The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics
Abstract
1. Introduction
2. Parabens and Their Associated Biological Risks
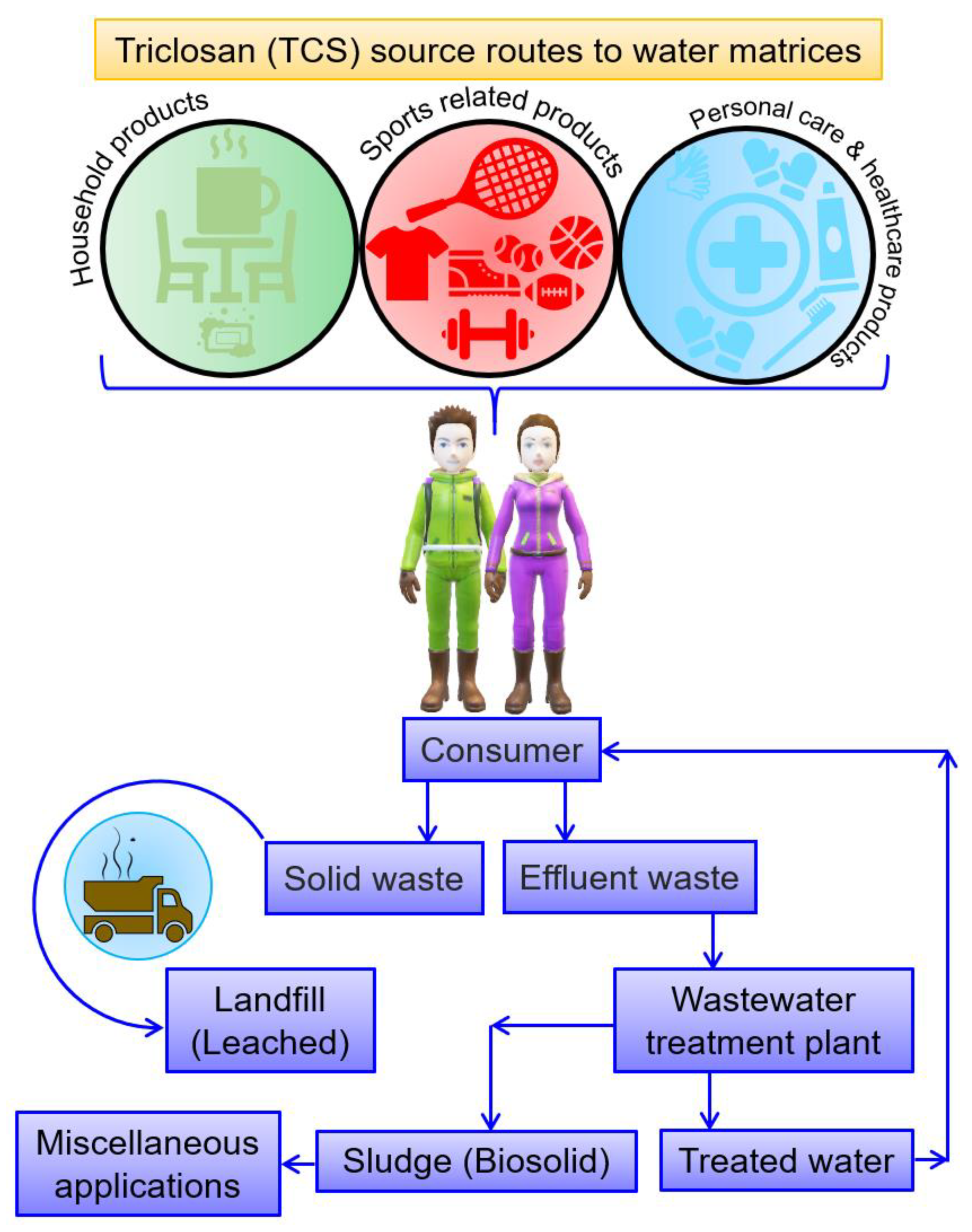
3. Triclosan Persistence and Ecological Impacts
4. Benzalkonium Chloride and its Cytotoxic Effects
5. 1,4-Dioxane, Uses and Adverse Impacts
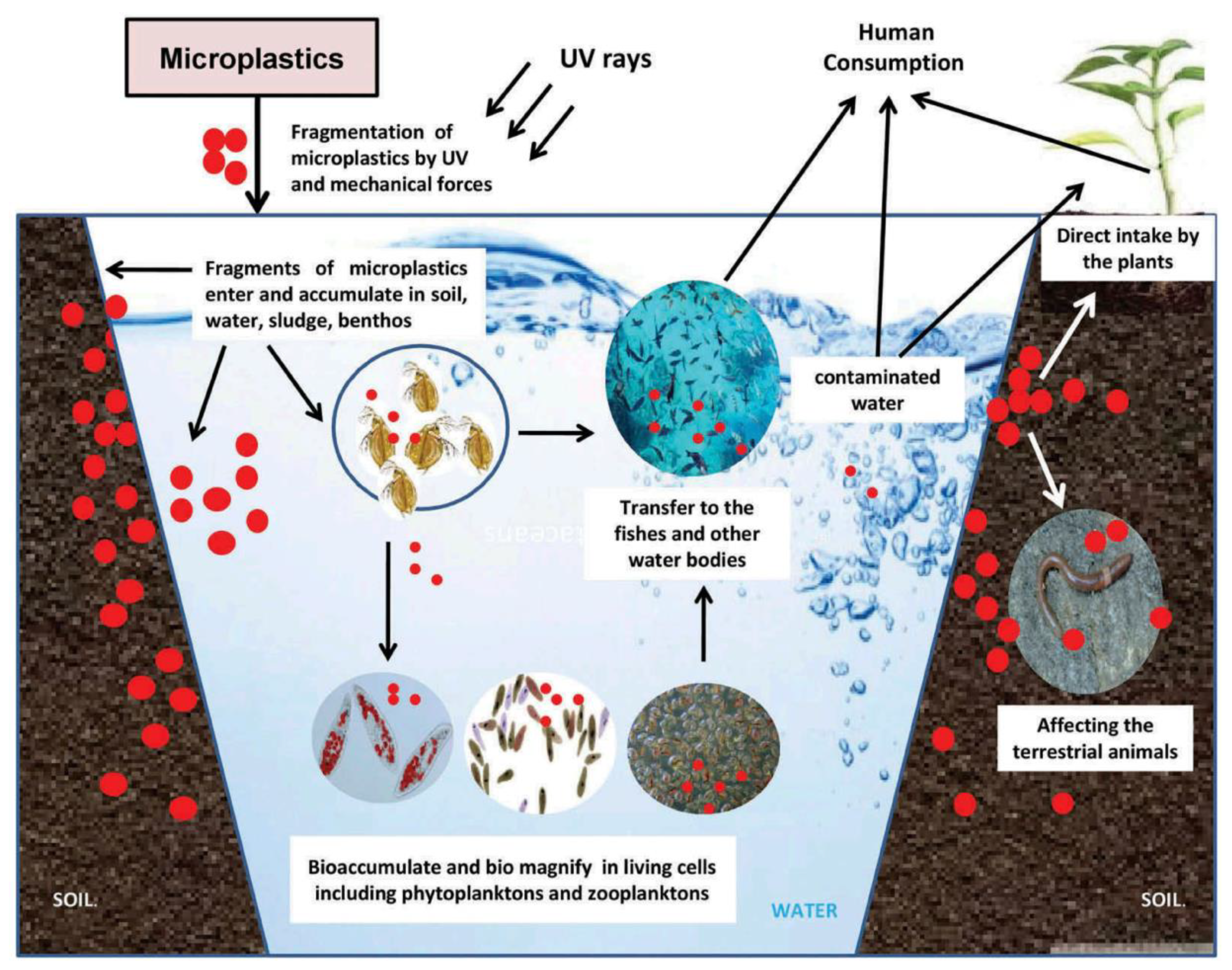
6. Microplastic Particles
7. Formaldehyde, Transmission Routes and Health-Related Effects
8. Imidazolidinyl Urea and Diazolidinyl Urea—Adverse Effects
9. Organic and Inorganic UV-Filters/Absorbers
10. Trace Metals and Their Adverse Effects
11. Synthetic Musk Compounds
12. Siloxanes and Silicones
13. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Market, V. Global Opportunity Analysis and Industry Forecast, 2017–2023; Allied Market Research: Pune, India, 2016. [Google Scholar]
- Chen, X.; Sullivan, D.A.; Sullivan, A.G.; Kam, W.R.; Liu, Y. Toxicity of cosmetic preservatives on human ocular surface and adnexal cells. Exp. Eye Res. 2018, 170, 188–197. [Google Scholar] [CrossRef]
- Karr, S.; Houtman, A.; Interlandi, J. Toxic bottles? On the trail of chemicals in our everyday lives. In American Environmental Science for a Changing World; Kate Ahr, P., Ed.; W. H. Freeman: New York, NY, USA, 2013; p. 54. [Google Scholar]
- Gao, P.; Lei, T.; Jia, L.; Yury, B.; Zhang, Z.; Du, Y.; Feng, Y.; Xing, B. Bioaccessible trace metals in lip cosmetics and their health risks to female consumers. Environ. Pollut. 2018, 238, 554–561. [Google Scholar] [CrossRef]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shi, Y.; Li, W.; Xu, L.; Cai, Y. Concentrations and distribution of synthetic musks and siloxanes in sewage sludge of wastewater treatment plants in China. Sci. Total Environ. 2014, 476, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; García-Galán, M.J.; Guerra, P.; Jelic, A.; Postigo, C.; Eljarrat, E.; Farré, M.; De Alda, M.L.; Petrovic, M.; Barceló, D. Analysis of selected emerging contaminants in sewage sludge. TrAC Trends Anal. Chem. 2009, 28, 1263–1275. [Google Scholar] [CrossRef]
- Ternes, T.A.; Joss, A.; Siegrist, H. Peer Reviewed: Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment; ACS Publications: Washington, DC, USA, 2004. [Google Scholar]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.-F. Occurrence, fate and behavior of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Evaluation of the occurrence and biodegradation of parabens and halogenated by-products in wastewater by accurate-mass liquid chromatography-quadrupole-time-of-flight-mass spectrometry (LC-QTOF-MS). Water Res. 2011, 45, 6770–6780. [Google Scholar] [CrossRef]
- Canosa, P.; Rodríguez, I.; Rubi, E.; Negreira, N.; Cela, R. Formation of halogenated by-products of parabens in chlorinated water. Anal. Chim. Acta 2006, 575, 106–113. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tamura, I.; Hirata, Y.; Kato, J.; Kagota, K.; Katsuki, S.; Yamamoto, A.; Kagami, Y.; Tatarazako, N. Aquatic toxicity and ecological risk assessment of seven parabens: individual and additive approach. Sci. Total Environ. 2011, 410, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Kannan, K. Accumulation profiles of parabens and their metabolites in fish, black bear, and birds, including bald eagles and albatrosses. Environ. Int. 2016, 94, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.; Metaxas, G.; Harbach, C.; Savoy, L.; Darbre, P. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 2012, 32, 219–232. [Google Scholar] [CrossRef]
- Guo, J.; Wu, C.; Lu, D.; Jiang, S.; Liang, W.; Chang, X.; Xu, H.; Wang, G.; Zhou, Z. Urinary paraben concentrations and their associations with anthropometric measures of children aged 3 years. Environ. Pollut. 2017, 222, 307–314. [Google Scholar] [CrossRef]
- Wang, L.; Asimakopoulos, A.G.; Kannan, K. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ. Int. 2015, 78, 45–50. [Google Scholar] [CrossRef]
- Ramaswamy, B.R.; Kim, J.-W.; Isobe, T.; Chang, K.-H.; Amano, A.; Miller, T.W.; Siringan, F.P.; Tanabe, S. Determination of preservative and antimicrobial compounds in fish from Manila Bay, Philippines using ultra high performance liquid chromatography tandem mass spectrometry, and assessment of human dietary exposure. J. Hazard. Mater 2011, 192, 1739–1745. [Google Scholar] [CrossRef]
- Darbre, P.D. Environmental oestrogens, cosmetics and breast cancer. Best Prac. Res. Clin. Endocrinol. Metab. 2006, 20, 121–143. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef]
- Routledge, E.J.; Parker, J.; Odum, J.; Ashby, J.; Sumpter, J.P. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharmacol. 1998, 153, 12–19. [Google Scholar] [CrossRef]
- Witorsch, R.J.; Thomas, J.A. Personal care products and endocrine disruption: A critical review of the literature. Crit. Rev. Toxicol. 2010, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Borgert, C.J.; Dietrich, D.; Rozman, K.K. Endocrine disruption: Fact or urban legend? Toxicol. Lett. 2013, 223, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yuan, C.; Tagmount, A.; Rudel, R.A.; Ackerman, J.M.; Yaswen, P.; Vulpe, C.D.; Leitman, D.C. Parabens and human epidermal growth factor receptor ligand cross-talk in breast cancer cells. Environ. Health Perspect. 2016, 124, 563–569. [Google Scholar] [CrossRef]
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect. 2011, 119, 252–257. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.; Yoo, Y.-M.; Choi, K.-C.; Jeung, E.-B. Potential estrogenic effect (s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod. Toxicol. 2010, 29, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Karpuzoglu, E.; Holladay, S.D.; Gogal, R.M., Jr. Parabens: Potential impact of low-affinity estrogen receptor binding chemicals on human health. J. Toxicol. Environ. Health Part B 2013, 16, 321–335. [Google Scholar] [CrossRef]
- Bayülken, D.G.; Bostancıoğlu, R.B.; Koparal, A.T.; Tüylü, B.A.; Dağ, A.; Benkli, K. Assessment of in vitro cytotoxic and genotoxic activities of some trimethoprim conjugates. Cytotechnology 2018, 70, 1051–1059. [Google Scholar] [CrossRef]
- Darbre, P.D.; Harvey, P.W. Parabens can enable hallmarks and characteristics of cancer in human breast epithelial cells: A review of the literature with reference to new exposure data and regulatory status. J. Appl. Toxicol. 2014, 34, 925–938. [Google Scholar] [CrossRef]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, S.; Qiao, P.; Dong, L.; Yu, M.; Wang, C.; Zhang, M.; Zhang, L.; Li, Y.; Tang, N. n-butylparaben induces male reproductive disorders via regulation of estradiol and estrogen receptors. J. Appl. Toxicol. 2016, 36, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Kralj, M.B.; Fortuna, A.; Abram, A.; Trebše, P. Dish handwashing: an overlooked source of contamination. Environ. Chem. Lett. 2020, 18, 181–185. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Ying, G.-G.; Liu, Y.-S.; Zhang, Q.-Q.; Zhao, J.-L.; Liu, S.-S.; Chen, J.; Peng, F.-J.; Lai, H.-J.; Pan, C.-G. Triclosan as a surrogate for household biocides: An investigation into biocides in aquatic environments of a highly urbanized region. Water Res. 2014, 58, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yueh, M.-F.; Tukey, R.H. Triclosan: A widespread environmental toxicant with many biological effects. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 251–272. [Google Scholar] [CrossRef]
- Olaniyan, L.; Mkwetshana, N.; Okoh, A. Triclosan in water, implications for human and environmental health. Springerplus 2016, 5, 1639. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Kim, J.-W.; Jang, H.-S.; Kim, J.-G.; Ishibashi, H.; Hirano, M.; Nasu, K.; Ichikawa, N.; Takao, Y.; Shinohara, R.; Arizono, K. Occurrence of pharmaceutical and personal care products (PPCPs) in surface water from Mankyung River, South Korea. J. Health Sci. 2009, 55, 249–258. [Google Scholar] [CrossRef]
- Dougherty, J.A.; Swarzenski, P.W.; Dinicola, R.S.; Reinhard, M. Occurrence of herbicides and pharmaceutical and personal care products in surface water and groundwater around Liberty Bay, Puget Sound, Washington. J. Environ. Qual. 2010, 39, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 19, 1044–1065. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kameda, Y.; Yamamoto, H.; Nakada, N.; Tamura, I.; Miyazaki, M.; Masunaga, S. Occurrence of preservatives and antimicrobials in Japanese rivers. Chemosphere 2014, 107, 393–399. [Google Scholar] [CrossRef]
- Chalew, T.E.; Halden, R.U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban 1. JAWRA J. Am. Water Resour. Assoc. 2009, 45, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.A.; Edziyie, R.E.; La Point, T.W.; Venables, B.J. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 2007, 67, 1911–1918. [Google Scholar] [CrossRef]
- Kookana, R.S.; Shareef, A.; Fernandes, M.B.; Hoare, S.; Gaylard, S.; Kumar, A. Bioconcentration of triclosan and methyl-triclosan in marine mussels (Mytilus galloprovincialis) under laboratory conditions and in metropolitan waters of Gulf St Vincent, South Australia. Mar. Pollut. Bull. 2013, 74, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Houtman, C.J.; van Oostveen, A.M.; Brouwer, A.; Lamoree, M.H.; Legler, J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ. Sci. Technol. 2004, 38, 6415–6423. [Google Scholar] [CrossRef]
- Coogan, M.A.; Point, T.W.L. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA, stream affected by wastewater treatment plant runoff. Environ. Toxicol. Chem. Int. J. 2008, 27, 1788–1793. [Google Scholar] [CrossRef]
- Balmer, M.E.; Poiger, T.; Droz, C.; Romanin, K.; Bergqvist, P.-A.; Müller, M.D.; Buser, H.-R. Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ. Sci. Technol. 2004, 38, 390–395. [Google Scholar] [CrossRef]
- Crofton, K.M.; Paul, K.B.; DeVito, M.J.; Hedge, J.M. Short-term in vivo exposure to the water contaminant triclosan: Evidence for disruption of thyroxine. Environ. Toxicol. Pharmacol. 2007, 24, 194–197. [Google Scholar] [CrossRef]
- Yang, L.H.; Ying, G.G.; Su, H.C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga pseudokirchneriella subcapitata. Environ. Toxicol. Chem. Int. J. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Drury, B.; Scott, J.; Rosi-Marshall, E.J.; Kelly, J.J. Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ. Sci. Technol. 2013, 47, 8923–8930. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Domingues, I.; Grisolia, C.K.; Soares, A.M. Effects of triclosan on zebrafish early-life stages and adults. Environ. Sci. Pollut. Res. 2009, 16, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.R.; Reemtsma, H.; Grimm, D.A.; Mitra, S. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci. Total Environ. 2003, 311, 135–149. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef]
- Unit, D.R.; Robertshaw, H. Contact dermatitis to triclosan in toothpaste. Contact Dermat. 2007, 57, 383–384. [Google Scholar]
- Miller, W.R. Motivational interviewing with problem drinkers. Behav. Cognit. Psychother. 1983, 11, 147–172. [Google Scholar] [CrossRef]
- Kumar, V.; Chakraborty, A.; Kural, M.R.; Roy, P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol. 2009, 27, 177–185. [Google Scholar] [CrossRef]
- Yueh, M.-F.; Taniguchi, K.; Chen, S.; Evans, R.M.; Hammock, B.D.; Karin, M.; Tukey, R.H. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc. Natl. Acad. Sci. USA 2014, 111, 17200–17205. [Google Scholar] [CrossRef]
- Tamura, I.; Kanbara, Y.; Saito, M.; Horimoto, K.; Satoh, M.; Yamamoto, H.; Oyama, Y. Triclosan, an antibacterial agent, increases intracellular Zn2+ concentration in rat thymocytes: Its relation to oxidative stress. Chemosphere 2012, 86, 70–75. [Google Scholar] [CrossRef]
- Adolfsson-Erici, M.; Pettersson, M.; Parkkonen, J.; Sturve, J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 46, 1485–1489. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- Siegert, W. Approved Preservatives for Cosmetics: A Review of Actives Listed in Regulation (EC) No 1223/2009 on Cosmetic Products-Annex V; Epubli: Berlin, Germany, 2014. [Google Scholar]
- Datta, S.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A.; Cortopassi, G.A. The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.-J.; Debbasch, C.; Hamard, P.; Creuzot-Garcher, C.; Rat, P.; Brignole, F.; Baudouin, C. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: An ex vivo and in vitro study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Tressler, C.S.; Beatty, R.; Lemp, M.A. Preservative use in topical glaucoma medications. Ocul. Surf. 2011, 9, 140–158. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Sarkar, J.; Chaudhary, S.; Namavari, A.; Ozturk, O.; Chang, J.-H.; Yco, L.; Sonawane, S.; Khanolkar, V.; Hallak, J.; Jain, S. Corneal neurotoxicity due to topical benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1792–1802. [Google Scholar] [CrossRef]
- Choi, S.M.; Roh, T.H.; Lim, D.S.; Kacew, S.; Kim, H.S.; Lee, B.-M. Risk assessment of benzalkonium chloride in cosmetic products. J. Toxicol. Environ. Health Part B 2018, 21, 8–23. [Google Scholar] [CrossRef]
- Antunes, S.; Nunes, B.; Rodrigues, S.; Nunes, R.; Fernandes, J.; Correia, A. Effects of chronic exposure to benzalkonium chloride in Oncorhynchus mykiss: Cholinergic neurotoxicity, oxidative stress, peroxidative damage and genotoxicity. Environ. Toxicol. Pharmacol. 2016, 45, 115–122. [Google Scholar] [CrossRef]
- Ryu, O.; Park, B.K.; Bang, M.; Cho, K.S.; Lee, S.H.; Gonzales, E.L.T.; Yang, S.M.; Kim, S.; Eun, P.H.; Lee, J.Y. Effects of several cosmetic preservatives on ROS-dependent apoptosis of rat neural progenitor cells. Biomol. Ther. 2018, 26, 608. [Google Scholar] [CrossRef]
- Johnson, N.F. Pulmonary toxicity of benzalkonium chloride. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, D.; Yoo, J.; Kwon, S. Effects of benzalkonium chloride on cell viability, inflammatory response, and oxidative stress of human alveolar epithelial cells cultured in a dynamic culture condition. Toxicol. Vitro 2019, 59, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-Y.; Wu, M.-C.; Lan, M.-Y.; Tan, C.-T.; Yang, A.-H. In vitro effects of preservatives in nasal sprays on human nasal epithelial cells. Am. J. Rhinol. 2008, 22, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Juhász, M.L.W.; Marmur, E.S. A review of selected chemical additives in cosmetic products. Dermatol. Ther. 2014, 27, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Occurrence and treatment of 1,4-dioxane in aqueous environments. Environ. Eng. Sci. 2003, 20, 423–432. [Google Scholar] [CrossRef]
- Godri, K.P.; Kim, J.-H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C. 1,4-Dioxane as an emerging water contaminant: State of the science and evaluation of research needs. The Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef]
- Gi, M.; Fujioka, M.; Kakehashi, A.; Okuno, T.; Masumura, K.; Nohmi, T.; Matsumoto, M.; Omori, M.; Wanibuchi, H.; Fukushima, S. In vivo positive mutagenicity of 1,4-dioxane and quantitative analysis of its mutagenicity and carcinogenicity in rats. Arch. Toxicol. 2018, 92, 3207–3221. [Google Scholar] [CrossRef]
- Mnif, W.; Pillon, A.; Balaguer, P.; Bartegi, A. Endocrine xenoestrogenics disrupters: Molecular mechanisms and detection methods. Therapie 2007, 62, 369–386. [Google Scholar] [CrossRef]
- Hashim, M.; Iqbal, R.; Afsheen, S.; Fahad, A.U.M.; Asghar, U.; Daman, M.; Ghazanfar, M. 8. Effect of 1,4-dioxane on rabbit (Oryctolagus cuniculus) reproductive hormonal level and histopathology of testes and ovaries. Pure Appl. Biol. (PAB) 2018, 7, 466–469. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S.S.; Parveen, N.; Zedan, H.S. Plastic microbeads: Small yet mighty concerning. Int. J. Environ. Health Res. 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.; Asch, R.G. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar. Ecol. Prog. Ser. 2011, 432, 173–180. [Google Scholar] [CrossRef]
- Van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.-L.; Heubeck, M.; Jensen, J.-K.; Le Guillou, G. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 2011, 159, 2609–2615. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Kȯhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Tagg, A.S.; do Sul, J.A.I. Is this your glitter? An overlooked but potentially environmentally-valuable microplastic. Mar. Pollut. Bull. 2019, 146, 50–53. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.; Barreiro, M.F. Cosmetics preservation: a review on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hou, J.; Xie, W.; Cheng, H. Investigation on formaldehyde release from preservatives in cosmetics. Int. J. Cosmet. Sci. 2015, 37, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Scheman, A.; Severson, D. American Contact Dermatitis Society contact allergy management program: An epidemiologic tool to quantify ingredient usage. Dermatitis 2016, 27, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Speit, G.; Schütz, P.; Högel, J.; Schmid, O. Characterization of the genotoxic potential of formaldehyde in V79 cells. Mutagenesis 2007, 22, 387–394. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Zhang, W.; Liu, J.; Wang, Y.-L.; Du, Z.; Song, B.; Xu, Z.P.; Yuan, J. “Dual-key-and-lock” ruthenium complex probe for lysosomal formaldehyde in cancer cells and tumors. J. Am. Chem. Soc. 2019, 141, 8462–8472. [Google Scholar] [CrossRef]
- Zhang, L.; Freeman, L.E.B.; Nakamura, J.; Hecht, S.S.; Vandenberg, J.J.; Smith, M.T.; Sonawane, B.R. Formaldehyde and leukemia: Epidemiology, potential mechanisms, and implications for risk assessment. Environ. Mol. Mutagen. 2010, 51, 181–191. [Google Scholar] [CrossRef]
- Tyihak, E.; Bocsi, J.; Timar, F.; Racz, G.; Szende, B. Formaldehyde promotes and inhibits the proliferation of cultured tumour and endothelial cells. Cell Prolif. 2001, 34, 135–141. [Google Scholar] [CrossRef]
- Li, Q.; Mei, Q.; Huyan, T.; Xie, L.; Che, S.; Yang, H.; Zhang, M.; Huang, Q. Effects of formaldehyde exposure on human NK cells in vitro. Environ. Toxicol. Pharmacol. 2013, 36, 948–955. [Google Scholar] [CrossRef]
- Lai, L.-J.; Hsu, W.-H.; Wu, A.M.; Wu, J.H. Ocular injury by transient formaldehyde exposure in a rabbit eye model. PLoS ONE 2013, 8, e66649. [Google Scholar] [CrossRef]
- De Groot, A.C.; Veenstra, M. Formaldehyde-releasers in cosmetics in the USA and in Europe. Contact Dermat. 2010, 62, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, C.; Ettorre, A.; Andreassi, M.; Centini, M.; Neri, P.; Di Stefano, A. In vitro induction of apoptosis vs. necrosis by widely used preservatives: 2-phenoxyethanol, a mixture of isothiazolinones, imidazolidinyl urea and 1, 2-pentanediol. Biochem. Pharmacol. 2002, 63, 437–453. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Buchholz, H.J.; Belsito, D.V.; Maibach, H.I.; Fowler Jr, J.F.; Rietschel, R.L.; Zug, K.A.; Mathias, C.T.; Pratt, M.D.; Sasseville, D. Allergic patch test reactions associated with cosmetics: Retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 2001–2004. J. Am. Acad. Dermatol. 2009, 60, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Facial skin care products and cosmetics. Clin. Dermatol. 2014, 32, 809–812. [Google Scholar] [CrossRef]
- An, J.H.; Lee, J.-S.; Chun, J.-R.; Oh, B.-K.; Kafi, M.; Choi, J.-W. Cell chip-based monitoring of toxic effects of cosmetic compounds on skin fibroblast cells. J. Nanosci. Nanotechnol. 2012, 12, 5143–5148. [Google Scholar] [CrossRef]
- Pfuhler, S.; Wolf, H.U. Effects of the formaldehyde releasing preservatives dimethylol urea and diazolidinyl urea in several short-term genotoxicity tests. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2002, 514, 133–146. [Google Scholar] [CrossRef]
- Volkovova, K.; Bilanicova, D.; Bartonova, A.; Letašiová, S.; Dusinska, M. Associations between environmental factors and incidence of cutaneous melanoma. Rev. Environ. Health 2012, 11, S12. [Google Scholar] [CrossRef]
- Chisvert, A.; Salvador, A. UV filters in sunscreens and other cosmetics. Regulatory aspects and analytical methods. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 83–120. [Google Scholar]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TrAC Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Zhang, P.-P.; Shi, Z.-G.; Yu, Q.-W.; Feng, Y.-Q. A new device for magnetic stirring-assisted dispersive liquid–liquid microextraction of UV filters in environmental water samples. Talanta 2011, 83, 1711–1715. [Google Scholar] [CrossRef]
- Jurado, A.; Gago-Ferrero, P.; Vàzquez-Suñé, E.; Carrera, J.; Pujades, E.; Díaz-Cruz, M.S.; Barceló, D. Urban groundwater contamination by residues of UV filters. J. Hazard. Mater. 2014, 271, 141–149. [Google Scholar] [CrossRef]
- Barón, E.; Gago-Ferrero, P.; Gorga, M.; Rudolph, I.; Mendoza, G.; Zapata, A.M.; Díaz-Cruz, S.; Barra, R.; Ocampo-Duque, W.; Páez, M. Occurrence of hydrophobic organic pollutants (BFRs and UV-filters) in sediments from South America. Chemosphere 2013, 92, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Oie, C.S.; Albaugh, C.E.; Peyton, B.M. Benzoate and salicylate degradation by Halomonas campisalis, an alkaliphilic and moderately halophilic microorganism. Water Res. 2007, 41, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.; Leung, H.; Wai, T.-C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Lee, C.-L.; Fang, M.-D. Emerging organic contaminants in coastal waters: Anthropogenic impact, environmental release and ecological risk. Mar. Pollut. Bull. 2014, 85, 391–399. [Google Scholar] [CrossRef]
- Ekpeghere, K.I.; Kim, U.-J.; Sung-Hee, O.; Kim, H.-Y.; Oh, J.-E. Distribution and seasonal occurrence of UV filters in rivers and wastewater treatment plants in Korea. Sci. Total Environ. 2016, 542, 121–128. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef]
- Faass, O.; Schlumpf, M.; Reolon, S.; Henseler, M.; Maerkel, K.; Durrer, S.; Lichtensteiger, W. Female sexual behavior, estrous cycle and gene expression in sexually dimorphic brain regions after pre-and postnatal exposure to endocrine active UV filters. Neurotoxicology 2009, 30, 249–260. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Vökt, C.C.; Birchler, M.; Durrer, S.; Faass, O.; Ehnes, C.; Fuetsch, M.; Gaille, C.; Henseler, M. Endocrine active UV filters: Developmental toxicity and exposure through breast milk. CHIMIA Int. J. Chem. 2008, 62, 345–351. [Google Scholar]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chimic. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wages, M.; Cox, S.B.; Maul, J.D.; Li, Y.; Barnes, M.; Hope-Weeks, L.; Cobb, G.P. Effect of titanium dioxide nanomaterials and ultraviolet light coexposure on African clawed frogs (Xenopus laevis). Environ. Toxicol. Chem. 2012, 31, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Castro, V.; Moura, M.; Jonsson, C.; Fraceto, L. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kabengi, N.; Bertsch, P.; Unrine, J.; Glenn, T.; Williams, P. Comparative phototoxicity of nanoparticulate and bulk ZnO to a free-living nematode Caenorhabditis elegans: The importance of illumination mode and primary particle size. Environ. Pollut. 2011, 159, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.; Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef]
- Lemaire, R.; Bianco, D.D.; Garnier, L.; Beltramo, J. Determination of lead in lipstick by direct solid sampling high-resolution continuum source graphite furnace atomic absorption spectrometry: Comparison of two digestion methods. Anal. Lett. 2013, 46, 2265–2278. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Foroghi, M.; Farhadkhani, M.; Vahid Dastjerdi, M. Assessment of lead and cadmium levels in frequently used cosmetic products in Iran. J. Environ. Public Health 2013, 2013. [Google Scholar] [CrossRef]
- Lin, S.-H.; Wang, X.-R.; Yu, I.T.S.; Tang, J.; Li, J.; Ba, O.; Liu, Y. Lead powder use for skin care and elevated blood lead level among children in a Chinese rural area. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 198–203. [Google Scholar] [CrossRef]
- Dickenson, C.A.; Woodruff, T.J.; Stotland, N.E.; Dobraca, D.; Das, R. Elevated mercury levels in pregnant woman linked to skin cream from Mexico. Am. J. Obstetr. Gynecol. 2013, 209, e4–e5. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Binkowski, Ł.J.; Błaszczyk, M.; Paluch, J.; Wojtaś, W.; Massanyi, P.; Stawarz, R. Cadmium, lead and mercury concentrations and their influence on morphological parameters in blood donors from different age groups from southern Poland. J. Trace Elem. Med. Biol. 2015, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Soussi, A.; Gargouri, M.; El Feki, A. Effects of co-exposure to lead and zinc on redox status, kidney variables, and histopathology in adult albino rats. Toxicol. Ind. Health 2018, 34, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, J.W.; Zheng, W. Distribution of lead and transthyretin in human eyes. J. Toxicol. Clin. Toxicol. 2000, 38, 377–381. [Google Scholar] [CrossRef]
- Erie, J.C.; Butz, J.A.; Good, J.A.; Erie, E.A.; Burritt, M.F.; Cameron, J.D. Heavy metal concentrations in human eyes. Am. J. Ophthalmol. 2005, 139, 888–893. [Google Scholar] [CrossRef]
- Saadatzadeh, A.; Afzalan, S.; Zadehdabagh, R.; Tishezan, L.; Najafi, N.; Seyedtabib, M.; Noori, S.M.A. Determination of heavy metals (lead, cadmium, arsenic, and mercury) in authorized and unauthorized cosmetics. Cutan. Ocul. Toxicol. 2019, 38, 207–211. [Google Scholar] [CrossRef]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: a status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- Hepp, N.M.; Mindak, W.R.; Gasper, J.W.; Thompson, C.B.; Barrows, J.N. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J. Cosmet. Sci. 2014, 65, 125. [Google Scholar]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Al-Enazi, S. Trace metals in lipsticks. Toxic. Environ. Chem. 2011, 93, 1149–1165. [Google Scholar] [CrossRef]
- Chan, T.Y. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin. Toxicol. 2011, 49, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.X.; Pereira, T.C. Cosmetics and its health risks. Glob. J. Med. Res. 2018, 18, 63–66. [Google Scholar] [CrossRef]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Robson, M.; Melymuk, L.; Shunthirasingham, C.; Alexandrou, N.; Shoeib, M.; Luk, E.; Helm, P.; Diamond, M.L.; Hung, H. Urban sources of synthetic musk compounds to the environment. Environ. Sci. Proc. Impacts 2019, 21, 74–88. [Google Scholar] [CrossRef]
- Nakata, H.; Hinosaka, M.; Yanagimoto, H. Macrocyclic-, polycyclic-, and nitro musks in cosmetics, household commodities and indoor dusts collected from Japan: Implications for their human exposure. Ecotoxicol. Environ. Saf. 2015, 111, 248–255. [Google Scholar] [CrossRef]
- Schmeiser, H.H.; Gminski, R.; Mersch-Sundermann, V. Evaluation of health risks caused by musk ketone. Int. J. Hyg. Environ. Health 2001, 203, 293–299. [Google Scholar] [CrossRef]
- Rimkus, G.G. Polycyclic musk fragrances in the aquatic environment. Toxicol. Lett. 1999, 111, 37–56. [Google Scholar] [CrossRef]
- Luckenbach, T.; Epel, D. Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environ. Health Perspect. 2005, 113, 17–24. [Google Scholar] [CrossRef]
- Hutter, H.-P.; Wallner, P.; Moshammer, H.; Hartl, W.; Sattelberger, R.; Lorbeer, G.; Kundi, M. Synthetic musks in blood of healthy young adults: Relationship to cosmetics use. Sci. Total Environ. 2009, 407, 4821–4825. [Google Scholar] [CrossRef]
- Lassen, C.; Hansen, C.; Mikkelsen, S.; Maag, J. Siloxanes–Consumption, Toxicity and Alternatives; Environmental Project No. 1031; Environment Protection Agency, Danish Ministry of the Environment: Copenhagen, Denmark, 2005. Available online: https://www2.mst.dk/Udgiv/publications/2005/87-7614-756-8/pdf/87-7614-757-6.pdf (accessed on 17 October 2018).
- Tran, T.M.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B. A survey of cyclic and linear siloxanes in indoor dust and their implications for human exposures in twelve countries. Environ. Int. 2015, 78, 39–44. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Jamrógiewicz, M.; Szymkowska, K.; Krenczkowska, D. Direct human contact with siloxanes (silicones)–safety or risk part 1. Characteristics of siloxanes (silicones). Front. Pharmacol. 2016, 7, 132. [Google Scholar] [CrossRef]
- Schalau, G.; Ulman, K. Silicone excipients in drug development. Contract Pharma 2009, 11, 2006–2009. [Google Scholar]
- Khan, A.D.; Alam, M.N. Cosmetics and their associated adverse effects: A review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Mehmood, S.; Iqbal, H.M.N. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics 2020, 7, 13. https://doi.org/10.3390/cosmetics7010013
Bilal M, Mehmood S, Iqbal HMN. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics. 2020; 7(1):13. https://doi.org/10.3390/cosmetics7010013
Chicago/Turabian StyleBilal, Muhammad, Shahid Mehmood, and Hafiz M. N. Iqbal. 2020. "The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics" Cosmetics 7, no. 1: 13. https://doi.org/10.3390/cosmetics7010013
APA StyleBilal, M., Mehmood, S., & Iqbal, H. M. N. (2020). The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics, 7(1), 13. https://doi.org/10.3390/cosmetics7010013





