Abstract
The preparation and selection of biobased materials compatible with skin is essential for producing innovative and highly eco-friendly beauty masks. The use of a commercial elastomeric poly(hydroxyalkanoate) and starch was fundamental to select materials for bioplastic films with the necessary resistance in wet conditions, skin compatibility and capacity for a fast release of polysaccharides and similar active and functional molecules. Micrometric calcium carbonate was also used to control the stickiness of film during moulding. Starch release in water was investigated by gravimetric and infrared analyses. The compatibility with skin was investigated via two different in vitro tests based on human keratinocytes and human mesenchymal stromal cells. The materials were highly cytocompatible with skin, enabled immune modulation by keratinocytes and starch release in water up to 49% by weight in 30 min. These outcomes are a good starting point for boosting the production of biobased and biodegradable beauty masks, thus decreasing the impact onto environment of cosmetic products that are currently still mainly produced using petrol-based substrates.
1. Introduction
Considering environmental concerns, it is necessary to produce more and more commercial products, especially those having a brief life-cycle, with biobased and biodegradable polymers [1,2,3]. Many of these products, such as cosmetics, are naturally oriented towards the use of biobased active molecules and materials and consumers demand is also following this direction. The growing demand for beauty masks is not only driven by aesthetical reasons but also by their capacity to preserve the skin health in a polluted environment [4]. The awareness about the necessity of preserving health and environment against pollution is growing in consumers on a global scale, much impacting the cosmetic sector [5].
Beauty masks are currently produced using wet non-woven tissues, often prepared with petrol-based fibres, packed in sealed total barrier packaging. After their use, these products are not selectively collected, hence they contribute to increase the non-differentiated part of the urban waste all over the world. The possibility of recycling beauty masks is highly limited because of the use of different plastic materials in diverse products. This general behaviour as well as the presence of contaminants inside the waste product due to the addition of functional molecules obstacle easy sorting operations. The high content of water is also negative for waste management. Therefore, it is difficult to obtain a selectively collected material with thermal resistance suitable for obtaining a secondary raw material from this kind of waste. Hence, landfill or incineration are beauty masks end-of-life options, both being considered as the last options for waste management by European Union [6,7].
Biobased and biodegradable polymeric materials with the capacity for a rapid release of molecules beneficial for skin and easily compostable after usage, represent highly eco-sustainable options and at the same time meet consumers’ expectations. From among biobased polymers already available on the market, poly(lactic acid) (PLA) as well as poly(3-hydroxybutyrate) (PHB) and poly(hydroxyalkanoates) (PHAs) in general are fully renewable [8,9]. Although PLA is produced on a higher scale and is currently less expensive than PHAs, some characteristics make PHAs more advantageous for this application: the very high biocompatibility [10,11], the lower Green House Gas emissions [12] and compostability both in soil and marine environment [13,14] of PHA. These properties make the PHA-based materials very promising for being used in applications where environmental concern and biocompatibility are fundamental.
PHAs are a family of polymers obtained from bacteria [15]. PHB is the polymer with the simplest structure in this group but copolymers with valerate or other repeating units can be considered belonging to the general family of PHAs. PHB shows properties comparable to petroleum-based polymers (e.g., polypropylene; PP), such as high melting temperature (175 °C) and relatively high tensile strength (30–35 MPa). However, pure PHB has had only a limited use mainly because of its intrinsic brittleness (presenting low strain at break) and a narrow processing temperature range [16,17,18]. Indeed, the elongation at break is different between PHB (5%) and currently used commodities. As an example, the elongation at break of PP is 400% [15].
Starch, being the major carbohydrate reserve in higher plants [19], is a very abundant biopolymer used in no-food applications (e.g., glues, gums, etc.). Starch can be suspended in water, having a portion soluble (i.e., amylose) and one insoluble (i.e., amylopectin). To enable its processability, the preparation of thermoplastic starch by gelatinization at least at 80 °C in the presence of water has been reported [20]. However, the presence of polar plasticizers and shear stress can anyway promote gelatinization in other experimental conditions [21].
When starch is present in a blend at high concentration, the peculiar morphology of the blend can allow starch domains to be exposed on the material surfaces, thus favouring the release of starch in aqueous solutions. This property is exploited in several sectors. As an example, the application of starch/polyvinyl alcohol films encapsulating fertilizers is reported in agriculture to thoroughly control the release of fertilizers [22]. Moreover, the blending of PHB with starch was indicated by Garrido-Miranda et al. as a strategy for preparing materials for active packaging showing anti-oxidant and antifungal properties [23].
For beauty masks, a flexible and hydrophilic material is necessary as a matrix to fit the facial curvature, which could be achieved using PHA/polysaccharide composites. Wu et al. reported about composites of PHA and polysaccharidic fillers prepared with rice husks [24]. The product that they developed showed a tensile strength of about 15 MPa and an elongation at break of 600%, which are typical of a flexible material. The commercial product used was EM 5400F, obtained from Shenzhen Ecomann Biotechnology Co. Successively, Wu and Liao prepared arrowroot starch/polyester-based membranes using this polymer and demonstrated that this membrane promotes cell viability [25]. Therefore, EM 5400F can be a good choice for preparing a polymeric flexible film based on PHA.
Interesting achievements with the extrusion of starch in the presence of PHA were described by Parulekar and Mohanty [26]. However, the amount of extruded starch in the blend was up to 14.4%.
In view of selecting materials suitable for beauty masks, the present paper describes the design, preparation and characterization of biobased films considering PHA and starch as main materials. Both these polymers are biocompatible and can be used for such an application. Micrometric calcium carbonate was used to control the stickiness of films during processing. The capacity of releasing starch in water was investigated by gravimetric and infrared analysis. The compatibility with skin was investigated by two different in vitro tests based on human keratinocytes and human mesenchymal stromal cells. The work is aimed at boosting the production of biobased and biodegradable beauty masks, in order to decrease the impact onto environment of these highly demanded cosmetic products.
2. Materials and Methods
2.1. Materials
PHA Ecomann EM F5400 F (further referred as EM, for brevity) is a commercial product of Shenzhen Ecomann Biotechnology Co., Ltd., Shandong, China. OmyaCarb 2AV (indicated as 2AV), with average diameter of 2.6 micron, with 38% of particles with a diameter less than 2 μm; Omya Smartfill® 55—OM (indicated as Smart), with 55% of particles with a diameter less than 2 μm; Wheat native starch is a commercial product of Sacchetto SPA (Lagnasco, CN, Italy). Poly(ethylene glycol), Mn 400, (PEG400) and Glycerol were purchased from Sigma-Aldrich (Milan, Italy). Human keratinocytes (HaCaT cells) were purchased from CLS (Cell Lines Service GmbH, Eppelheim, Germany), human mesenchymal stromal cells (hMSCs) were obtained from Merck S.p.A. (Milan, Italy). Absolute ethanol was purchased by Bio Optica S.p.A. (Milan, Italy). Resazurin sodium salt (used to prepare AlamarBlue test reagent), phosphate buffered saline (PBS), fungizone, trypsin, gelatin (from bovine skin, type B), were provided by Sigma-Aldrich (Milan, Italy). Dulbecco’s modified Eagle’s Medium (D-MEM), l-glutamine, penicillin, streptomycin and heat-inactivated foetal bovine serum (FBS) were purchased from Life Technologies–Thermo Fisher Scientific (Waltham, MA, USA). Foetal calf serum (FCS) was obtained from Invitrogen (Carlsbad, CA, USA). Differentiation medium-osteogenic was obtained by Lonza (Milan, Italy). Tri Reagent® was purchased by Sigma-Aldrich/Merck (Darmstadt, Germany). LC Fast Start DNA Master SYBR Green kit was provided by Roche Applied Science (Euroclone S.p.A., Pero, Italy).
2.2. Preparation of Films
A methodology for preparing PHA/starch films was set up consisting in different steps: (1) Starch pre-gelatinization using glycerol and PEG 400 in oven for 16 h; (2) Mixing with PHA powder to have a homogenous powder; (3) Compression moulding (1 min at 190 °C).
Starch pre-plasticization was achieved by mixing wheat starch (60 g), previously conditioned at controlled humidity of 75%, with 10 g of PEG400 and 30 g of glycerol. The mix was homogenized in a mortar and kept overnight in a ventilated oven at 80 °C to allow starch gelatinization. The 100 g of pre-plasticized starch (P-PLS) were then mixed with 100 g of EM in powder in a mortar using a pestle to have a homogenous powder containing 50% of EM. The obtained powder (4 g), after eventual addition of calcium carbonate powder, was then transferred, in between Teflon square sheets, in the compression moulding equipment to prepare a film. Several films were produced for the successive tests. The films were produced at 190 °C, with no pressure applied for 30 s, followed by the application of 4 metric tons for 30 s. Each film was rapidly removed from the press and quenched with a cold air flow and finally detached from Teflon sheets.
The sample E-BM SMART was obtained by extruding the powder containing EM, P-PLST and SMART in a Minilab II HaakeTMRheomex CTW 5 conical twin-screw extruder (Haake, Vreden, Germany) at 140 °C and 60 rpm. The extrusion was performed for 1 min and the torque was recorded along the extrusion trial. The ribbon-like extruded strands recovered after the extrusion were not elastic and the final torque value was 131 N∙cm.
2.3. Characterization
2.3.1. Material Characterization
Small square specimens of about 20 mm side were cut out from the films in two replicates. The samples were immersed at RT in water and left 30 min. After soaking with water, the specimens were dried in oven at 60 °C until constant weight. The replicates were left in water for 16 h and then washed and dried in the same way.
Before and after release in water, the films were analysed by stereomicroscopy using a Wild Heerbrugg M3 microscope equipped with a Pulnix TMC-6 camera (Heerbrugg, Switzerland), to observe the surficial morphology. Furthermore, before and after tests of release in water, films were characterized by infrared spectroscopy using a Nicolet T380 Thermo Scientific instrument equipped with a Smart ITX ATR accessory with diamond plate (Thermo Fisher Scientific, Waltam, MA, USA). The spectra were normalized in intensity with respect to the band at 1720 cm−1 typical of EM polymer.
2.3.2. Cytocompatibility
BM 2AV and BM SMART films were investigated via in vitro tests using hMSCs. HMSCs were used as a (mesoderm) stem cell model to assess the influence of the produced materials towards non-terminally differentiated cells, which thus retain multipotent regenerative capacity, being invoked in tissue homeostasis, turnover and replacement. AlamarBlue test was used to monitor cell viability in terms of cell metabolic activity. As a possible differentiation pathway, the osteogenic potential was evaluated, after the hMSCs were cultured in contact with the films.
Each film was cut into small square pieces (about 10 mm side) and sterilized with absolute ethanol overnight, then washed with PBS containing 3× antibiotics and antimycotics (penicillin-streptomycin and fungizone, 3 washings for 10 min). Briefly, the cells were resuscitated from liquid nitrogen storage and cultured in low-glucose D-MEM, supplemented with 2 mM l-glutamine, 100 IU/mL penicillin, 100 mg/mL streptomycin and 10% heat-inactivated FBS, seeded at low density (2000–3000 cells/cm2) in 75 cm2 tissue culture (TC) flasks for further expansion and placed in an incubator set at 37 °C in air and 5% CO2. Once 70%–80% confluence was reached, the cells were detached using trypsin and suspended in a sterile-filtered 2% gelatin aqueous solution at 150.000 cells/20 µL. The hMSCs were seeded on the films placed in 6-well TC plates in triplicate (n = 3).
The day after seeding, the scaffolds were moved into 24-well TC plates and AlamarBlue test was performed. The culture was carried out for 1 week and AlamarBlue test was repeated at day 4 and at day 8. Metabolic activity (which is an indirect measurement of viability and cellular proliferation) of the cells grown on the scaffolds was monitored along the culture time using the AlamarBlue assay (the stock solution was obtained by appropriate dilution of Resazurin sodium salt into sterile PBS). This bioassay incorporates a REDOX indicator resulting in a colour change of the culture medium (CM) according to cell metabolism. Moreover, it can be performed multiple times on the same samples due to its negligible toxicity. Data were acquired according to manufacturing instructions and expressed as a percentage of reduced AlamarBlue (%ABred). Briefly, samples (n = 3) and blank controls (n = 3), were incubated for 3 h at 37 °C with the AlamarBlue dye diluted in 0.5 mL of CM for each sample. At each time-point, 100 μL of supernatant from sample or control was loaded in 96-well TC plates; then, excess supernatant was removed from the cultures and replaced with fresh CM. The absorbance (λ) of supernatants was measured with a spectrophotometer (Victor 3; PerkinElmer, Waltham, MA, USA) under a double wavelength reading (570 nm and 600 nm). Finally, %ABred was calculated correlating the absorbance values and the molar extinction coefficients of the dye at the selected wavelengths, following the protocol provided by the manufacturer. The equation applied (Equation (1)) is shown below, in which: λ = absorbance, s = sample and c = control:
On day 8, the culture of one representative sample for each beauty mask type was switched to osteo-differentiating medium (Osteodiff), whereas the culture of their counterparts was continued in basal (i.e., non-differentiating) medium as control. After 1 week the scaffolds were fixed in 4% formalin followed by PBS washing, the samples were dehydrated with a graded series of ethanol up to anhydrous ethanol. The specimens were then air dried in a vacuum oven at 37 °C for 24 h. The specimens were analysed via scanning electron microscopy (SEM; Phillips XL30 ESEM-FEG). The samples were sputter-coated with gold (Emitech K550, Quorum Technologies Ltd., West Sussex, UK) for 60 s prior to SEM analysis. SEM analysis was carried out to assess cell morphology and adhesion to the film surface.
2.3.3. Immunomodulatory Properties
The immunomodulatory properties of BM-SMART films were assayed using immortalized human keratinocyte HaCaTcell line (purchased from CLS–Cell Lines Service, Eppelheim, Germany), cultured in D-MEM supplemented with 1% Pen-strep, 1% l-glutamine and 10% FCS at 37 °C in air and 5% CO2. The HaCaT cells seeded inside 12-well TC plates until 80% of confluence, were incubated for 24 h with the films for 6 h and 24 h (n = 3). At these endpoints, total RNA was isolated with TRizol and 1 µm of RNA was reverse-transcribed into complementary DNA (cDNA) using random hexamer primers, at 42 °C for 45 min, according to the manufacturer’s instructions. Real time polymer chain reaction (PCR) was carried out with the LC Fast Start DNA Master SYBR Green kit using 2 µL of cDNA, corresponding to 10 ng of total RNA in a 20 µL final volume, 3 mM MgCl2 and 0.5 µM sense and antisense primers (Table 1). Real-Time PCR was used to evaluate the expression of interleukins (ILs) IL-1α, IL 1β, IL-6 and IL-8, tumour necrosis factor alfa (TNF-α), transforming growth factor beta (TGF-β) and human beta defensin 2 (hBD-2).

Table 1.
Real-time PCR conditions for HaCaT cells.
3. Results
The first preparation of films by compression moulding using the pre-plasticized starch and EM was carried out considering mixtures with a high percentage of starch but trials made considering more than 60% by weight of starch resulted in a discontinuous and brittle film. Hence, the content of starch was reduced to 50% by weight. This composition was suitable for obtaining continuous flexible films. The films could not be easily detached from the Teflon sheet after rapid cooling because of their stickiness, shown especially on films edges. Moreover, after some hours from preparation the surface of the film resulted in some cases slightly but evidently oily, probably because of the slight release of plasticizers. To reduce this inconvenient, the addition of micrometric calcium carbonate was attempted at 7% by weight (BM 2AV and BM SMART) (Table 2). Calcium carbonate was selected because it is a safe and cheap material largely available in different granulometries [27,28] and can be modified by surface treatments. Indeed, it is used in biopolyester formulations without detrimental loss of properties [29]. Two different products were tested: OmyaCarb 2AV (indicated as 2AV), with average diameter of 2.6 µm, with 38% of particles with diameter lower than 2 µm and Omya Smartfill® 55—OM (indicated as SMART), with 55% of particles with diameter lower than 2 µm. Both the additives resulted beneficial to reduce the stickiness, although the BM SMART films appeared slightly better than those based on BM 2AV.

Table 2.
Compositions of investigated materials and evaluation of stickiness during processing (detaching from Teflon sheets) and oiliness to the touch.
The extrusion of the composition typical of the BM SMART resulted in a continuous extruded strand (Figure 1e) that was cut and compression moulded to obtain E-BM SMART films (Figure 1d). These films were more homogenous than the ones obtained by compression moulded powder but still flexible.
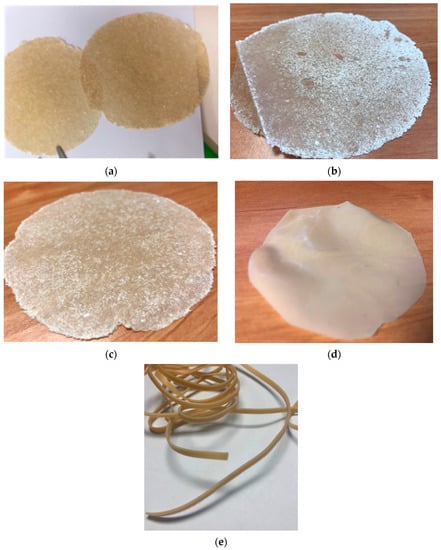
Figure 1.
Appearance of BM films (a,b), BM SMART film (c) and E-BM SMART film (d). The diameter of films is about 150 mm and the thickness 150 µm. (e) extruded strand of E-BM SMART recovered from a mini-extruder.
Considering the tests of mass release in water, it was observed that 24.6% of the starting BM films was lost in 30 min (Table 3). The mass lost after 16 h was quite similar. Hence, the plasticized starch is released rapidly, within 30 min. When the calcium carbonate was used, the mass loss at 30 min was slightly reduced but it is acceptable, because in both cases more than 70% of the mass was lost in 30 min. The film obtained after extrusion (E-BM SMART) has not a different behaviour with respect to the one obtained without extruding (BM SMART), indicating in both cases a similar availability of starch in correspondence of film surface. Anyhow, it must be noticed that the content of calcium carbonate cannot be further increased, because it can be detrimental for both mass loss kinetic and flexibility, due to the fact that usually a micrometric filler increases material stiffness thus decreasing flexibility.

Table 3.
Mass percentage of release from film in water at different times.
In Figure 2 the infrared ATR spectra of wheat native starch and EM PHA are reported. The spectrum of starch showed the typical peaks at 3300 cm−1, 1610 cm−1, 1350 cm−1and 1000 cm−1 [30]. The spectrum of EM showed the typical C=O stretching peak at 1720 cm−1, the C-H stretching at 2977 cm−1 and the main C-O stretching at 1274 cm−1, 1174 cm−1and 1051 cm−1 [31]. The high number of peaks in the region 1000–1300 cm−1 due to C-O is in agreement with a copolymeric disordered structure.
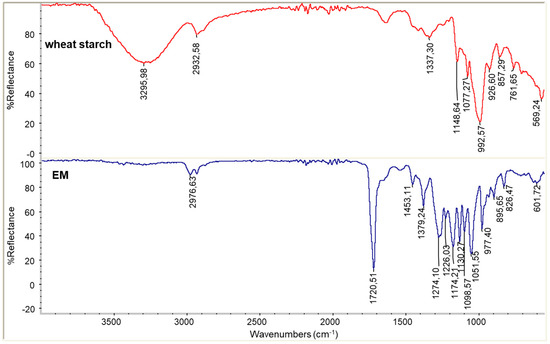
Figure 2.
Infrared ATR spectra of wheat starch powder and EM PHA.
In Figure 3 the infrared ATR spectra recorded on the films surface, the film recovered from water immersion trials at 30 min and 16 h is reported for BM and BM 2AV. In the two cases, it is evident the decrease in intensity of the peaks typical of starch at 3300 cm−1 and 1000 cm−1 as a function of immersion time, in agreement with the loss of starch from the surface of the film. In the BM 2AV spectra the bands typical of calcium carbonate at 1455 cm−1 and 850 cm−1 could be observed. Interestingly, their intensity increased as a function of releasing time, indicating that calcium carbonate is not removed by water from the film surfaces.
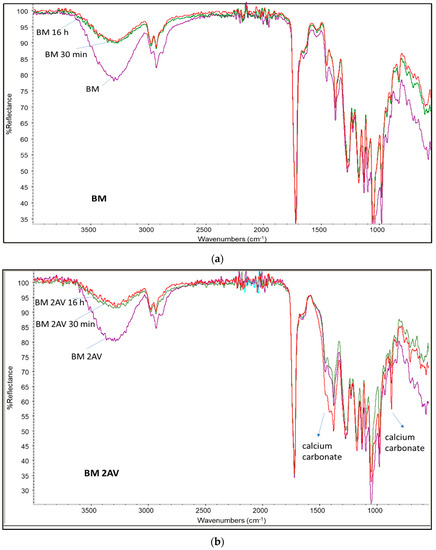
Figure 3.
Infrared spectra of films before and after immersion in water at different times (30 min and 16 h): (a) BM (purple) and BM after water extraction of 30 min (green) and 16 h (red); (b) spectrum of BM 2AV (purple) and the same after water extraction of 30 min (green) and 16 h (red); (a) spectrum of BM SMART before (blue) and after water extraction of 30 min (red) and 16 h (green).
In Figure 4 the infrared ATR spectra recorded on the films surface, the film recovered from water immersion trials at 30 min and 16 h is reported for BM SMART and E-BM SMART. Again, in the two cases, it is evident the decrease in intensity of the peaks typical of starch as a function of immersion time, in agreement with the loss of starch from the surface of the film. For these films a similar trend regarding the intensity of calcium carbonate bands are observed.
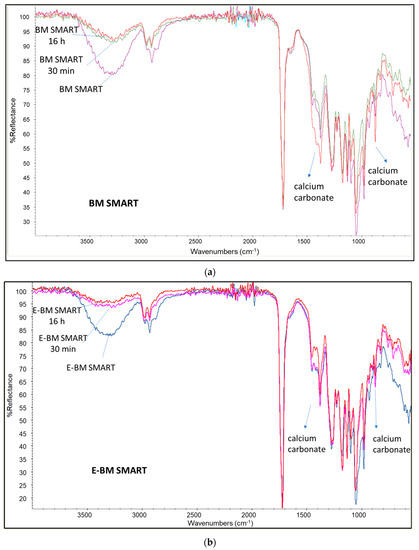
Figure 4.
Infrared spectra of films before and after immersion in water at different times (30 min and 16 h): (a) spectrum of BM SMART (purple) and the same after water extraction of 30 min (green) and 16 h (red); (b) spectrum of E-BM SMART (blue) before and after water extraction of 30 min (pink) and 16 h (red).
The films, which showed an inhomogeneous appearance (Figure 1), were analysed by stereomicroscopy before and after release in water (Figure 5). The lowest magnification (6.4×) was sufficient to highlight the significant differences between the starting film and the film after extraction with water. In particular, evident cavities were imaged on the film surfaces. The morphology is therefore in agreement with the loss of plasticized starch from their surface. The colour of the pictures is changed as a consequence of the applied light intensity, which was optimized each time to highlight the presence of surface patterning on a millimetric scale.
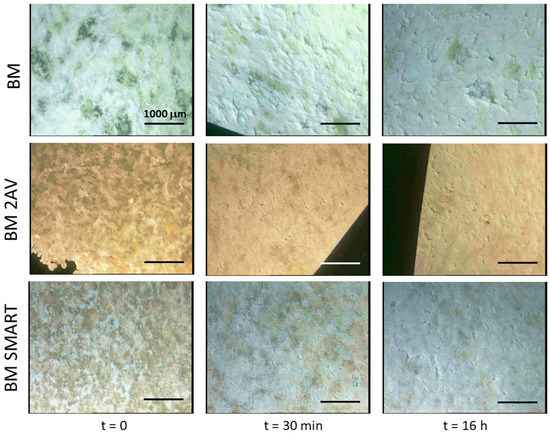
Figure 5.
Stereomicroscopy images of films before and after immersion in water for 30 min and 16 h. Magnification is 6.4×.
Regarding the analysis of the surface of E-BM SMART films by stereomicroscopy, the surface appeared smooth and homogeneous before the treatment in water. After the treatment for 30 min, some small holes and surface roughness changes can be observed, without a significative change after 16 h (Figure 6). It is important to evidence that in Figure 6 the magnification of the images reported is 40×, differently from Figure 5, in which it was only 6.4×. Therefore, as noticed by observing visually the improved homogeneity, the extrusion improved the homogeneity of the EM/starch blend by dispersing starch finely in the polymeric melt. In good agreement, the surface patterning observed because of starch release from the surface at 30 min and 16 h were in the micrometric scale. Moreover, the porosity of BM 2AV can be visible in the first two pictures of Figure 5.
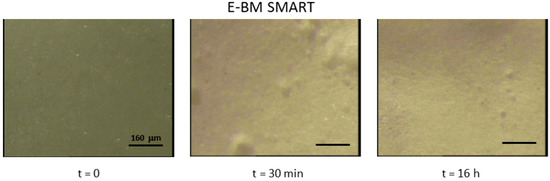
Figure 6.
Stereomicroscopy images of films before and after immersion in water for 30 min and 16 h. Magnification is 40×.
The cytocompatibility of BM 2AV and BM SMART films was investigated using hMSCs as a mesoderm stem cell model. The bar graph in Figure 7, showed high metabolic activity of hMSCs cultured on BM 2AV and BM SMART. Moreover, AlamarBlue assay revealed the best hMSC viability on BM 2AV. Osteogenic induction performed on the BM 2AV and BM SMART revealed a granular matrix compatible with calcium deposition in both the scaffolds if compared to the undifferentiated samples (maintained inside basal medium), as appreciable by SEM analysis (Figure 8).
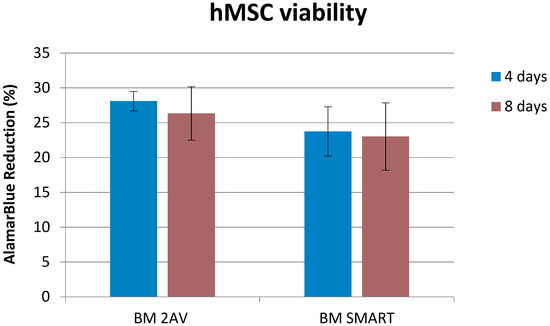
Figure 7.
HMSC metabolic activity after being in contact with films of BM 2AV and BM SMART for 4 (blue) and 8 (brown) days.
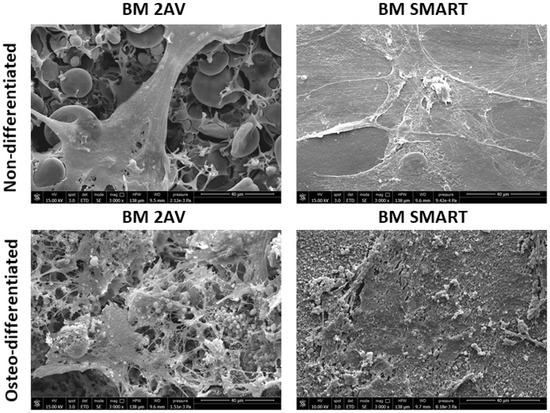
Figure 8.
SEM micrographs of BM 2AV and BM SMART films cultured with hMSCs under basal (undifferentiated) and osteodifferentiated conditions.
The results of gene expression analysis by keratinocytes (Figure 9) showed that BM SMART possessed a significant immunomodulatory activity; indeed, it was capable of upregulating the expression of both pro-and anti-inflammatory cytokines (with the exception of IL-6) but it did not upregulate the expression of antimicrobial peptide hBD-2.
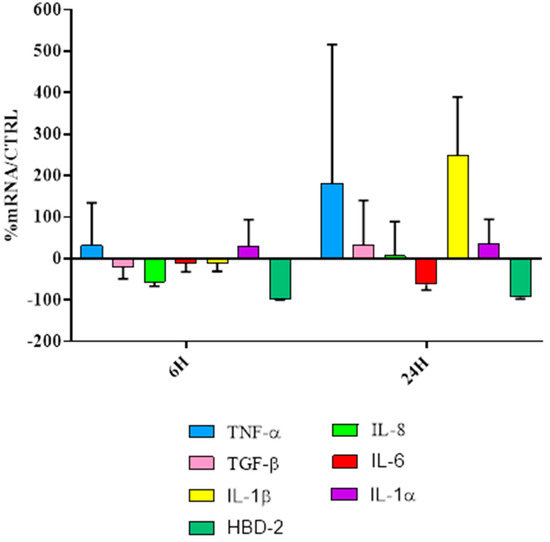
Figure 9.
Real-time PCR results of HaCaT cell line and BM SMART film, at 6 h and 24 h of exposure calculated as mRNA% of the sample (cells treated with BM SMART) with respect to control (CTRL; only cells).
4. Discussion
The treatment of wheat native starch with glycerol [32,33] and PEG400 at 80 °C allowed a pre-plasticization of starch before the compression moulding. Only during compression moulding the plasticization occurred but the pre-treatment at 80 °C was necessary to prepare the starch grains [26,30] to the subsequent brief step of 1 min at the higher temperature of 190 °C. Under compression moulding, both the EM powder and the P-PLST were molten and flowed under the applied pressure with plasticization conditions quite mild. As the process was brief and the applied shear stress very low, it was not possible to reach a high homogeneity of the film, in which the transparent brown part of EM and the white part of starch are macroscopically visible. In any case, the films were flexible and resistant, in accordance with a good material compatibility reached in this short process and did not disaggregate if immersed in water. To have a homogenous morphology of the film, a preliminary extrusion was necessary. In each case, the prepared films showed a good flexibility both in dry and wet conditions. These conditions are fundamental to adapt the treatment to the face profile.
The use of calcium carbonate resulted useful for reducing surface stickiness. Slight differences were observed for the two tested products, being also similar in the granulometry. Nevertheless, it must be considered that the size of calcium carbonate being less than 2 µm as dimension is 55% for SMART and 38% for 2AV products [29]. As a result, the surface area of SMART is higher than that of 2AV. Therefore, the former can better interact with polymers present in the sample and plasticizers than the latter calcium carbonate. For the same reason, the release of starch was slightly but significantly decreased when the calcium carbonate was present in the film. Hence, the content of calcium carbonate must be well balanced to avoid an insufficient release of starch and an increase in undesired rigidity.
It must be noticed that the plasticized starch was not completely released from the film, also in the long time. This can be ascribed to the fact that some starch domains are embedded in the EM matrix and not available on the surface. The in-water swelling was in fact reduced because the EM polymer is mainly hydro repellent. Its polarity is anyway improved with respect to that of petrol-based plastics, like poly(ethylene) or PP [10,11] and for this reason it is more suitable for contact with skin, as evidenced also in cytocompatibility tests. Moreover, some of the plasticizers can be present in the PHA phase, since the migration from the starch to EM powder may occur in oven treatment at 80 °C as well as in the subsequent processing steps (i.e., compression moulding or combination of extrusion and compression moulding). This fraction of plasticizers entrapped in the PHA phase cannot be released into water.
The alternative preparation by compression moulding or by extrusion and compression moulding was carried out to investigate about the possibility of modulating the appearance and surface texture of the support. Interestingly conditions for extruding starch/PHA blends with a high starch content were assessed. Moreover, it was demonstrated that the film texture can be well-modulated by selecting a different production methodology.
In vitro tests carried out with hMSCs and HaCaT cells showed a good cytocompatibility of the beauty mask films containing calcium carbonate. Both BM 2AV and BM SMART films supported the viability of hMSCs up to 2 weeks in culture, as confirmed by AlamarBlue assay (up to 1 week in culture) and SEM analysis (endpoint at 1 weeks in culture). The undifferentiated hMSCs were found very well spread onto both films, thus corroborating the high biocompatibility of the selected ingredients of the blend. The films thus showed chemical-physical properties (including sufficient hydrophilicity) and surface texture suitable for a beneficial interaction with hMSCs. In addition, the materials did not inhibit the osteogenic potential of hMSCs, namely, their multipotency.
In fact, wound healing is characterized by two main phases: in the first phase a number of overlapping events occur, including the production of pro-inflammatory cytokines. IL-1α and TNF-α represent the primary cytokines for pro-inflammatory responses. A direct effect of IL-1 release is the upregulation of IL-6 and IL-8 production, chemokine with angiogenic properties. The second phase is associated with growth-oriented cytokines and factors, among which TGF-β. This anti-inflammatory cytokine has multiple functions throughout wound healing, including a critical role in the deposition of extracellular matrix proteins and prevention of hyper proliferation of keratinocytes after wound closure. However, these materials for beauty masks are not able to stimulate human beta-defensin-2 (HBD-2) production, therefore we can assume that they are not potentially endowed with an indirect antimicrobial activity. This finding seems in agreement with the film composition, since the used blends do neither contain any anti-microbial molecules, nor any specific polymers with anti-microbial properties. However, due to their high cytocompatibility and immunomodulatory activity, these films can be applied as beauty mask substrates for releasing a variety of bioactive molecules to be incorporated or applied onto their surface.
The use of biobased formulations for beauty masks can thus promote both cosmetic performance and environment sustainability.
5. Conclusions
A commercial elastomeric PHA (EM) and starch were selected for preparing films to be used as beauty mask substrates. The materials were selected considering the necessary resistance in wet conditions, skin compatibility and capacity for a rapid release of polysaccharides and similar active and functional molecules. Starch can be considered as the phase where the active molecules, needed for imparting specific functions to the mask (e.g., anti-aging, anti-microbial, anti-pollution and so on) will be mixed and then delivered to the skin in wet conditions.
The preparation of flexible films by compression moulding or extrusion (followed by compression moulding) was attained by considering a mixture consisting of 50% EM and 50% P-PLST. Micrometric calcium carbonate was also added to successfully control the stickiness of film during moulding. The capacity of releasing starch in water was investigated by gravimetric and infrared analysis and it was found that a 37%–49% P-PLST release occurred in 30 min.
The compatibility with skin, in terms of interaction with mesoderm stem cells and epithelial cells, was investigated via a panel of in vitro tests using hMSCs and HaCaT cells, respectively. The produced films resulted highly cytocompatible, did not interfere with multi-potency potential of hMSCs and induced immunomodulatory activity in keratinocytes.
As the adopted methodology for films production was found to much influence the films patterning, it could be interesting in the future to better deepen the effect of the texture on cells compatibility.
The achieved results can be a starting point for boosting the production of bio-based and biodegradable beauty masks, aimed to decrease the impact onto environment of these cosmetic products that are currently mainly produced using not renewable nonwoven tissues.
Author Contributions
Conceptualization, M.-B.C. and S.D.; Data curation, M.-B.C., L.T., G.D. and A.F.; Formal analysis, M.-B.C., L.T. and A.F.; Funding acquisition, M.-B.C. and A.L.; Methodology, M.-B.C., L.T. and G.D.; Supervision, G.D., A.B. and A.L.; Writing—original draft, M.-B.C.; Writing—review & editing, S.D., P.M., G.D., A.B. and A.F.
Funding
This research was funded by EU by the H2020 POLYBIOSKIN project (High performance functional bio-based polymers for skin-contact products in biomedical, cosmetic and sanitary industry, G.A. no. 745839)
Acknowledgments
Doriana Morganti, of the PLANET BIOPLASTICS company (Pisa, Italy) is kindly acknowledged for supporting the obtaining of samples of poly(hydroxyalcanoates). Claudio Cantoni of OMYA (Avenza, MS, Italy) was thanked for kind discussion and for providing samples of calcium carbonate commercial products. Angelo Cobalto of Sacchetto S.p.A. (Lagnasco, CN, Italy) is kindly acknowledged for providing supporting information on starch products and providing samples of wheat native starch. Delfo D’Alessandro (University of Pisa, Pisa, Italy) is kindly acknowledged for his valuable technical support with the SEM and stem cell characterization. European Union, financing the project POLYBIOSKIN (High performance functional bio-based polymers for skin-contact products in biomedical, cosmetic and sanitary industry, G.A. n°. 745839) is acknowledged for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable biocomposites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Razza, F.; Degli Innocenti, F. Bioplastics from renewable resources: The benefits of Biodegradability. Asia-Pac. J. Chem. Eng. 2012, 7 (Suppl. 3), S301–S309. [Google Scholar] [CrossRef]
- Miller, S.A.; Billington, S.L.; Lepech, M.D. Influence of carbon feedstock on potentially net beneficial environmental impacts of bio-based composites. J. Clean. Prod. 2016, 132, 266–278. [Google Scholar] [CrossRef]
- Schlay, S.; Slotta, U. Efficient Skin Protection against Negative Environmental Influences by Breathable, Vegan Silk Polypeptides. Sofw. J. 2016, 4, 14–17. [Google Scholar]
- Liu, Z.L; Anderson, T.D.; Cruz Jose, M. Consumer environmental awareness and competition in two-stage supply chains. Eur. J. Oper. Res. 2012, 218, 602–613. [Google Scholar] [CrossRef]
- Directive 2008/98/EC on Waste (Waste Framework Directive). Available online: https://eur-lex.europa.eu/legal-content/IT/ALL/?uri=CELEX%3A32008L0098 (accessed on 29 March 2019).
- Coltelli, M.B.; Aglietto, M. Riutilizzo dei Materiali Polimerici; Edizioni Nuova Cultura: Rome, Italy, 2015. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, A. Biosynthesis of planet friendly bioplastics using renewable carbon source. J. Environ. Health Sci. Eng. 2015, 13. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P. Applications of PHAs in Medicine and Pharmacy. In Biopolymers Online; Wiley: Hoboken, NJ, USA, 2005; Chapter 20. [Google Scholar]
- Chen, Y.; Tsai, Y.-H.; Chou, I.-N.; Tseng, S.-H; Wu, H.-S. Application of Biodegradable Polyhydroxyalkanoates as Surgical Films for Ventral Hernia Repair in Mice. Int. J. Polym. Sci. 2014, 2014, 789681. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.X.L. The Greenhouse Gas Emissions and Fossil Energy Requirement of Bioplastics from Cradle to Gate of a Biomass Refinery. Environ. Sci. Technol. 2008, 42, 6961–6966. [Google Scholar] [CrossRef]
- Seggiani, M.; Cinelli, P.; Balestri, E.; Mallegni, N.; Stefanelli, E.; Rossi, A.; Lardicci, C.; Lazzeri, A. Novel Sustainable Composites Based on Poly(hydroxybutyrate-co-hydroxyvalerate and Seagrass Beach-CAST Fibers: Performance and Degradability in Marine Environments. Materials 2018, 11, 772. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly(3-hydroxybutyrateco-3-hydroxyhexanoate)(PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. eXPRESS Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Doi, Y.; Mukai, K.; Kasuya, K.; Yamada, K. Biodegradation of biosynthetic and chemosynthetic polyhydroxyalkanoates. Stud. Polym. Sci. 1994, 12, 39–51. [Google Scholar]
- Grassie, N.; Murray, E.J.; Holmes, P.A. The thermal degradation of poly(-(d)-hydroxybutyric acid): Part 1—Identification and quantitative analysis of products. Polym. Degrad. Stab. 1984, 6, 47–61. [Google Scholar] [CrossRef]
- Grassie, N.; Murray, E.J.; Holmes, P.A. The thermal degradation of poly(-(d)-%-hydroxybutyric acid): Part 2—Changes in molecular weight. Polym. Degrad. Stab. 1984, 6, 95–103. [Google Scholar] [CrossRef]
- Carvalho, A.J.F. Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 15; pp. 321–342. [Google Scholar]
- Ratnayake, W.S.; Jackson, D.S. Gelatinization and Solubility of Corn Starch during Heating in Excess Water: New Insights. J. Agric. Food Chem. 2006, 54, 3712–3716. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liu, H.; Chen, P.; Xue, T.; Chen, L.; Yu, L.; Corrigan, P. Starch Gelatinization under Shearless and Shear Conditions. Int. J. Food Eng. 2006, 2, 6. [Google Scholar] [CrossRef]
- Hana, X.; Chen, S.; Hu, X. Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating. Desalination 2009, 240, 2126. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez -Rivera, M.A.; Sanfuentes, E.A.; Peña-Farfal, C. Antioxidant and antifungal effects of eugenol incorporated in bionanocomposites of poly(3-hydroxybutyrate)-thermoplastic starch. LWT Food Sci. Technol. 2018, 98, 260–267. [Google Scholar] [CrossRef]
- Wu, C.-S. Preparation and Characterization of PolyhydroxyalkanoateBioplastic-Based Green Renewable Composites from Rice Husk. J. Polym. Environ. 2014, 22, 384–392. [Google Scholar] [CrossRef]
- Wu, C.-S; Liao, H.-T. Interface design and reinforced features of arrowroot (Marantaarundinacea) starch/polyester-based membranes: Preparation, antioxidant activity, and cytocompatibility. Mater. Sci. Eng. 2017, 70, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, Y.; Mohanty, A.K. Extruded Biodegradable Cast Films from Polyhydroxyalkanoate and Thermoplastic Starch Blends: Fabrication and Characterization. Macromol. Mater. Eng. 2007, 292, 1218–1228. [Google Scholar] [CrossRef]
- Shi, X.; Rosa, R.; Lazzeri, A. On the Coating of Precipitated Calcium Carbonate with Stearic Acid in Aqueous Medium. Langmuir 2010, 26, 8474–8482. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.B.; Paolucci, D.; Castelvetro, V.; Bianchi, S.; Mascha, E.; Panariello, L.; Pesce, C.; Weber, J.; Lazzeri, A. Preparation of Water Suspensions of Nanocalcite for Cultural Heritage Applications. Nanomaterials 2018, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rigid filler toughening in PLA-Calcium Carbonate composites: Effect of particle surface treatment and matrix plasticization. Eur. Polym. J. 2019, in press. [Google Scholar]
- Iizuka, K.; Aiashima, T. Starch gelation process observed by FT-IR/ATR spectroscopy with multivariate data analysis. J. Food Sci. 1999, 64, 653–658. [Google Scholar] [CrossRef]
- Kansiz, M.; Domínguez-Vidal, A.; McNaughton, D.; Lendl, B. Fourier-transform infrared (FTIR) spectroscopy for monitoring and determining the degree of crystallization of polyhydroxyalkanoates (PHAs). Anal. Bioanal. Chem. 2007, 388, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Altayan, M.M.; Al Darouich, T.; Karabet, F. On the Plasticization Process of Potato Starch: Preparation and Characterization. Food Biophys. 2017, 12, 397–403. [Google Scholar] [CrossRef]
- Mikusa, P.-Y.; Alix, S.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).