Simple Summary
Afforestation is widely recognized as an effective strategy for enhancing soil properties and ecosystem functions in coastal saline–alkali regions. However, the dynamic relationship between soil quality and ecosystem multifunctionality, as well as the underlying mechanisms driving this relationship, remains poorly understood. To fill this knowledge gap, we investigated four stand ages (6, 12, 22, and 36 years) of Robinia pseudoacacia in the Yellow River Delta, a representative coastal ecosystem in China. Our findings indicate that afforestation significantly enhanced soil quality compared to non-afforested sites. Afforestation also stimulated the activities of carbon-, nitrogen-, phosphorus-acquiring enzymes, and alleviated microbial N limitation with stand age. Additionally, EMF showed significant improvement across the four stand ages. Notably, we found a strong and positive correlation between SQI and EMF at all stand ages, with soil salinity and nutrients emerging as the most significant predictors of EMF. These results provide useful guidance and scientific support for improving forest management and ecological restoration efforts in this region.
Abstract
Afforestation is critical for enhancing soil quality and optimizing land use in coastal saline–alkali regions. However, the dynamics of soil quality and ecosystem multifunctionality (EMF) during afforestation, as well as their coupling relationships, remain poorly understood. In this study, the effects of four stand ages (6, 12, 22, and 36 years) of a leguminous tree species (Robinia pseudoacacia) on the soil quality index (SQI), enzymatic stoichiometry, and soil ecosystem multifunctionality (EMF) were investigated in coastal saline–alkaline lands of the Yellow River Delta, China. Results showed that compared to non-afforestation, afforestation increased the SQI by 81%, 74%, 146%, and 184% in the 6-, 12-, 22-, and 36-year-old stands, respectively. Concurrently, afforestation increased the activities of the carbon acquisition (Cacq), nitrogen acquisition (Nacq), and phosphorus acquisition (Pacq) enzymes and alleviated microbial N limitation. Afforestation increased the soil EMF by 182%, 243%, 263%, and 295% in the 6-, 12-, 22-, and 36-year-old stands, respectively. The SQI was significantly positively correlated with soil EMF, regardless of afforestation age. Soil salinity, SOC, TN, and TP were the best predictors of soil EMF. The afforestation of Robinia pseudoacacia improved the soil quality and stimulated enzyme activity, thereby increasing the soil EMF. Our findings provide a theoretical foundation for the sustainable management of stands in coastal saline–alkali lands.
1. Introduction
The Yellow River Delta is a fast-growing region, and its ecosystem is highly vulnerable. Soil salinization is particularly prominent in the region, with over 60% of the land affected by different degrees of salinization. The resulting chain of ecological consequences, such as soil quality degradation, biodiversity decline, and soil carbon sink reduction, seriously threatens ecosystem multifunctionality (EMF) [1,2]. Consequently, improving saline soil, enhancing soil quality, and achieving sustainable use are key to constructing an eco-economic zone in the Yellow River Delta. Phytoremediation can improve soil quality and significantly affect aboveground and belowground ecosystems and their ecological benefits [3]. Afforestation is a key pathway for rehabilitating saline–alkali lands in the Yellow River Delta. Afforestation effectively decreases salinity and pH levels in the soil while increasing soil fertility [4,5]. Additionally, afforestation affects microorganisms by altering microenvironmental conditions and soil properties. Microorganisms are highly sensitive to environmental changes, and their responses can influence multiple ecosystem functions [6,7]. However, the biological mechanisms by which afforestation affects soil quality and EMF in coastal saline–alkali lands in the Yellow River Delta remain unclear.
Soil quality is the comprehensive capacity of soil to maintain biological productivity, improve animal and plant health, and preserve environmental quality [8]. Soil quality is assessed using the soil quality index (SQI), which is calculated based on indicators of soil physical, chemical, and biochemical characteristics [9]. Soil EMF is generally used to evaluate complex and interacting biological, geochemical, and physical processes [10,11]. EMF is associated with soil nutrient cycling and the soil enzyme activities that catalyze these cycling reactions [12]. Specifically, soil enzymes promote organic matter turnover and activate unstable substances, thereby facilitating energy flow and nutrient cycling. Additionally, soil enzymes strongly influence cell metabolism, which can modulate microbial community composition by modulating resource availability for microbial growth and energy production [13,14]. Additionally, soil enzymatic stoichiometry is used to determine the relationship between microbial nutrient demands and the soil nutrient supply, as well as to evaluate microbial resource limitations, particularly for carbon (C), nitrogen (N), and phosphorus (P) [15]. Soil properties probably influence the apparent affinity and catalytic performance of element cycling-related enzymes, thereby influencing the soil EMF [16,17]. Long-term afforestation can enhance soil quality by increasing aboveground litter input, belowground root biomass, and root secretions, leading to a general increase in soil organic C, total N contents with stand age [18,19,20]. Afforestation is speculated to improve soil quality, thereby promoting microbial nutrient cycling. The changes in enzymatic stoichiometry induced by afforestation alter microbial nutrient demands, inhibiting or stimulating microbially mediated soil ecosystem functions [21,22]. Therefore, understanding the mechanism underlying the effect of afforestation on enzyme stoichiometry changes is crucial for determining effective stand age management measures to maintain soil quality and EMF in the coastal saline–alkali forest ecosystem of the Yellow River Delta.
Robinia pseudoacacia is a eurytopic pioneer tree species that alters soil properties through specific biological mechanisms. Its rapid growth promotes the development of a canopy and litter layer, which improves the soil microclimate. Furthermore, litter inputs and root activity accelerate the humification process and improve soil structure. As a legume, Robinia pseudoacacia forms symbiotic relationships with rhizobia, fixing atmospheric N and increasing soil N content. In addition, it accumulates osmolytes, such as proline and soluble sugars, to maintain cellular osmotic balance and mitigate stress-induced damage [23]. Owing to these traits, Robinia pseudoacacia has been widely used in the reclamation of various degraded lands worldwide, such as the Yellow River Delta [24,25]. However, stands generally decline as stand age increases, when certain limiting factors (e.g., persistent salinity and nutrient imbalance) may become dominant in this specific delta environment [26]. Whether stand decline affects soil quality and EMF, particularly its relationship with soil enzymatic stoichiometry, remains unclear. Therefore, Robinia pseudoacacia stands of different ages, along with un-afforested stands (considered as controls), were selected in the Yellow River Delta. In this study, we examined the relationship between soil quality and EMF by analyzing enzymatic stoichiometry and associated soil nutrients. We hypothesized that (1) afforestation on saline–alkali land increases litter and root inputs, accelerates organic matter humification, improves nutrient availability, and reduces soil salinity and pH, thereby enhancing the SQI with increasing stand age. (2) By improving soil physicochemical properties and nutrient conditions, afforestation stimulates soil enzyme activities and nutrient acquisition, leading to a significant increase in soil EMF. (3) Considering these mechanisms and the coastal saline–alkali context, soil salinity and pH are identified as the key limiting factors regulating and predicting soil EMF.
2. Materials and Methods
2.1. Study Site
This study was conducted in Gudao Town (37°35′ to 38°12′ N, 118°33′ to 119°20′ E), Dongying City, Shandong Province, China. It has a warm temperate continental monsoon type of climate, and the mean annual temperature, precipitation, and evaporation are 12.3 °C, 555.9 mm, and 1962.1 mm, respectively. The soil is classified as Solonchaks in the FAO system [27]. Owing to unique depositional settings and soil parent materials, most lands in this region have different degrees of salinization dominated by chloride salts (Cl−).
2.2. Experimental Design and Soil Sampling
Four Robinia pseudoacacia stands aged 6, 12, 22, and 36 years were selected in July 2024. These stand ages correspond to documented periods of major afforestation activities in Gudao Town. Unafforested wasteland was used as a control. Four plots (20 × 20 m) were established in Robinia pseudoacacia stands of different ages. Each plot was spaced at least 20 m apart to ensure spatial independence.
Soil was sampled at a depth of 0–20 cm with an auger (5 cm in diameter) from five random points per plot and blended to obtain the composite sample. The soil sample was subsequently separated into three portions and passed through a 2 mm sieve to remove all visible debris. One portion was frozen at 4 °C for measuring soil mineral N (NH4+-N and NO3−-N), dissolved organic carbon (DOC), and available phosphorus (AP) contents. The second portion was preserved at −20 °C for measuring the activities of hydrolytic soil enzymes associated with C-, N-, and P-acquisition. The third portion was air-dried to determine the soil salinity, pH, SOC, TN, and TP contents.
2.3. Edaphic Properties
The soil salinity was analyzed via gravimetry based on the mass of the residue following oven-drying at 105 °C. A glass electrode was used to measure the soil pH–water ratio at 1:2.5 (v/w). The SOC and TN contents were measured through combustion using an elemental analyzer (multi-EA 5000 Analytik, Jena AG, Jena, Germany). The soil TP content was analyzed using the molybdenum-blue approach following HClO4-H2SO4 digestion [28]. The soil DOC concentration was determined in fresh soil at a soil–ultrapure water ratio of 1:2 (w/v) before 5 min of centrifugation at 3000 rpm; the soil DOC concentration was later analyzed using a TOC analyzer (multiN/C3100, Jena AG, Jena, Germany). The soil mineral N concentration (NH4+-N, NO3−-N) was determined using 2 M KCl (soil–solution = 1:5 (w/v)) and examined using a continuous flow analyzer (Skalar SA-40, Skalar, Breda, The Netherlands). The soil AP concentration was determined through sodium bicarbonate extraction (soil–solution = 1:4 (w/v)) using the molybdenum-blue method [29].
2.4. Soil Enzyme Activities
The activities of four hydrolytic soil enzymes were examined by conducting high-throughput fluorometric assays using 96-well microtiter plates (Solarbio, Beijing, China) [30]. The soil slurry was obtained by homogenizing 2.75 g of soil with 91 mL of Tris-NaOH buffer (50 mM, pH = 8.0). The soil suspension (800 µL) was dispensed in the wells of a deep-well microplate. The enzymatic reaction was initiated by adding 200 µL of 200 mM fluorogenic substrate solution to each well. Following 4 h of incubation at 25 °C in the dark, the reaction mixtures were centrifuged for 3 min at 2960× g (4800 rpm). The clarified supernatant (250 µL) in each well was added to the respective wells of a black, flat-bottomed 96-well plate. Fluorescence was quantified using a TECAN Infinite M200 multifunctional microplate reader (Grödig, Salzburg, Austria) at excitation and emission wavelengths of 365 nm and 450 nm, respectively.
2.5. Data Analysis
The normality and homogeneity of variance of the data were analyzed by conducting the Shapiro–Wilk test and Levene’s test. The changes in each edaphic property (salinity, pH, SOC, TP, TN, DOC, NH4+-N, NO3−-N, and AP) induced by afforestation were indicated as Ln (Variableafforestation/VariableWithout afforestation). The C-acquisition enzyme activity (Cacq) was indicated as BG, the N-acquisition enzyme activity (Nacq) was indicated as , and the P-acquisition enzyme activity (Pacq) was indicated as ALP [31].
Second, microbial resource limitations were evaluated based on the vector characteristics of the enzymatic stoichiometry [32]:
Here, x indicates the relative ratio of Cacq to Pacq, and indicates the relative ratio of Cacq to Nacq.
For the SQI after afforestation, every edaphic indicator was transformed into a score between 0 and 1 [33]:
Here, SLi represents the linear score of parameters i within 0–1, and X, Xmax, and Xmin represent the analyzed values and the maximum and minimum means of parameter i, respectively. The soil indicators were classified into two groups: “more is better” (SOC, TP, TN, DOC, NH4+-N, NO3−-N, and AP) and “less is better” (salinity and pH).
Soil EMF was assessed based on the activities of carbon-cycling enzymes (β-glucosidase, BG), nitrogen-cycling enzymes (N-acetylglucosaminidase, NAG; leucine aminopeptidase, LAP), and a phosphorus-cycling enzyme (alkaline phosphatase, ALP). These enzymes participate in elemental cycling processes and were therefore used as indicators of ecosystem functions [22,34].
The EMF was quantified by normalizing soil enzyme activities with the Z-score method, which is widely employed in contemporary studies on ecosystem multifunctionality [35,36,37].
Here, x represents the analyzed enzyme activity, the mean represents the mean of enzyme i, and SD represents the standard deviation of enzyme i.
Third, the effects of afforestation on edaphic properties (salinity, pH, SOC, TP, TN, DOC, NH4+-N, NO3−-N, and AP), soil enzyme activities (Cacq, Nacq, and Pacq), soil enzymatic stoichiometric characteristics (vector length and vector angle), SQI, and soil EMF were tested via one-way ANOVA. In the case of significant effects, we performed post hoc tests with Fisher’s least significant difference (LSD) test, with a significance level of α = 0.05. Pearson correlation analysis was performed to determine the relationships of edaphic properties with soil enzyme activity and stoichiometric characteristics. The relationships between the SQI and the soil EMF were tested by conducting regression analysis. Random forest analysis was performed to analyze the importance and statistical significance of edaphic properties on the soil EMF. All statistical analyses were performed using R Version 4.0.5.
3. Results
3.1. Influence of Afforestation on Edaphic Properties
Afforestation significantly altered nearly all edaphic properties, but the magnitude of these effects differed among the variables. The soil salinity, pH, and AP were significantly lower with afforestation than without afforestation. The soil salinity decreased by 38%, 63%, 53%, and 44% in the 6-, 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 1a–d). The soil pH decreased by 7%, 2%, 4%, and 2% in the 6-, 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 1a–d). The soil AP decreased substantially by 166%, 268%, 213%, and 267% in the 6-, 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 1a–d). However, afforestation significantly increased the SOC and TN contents. In the 6-, 12-, 22-, and 36-year-old stands, the SOC content increased by 29%, 45%, 83%, and 88%, respectively, and the TN content increased by 31%, 128%, 138%, and 165%, respectively (p < 0.05, Figure 1a–d). Moreover, the soil NO3−-N content increased by 127%, 40%, and 59% in the 6-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 1a,c,d). The soil TP content increased by 13%, 16%, and 15% in the 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 1b–d). Additionally, the soil DOC content increased by 22% in the 36-year-old stand (p < 0.05, Figure 1d), whereas the soil NH4+-N content increased by 25% in the 22-year-old stand (p < 0.05, Figure 1c).
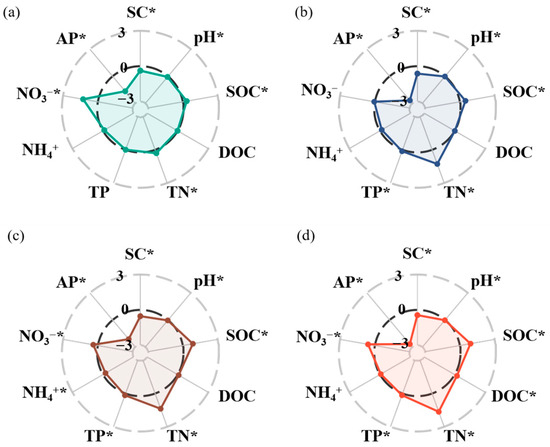
Figure 1.
Radar graphs reflecting the responses of soil physical and chemical properties of soils to different stand ages ((a) 6 years; (b) 12 years; (c) 22 years; (d) 36 years). * indicates significant differences between soils without and with Robinia pseudoacacia (p < 0.05). An effect size > 0 indicates a positive response to stand age of Robinia pseudoacacia, and an effect size < 0 indicates a negative response to stand age of Robinia pseudoacacia.
3.2. Influence of Afforestation on Soil Enzyme Activities and Stoichiometric Characteristics
Soil enzyme activities were significantly influenced by afforestation. Compared to that without afforestation, the soil Cacq enzyme activity increased by 35%, 58%, and 88% in the 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 2a). The soil Nacq enzyme activity increased by 21%, 32%, 39%, and 52% in the 6-, 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 2b). The soil Pacq enzyme activity increased by 29%, 60%, 62%, and 68% in the 6-, 12-, 22-, and 36-year-old stands, respectively (p < 0.05, Figure 2c). All results of the soil enzyme stoichiometry vectors were under the dashed line (1:1 line), suggesting the strong N limitation of the soil microbial community (Figure 3a,b). The vector angle increased with stand age, which intensified N limitation (Figure 3c). The vector length initially decreased but then increased, which induced an increase in C limitation (Figure 3d). The activities of the soil Cacq, Nacq, and Pacq enzymes were significantly negatively related to the soil salinity and AP content and significantly positively related to the SOC, DOC, TN, and TP contents (Figure 4). The vector angle was significantly positively related to the soil salinity, NH4+-N, NO3−-N, and AP contents but significantly negatively related to the SOC, DOC, TN, and TP contents (Figure 4). The vector length was significantly positively related to the SOC, DOC, and TN contents (Figure 4).
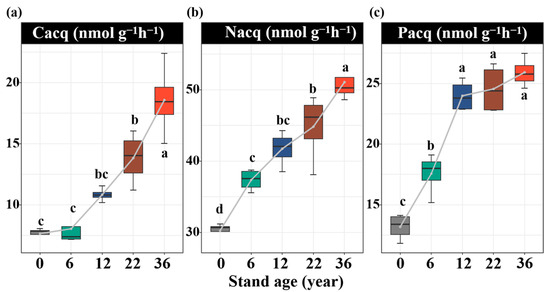
Figure 2.
Variations in carbon acquisition (Cacq, (a)), nitrogen acquisition (Nacq, (b)), and phosphorus acquisition (Pacq, (c)) enzyme activities with stand ages. Values are shown as means ± standard error (n = 4). Bars with different lowercase letters are significantly different (p < 0.05) based on Tukey’s HSD tests.
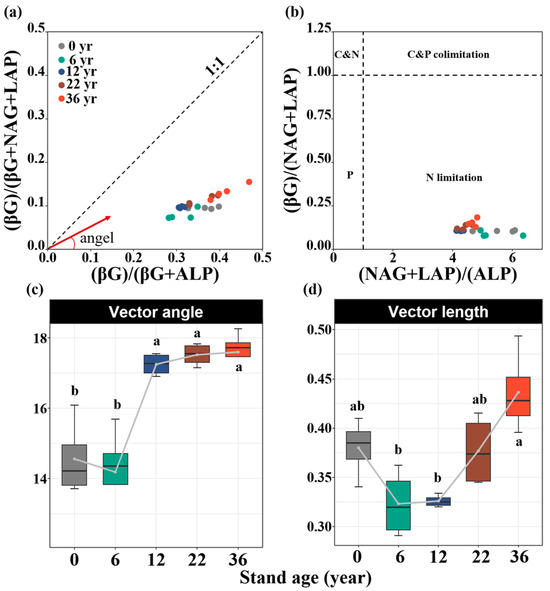
Figure 3.
Extracellular enzymatic stoichiometry of the relative proportions of Cacq to Nacq versus Cacq to Pacq in response to different stand ages (a). Stoichiometric analysis of enzyme activities to identify the C, N, and P limitations for microorganisms in soil (b). Using 1.0 as a horizontal and vertical baseline along the axes of enzyme activity ratios ((NAG + LAP)/ALP on the x-axis and (BG)/(NAG + LAP) on the y-axis), four groups of microbial resource limitations were identified: N limitation, P limitation, C and N co-limitation, and C and P co-limitation. Vector angle (c) and vector length (d) in response to different stand ages. Values are shown as means ± standard error (n = 4). Bars with different lowercase letters are significantly different (p < 0.05) based on Tukey’s HSD tests.
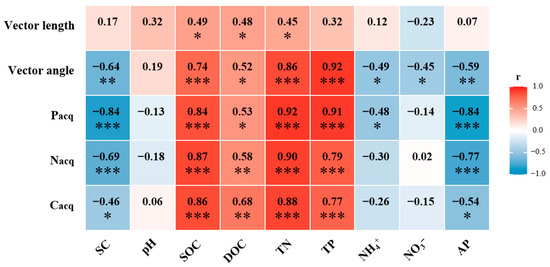
Figure 4.
Relationships between vector length, vector angle, Cacq, Nacq, and Pacq enzyme activities and edaphic properties. Significance levels are indicated as follows: * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Influence of Afforestation on SQI and EMF
Afforestation affected the SQI and EMF. Compared to that without afforestation, the SQI increased by 81%, 74%, 146%, and 184% in the 6-, 12-, 22-, and 36-year-old stands, respectively (Figure 5a). The soil EMF increased by 182%, 243%, 263%, and 295% in the 6-, 12-, 22-, and 36-year-old stands, respectively (Figure 5b). The soil EMF was strongly correlated with the SQI (Figure 6a). Based on random forest model analysis, the contributions of edaphic properties accounted for 85% of the soil EMF variability (Figure 6b). Soil salinity, TN, SOC, and TP were the key factors driving the soil EMF (Figure 6b).

Figure 5.
Variations in soil quality index (a) and ecosystem multifunctionality (b) with stand ages. Values are means ± standard error (n = 4). Bars with different lowercase letters are significantly different (p < 0.05) based on Tukey’s HSD tests.
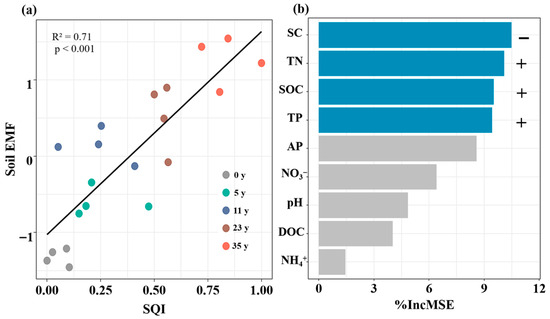
Figure 6.
Relationship between soil ecosystem multifunctionality and soil quality index via line regression analysis (a). Random Forest mean predictor importance (% increase in MSE) of edaphic properties on soil EMF (b). Blue bars indicate significant relationships, gray bars indicate insignificant relationships. + indicates positive relationships, − indicates negative relationships.
4. Discussion
4.1. Effect of Afforestation on Edaphic Properties with Afforestation Age
These findings support our first hypothesis that soil salinity and pH decrease significantly with increasing stand age (Figure 1). This phenomenon stems from a series of interconnected physical, biological, and biogeochemical processes initiated by afforestation. First, afforestation increases ground cover, reducing surface evaporation and consequently decreasing salt accumulation in the topsoil [38,39]. As stand age increased, the canopy density of Robinia pseudoacacia and the thickness of the surface litter layer increased, which increased the water-retention capacity of the soil, creating favorable conditions for salt leaching [4]. Second, afforestation markedly improved the soil physical structure, as indicated by an increase in the proportion of macroaggregates [40]. This structural change could reduce surface runoff and increase water infiltration. More importantly, the production of humic acids and other organic acids was increased during litter decomposition, altering the soil chemical environment [41]. The H+ produced during litter decomposition exchangeable basic cations (e.g., Ca2+, Mg2+, and Na+) from soil colloids, allowing them to enter the soil. Meanwhile, improvements in soil physical structure and infiltration capacity are an effective way for displaced cations and their associated anions (e.g., Cl− and SO42−) to be leached out of the soil [42]. Acidification makes basic ions mobile, while enhanced infiltration and leaching remove them; the two work synergistically to effectively transform the soil from a slightly alkaline to a slightly acidic state [43]. Additionally, the SOC and TN contents increased significantly with stand age, whereas the AP content decreased significantly (Figure 1). Litter inputs and root-derived carbon are the primary sources of SOC accumulation in forests. The aboveground productivity of Robinia pseudoacacia increased with stand age, leading to greater litter input, which directly contributed to the accumulation of SOC and TN [44]. Concurrently, root turnover and root exudates contributed substantial amounts of labile carbon compounds to the soil, further contributing to the accumulation of SOC and TN [45]. Furthermore, as a legume, Robinia pseudoacacia efficiently fixes atmospheric N through symbiotic rhizobia [46]. Both root biomass and N-fixing capacity increased with stand age, providing a significant supplementary source of soil N. In contrast, the AP content decreased with stand age, likely due to increased P uptake by both the Robinia pseudoacacia and soil microbial biomass, which likely gradually offset and exceeded P inputs derived from litter decomposition and root exudates [47,48]. In addition, the soil acidification may enhance the chemical fixation of P, further reducing the soil AP pool. Our findings indicate that Robinia pseudoacacia afforestation effectively enhances soil quality, with this positive effect becoming more pronounced as stand age.
4.2. Effect of Afforestation on Soil Enzyme Activity and Stoichiometry with Afforestation Age
Afforestation significantly increases the activities of the Cacq, Nacq, and Pacq enzymes, which are driven primarily by the evolution of edaphic properties with stand age. Soil enzyme activity is largely regulated by changes in soil nutrient availability [49]. An increase in litter input elevated the amount of soil microbial biomass carbon and stimulated microbial metabolic activity [50]. Soil microorganisms accelerate the mineralization and utilization of nutrients (N and P) by increasing the production of corresponding hydrolases and oxidases to assimilate and use these nutrients from litter for growth [51]. However, our findings revealed that despite an increase in litter-derived C inputs, the vector length increased, indicating that microbial C limitation intensified with stand age (Figure 3d), which may be explained in three ways. First, while increased litter input stimulates enzyme production, the synthesis of enzymes demands substantial amounts of carbon and energy [52]. Second, the accumulation of recalcitrant compounds in litter may require more specialized hydrolases and oxidases for decomposition, further increasing C resource demand [53]. Finally, the litter and root exudates of Robinia pseudoacacia plants contain relatively high N contents, and microorganisms consume additional C and energy to metabolize and utilize this N [44]. Consequently, the afforestation of Robinia pseudoacacia stimulated microbial metabolism, combined with the microbial demand for N and P, which contributed to an increase in enzyme activity. The vector length was positively associated with the NH4+-N, NO3−-N, and AP contents, suggesting that a relatively high soil N content was associated with greater microbial C limitation, reflecting an increase in C consumption to meet N metabolic demands [54]. Moreover, the vector angles were smaller than 45° at all stand ages, indicating microbial N limitation in coastal saline–alkali land (Figure 3a), which is consistent with findings in temperate forest ecosystems [55]. This persistent N limitation aligns with the consistently high activities of NAG and LAP enzymes observed in our study, demonstrating a microbial strategy to increase Nacq enzyme production in response to N scarcity. The vector angle was negatively related to soil salinity but positively related to C and nutrient availability, suggesting that the afforestation of Robinia pseudoacacia can alleviate microbial N limitation by reducing soil salinity and increasing the SOC, N, and P contents with stand age (Figure 4). Therefore, the afforestation of Robinia pseudoacacia effectively increases the activity of soil enzymes driving key biogeochemical cycles, potentially contributing significantly to the improvement in soil EMF, which supports our second hypothesis.
4.3. Main Factors Affecting EMF with Afforestation Age
In contrast to our third hypothesis, soil salinity, TN, SOC, and TP, rather than soil pH, are key predictors of soil EMF. In this study, the activities of the Cacq, Nacq, and Pacq enzymes increased significantly with stand age, likely driven by a decrease in soil salinity and an increase in nutrient availability. These changes collectively stimulate microbial activity and enzyme production, ultimately increasing the soil EMF [2]. Random forest analysis confirmed that an increase in SQI enhances enzyme activities, supporting key biogeochemical processes and thereby promoting soil EMF. Additionally, microenvironments with a greater soil quality probably activate microorganisms, increasing their diversity and richness, thereby promoting the formation of microbial hotspots related to nutrient cycling and soil organic matter decomposition, ultimately synergistically enhancing EMF [35]. This was supported by the positive relationship between the SQI and the soil EMF (Figure 6a). Thus, we speculated that during the phytoremediation of coastal saline–alkali land, the progressive improvement in soil quality with stand age not only effectively activates key ecosystem functions (such as nutrient cycling and organic matter decomposition) but also synergistically promotes multiple ecosystem functions.
4.4. Limitations and Outlook
In this study, we used a space-for-time substitution approach to analyze the relationship between soil quality and EMF across different stand ages, with a focus on soil microbial enzyme stoichiometry and associated soil nutrient dynamics. However, the relationship was limited, and the limited range of stand ages may have influenced our findings. Therefore, large-scale studies with more data are needed to validate and expand our findings. First, long-term studies that cover broader stand age gradients are needed to comprehensively understand the relationship of the SQI with the soil EMF and identify the inflection points in the SQI and the key ecological thresholds [56,57]. Second, soil enzyme activities are regulated not only by resource inputs and nutrient limitations but also closely by structural and functional shifts in soil microorganisms. We did not investigate the successional processes of soil microorganisms with stand age or their linkages to enzyme stoichiometry and EMF [58]. The application of high-throughput sequencing and functional gene quantification techniques can elucidate the mechanisms linking the abundance and diversity of specific functional microbial groups (e.g., P-solubilizing bacteria, N-fixing bacteria, and C-degrading bacteria) with enzyme activity, nutrient cycling, and EMF. Finally, microorganisms in deep soil are significantly distinct and may differ fundamentally from those in topsoil, which serves as the primary microbial habitat [59]. Future research should investigate the relationship between microbial diversity and soil EMF in deep soil to provide a scientific basis for comprehensively assessing the ecological functions of plantation soils and developing ecological restoration strategies based on stand age.
5. Conclusions
The afforestation of Robinia pseudoacacia initiated key pedogenic and biological processes that enhanced soil quality and ecosystem multifunctionality in coastal saline–alkali soils. As the stand ages, afforestation promoted humification and soil structure improvement by increasing litter input and enhancing root–soil interactions, which in turn facilitated salt leaching and led to reduced soil salinity and pH. Concurrently, afforestation stimulated microbial activity, which was reflected in increased enzyme activity and an alleviation of microbial N limitation. The decomposition and nutrient mineralization mediated by enzymes could strengthen soil EMF, with reduction in salinity and increase in soil nutrients. The management strategy of combining optimal stand age with N fertilization could be a viable approach for improving soil quality and ecosystem multifunctionality in coastal saline–alkaline lands.
Author Contributions
Conceptualization, methodology, funding acquisition, J.S. and H.X.; validation, formal analysis, H.L. and T.Z.; investigation, J.Y., X.W. and H.X.; writing—original draft preparation, writing—review and editing, J.S.; All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the Shandong Provincial Natural Science Foundation (No. ZR2023QC070); the Ph.D. Research Startup Foundation of Shandong University of Aeronautics (Nos. 2025Y16 and 2023Y15); and the College Student Innovation Training Program Plan (Nos. 202510449030 and 202410449004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Schäfer, R.B.; Bundschuh, M.; Rouch, D.A.; Szöcs, E.; von der Ohe, P.C.; Pettigrove, V.; Schulz, R.; Nugegoda, D.; Kefford, B.J. Effects of pesticide toxicity, salinity and other environmental variables on selected ecosystem functions in streams and the relevance for ecosystem services. Sci. Total Environ. 2012, 415, 69–78. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, J.; Li, Y.; Koziol, L.; Podzikowski, L.; Delgado-Baquerizo, M.; Wang, G.; Zhang, J. Relationships between soil bio-diversity and multifunctionality in croplands depend on salinity and organic matter. Geoderma 2023, 429, 116273. [Google Scholar] [CrossRef]
- Bharti, P.; Singh, B.; Bauddh, K.; Dey, R.K.; Korstad, J. Efficiency of bioenergy plant in phytoremediation of saline and sodic soil. In Phytoremediation Potential of Bioenergy Plants; Springer: Singapore, 2017; pp. 353–369. [Google Scholar] [CrossRef]
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, H.; Xiang, Y.; Wu, J. Soil pH neutralization by conversion of cropland to forest in China: A meta-analysis. CATENA 2025, 258, 109263. [Google Scholar] [CrossRef]
- Jing, C.; Xu, Z.; Zou, P.; Tang, Q.; Li, Y.; You, X.; Zhang, C. Coastal halophytes alter properties and microbial community structure of the saline soils in the Yellow River Delta, China. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. Microbiol. 2021, 23, 1020–1037. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamerb, R.E.; Deynb, G.D.; Goedeb, R.D.; Fleskensd, L.; Geissend, V.; Kuyperb, T.W.; Madera, P.; et al. Soil quality-A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Gunina, A.; Zamanian, K.; Tian, J.; Luo, Y.; Xu, X.; Yudina, A.; Aponte, H.; Alharbi, H.; Ovsepyan, L.; et al. New approaches for evaluation of soil health, sensitivity and resistance to degradation. Front. Agric. Sci. Eng. 2020, 7, 282–288. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Manning, P.; van der Plas, F.; Soliveres, S.; Allan, E.; Maestre, F.T.; Mace, G.; Whittingham, M.J.; Fischer, M. Redefining ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 427–436. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Lalanne, J.B.; Taggart, J.C.; Guo, M.S.; Herzel, L.; Schieler, A.; Li, G.W. Evolutionary convergence of pathway-specific enzyme expression stoichiometry. Cell 2018, 173, 749–761. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.; Dou, Y.; Cheng, H.; Liu, L.; An, S. Linkage between soil ectoenzyme stoichiometry ratios and microbial diversity following the conversion of cropland into grassland. Agric. Ecosyst. Environ. 2021, 314, 107418. [Google Scholar] [CrossRef]
- Zheng, H.; Vesterdal, L.; Schmidt, I.K.; Rousk, J. Ecoenzymatic stoichiometry can reflect microbial resource limitation, substrate quality, or both in forest soils. Soil Biol. Biochem. 2022, 167, 108613. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Yan, G.; Wang, M.; Jiang, S.; Wang, X.; Xue, J.; Xu, M.; Xing, Y.; Wang, Q. Soil enzyme activities and microbial nutrient limitation during the secondary succession of boreal forests. CATENA 2023, 230, 107268. [Google Scholar] [CrossRef]
- Zhou, T.; Lv, Q.; Zhang, L.; Fan, J.; Wang, T.; Meng, Y.; Xia, H.; Ren, X.; Hu, S. Converted paddy to upland in saline-sodic land could improve soil ecosystem multifunctionality by enhancing soil quality and alleviating microbial metabolism limitation. Sci. Total Environ. 2024, 924, 171707. [Google Scholar] [CrossRef]
- Haghverdi, K.; Kooch, Y. Long-term afforestation effect and help to optimize degraded forest lands and reducing climate changes. Ecol. Eng. 2020, 142, 105656. [Google Scholar] [CrossRef]
- Wang, J.; Delang, C.O.; Hou, G.; Gao, L.; Yang, X.; Lu, X. Carbon sequestration in biomass and soil following reforestation: A case study of the Yangtze River Basin. J. For. Res. 2022, 33, 1663–1690. [Google Scholar] [CrossRef]
- Tang, S.; Xu, X.; Wu, Y.; Meng, L.; Tawaraya, K.; Cheng, W. Long-term afforestation of black pine over two centuries asymptotically enhanced SOC and TN stocks in a typical coastal sand dune of Japan. CATENA 2025, 249, 108697. [Google Scholar] [CrossRef]
- Kong, W.; Wei, X.; Wu, Y.; Shao, M.; Zhang, Q.; Sadowsky, M.J.; Ishii, S.; Reich, P.B.; Wei, G.; Jiao, S.; et al. Afforestation can lower microbial diversity and functionality in deep soil layers in a semiarid region. Glob. Change Biol. 2022, 28, 6086–6101. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liao, G.; Banerjee, S.; Gu, S.; Liang, J.; Guo, X.; Zhao, H.; Liang, Y.; Li, T. Long-term organic fertilization promotes the resilience of soil multifunctionality driven by bacterial communities. Soil Biol. Biochem. 2023, 177, 108922. [Google Scholar] [CrossRef]
- Meng, F.; Wang, Q.; Yang, C.; Liu, J. Investigation of anti-salt stress on tetraploid Robinia pseudoacacia. Front. For. China 2009, 4, 227–235. [Google Scholar] [CrossRef]
- Guo, L.; Pang, Y.; Cao, B.; Fan, Z.; Mao, P.; Li, Z.; Liu, W.; Li, P. Robinia pseudoacacia decline and fine root dynamics in a plantation chronosequence in the Yellow River Delta, China. For. Sci. 2022, 68, 425–433. [Google Scholar] [CrossRef]
- Wang, S.; Lv, C.; Tang, B.; Wang, M.; Cao, B.; Wu, K. Dynamics of Soil N and P Nutrient Heterogeneity in Mixed Forest of Populus × Euramercana ‘Neva’ and Robinia pseucdoacacia in Coastal Saline-Alkali Land. Forests 2024, 15, 2226. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, C.; Liu, J.; Li, K.; Wang, B.; Chen, M. Increased salinity and groundwater levels lead to degradation of the Robinia pseudoacacia forest in the Yellow River Delta. J. For. Res. 2022, 33, 1233–1245. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 150–152. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Chinese Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-Throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. J. Vis. Exp. 2013, 81, e50961. [Google Scholar] [CrossRef]
- Luo, G.; Rensing, C.; Chen, H.; Liu, M.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct. Ecol. 2018, 32, 1103–1116. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, H.; Xie, Y.; Jia, X.; Su, T.; Li, J.; Shen, Y. Assessment of soil quality indexes for different land use types in typical steppe in the loess hilly area, China. Ecol. Indic. 2020, 118, 106743. [Google Scholar] [CrossRef]
- Han, S.; Delgado-Baquerizo, M.; Luo, X.; Liu, Y.; Van Nostrand, J.D.; Chen, W.; Zhou, J.; Huang, Q. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality. Soil Biol. Biochem. 2021, 154, 108143. [Google Scholar] [CrossRef]
- Jia, R.; Zhou, J.; Chu, J.; Shahbaz, M.; Yang, Y.; Jones, D.L.; Zang, H.; Razavi, B.S.; Zeng, Z. Insights into the associations between soil quality and ecosystem multifunctionality driven by fertilization management: A case study from the North China Plain. J. Clean. Prod. 2022, 362, 132265. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Gundale, M.J.; Farrell, M.; Ciobanu, M.; Baldock, J.A.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Consistent effects of bio-diversity loss on multifunctionality across contrasting ecosystems. Nat. Ecol. Evol. 2018, 2, 269–278. [Google Scholar] [CrossRef]
- He, B.; Cai, Y.; Ran, W.; Zhao, X.; Jiang, H. Spatiotemporal heterogeneity of soil salinity after the establishment of vegetation on a coastal saline field. CATENA 2015, 127, 129–134. [Google Scholar] [CrossRef]
- Chu, L.; Yuan, S.; Chen, D.; Kang, Y.; Shaghaleh, H.; Okla, M.K.; AbdElgawad, H.; Hamoud, Y.A. Changes in salinity and vegetation growth under different land use types during the reclamation in coastal saline soil. Chemosphere 2024, 366, 143427. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Wei, X.; Liu, X.; Shao, M. Long-term afforestation significantly improves the fertility of abandoned farmland along a soil clay gradient on the Chinese Loess Plateau. Land Degrad. Dev. 2018, 29, 3521–3534. [Google Scholar] [CrossRef]
- Zech, W. Litter decomposition and humification in forest soils. In Decomposition and Accumulation of Organic Matter in Terrestrial Ecosystems: Research Priorities and Approaches; Van Breemen, N., Ed.; Ecosystem Research Report No. 1.; CEC: Brussels, Belgium, 1991; pp. 46–51. [Google Scholar]
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Fox, D., Ed.; Pearson: Columbus, OH, USA, 2016. [Google Scholar]
- Bauhus, J.; Pare, D. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biol. Biochem. 1998, 30, 1077–1089. [Google Scholar] [CrossRef]
- Feng, D.; Yao, Y.; Zhou, J.; Kong, W.; Gao, J.; Zhang, Q.; Jia, X.; Shao, M.; Wei, X.; Qiu, L. Responses of soil nutrients and enzyme activities to afforestation species and age on China’s Loess Plateau: An investigation from soil aggregates aspect. Agric. Ecosyst. Environ. 2025, 393, 109804. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, N.; Liao, X.; Wang, Z.; Wei, X.; Jia, X. Long-term afforestation accelerated soil organic carbon accumulation but decreased its mineralization loss and temperature sensitivity in the bulk soils and aggregates. CATENA 2021, 204, 105405. [Google Scholar] [CrossRef]
- Sun, H.; Liu, R.; Yuan, H.; Zhou, M.; Liu, Z.; Hu, B.; Rennenberg, H. Interaction of nitrogen availability in the soil with leaf physiological traits and nodule formation of Robinia pseudoacacia-rhizobia symbiosis depends on provenance. Plant Soil 2023, 490, 239–259. [Google Scholar] [CrossRef]
- Zhu, H.; Gong, L.; Luo, Y.; Tang, J.; Ding, Z.; Li, X. Effects of Litter and Root Manipulations on Soil Bacterial and Fungal Community Structure and Function in a Schrenk’s Spruce (Picea schrenkiana) Forest. Front. Plant Sci. 2022, 13, 849483. [Google Scholar] [CrossRef]
- Luo, X.; Hou, E.; Zhang, L.; Kuang, Y.; Wen, D. Altered soil microbial properties and functions after afforestation increase soil carbon and nitrogen but not phosphorus accumulation. Biol. Fertil. Soils 2023, 59, 645–658. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Liu, L.; King, J.S.; Booker, F.L.; Giardina, C.P.; Allen, H.L.; Hu, S. Enhanced litter input rather than changes in litter chemistry drive soil carbon and nitrogen cycles under elevated CO2: A microcosm study. Glob. Change Biol. 2009, 15, 441–453. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Moorhead, D. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Zhang, S. Soil enzyme activity and stoichiometry indicates that litter quality regulates soil microbial nutrient demand in a Tibetan alpine meadow. Eur. J. Soil Biol. 2024, 123, 103686. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Change Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Xu, H.; Yu, M.; Cheng, X. Abundant fungal and rare bacterial taxa jointly reveal soil nutrient cycling and multifunctionality in uneven-aged mixed plantations. Ecol. Indic. 2021, 129, 107932. [Google Scholar] [CrossRef]
- Yue, Q.; Hao, M.; Geng, Y.; Wang, X.; von Gadow, K.; Zhang, C.; Zhao, X.; Gao, L. Evaluating alternative hypotheses behind biodiversity and multifunctionality relationships in the forests of Northeastern China. For. Ecosyst. 2022, 9, 100027. [Google Scholar] [CrossRef]
- Huang, H.; Tian, D.; Zhou, L.; Su, H.; Ma, S.; Feng, Y.; Tang, Z.; Zhu, J.; Ji, C.; Fang, J. Effects of afforestation on soil microbial diversity and enzyme activity: A meta-analysis. Geoderma 2022, 423, 115961. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).