Preparation and Characterization of Callus Extract from Pyrus pyrifolia and Investigation of Its Effects on Skin Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of P. pyrifolia Callus Extract
2.2.1. Preparation of Plant Material
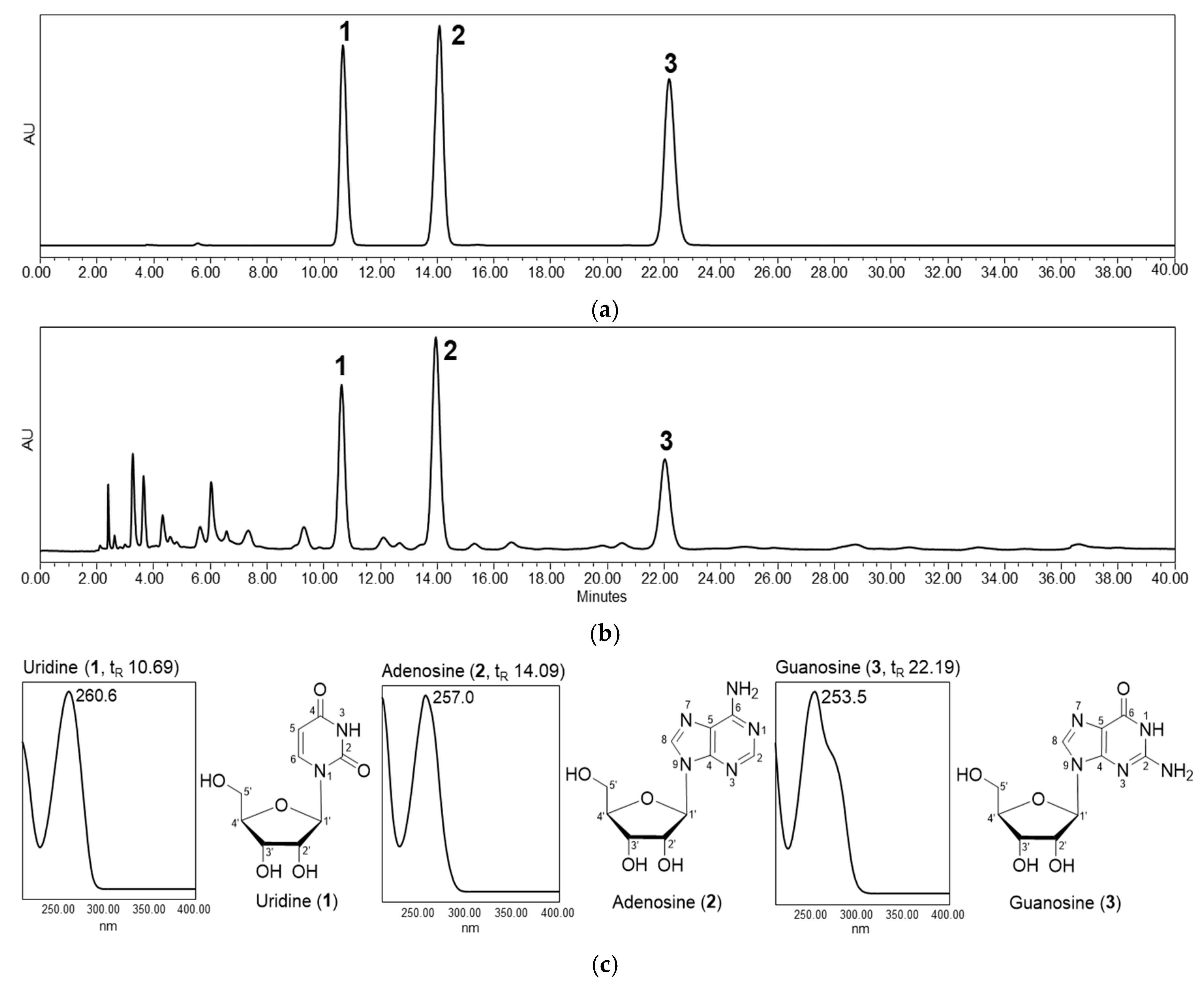
2.2.2. Isolation and Identification Procedure
2.3. Antioxidant Activity of P. pyrifolia Callus Extract
2.3.1. DPPH Radical Scavenging Assay
2.3.2. ABTS Radical Scavenging Assay
2.3.3. FRAP Assay
2.3.4. Protein Glycation Assay
2.4. Antiaging Potential of a Callus Extract of P. pyrifolia
2.4.1. Cell Proliferation Assay
2.4.2. Procollagen Synthesis Assay
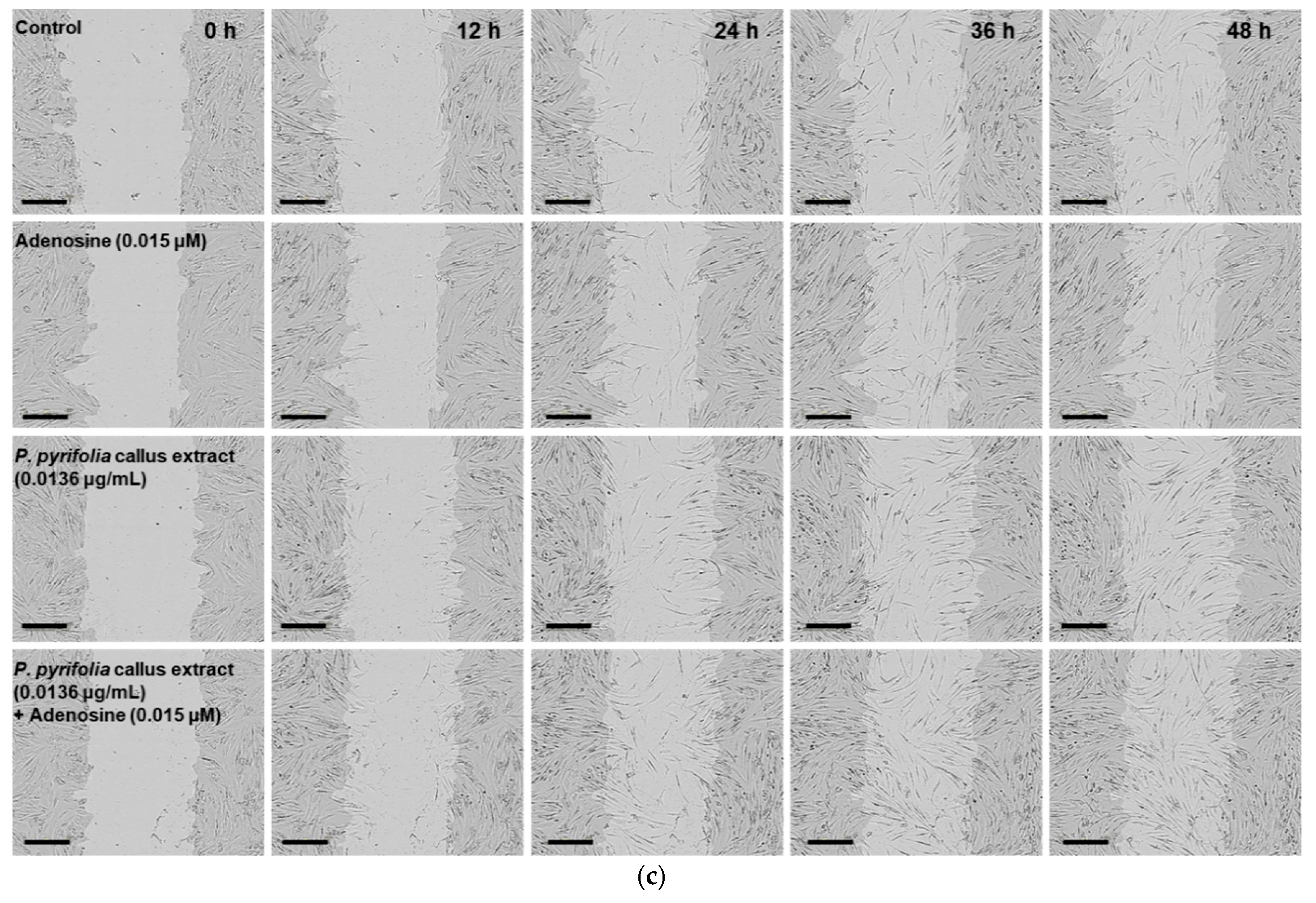
2.4.3. In Vitro Scratch Wound Recovery Assay
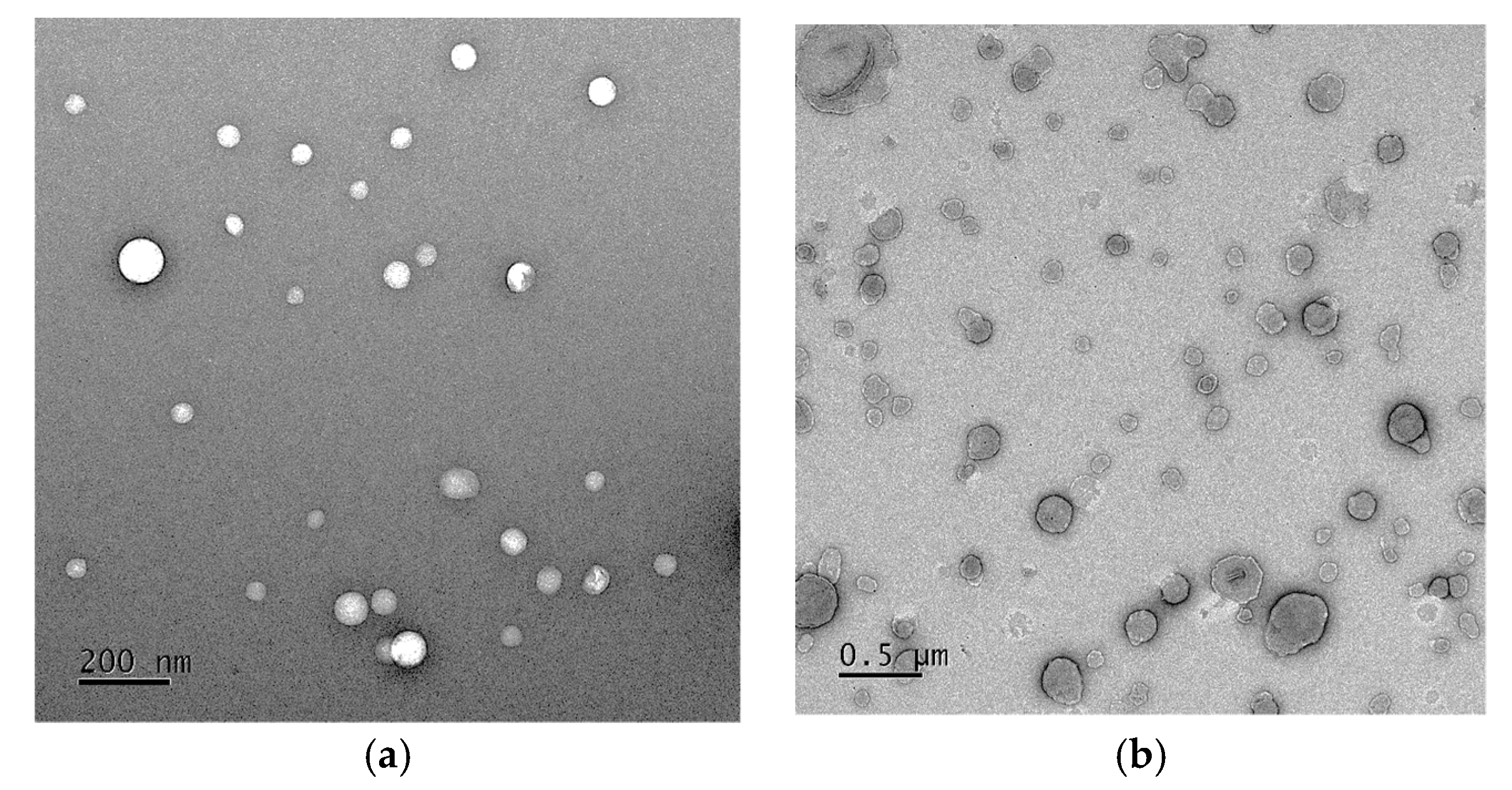
2.5. Preparation and Characterization of P. pyrifolia Callus Extract-Loaded NLs
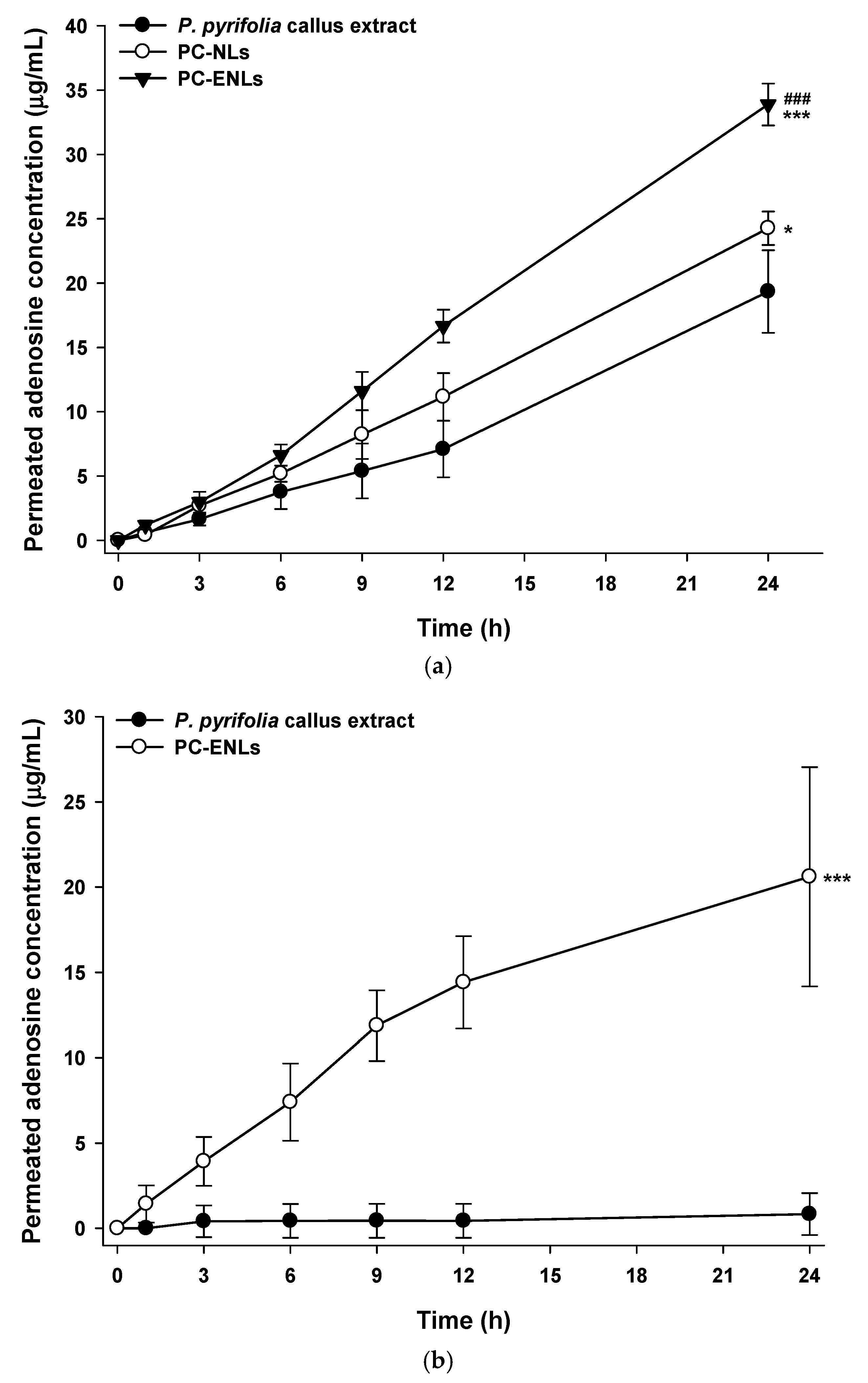
2.6. In Vitro Skin Permeability of P. pyrifolia Callus Extract-Loaded NLs
2.7. Statistics
3. Results and Discussion
3.1. Preparation and Characterization of Callus Extract from P. pyrifolia
3.2. Antioxidant Activities of P. pyrifolia Callus Extract
3.2.1. Antioxidant Activities of P. pyrifolia Callus Extract
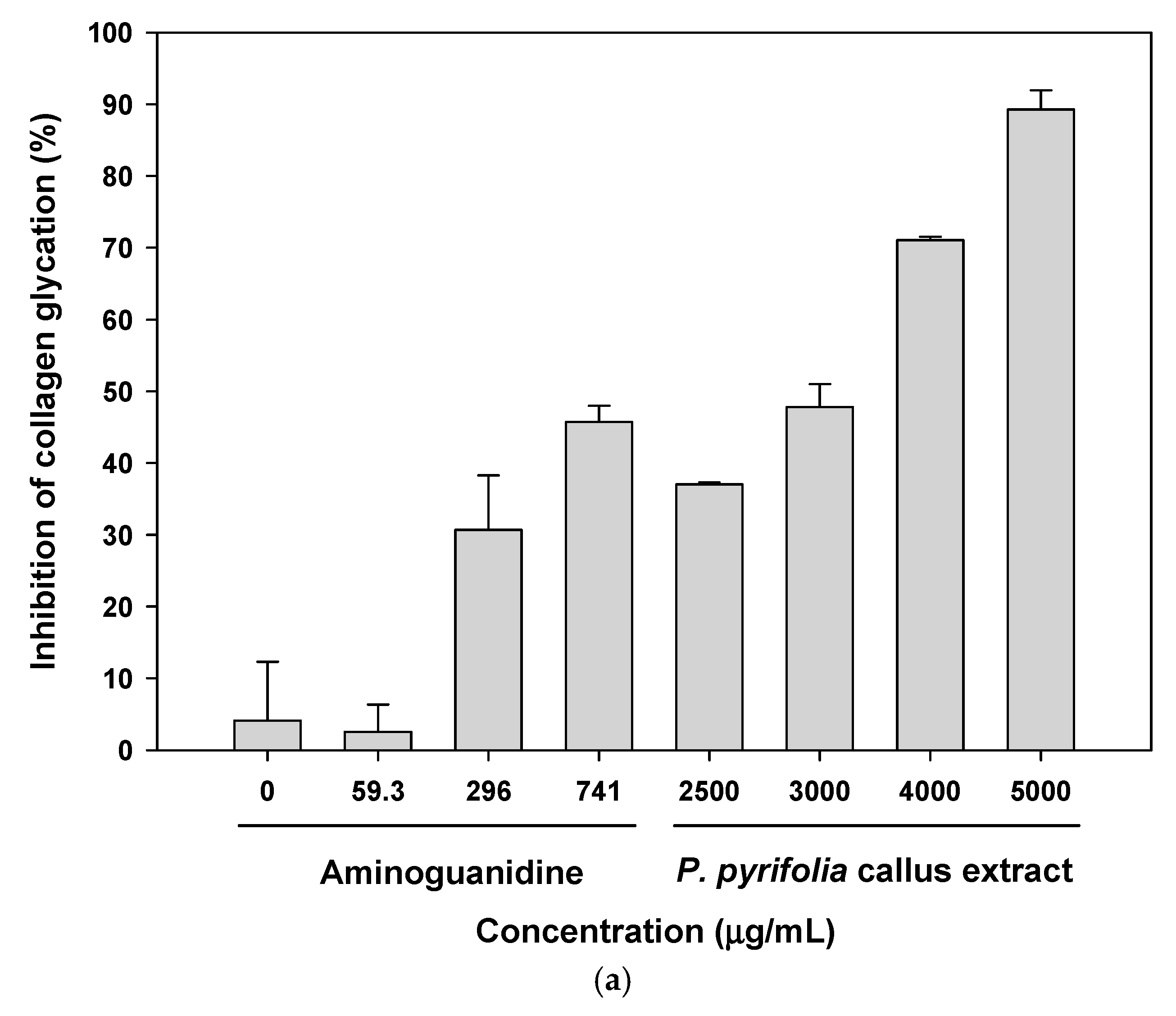
3.2.2. Antiglycation Potential of P. pyrifolia Callus Extract
3.3. Skin Regeneration Activities of P. pyrifolia Callus Extract
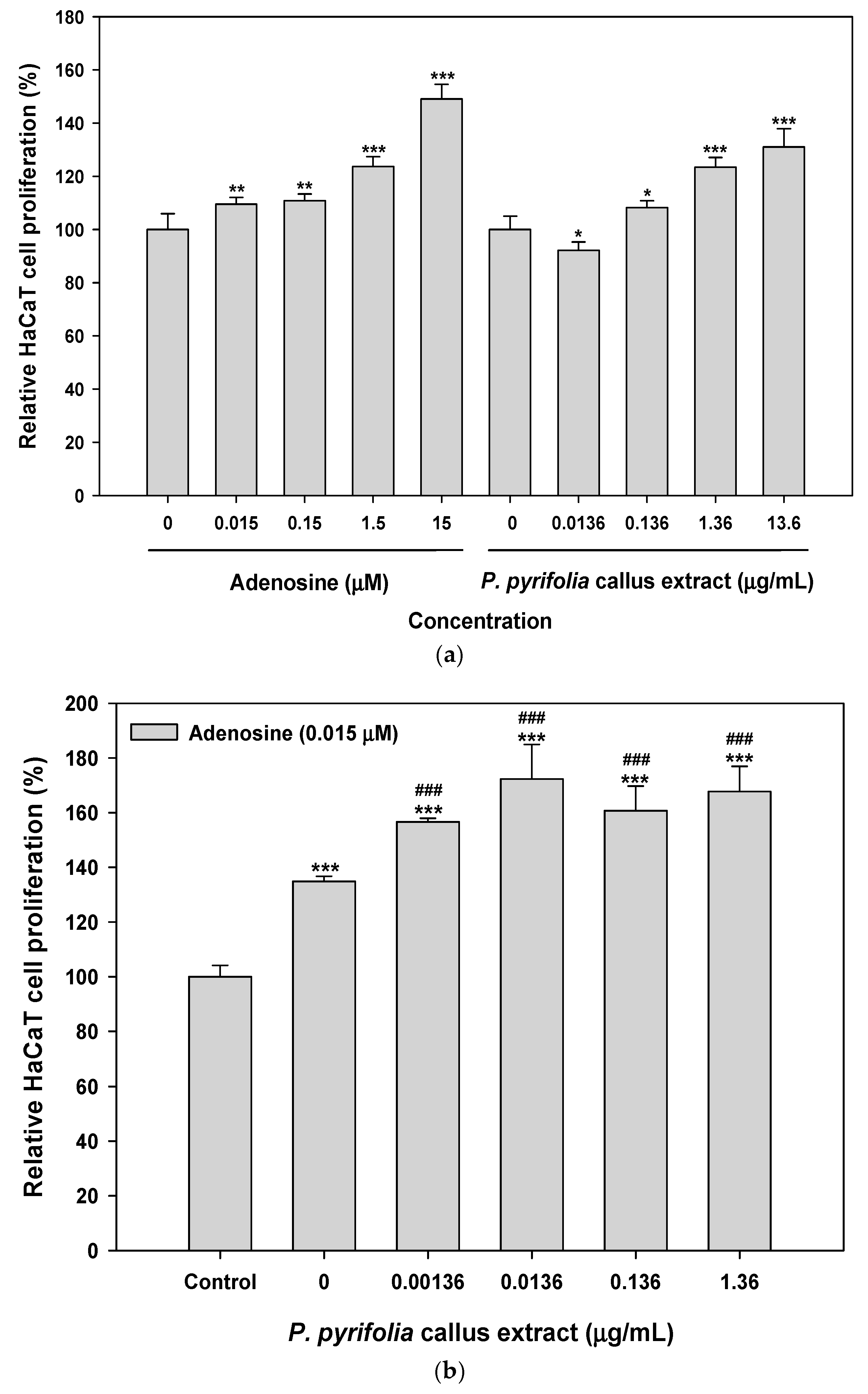
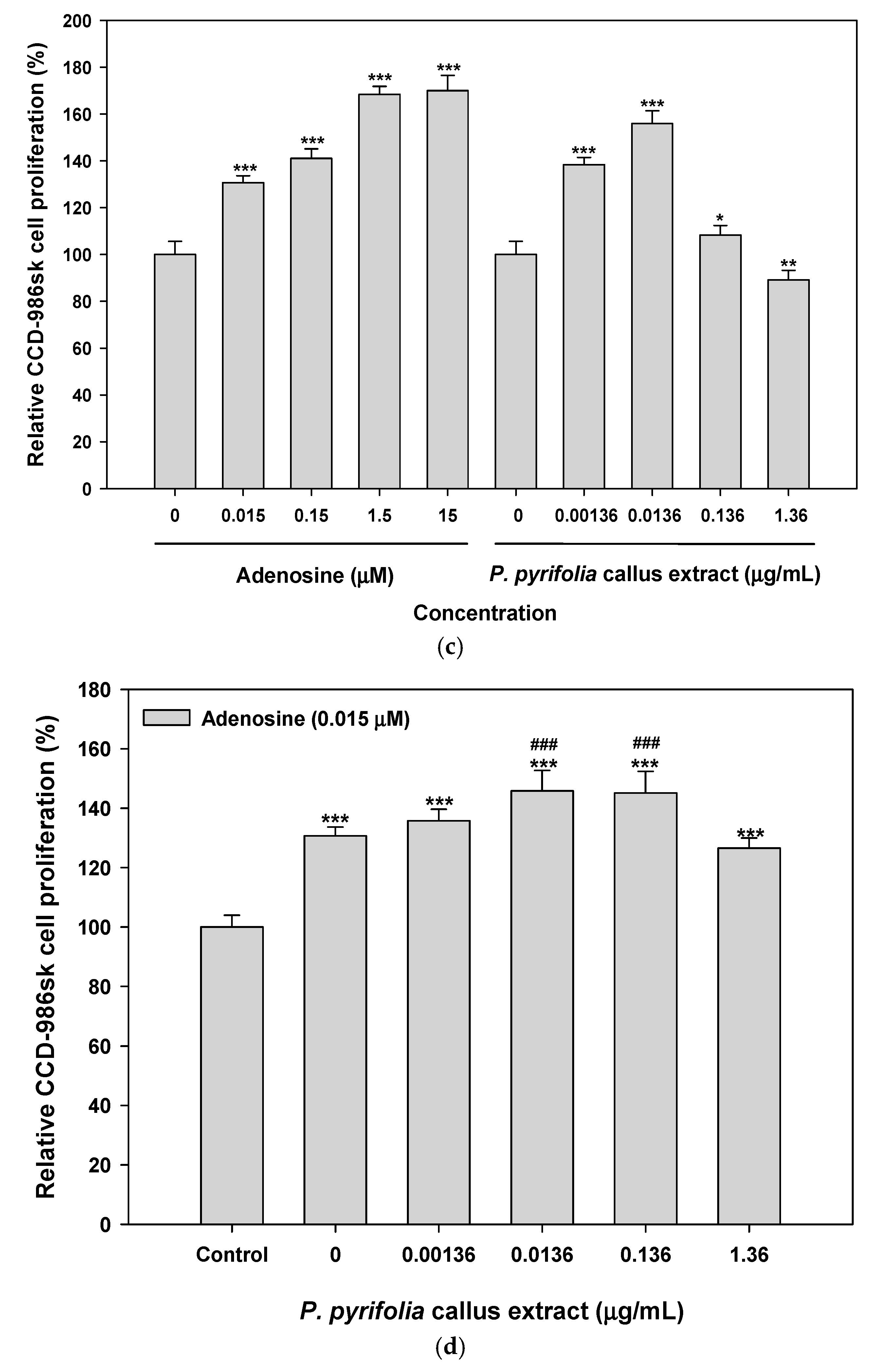
3.3.1. Effects of Callus Extract and Combination of Callus Extract and Adenosine on Keratinocyte and Fibroblast Cell Proliferation
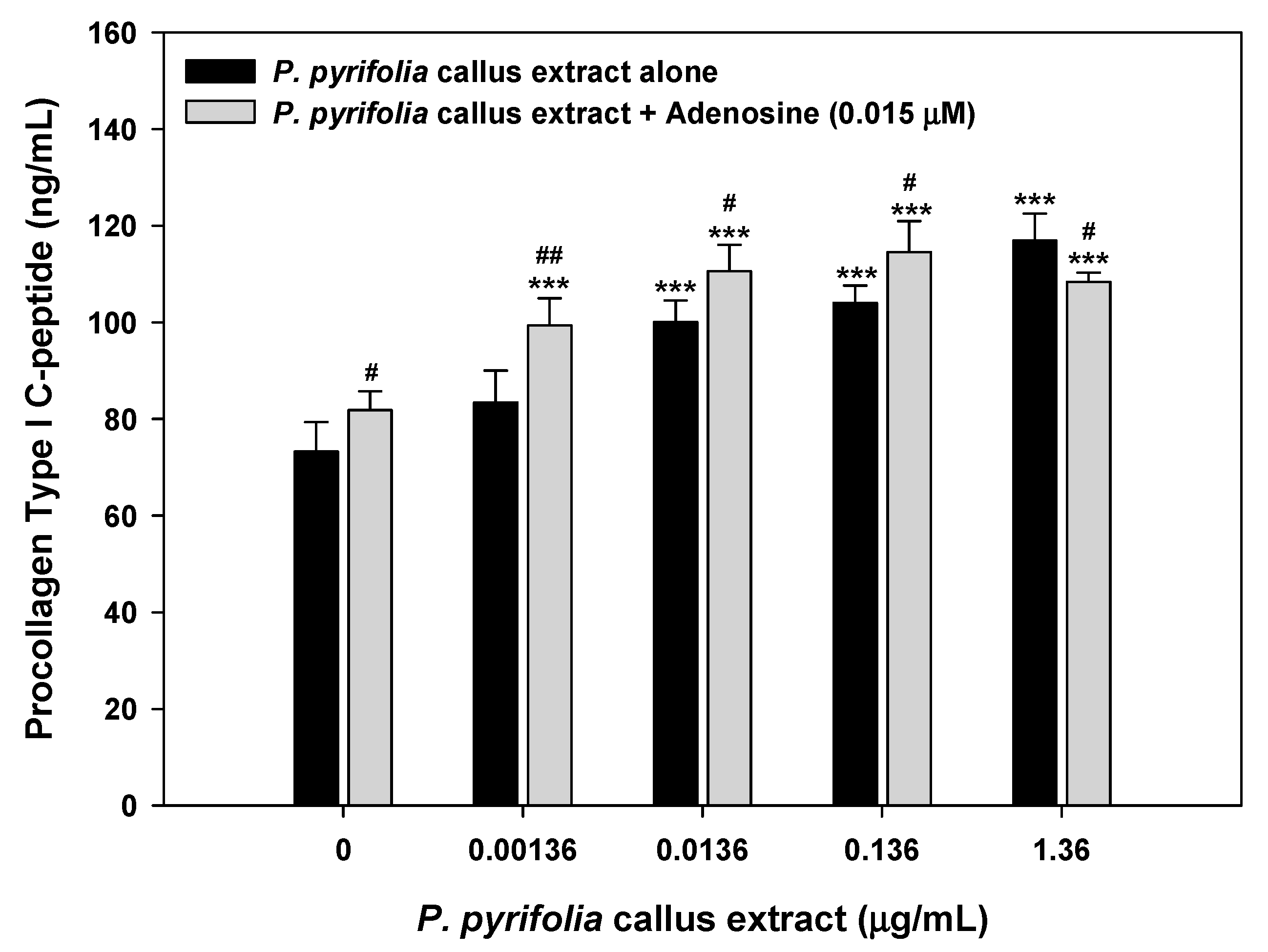
3.3.2. Procollagen Synthesis Effects of Treatment with P. pyrifolia Callus Extract Alone and in Combination with Adenosine
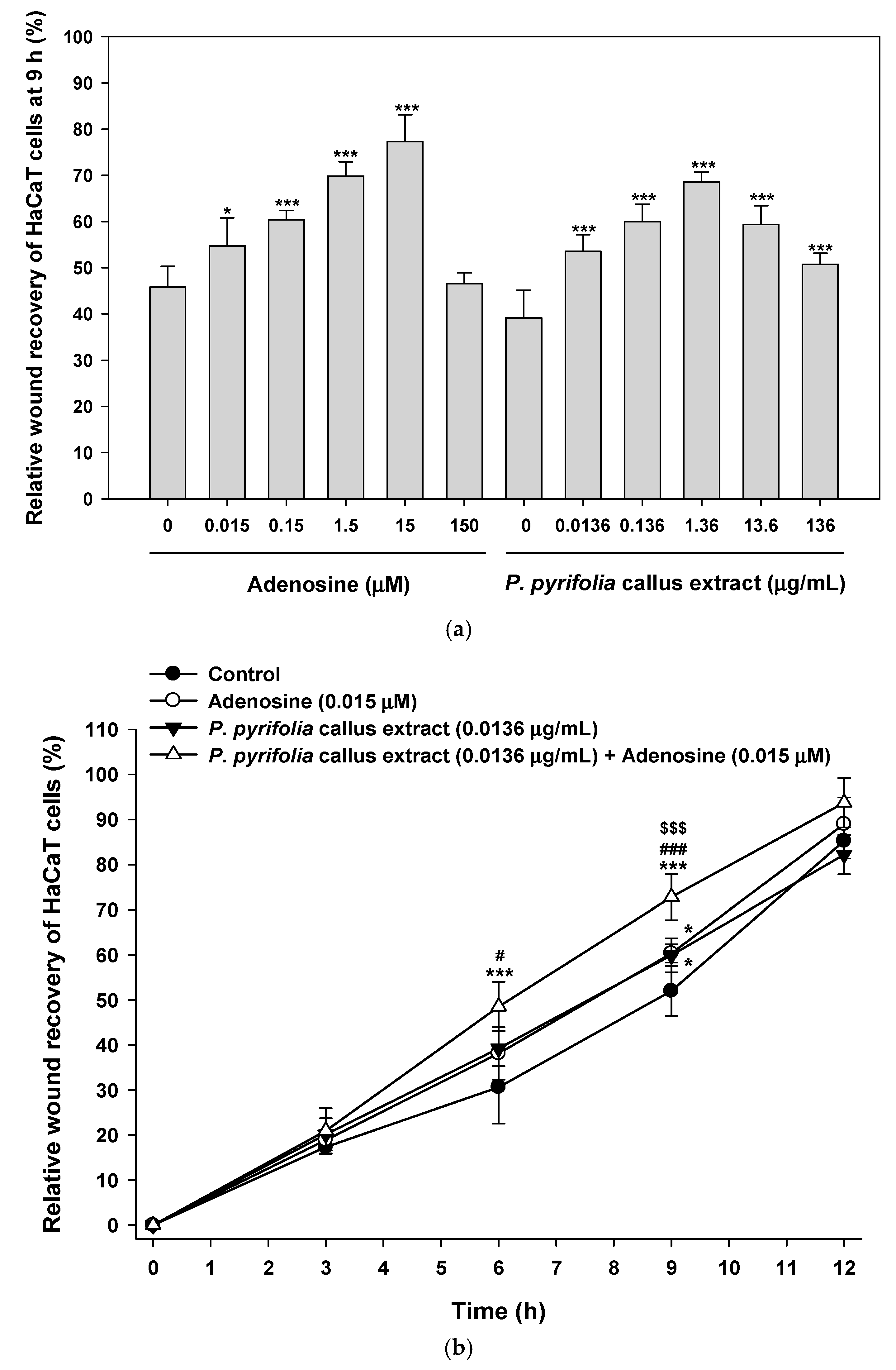
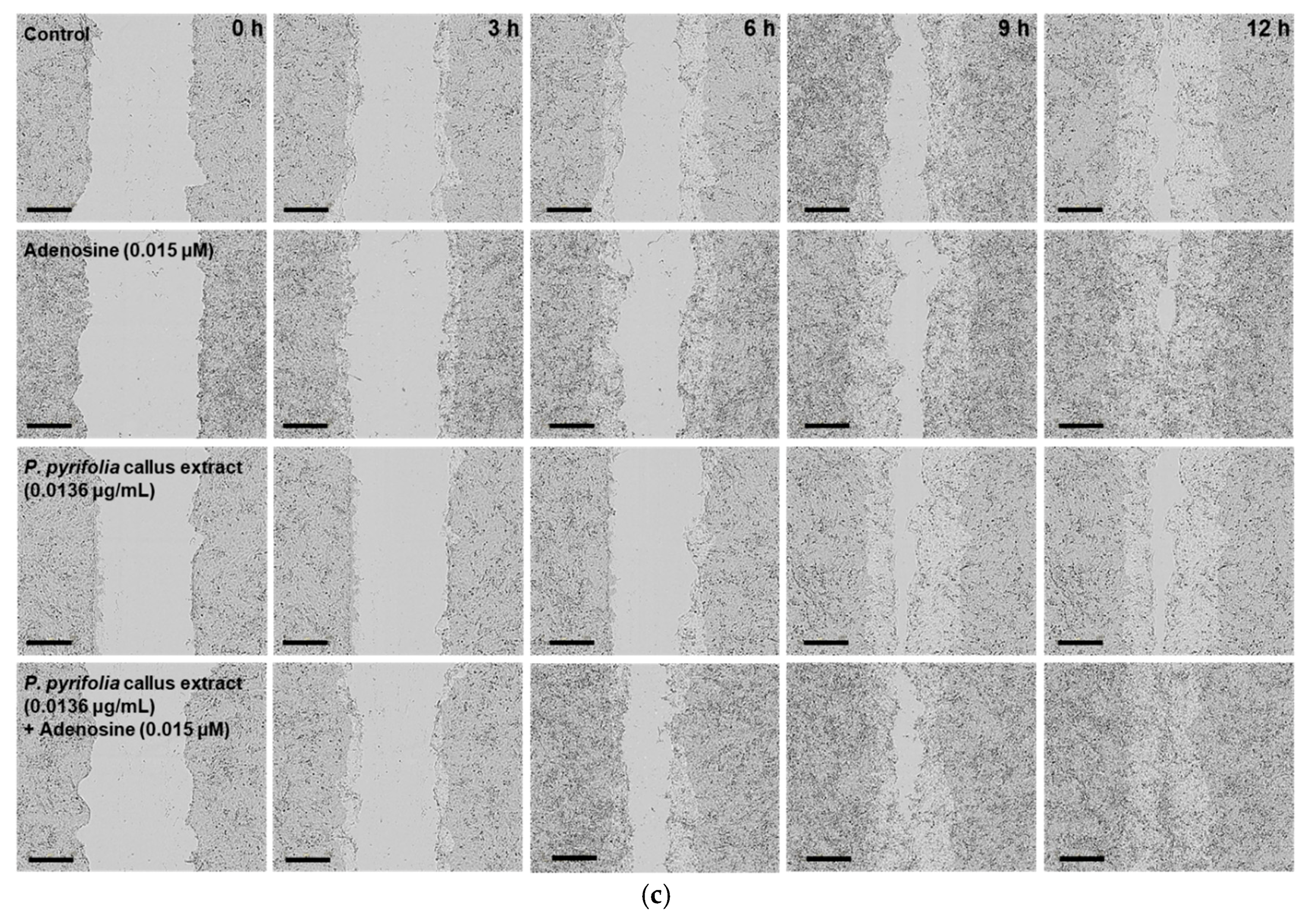
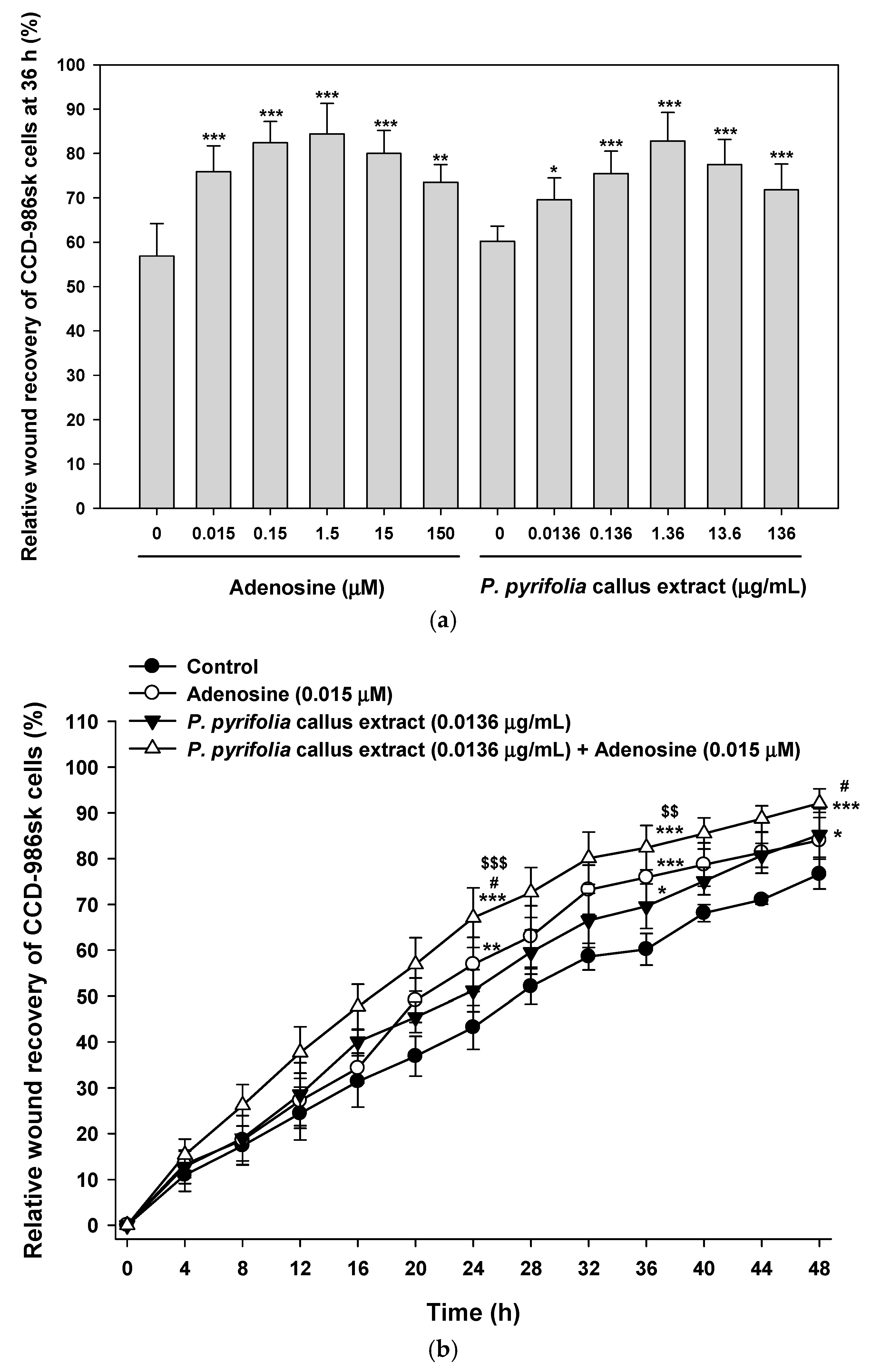
3.3.3. Effects of P. pyrifolia Callus Extract Alone and in Combination with Adenosine on In Vitro Scratch Wound Recovery
3.4. Characterization and Skin Permeability of P. pyrifolia Callus Extract-Loaded NLs
3.4.1. Characterization of NLs Containing P. pyrifolia Callus Extract
3.4.2. In Vitro Skin Permeability of NLs Containing P. pyrifolia Callus Extract
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of oxidative stress and skin aging-related enzymes by prenylated chalcones and other flavonoids from helichrysum teretifolium. Molecules 2015, 20, 7143–7155. [Google Scholar] [CrossRef] [PubMed]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, in vitro photoprotective and skin-aging related enzyme inhibitory activities of malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B 2017, 173, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of phyllanthus emblica, manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Bravo, K.; Alzate, F.; Osorio, E. Fruits of selected wild and cultivated andean plants as sources of potential compounds with antioxidant and anti-aging activity. Ind. Crop Prod. 2016, 85, 341–352. [Google Scholar] [CrossRef]
- Tu, P.T.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. The role of cellular senescence in skin aging. J. Investig. Dermatol. Symp. Proc. 1998, 3, 1–5. [Google Scholar]
- Faragher, R.G.A. Aging & the immune system. Biochem. Soc. Trans. 2000, 28, 221–226. [Google Scholar]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Kasper, M.; Funk, R.H.W. Age-related changes in cells and tissues due to advanced glycation end products (ages). Arch. Gerontol. Geriatr. 2001, 32, 233–243. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Bakhti, M.; Habibi-Rezaei, M. Nicotine reduces the cytotoxic effect of glycated proteins on microglial cells. Neurochem. Res. 2010, 35, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Stern, D. Atherosclerosis and diabetes: The rage connection. Curr. Atheroscler. Rep. 2000, 2, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of maillard reaction products in skin collagen in diabetes and aging. Ann. N. Y. Acad. Sci. 1992, 663, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Verzijl, N.; DeGroot, J.; Oldehinkel, E.; Bank, R.A.; Thorpe, S.R.; Baynes, J.W.; Bayliss, M.T.; Bijlsma, J.W.; Lafeber, F.P.; Tekoppele, J.M. Age-related accumulation of maillard reaction products in human articular cartilage collagen. Biochem. J. 2000, 350 Pt 2, 381–387. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.-Y.; Gao, W.-Y.; Wang, Y.; Wang, H.-Y.; Cao, J.-G.; Huang, L.-Q. Chemical composition and anti-inflammatory and antioxidant activities of eight pear cultivars. J. Agric. Food Chem. 2012, 60, 8738–8744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Gao, W.; Wang, H. Study on chemical composition, anti-inflammatory and anti-microbial activities of extracts from chinese pear fruit (pyrus bretschneideri rehd.). Food Chem. Toxicol. 2012, 50, 3673–3679. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, J.H.; Han, H.S.; Son, J.H.; Choi, C.; Son, G.M.; Bae, J.H. Effect of polyphenol compound from korean pear (pyrus pyrifolia nakai) on lipid metabolism. J. Korean Soc. Food Sci. Nutr. 2004, 33, 229–304. [Google Scholar]
- Hamauzu, Y.; Forest, F.; Hiramatsu, K.; Sugimoto, M. Effect of pear (pyrus communis l.) procyanidins on gastric lesions induced by hcl/ethanol in rats. Food Chem. 2007, 100, 255–263. [Google Scholar] [CrossRef]
- Huang, L.-J.; Gao, W.-Y.; Li, X.; Zhao, W.-S.; Huang, L.-Q.; Liu, C.-X. Evaluation of the in vivo anti-inflammatory effects of extracts from pyrus bretschneideri rehd. J. Agric. Food Chem. 2010, 58, 8983–8987. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, W.; Amiot, M.J.; Aubert, S.Y. Identification of some phenolics in pear fruit. J. Agric. Food Chem. 1994, 42, 1261–1265. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.Y.; Lee, H.J.; Ma, Y.K.; Kwon, J.; Park, S.H.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Park, K.H.; et al. Hydroxycinnamoylmalic acids and their methyl esters from pear (pyrus pyrifolia nakai) fruit peel. J. Agric. Food Chem. 2011, 59, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cho, J.-Y.; Lee, H.J.; Park, K.Y.; Ma, Y.-K.; Lee, S.-H.; Cho, J.A.; Kim, W.-S.; Park, K.-H.; Moon, J.-H. Isolation and identification of phenolic compounds from an asian pear (pyrus pyrifolia nakai) fruit peel. Food Sci. Biotechnol. 2011, 20, 1539–1545. [Google Scholar] [CrossRef]
- Ma, J.-N.; Wang, S.-L.; Zhang, K.; Wu, Z.-G.; Hattori, M.; Chen, G.-L.; Ma, C.-M. Chemical components and antioxidant activity of the peels of commercial apple-shaped pear (fruit of pyrus pyrifolia cv. Pingguoli). J. Food Sci. 2012, 77, C1097–C1102. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, K. Protein glycation inhibitory and antioxidative activities of some plant extracts in vitro. J. Agric. Food Chem. 2003, 51, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Apone, F.; Colucci, G. Plant cell cultures as source of cosmetic active ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N. Optimization of adventitious root culture for production of biomass and secondary metabolites in prunella vulgaris L. Appl. Biochem. Biotechnol. 2014, 174, 2086–2095. [Google Scholar] [CrossRef]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of stevia rebaudiana (bert). J. Photochem. Photobiol. B 2016, 154, 51–56. [Google Scholar] [CrossRef]
- Grąbkowska, R.; Matkowski, A.; Grzegorczyk-Karolak, I.; Wysokińska, H. Callus cultures of harpagophytum procumbens (burch.) dc. Ex meisn.; production of secondary metabolites and antioxidant activity. S. Afr. J. Bot. 2016, 103, 41–48. [Google Scholar] [CrossRef]
- Debnath, M.; Malik, C.P.; Bisen, P.S. Micropropagation: A tool for the production of high quality plant-based medicines. Curr. Pharm. Biotechnol. 2006, 7, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Vasilev, N.; Twyman, R.M.; Schillberg, S. High-value products from plants: The challenges of process optimization. Curr. Opin. Biotechnol. 2015, 32, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for delivery of antioxidants on the skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Adhikari, D.; Panthi, V.K.; Pangeni, R.; Kim, H.J.; Park, J.W. Preparation, characterization, and biological activities of topical anti-aging ingredients in a citrus junos callus extract. Molecules 2017, 22, 2198. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; In, Y.S.; Kim, M.K.; Ko, J.H.; Lee, H.J.; Shin, K.C.; Lee, S.M.; Wee, W.R.; Lee, J.H.; Park, M. Protective effect of uridine on cornea in a rabbit dry eye model. Invest. Ophthalmol. Vis. Sci. 2007, 48, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Abella, M.L. Evaluation of anti-wrinkle efficacy of adenosine-containing products using the foits technique. Int. J. Cosmet. Sci. 2006, 28, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.G.; Bellaver, B.; Bobermin, L.D.; Souza, D.O.; Quincozes-Santos, A. Anti-aging effects of guanosine in glial cells. Purinergic Signal. 2016, 12, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Hariprasath, L.; Jegadeesh, R.; Arjun, P.; Raaman, N. In vitro propagation of senecio candicans dc and comparative antioxidant properties of aqueous extracts of the in vivo plant and in vitro-derived callus. S. Afr. J. Bot. 2015, 98, 134–141. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- López-Laredo, A.R.; Gómez-Aguirre, Y.A.; Medina-Pérez, V.; Salcedo-Morales, G.; Sepúlveda-Jiménez, G.; Trejo-Tapia, G. Variation in antioxidant properties and phenolics concentration in different organs of wild growing and greenhouse cultivated castilleja tenuiflora benth. Acta Physiol. Plant. 2012, 34, 2435–2442. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, J.-Y.; Jeong, H.Y.; Jeong, D.E.; Kim, D.; Cho, S.-Y.; Kim, W.-S.; Moon, J.-H. Comparison of bioactive compound contents and in vitro and ex vivo antioxidative activities between peel and flesh of pear (pyrus pyrifolia nakai). Food Sci. Biotechnol. 2015, 24, 207–216. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef]
- Valls, M.D.; Cronstein, B.N.; Montesinos, M.C. Adenosine receptor agonists for promotion of dermal wound healing. Biochem. Pharmacol. 2009, 77, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Fernandez, P.; Merchant, A.A.; Montesinos, M.C.; Trzaska, S.; Desai, A.; Tung, C.F.; Khoa, D.N.; Pillinger, M.H.; Reiss, A.B.; et al. Adenosine a2a receptors in diffuse dermal fibrosis: Pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006, 54, 2632–2642. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, J.; Vesely, P.; Netikova, I.; Matouskova, E.; Petruzelka, L. The protective effect of pyrimidine nucleosides on human hacat keratinocytes treated with 5-fu. Anticancer Res. 2015, 35, 1303–1310. [Google Scholar] [PubMed]
- Jin, H.; Seo, J.; Eun, S.Y.; Joo, Y.N.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2y2 r activation by nucleotides promotes skin wound-healing process. Exp. Dermatol. 2014, 23, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.N.; Youkey, R.; Liu, X.; Jia, L.; Blatt, R.; Day, Y.J.; Sullivan, G.W.; Linden, J.; Tucker, A.L. A1 adenosine receptor activation promotes angiogenesis and release of vegf from monocytes. Circ. Res. 2007, 101, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef]
- Subongkot, T.; Pamornpathomkul, B.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Investigation of the mechanism of enhanced skin penetration by ultradeformable liposomes. Int. J. Nanomed. 2014, 9, 3539–3550. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.E.; Adhikari, D.; Pangeni, R.; Panthi, V.K.; Kim, H.J.; Park, J.W. Preparation and Characterization of Callus Extract from Pyrus pyrifolia and Investigation of Its Effects on Skin Regeneration. Cosmetics 2018, 5, 71. https://doi.org/10.3390/cosmetics5040071
Park DE, Adhikari D, Pangeni R, Panthi VK, Kim HJ, Park JW. Preparation and Characterization of Callus Extract from Pyrus pyrifolia and Investigation of Its Effects on Skin Regeneration. Cosmetics. 2018; 5(4):71. https://doi.org/10.3390/cosmetics5040071
Chicago/Turabian StylePark, Dae Eung, Deepak Adhikari, Rudra Pangeni, Vijay Kumar Panthi, Hyun Jung Kim, and Jin Woo Park. 2018. "Preparation and Characterization of Callus Extract from Pyrus pyrifolia and Investigation of Its Effects on Skin Regeneration" Cosmetics 5, no. 4: 71. https://doi.org/10.3390/cosmetics5040071
APA StylePark, D. E., Adhikari, D., Pangeni, R., Panthi, V. K., Kim, H. J., & Park, J. W. (2018). Preparation and Characterization of Callus Extract from Pyrus pyrifolia and Investigation of Its Effects on Skin Regeneration. Cosmetics, 5(4), 71. https://doi.org/10.3390/cosmetics5040071





