the use of SLN as a carrier enhanced the bioavailability of fucoxanthin and showed that SLN could be a promising carrier for the production of sunscreen products by allowing the scaling-up of production.

Table 3.
A formulation of SLN samples, including general sunscreen agents for comparing SPF (SLN-T: samples with ethylhexyl methoxycinnamate, bis-ethylhexyphenol methoxyphenol triazine, ethylhexyl salicylate, and fucoxanthin; SLN-F: samples with ethylhexyl methoxycinnamate, bis-ethylhexyphenol methoxyphenol triazine, and ethylhexyl salicylate).

Table 4.
Formulation of lotion and cream samples for SPF analysis with SLN samples. Three sunscreen chemicals (ethylhexyl methoxycinnamate, bis-ethylhexyphenol methoxyphenol triazine, and ethylhexyl salicylate) are included with or without fucoxanthin, respectively.
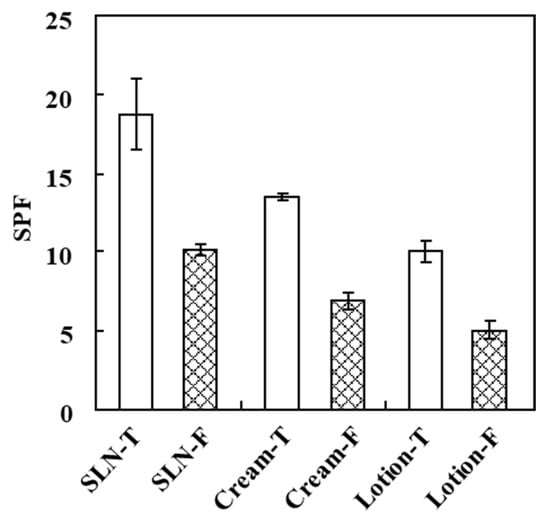
Figure 6.
The SPF analysis of SLM, cream, and lotion. The left bar shows the SPF value of each formula, including the three types of sunscreen chemicals, ethylhexyl methoxycinnamate, bis-ethylhexyphenol methoxyphenol triazine, and ethylhexyl salicylate with fucoxanthin. The right bar shows the SPF value of each formula including only the three types of sunscreen chemicals.
4. Conclusions
SLN and NLC were used to increase the water content of the skin [13] and showed an effective UV-blocking potential [14]. Because of their particulate character and adhesive properties, SLNs are known to be highly suitable as carriers for sunscreen agents [14]. In this study, the SLN formula that was loaded with fucoxanthin was successfully employed to evaluate the sunscreen-boosting effect. The experimental results indicate that fucoxanthin-loaded SLN presented a nanometer size, structural stability, and a synergistic effect on the protective activity of the particulate carrier and molecular sunscreen. Moreover, the SLN formulation that was loaded with fucoxanthin seems to be a good application process for the production of sunscreen products via the scale-up process. Although additional experiments are required for the determination of the proper concentration of fucoxanthin and combinations of sunscreen chemical agents for the optimization of sunscreen-boosting effect in the SLN formulation, this study can offer the possibility of creating more effective and safer sunscreen formulations with reduced sunscreen agent.
Author Contributions
Y.-J.L. and G.-W.N. designed and performed the experiments; G.-W.N. analyzed the data and interpreted data; Y.-J.L. wrote the paper and G.W.N. corrected the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Kee-Yeol Kyoung for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Derm. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, A.; Lorrio, S.; Gonzalez, S.; Juarranz, A. Fernblock Prevents Dermal Cell Damage Induced by Visible and Infrared A Radiation. Int. J. Mol. Sci. 2018, 19, 2250. [Google Scholar] [CrossRef]
- Tomaino, A.; Cristani, M.; Cimino, F.; Speciale, A.; Trombetta, D.; Bonina, F.; Saija, A. In vitro protective effect of a Jacquez grapes wine extract on UVB-induced skin damage. Toxicol In Vitr. 2006, 20, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 196–199. [Google Scholar] [PubMed]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of Fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci Biotechnol Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Jenning, V.; Thunemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Anselmi, C.; Centini, M.; Muller, R.H. Preparation and characterization of n-dodecyl-ferulate-loaded solid lipid nanoparticles (SLN). Int. J. Pharm 2005, 295, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Netto, M.G.; Jose, J. Development, characterization, and evaluation of sunscreen cream containing solid lipid nanoparticles of silymarin. J. Cosmet Derm. 2018, 17, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Leach, W.T.; Simpson, D.T.; Val, T.N.; Yu, Z.; Lim, K.T.; Park, E.J.; Williams, R.O., 3rd; Johnston, K.P. Encapsulation of protein nanoparticles into uniform-sized microspheres formed in a spinning oil film. AAPS PharmSciTech 2005, 6, E605-17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jose, J.; Netto, G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J. Cosmet Derm. 2019, 18, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.; Lippacher, A.; Muller, R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet Sci. 2001, 52, 313–324. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).